Introduction
Studies have shown that high fluoride environments
have adverse effects on soft and hard body tissues (1,2).
Periodontal tissue comprises hard tissues, such as alveolar bone
and cementum, as well as soft tissues, including the gingiva and
periodontal ligaments (3). The
biological mechanism of orthodontic tooth movement includes the
conversion of mechanical force signals into biological force
signals, which stimulate various growth factors in periodontal
tissue, thereby promoting alveolar bone remodeling and ultimately
resulting in changes in tooth movement (4,5).
Therefore, exploration of the changes in periodontal tissue lesions
in a high-fluoride environment may assist in the development of
targeted diagnosis and treatment plans for orthodontic patients who
have dental fluorosis. It has been reported that the periodontal
health of individuals in high-fluoride areas has declined, and
patients with dental fluorosis tend to require prolonged
orthodontic treatment (6). The
present research team has shown that the orthodontic tooth movement
rate of rats in a high-fluoride environment slows down and the
rhythm of the tooth movement cycle changes (7). The research team also observed
changes in the periodontal tissue of Sprague-Dawley (SD) rats with
chronic fluorosis from multiple perspectives, such as alveolar bone
histology, alveolar bone microstructure, bone biomechanics, bone
metabolism levels and oxidative stress (8,9). The
results of these studies suggest that, during orthodontic tooth
movement, fluoride exposure results in the disordered arrangement
of periodontal ligament cells, increased periodontal fiber
breakage, uneven distribution of vascular endothelial cells, and
decreased protein expression levels of vascular endothelial growth
factor (VEGF) and endothelial nitric oxide synthase (eNOS) in
periodontal tissue. In addition, the gingiva participates in the
organization structure of periodontal tissue and circulatory
nourishment (10). The gingiva is
a component of periodontal tissue with abundant blood vessels;
however, it remains to be determined how the vascular response in
gingival tissue manifests during orthodontic tooth movement in an
environment of chronic fluorosis. Therefore, the present study
aimed to investigate the effects of a high-fluoride environment on
gingival angiogenesis in rats undergoing orthodontic tooth movement
by detecting the protein and gene expression levels of VEGF,
phosphatidylinositol-3 kinase (PI3K), AKT (also known as protein
kinase B) and eNOS, which are involved in the gingival
microvascular generation pathway.
Materials and methods
Laboratory animals, reagents and
instruments
SPF-grade male rats (age, 3 weeks; body weight, 60±5
g) were obtained from Guizhou Medical University Experimental
Animal Center [license no. SCXK (Xiang) 2019-0014]. NaF of superior
purity grade was purchased from Sigma Technologies, Inc. The
drinking water was tap water from Guizhou, China, in which has a
fluoride ion level of 0.08 mg/kg lower compared with the Chinese
national standard. Orthodontic nickel titanium tension springs
(0.012 inch) were obtained from Shenzhen Suhang Technology
Development Co., Ltd. Ti-Ni brackets were obtained from Hangzhou
Jiali Trading Co., Ltd. The hematoxylin and eosin (H&E)
staining kit (cat. no. G1120) was purchased from Beijing Solarbio
Science & Technology Co., Ltd., the SYBR Green Master Mix kit
was from Vazyme Biotech Co., Ltd., the RIPA lysis buffer (cat. no.
HJ202804) was from Wuhan Servicebio Technology Co., Ltd., the Total
RNA Extraction kit (cat. no. R1200) was from Beijing Solarbio
Science & Technology Co., Ltd., theRevertAid First Strand cDNA
Synthesis kit (cat. no. K1622) was from Thermo Fisher Scientific,
Inc., the protease inhibitor cocktail (cat. no. 4693132001) was
from Merck KGaA and the microscope (XSP-C204) was from Chongqing
Liuhui Technology Co., Ltd. The following antibodies were also
used: Anti-VEGF (cat. no. AF5131; Affinity Biosciences), anti-PI3K
(cat. no. abs148658; Absin Bioscience, Inc.), anti-AKT (cat. no.
C67E7; Cell Signaling Technology, Inc.), anti-eNOS (cat. no.
AF0096; Affinity Biosciences), anti-GAPDH (cat. no. ab8245; Abcam),
TBST solution (cat. no. G0004; Wuhan Servicebio Technology Co.,
Ltd.) and horseradish peroxidase-conjugated secondary antibody
(cat. no. E-AB-1003; Elabscience Biotechnology, Inc.), ECL (cat.
no. 170-5060; Bio-Rad Laboratories, Inc.). In addition, a CFX
Connect Real-Time PCR detection system from Bio-Rad Laboratories,
Inc., a refrigerated centrifuge (Microfuge 20R) from Beckman
Coulter, Inc., an electrophoresis instrument (DYY-6C) from Beijing
Liuyi Biotechnology Co., Ltd., a microplate reader (SMR16.1) from
USCN Kit, Inc., a QuickChemi Imager (QuickChemi 5100) from Monad
Biotech Co., Ltd., and ImageJ 1.53 software (Bio-Rad Laboratories,
Inc.) were used.
