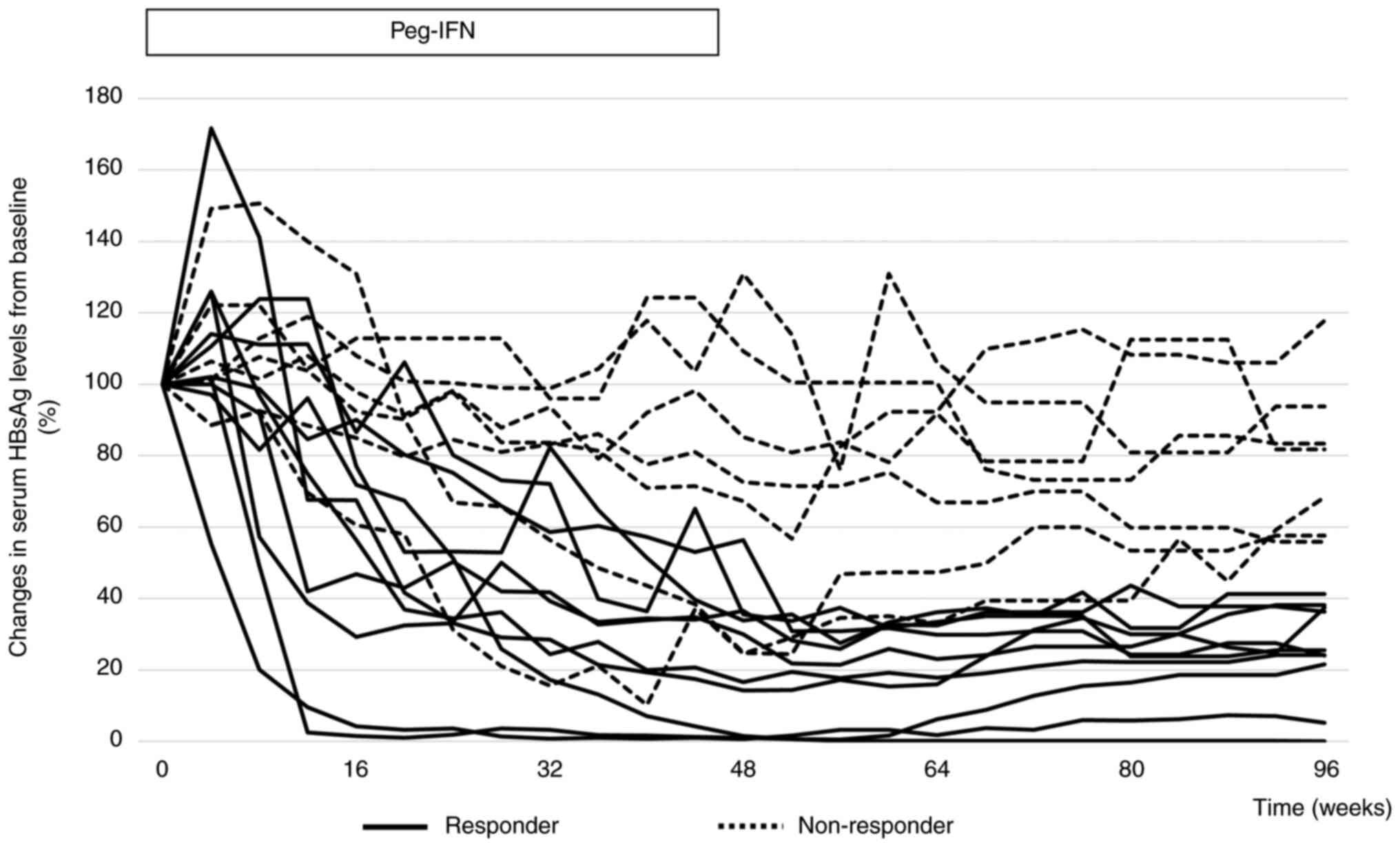
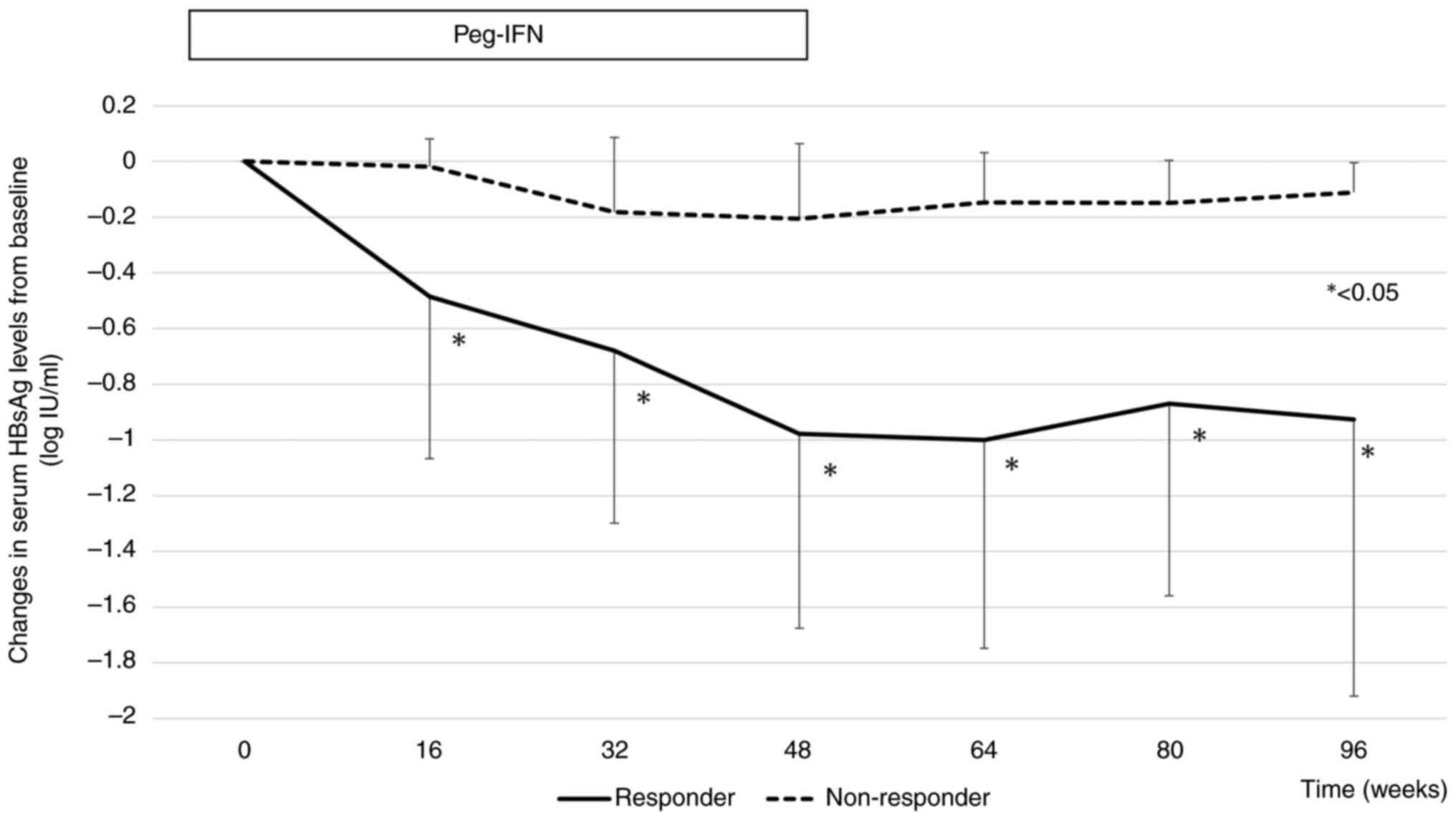
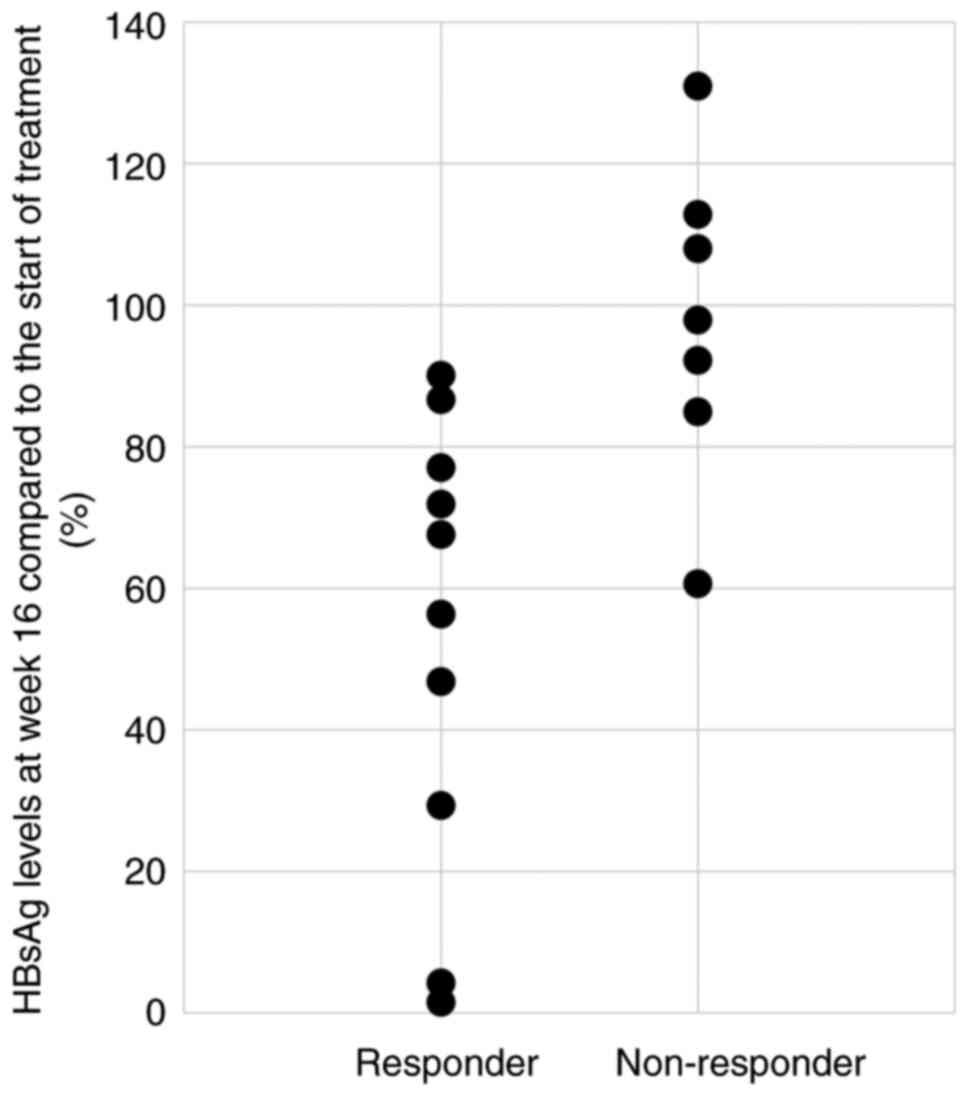
|
1
|
Wigfield P, Sbarigia U, Hashim M, Vincken
T and Heeg B: Are published health economic models for chronic
hepatitis B appropriately capturing the benefits of HBsAg Loss? A
systematic literature review. Pharmacoecon Open. 4:403–418.