Group design and experimental
methods
In a laboratory room with temperature 22-24˚C,
humidity 55-60%, alternating light and dark lighting environment
for 12 h. The 60 SD rats were divided into the orthodontic group (O
group) and the fluorosis orthodontic group (FO group), with 30 rats
per group. In the FO group, 150 mg/l NaF aqueous solution was
administered to the rats daily for 3 months. Subsequently, all rats
in both groups were equipped with a nickel-titanium tension spring
orthodontic force-applying device as follows: The rats were
intraperitoneally injected with 2% sodium pentobarbital (30 mg/kg)
and fixed on a fixator; acid etching of the labial surfaces of the
maxillary mesial incisors was performed for 1 min; adhesive was
applied and Ti-Ni brackets were bonded to the surfaces of the teeth
with green orthodontic adhesive by light fixing. A 0.25-mm ligature
wire was threaded through the gap adjacent to the first two molars,
and ligated and fixed to one end of a binaural tension spring. The
other end of the tension spring was ligated and fixed to the
anterior bracket of the device. After the application of the
device, the rats were fed a soft diet, and the device was inspected
daily to ensure that it remained in place for the duration of the
study. The bilateral maxillary first molars were moved mesially
with 70-g force, and each group was divided into 0-, 3-, 7-, 14-
and 21-day groups (each n=6) according to the duration of
orthodontic force application. A total of six rats from each group
were placed in the same metabolic cage, with 240 ml of tap water (O
group) or NaF aqueous solution (FO group) and 240 g of feed per
cage per day. All rats were sacrificed after reaching the time
point appropriate for their group. The rats were intraperitoneally
injected with 2% sodium pentobarbital (30 mg/kg) and sacrificed by
exsanguination. Blood and urine samples were collected for the
measurement of fluoride ion levels by the fluoride ion selective
electrode method. Gingival tissue samples were also taken to
prepare specimen sections for H&E staining, reverse
transcription-quantitative polymerase chain reaction (RT-qPCR) and
western blot analysis.
H&E staining
The gingival tissue samples were dehydrated,
embedded, cut into 5-µm tissue slices, routinely deparaffinized
with xylene and dehydrated with a gradient alcohol series. The
sections were then stained with hematoxylin for 4 min at 35˚C,
after which they were rinsed with tap water, soaked in 1%
hydrochloric acid alcohol for 3 sec, rinsed with tap water, soaked
in 1% aqueous ammonia for 3 sec and rinsed with tap water again.
After soaking in 85-90-95% alcohol for 5 min per percentage, they
were then stained with eosin for 4 min at 35˚C. Finally, the
sections were dehydrated with a routine gradient alcohol series,
permeabilized with xylene, sealed with neutral gum and observed
under an optical light microscope.
RT-qPCR
Total ribonucleic acid (RNA) was extracted from the
gingival tissue samples according to the instructions of the Total
RNA Extraction kit, after which the extracted RNA was reverse
transcribed into complementary deoxyribonucleic acid (cDNA)
according to the instructions of the RevertAid First Stand cDNA
kit. The cDNA was subjected to qPCR using the 20-µl amplification
reaction system from the SYBR Green Master Mix kit and the
following reaction conditions: Denaturation at 95˚C for 3 min,
followed by 40 cycles at 95˚C for 15 sec and 60˚C for 1 min. The
relative expression levels of mRNA were analyzed using the
2-∆∆Cq method (11).
The primer sequences used are shown in Table I.
 | Table IPrimer sequences. |
Table I
Primer sequences.
| Name | Primer sequences
(5'-3') |
|---|
| VEGF | F:
ACTGGACCCTGGCTTTACTG |
| | R:
GCTTTCTGCTCCCCTTCTGT |
| PI3K | F:
CAACGGAGTGGAGTGAGC |
| | R:
GGTCCCATCAGCAGTGTC |
| AKT | F:
GCTGGAGAACCTCATGCTG |
| | R:
GTGTCCCGCAGAACGTC |
| eNOS | F:
TACTCCAGGCTCCCGATG |
| | R:
AAGGGCAGCAAACCACTC |
| GAPDH | F:
ACGGCAAGTTCAACGGCACAG |
| | R:
GAAGACGCCAGTAGACTCCACGAC |
Western blotting
Tissues were added to a solution comprising RIPA
lysis buffer and protease inhibitor cocktail, and lysed by
incubation in an ice bath for 60 min. After centrifugation at
12,000 x g for 5 min at 4˚C, the supernatant was collected and the
protein content was quantified using the bicinchoninic acid method
by microplate reader. The proteins were separated by sodium dodecyl
sulfate polyacrylamide gel electrophoresis (10 µg per lane; 10%
separation gel and 5% concentrated gel) and transferred to
polyvinylidene fluoride membranes, which had been blocked with 5%
skimmed milk at 20˚C for 1 h. Then, the membranes were incubated
with anti-VEGF, PI3K, AKT, eNOS and GAPDH primary antibodies
(1:1,000) at 4˚C for 20 h. then washed the membrane with TBST
solution for 5 times, 5 min each time. followed by the horseradish
peroxidase-labeled secondary antibody (1:3,000) at 37˚C for 1 h.
Finally, the membranes were placed in ECL and developed in the dark
and visualized using QuickChemi Imager. Quantification of western
blot results was performed using ImageJ software.
Statistical analysis
The experimental data were statistically analyzed
using SPSS 26.0 statistical software (IBM Corp.). Normally
distributed data are presented as the mean ± standard deviation and
non-normally distributed data are presented as the median
(interquartile interval). The unpaired t-test was used to compare
normally distributed data between two groups, whereas a
Mann-Whitney U test was used to analyze data that were not normally
distributed. Two-way ANOVA and the Bonferroni post hoc test were
used to compare multiple groups. P<0.05 was considered to
indicate a statistically significant difference.
Results
Fluorosis model identification
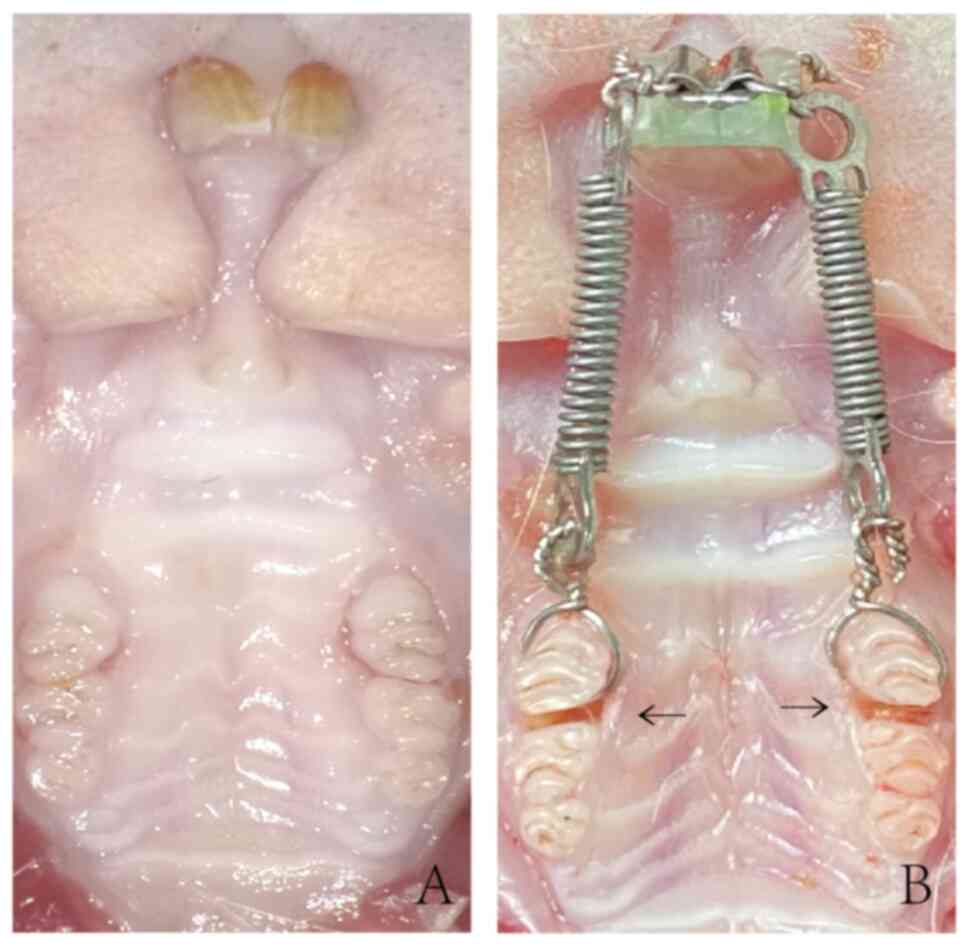
After 3 months of fluoride feeding, the rats in the
FO group exhibited dental fluorosis, which presented as
yellowish-white teeth with chalky streaks (Fig. 1). In addition, the concentrations
of fluoride ions in the blood and urine were significantly
increased in the FO group compared with those in the O group
(Table II).
 | Table IIFluoride ion concentration in the
serum and urine of rats (mean ± standard deviation; n=20). |
Table II
Fluoride ion concentration in the
serum and urine of rats (mean ± standard deviation; n=20).
| | Concentration,
mg/l | |
|---|
| Analyte | O group | FO group | t-value | P-value |
|---|
| Urine fluoride
ion | 1.755±0.370 |
4.698±0.443a | -12.483 | <0.001 |
| Blood fluoride
ion | 0.045±0.002 |
0.087±0.008a | -13.017 | <0.001 |
Orthodontic tooth movement model
identification
Following orthodontic tooth movement, a gap could be
observed between the maxillary bilateral first and second molars in
the rats (Fig. 2), indicating that
the first molar was moved in the direction of the load and the
tooth movement model was successfully established.