2020.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Sarin SK, Choudhury A, Sharma MK, Maiwall
R, Al Mahtab M, Rahman S, Saigal S, Saraf N, Soin AS, Devarbhavi H,
et al: Acute-on-chronic liver failure: Consensus recommendations of
the Asian Pacific association for the study of the liver (APASL):
An update. Hepatol Int. 13:353–390. 2019.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Chevaliez S, Hézode C, Bahrami S, Grare M
and Pawlotsky JM: Long-term hepatitis B surface antigen (HBsAg)
kinetics during nucleoside/nucleotide analogue therapy: Finite
treatment duration unlikely. J Hepatol. 58:676–683. 2013.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Zoutendijk R, Hansen BE, Van Vuuren AJ,
Boucher CA and Janssen HL: Serum HBsAg decline during long-term
potent nucleos(t)ide analogue therapy for chronic hepatitis B and
prediction of HBsAg loss. J Infect Dis. 204:415–418.
2011.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Boni C, Penna A, Bertoletti A, Lamonaca V,
Rapti I, Missale G, Pilli M, Urbani S, Cavalli A, Cerioni S, et al:
Transient restoration of anti-viral T cell responses induced by
lamivudine therapy in chronic hepatitis B. J Hepatol. 39:595–605.
2003.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Tjwa ET, Van Oord GW, Hegmans JP, Janssen
HL and Woltman AM: Viral load reduction improves activation and
function of natural killer cells in patients with chronic hepatitis
B. J Hepatol. 54:209–218. 2011.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Swiecki M and Colonna M: Type I
interferons: Diversity of sources, production pathways and effects
on immune responses. Curr Opin Virol. 1:463–475. 2011.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Wursthorn K, Lutgehetmann M, Dandri M,
Volz T, Buggisch P, Zollner B, Longerich T, Schirmacher P, Metzler
F, Zankel M, et al: Peginterferon alpha-2b plus adefovir induce
strong cccDNA decline and HBsAg reduction in patients with chronic
hepatitis B. Hepatology. 44:675–684. 2006.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Brouwer WP, Xie Q, Sonneveld MJ, Zhang N,
Zhang Q, Tabak F, Streinu-Cercel A, Wang JY, Idilman R, Reesink HW,
et al: Adding pegylated interferon to entecavir for hepatitis B e
antigen-positive chronic hepatitis B: A multicenter randomized
trial (ARES study). Hepatology. 61:1512–1522. 2015.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Li GJ, Yu YQ, Chen SL, Fan P, Shao LY,
Chen JZ, Li CS, Yi B, Chen WC, Xie SY, et al: Sequential
combination therapy with pegylated interferon leads to loss of
hepatitis B surface antigen and Hepatitis B e Antigen (HBeAg)
seroconversion in HBeAg-positive chronic hepatitis B patients
receiving long-term entecavir treatment. Antimicrob Agents
Chemother. 59:4121–4128. 2015.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Brouwer WP, Chan HLY, Lampertico P, Hou J,
Tangkijvanich P, Reesink HW, Zhang W, Zhang W, Mangia A, Tanwandee
T, et al: Genome-wide association study identifies genetic variants
associated with early and sustained response to (Pegylated)
interferon in chronic hepatitis B patients: The GIANT-B study. Clin
Infect Dis. 69:1969–1979. 2019.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Hu P, Shang J, Zhang W, Gong G, Li Y, Chen
X, Jiang J, Xie Q, Dou X, Sun Y, et al: HBsAg Loss with
Peg-interferon Alfa-2a in Hepatitis B patients with partial
response to nucleos(t)ide Analog: New switch study. J Clin Transl
Hepatol. 6:25–34. 2018.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Hsu YC, Yeh ML, Wong GL, Chen CH, Peng CY,
Buti M, Enomoto M, Xie Q, Trinh H, Preda C, et al: Incidences and
determinants of functional cure during entecavir or tenofovir
disoproxil fumarate for chronic hepatitis B. J Infect Dis.
224:1890–1899. 2021.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Papatheodoridis G, Goulis J,
Manolakopoulos S, Margariti A, Exarchos X, Kokkonis G, Hadziyiannis
E, Papaioannou C, Manesis E, Pectasides D and Akriviadis E: Changes
of HBsAg and interferon-inducible protein 10 serum levels in naive
HBeAg-negative chronic hepatitis B patients under 4-year entecavir
therapy. J Hepatol. 60:62–68. 2014.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Tenney DJ, Rose RE, Baldick CJ,
Pokornowski KA, Eggers BJ, Fang J, Wichroski MJ, Xu D, Yang J,
Wilber RB and Colonno RJ: Long-term monitoring shows hepatitis b
virus resistance to entecavir in nucleoside-naïve patients is rare
through 5 years of therapy. Hepatology. 49:1503–1514.