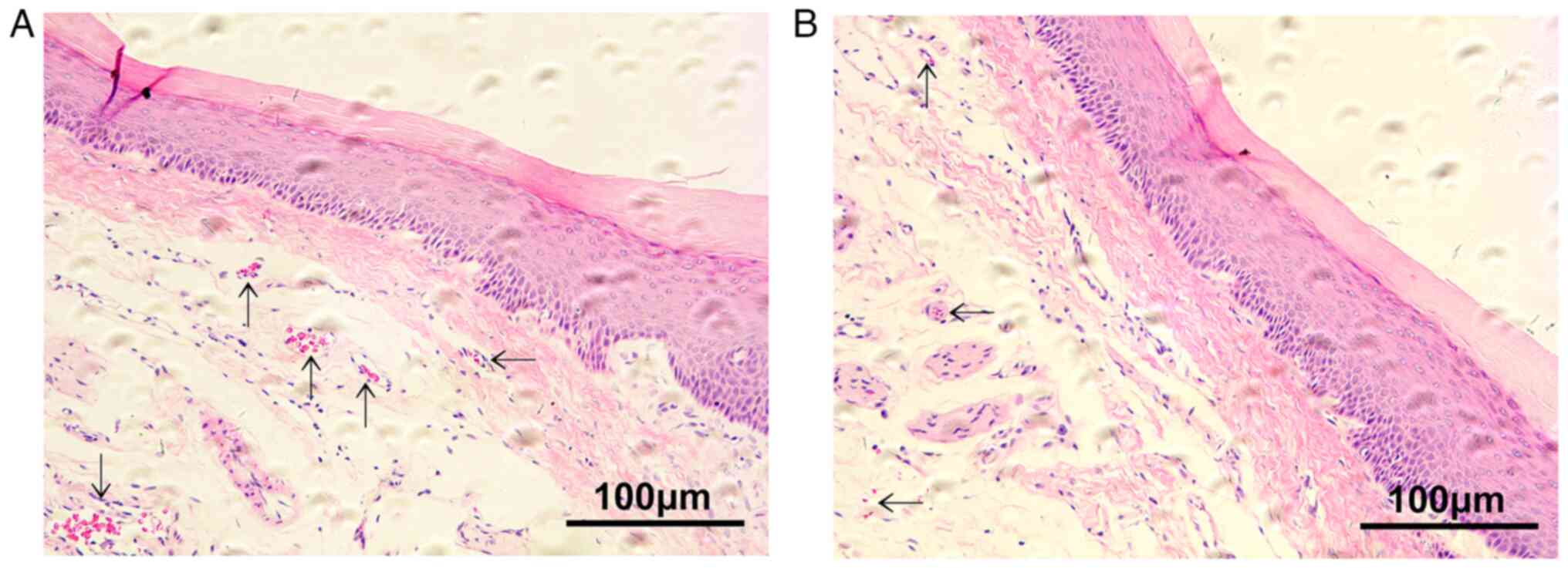
H&E staining of gingival
tissue
The blood vessel distribution in the O group was
relatively uniform, with large lumens. By contrast, the blood
vessel distribution in the FO group was sparse with smaller lumens
and less evident endothelial cells compared with those in the O
group (Fig. 3).
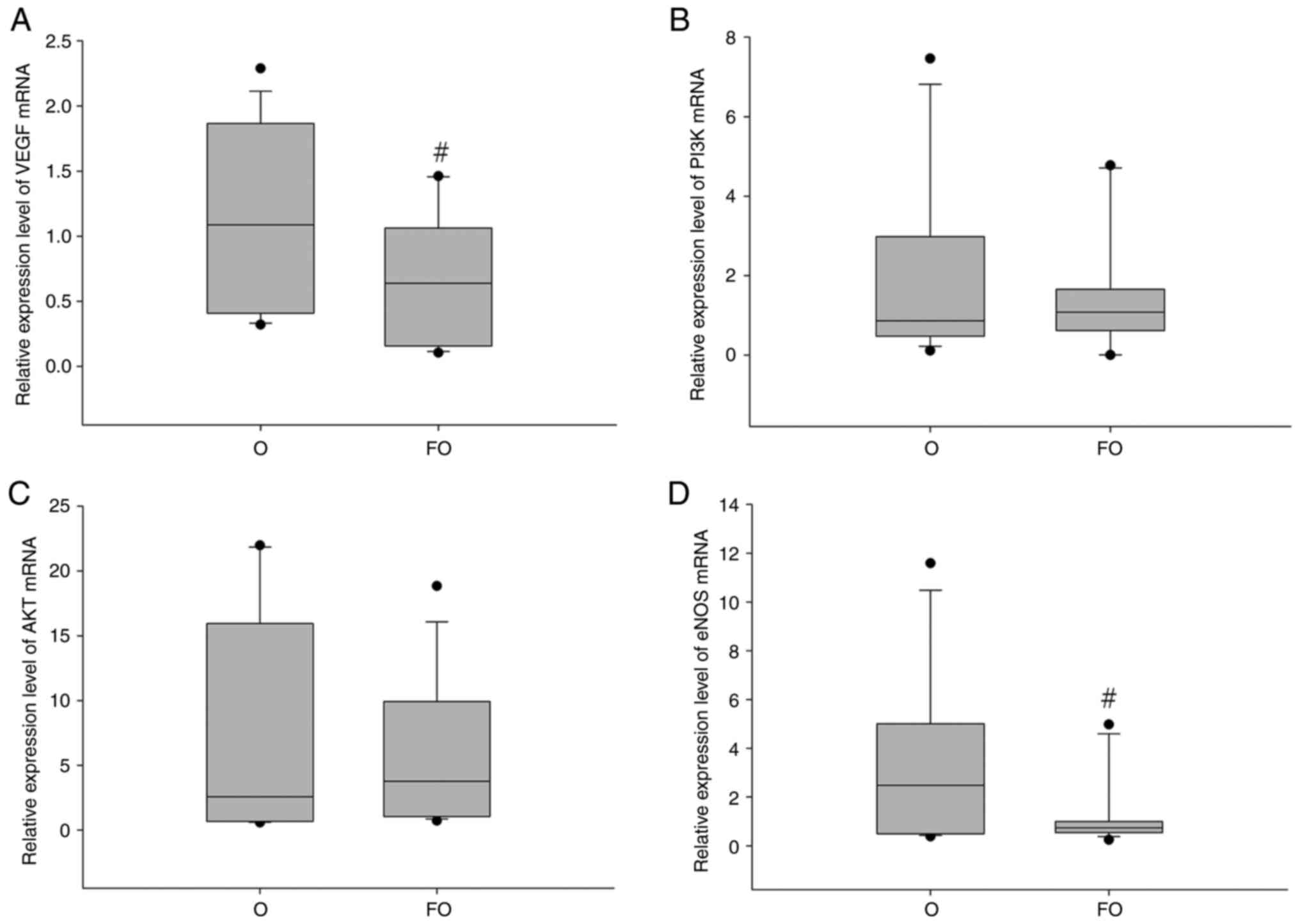
Average mRNA expression levels of
VEGF, PI3K, AKT and eNOS over all the time points
The average mRNA expression levels of VEGF and eNOS
were significantly reduced in the FO group compared with those in
the O group. These findings suggested that chronic fluorosis
inhibits gingival VEGF and eNOS mRNA expression during orthodontic
tooth movement (Fig. 4).
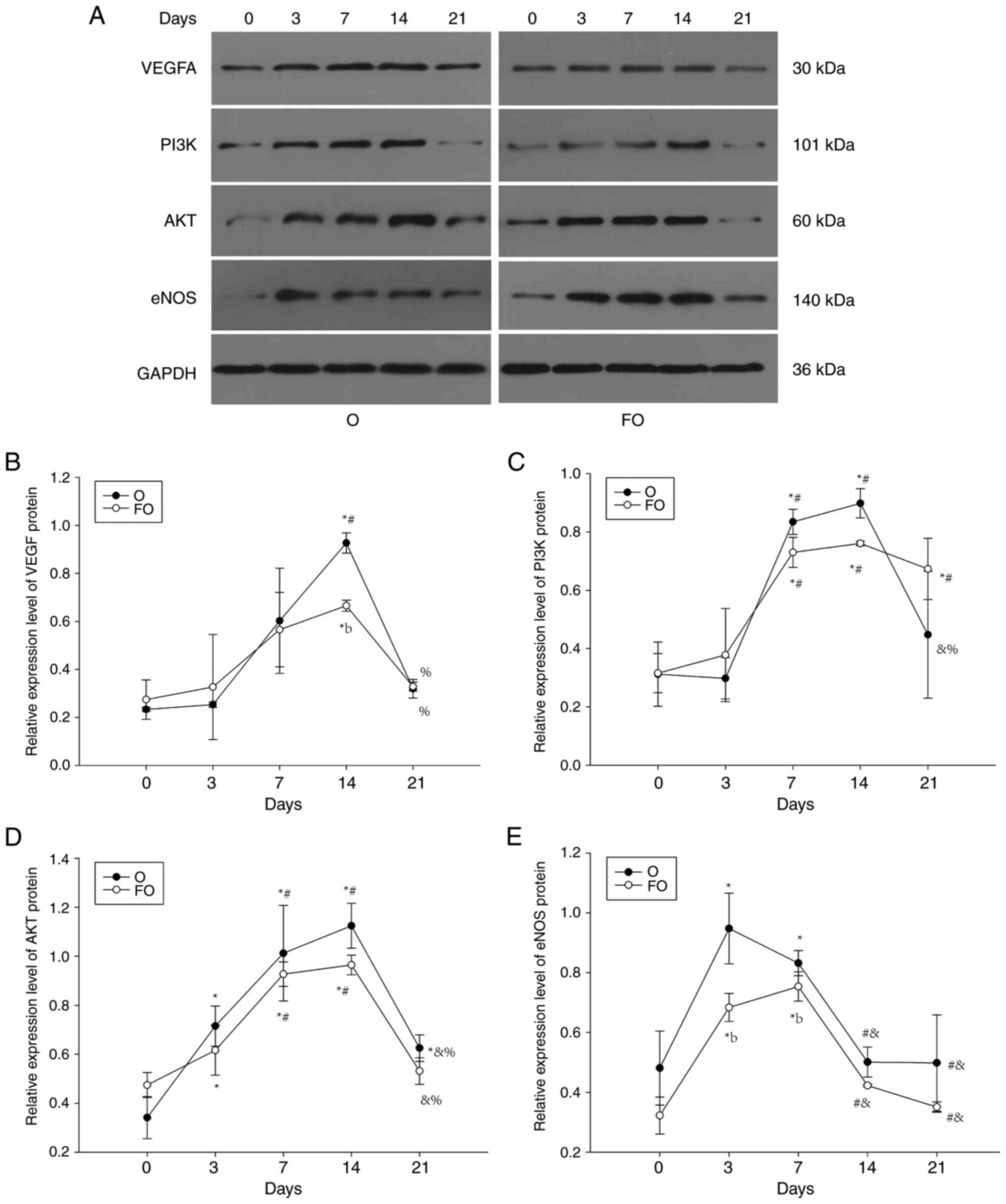
Average protein expression levels of
VEGF, PI3K, AKT and eNOS over all the time points
The overall protein expression levels of AKT and
eNOS were decreased in the FO group compared with the O group, but
a statistically significant difference was only observed for eNOS.
These findings indicated that chronic fluorosis inhibits the
protein expression levels of eNOS in the gingiva during orthodontic
tooth movement (Fig. 5).