2009.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Chang TT, Liaw YF, Wu SS, Schiff E, Han
KH, Lai CL, Safadi R, Lee SS, Halota W, Goodman Z, et al: Long-term
entecavir therapy results in the reversal of fibrosis/cirrhosis and
continued histological improvement in patients with chronic
hepatitis B. Hepatology. 52:886–893. 2010.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Yang SC, Lee CM, Hu TH, Wang JH, Lu SN,
Hung CH, Changchien CS and Chen CH: Virological response to
entecavir reduces the risk of liver disease progression in
nucleos(t)ide analogue-experienced HBV-infected patients with prior
resistant mutants. J Antimicrob Chemother. 68:2154–2163.
2013.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Liaw YF: Antiviral therapy of chronic
hepatitis B: Opportunities and challenges in Asia. J Hepatol.
51:403–410. 2009.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Wu FP, Yang Y, Li M, Liu YX, Li YP, Wang
WJ, Shi JJ, Zhang X, Jia XL and Dang SS: Add-on pegylated
interferon augments hepatitis B surface antigen clearance vs
continuous nucleos(t)ide analog monotherapy in Chinese patients
with chronic hepatitis B and hepatitis B surface antigen ≤ 1500
IU/mL: An observational study. World J Gastroenterol. 26:1525–1539.
2020.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Yoshida K, Enomoto M, Tamori A, Nishiguchi
S and Kawada N: Combination of entecavir or tenofovir with
pegylated interferon-α for long-term reduction in hepatitis B
surface antigen levels: Simultaneous, sequential, or add-on
combination therapy. Int J Mol Sci. 2021; 22: 1456, 2021.
|
|
21
|
Brunetto MR, Moriconi F, Bonino F, Lau GK,
Farci P, Yurdaydin C, Piratvisuth T, Luo K, Wang Y, Hadziyannis S,
et al: Hepatitis B virus surface antigen levels: A guide to
sustained response to peginterferon alfa-2a in HBeAg-negative
chronic hepatitis B. Hepatology. 49:1141–1150. 2009.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Moucari R, Mackiewicz V, Lada O, Ripault
MP, Castelnau C, Martinot-Peignoux M, Dauvergne A, Asselah T, Boyer
N, Bedossa P, et al: Early serum HBsAg drop: A strong predictor of
sustained virological response to pegylated interferon alfa-2a in
HBeAg-negative patients. Hepatology. 49:1151–1157. 2009.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Rijckborst V, Hansen BE, Cakaloglu Y,
Ferenci P, Tabak F, Akdogan M, Simon K, Akarca US, Flisiak R,
Verhey E, et al: Early on-treatment prediction of response to
peginterferon alfa-2a for HBeAg-negative chronic hepatitis B using
HBsAg and HBV DNA levels. Hepatology. 52:454–461. 2010.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Boglione L, Cusato J, Cariti G, Di Perri G
and D'Avolio A: Role of HBsAg decline in patients with chronic
hepatitis B HBeAg-negative and E genotype treated with
pegylated-interferon. Antiviral Res. 136:32–36. 2016.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Mimura S, Fujita K, Takuma K, Nakahara M,
Oura K, Tadokoro T, Kobara H, Tani J, Morishita A, Himoto T and
Masaki T: Effect of pegylated interferon alfa-2a in HBeAg-negative
chronic hepatitis B during and 48 weeks after off-treatment
follow-up: the limitation of pre-treatment HBsAg load for the
seroclearance of HBsAg. Intern Emerg Med. 16:1559–1565.
2021.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Brunetto MR and Bonino F: Interferon
therapy of chronic hepatitis B. Intervirology. 57:163–170.
2014.PubMed/NCBI View Article : Google Scholar
|