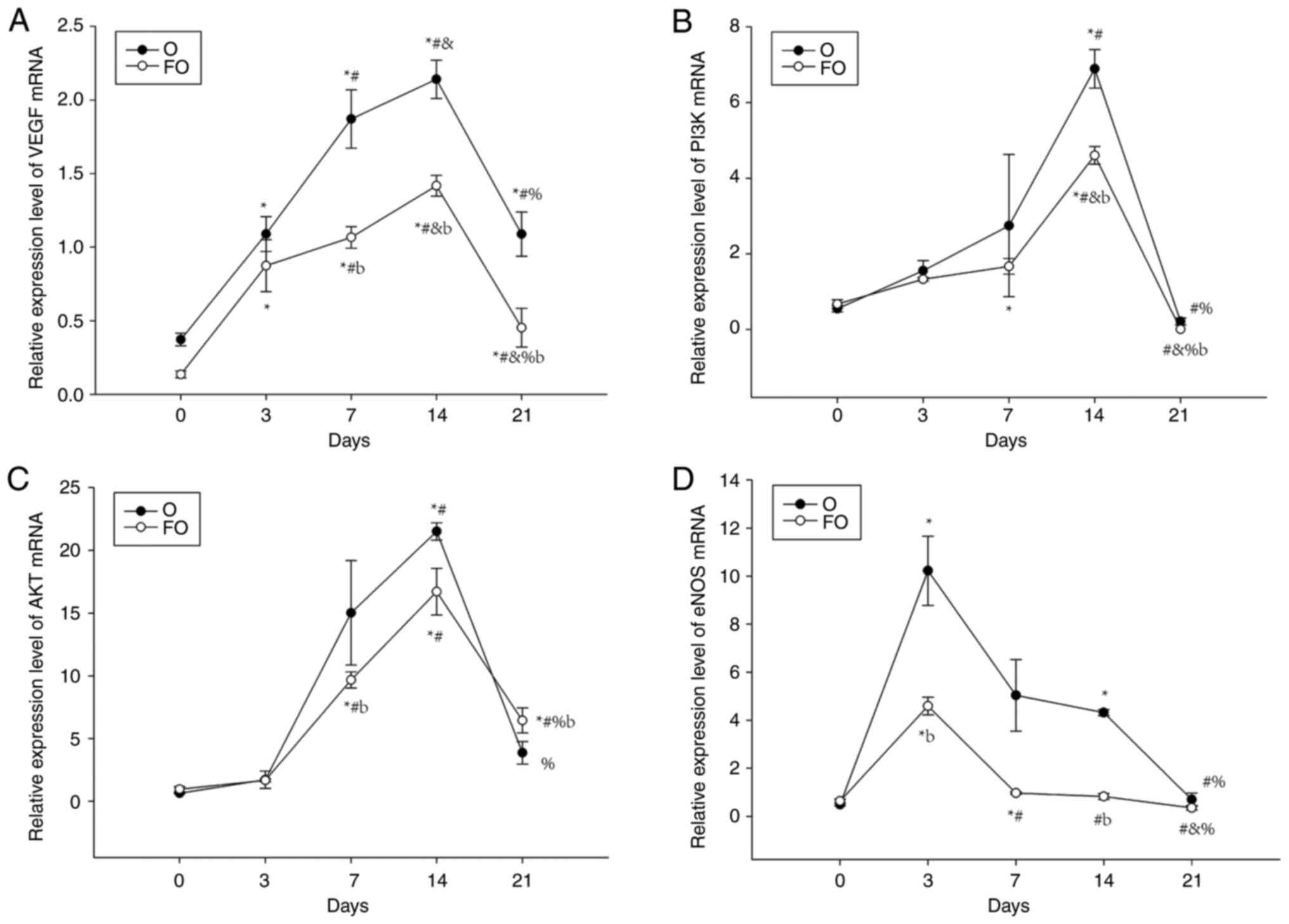
Expression profiles of VEGF, PI3K, AKT
and eNOS mRNA over time
The gingival tissue mRNA expression levels of VEGF,
PI3K, AKT and eNOS in the O group exhibited large fluctuations with
increasing time, showing an increasing and then decreasing trend.
The peak in eNOS expression appeared the earliest, on day 3,
whereas VEGF, PI3K and AKT expression peaked on day 14. The
expression levels at the peaks were statistically different when
compared with the expression levels at time points before and after
the peaks. The variation in VEGF, PI3K, AKT and eNOS mRNA
expression over time in the FO group was consistent with that in
the O group, increasing at first and then decreasing, and the peaks
appeared at the same time points as those of the same mRNA in the O
group. When the two groups were compared at the same time points,
VEGF, PI3K, AKT and eNOS mRNA expression in the FO group was lower
than that in the O group on days 7 and 14, and there were
significant differences in the expression of mRNA for VEGF and AKT
on day 7, and VEGF, PI3K and eNOS on day 14 (Fig. 6).
Expression profiles of VEGF, PI3K, AKT
and eNOS protein over time
The protein expression levels of VEGF, PI3K, AKT and
eNOS in the gingival tissue of the O group showed a trend
consistent with that of mRNA expression over time, increasing at
first and then decreasing. The peak in eNOS expression appeared
earliest, on day 3, whereas the peaks in VEGF, PI3K and AKT
expression appeared on day 14, with statistically significant
differences between the expression levels at the peak and those at
the pre- and post-peak time points. The effect of time on VEGF,
PI3K, AKT and eNOS protein expression in the FO group was
consistent with that in the O group, showing a trend of first
increasing and then decreasing. The time at which expression peaked
was also similar to that in group O, with VEGF, PI3K and AKT
peaking on day 14, and eNOS peaking on day 7. Statistically
significant differences were identified between the expression
values at the peak and at time points before and after the peak.
When compared at the same time point, the protein expression levels
of VEGF, PI3K, AKT and eNOS were lower in the FO group than those
in the O group on days 7 and 14, and there were significant
differences in the expression of protein for eNOS on day 7, and
VEGF on day 14 (Fig. 7).
Discussion
Fluorine is an essential trace element in the human
body; however, the long-term excessive intake of fluorine can lead
to fluorosis. Chronic fluorosis, a type of systemic chronic
cumulative poisoning, can damage various tissues, organs and
systems of the body, and induce vascular damage (12,13).
Wu et al (14) studied the
mRNA expression levels of methyltransferase and mismatch repair
genes in the blood cells of rats with chronic fluorosis, and found
that they were significantly reduced compared with those of control
rats, indicating that excessive fluoride may inhibit the DNA repair
function of blood cells, thus leading to vascular damage.
A high-fluoride environment has been shown to
inhibit the expression of molecules in the VEGF/PI3K/AKT/eNOS
pathway. Huang et al (15)
treated human umbilical vein endothelial cells with 1.2 µg/ml
sodium fluoride for 24 h, and evaluated the level of nitric oxide
(NO) in the culture medium, and the protein levels of eNOS,
phosphorylated (p)-eNOS, PI3K, AKT and p-AKT. The results suggested
that excess fluoride exposure inhibited NO synthesis and that the
PI3K/AKT/eNOS pathway played a key role in the reduction of NO
expression.
It has previously been shown that under the action
of orthodontic treatment force, local ischemia and hypoxia in
periodontal tissues can cause the upregulation of VEGF, which in
turn increases angiogenesis (16).
The binding of VEGF to tyrosinase receptors on the surface of
endothelial cells activates intracellular PI3K, which subsequently
activates AKT. Furthermore, the phosphorylation of AKT activates
the eNOS that is distributed in vascular endothelial cells, and
activated eNOS produces NO in the vasculature, which assists
vascular endothelial cell outgrowth, proliferation and migration,
and promotes vascular neovascularization (17).
When chronic fluorosis is present, it has not yet
been determined how orthodontic force affects the angiogenesis of
periodontal tissue. Our previous study found that during
orthodontic tooth movement in rats with chronic fluorosis, the
number of microvessels in the periodontal ligament near the
alveolar bone decreased, and the expression of VEGF and eNOS in the
periodontal tissue was significantly lower than that in the control
group without fluorosis (18).
Similar changes were observed in gingival tissue in the present
study. The present study also found that the expression of VEGF,
PI3K, AKT and eNOS in the gingiva was inhibited, indicating that
angiogenesis activity was weakened in rats undergoing orthodontic
tooth movement in a high-fluoride environment, which suggests that
the fluoride-induced damage of gingival blood vessels might be
associated with the slowdown of orthodontic tooth movement.
The present study also found that chronic fluorosis
had a more marked inhibitory effect on gingival VEGF and eNOS
expression than on other analytes. As upstream and downstream
signaling molecules of the angiogenesis pathway, significant
changes in their expression may occur as a sensitive response to
fluoride ion stimulation. VEGF serves to create the initiation
signal of the pathway, causing downstream reactions (19), whereas eNOS ultimately produces NO,
thus promoting angiogenesis (20).
The synchronous expression of VEGF and eNOS in response to
excessive fluoride ions may be considered to indicate the
relatively stable expression of this vascular pathway in the
mechanism of gingival fluorosis.
Under conditions of chronic fluoride toxicity, in
the present study, the expression of VEGF, PI3K, AKT and eNOS,
which are regulators of gingival angiogenesis in rats, was
inhibited at the mRNA and protein levels, and the trends were very
similar. However, there were some differences in the degree of
inhibition, and the reduction in mRNA expression was more marked.
It is suggested that inhibition of the PI3K vascular pathway by
excess fluoride ions may be interfered with by multiple factors,
leading to the inhibition of protein function being weakened and
delayed. Therefore, it is necessary to conduct an in-depth analysis
on the regulation of this vascular pathway in future studies to
elucidate the intrinsic mechanism underlying the inhibition of
neovascularization induced by fluoride toxicity.
Orthodontic tooth movement has a cyclic pattern of
change, which generally comprises three stages (21). Stage 1 is the initial movement of
the tooth within the periodontal membrane and supporting bone
tissues, which results mainly in elastic changes in the periodontal
membrane and mechanical displacement of the tooth. In addition, the
blood vessels in the periodontal membrane are squeezed and pulled,
the lumen becomes thinner and the blood flow is reduced, thereby
reducing the local oxygen concentration and promoting
vascularization. This stage lasts for 1-3 days. Stage 2 refers to
the delayed stage of degeneration, where the elastic deformation of
the periodontal membrane reaches its limit, the local oxygen
concentration decreases to the minimum and vitreous tissue appears.
In addition, periodontal tissue undergoes active remodeling, active
vascularization occurs and the neovascularization extends into the
vitreous tissue and finally clears it. This stage generally lasts
for 2-3 weeks. Stage 3 is when the removal of the vitreous tissue
is completed, the periodontal membrane stress is released, blood
circulation improves, vascularization is reduced to normal and the
tooth enters the stage of continuous movement. Periodontal tissue
resorption alternates with new growth, and the dynamic balance of
tissue remodeling is maintained. This remodeling process has been
shown to be dependent on the periodontal tissue vascularization
response (22). The observation
time points of the present study were designed using the tooth
movement cycle as a reference, in an attempt to observe the
potential relationship between changes in the temporal effect of
gingival vascularization and orthodontic tooth movement.
The results of the present study showed that there
was a certain temporal rhythm in the mRNA and protein expression
levels of VEGF, PI3K, AKT and eNOS in the gingiva of the O and FO
groups, which corresponded with the vascular response during the
tooth movement cycle. The gene and protein expression levels of
VEGF, PI3K, AKT and eNOS in the gingiva of the O group began to
increase after the application of stress, and then decreased once
they had reached their peak. This finding indicates that, after
being stimulated by orthodontic force, the body triggers a vascular
response, and as time goes on and the mechanical force stimulation
persists, the vascular response intensifies and hyalinization
occurs. Afterwards, as the glassy tissue is cleared, the vascular
response gradually weakens and eventually reduces to normal. The
trends observed with fluoride exposure in the FO group were
basically consistent with those in the O group, showing an upward
and then downward trend, but the upward and downward changes in
amplitude occurred more slowly and the peak expression levels were
lower than those in group O. This suggests that fluorosis
interferes with the vascular remodeling of orthodontic tooth
movement during the delayed tooth movement period, reduces vascular
reactivity, prolongs the duration of vascular remodeling, and may
prevent the complete removal of glassy tissue, which has a negative
impact on the stability of orthodontic tooth movement (23).
The peak eNOS expression on days 3 and 7 in the O
and FO groups occurred earlier than the peak expression of VEGF,
PI3K and AKT on days 7 and 14. This may be attributed to the
negative association between eNOS and vasoactivity, with eNOS
significantly elevated when the blood vessels were only slightly
stimulated during the early stage of orthodontic tooth movement,
and suppressed with the prolongation of the force application and
the continuous intensification of stress stimulation. In addition,
the changes in the expression of VEGF, PI3K, AKT and eNOS pathway
molecules over time were not completely consistent with each other,
likely because PI3K/AKT not only promotes vascular remodeling
during tooth movement as a downstream signaling molecule of VEGF
but also participates in other signaling pathways to regulate
activities such as osteogenesis and osteoblastogenesis, which are
closely associated with cell proliferation, migration and
differentiation, and protein synthesis (24).
Gingival tissue covers the alveolar bone cortex,
which is different from periodontal ligament tissue and is not
directly associated with alveolar bone remodeling during
orthodontic tooth movement. Therefore, previous studies have
focused more on changes to the alveolar bone and periodontal
ligament tissue. The present study revealed that the gingival
component of periodontal tissue is rich in fibrous tissue and may
be susceptible to fluoride. It is likely to cause persistent
fluoride accumulation in periodontal bone tissue by connecting the
periodontal bone tissue with saliva and the high-fluoride
environment of the whole body through rich microcirculation
channels; this has been confirmed in the previous research of the
present study group (9).
Therefore, it is necessary to further explore the possible role of
gingival fluorosis injury in orthodontic tooth movement in future
studies.
Acknowledgements
Not applicable.
Funding
Funding: The study was supported by the National Natural Science
Foundation of China (grant no. 81860795).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
YJ designed the experiments. XD and LL established
the orthodontic tooth movement model. XL and JH performed
immunohistochemistry and H&E staining. JH and WC were
responsible for RT-qPCR and western blotting. YJ, XD, LL, XL, WC
and JH confirm the authenticity of all the raw data. All authors
read and approved the final version of the manuscript.
Ethics approval and consent to
participate
The study was approved by the Ethics Committee of
Guizhou Medical University (Guiyang, China; approval no.
2000890).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Srivastava S and Flora SJS: Fluoride in
drinking water and skeletal fluorosis: A review of the global
impact. Curr Environ Health Rep. 7:140–146. 2020.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Meena L and Gupta R: Skeletal fluorosis. N
Engl J Med. 385(1510)2021.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Vandana KL, Srishti Raj B and Desai R:
Dental fluorosis and periodontium: An original research report of
in vitro and in vivo institutional studies. Biol Trace Elem Res.
199:3579–3592. 2021.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Yue Y, Chen Z, Xie B and Yao HL:
Expression of vascular endothelial growth factor in periodontal
tissues during orthodontic tooth movement and its role in bone
remodeling. Shanghai Kou Qiang Yi Xue. 27:18–21. 2018.PubMed/NCBI(In Chinese).
|
|
5
|
Narimiya T, Wada S, Kanzaki H, Ishikawa M,
Tsuge A, Yamaguchi Y and Nakamura Y: Orthodontic tensile strain
induces angiogenesis via type IV collagen degradation by matrix
metalloproteinase-12. J Periodontal Res. 52:842–852.
2017.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Wang XQ, Zheng LL and Ma HF: The effect of
dental fluorosis on its tooth movement distance and extraction
wound alveolar bone remodeling during orthodontic extraction
treatment. Chin J Endemic Dis Control. 33:393–394. 2018.
|
|
7
|
Ding X, Jia Y, Liu C, Yang SR, Lai LY,
Yang H and Ding Q: Changes in displacement and rate of orthodontic
tooth movement in SD rats with chronic fluorosis. Chin J Tissue Eng
Res. 26:4687–4692. 2022.
|
|
8
|
Lai LY, Jia Y, Ding X, Hu J and Chen B:
Changes in the internal environment and shear mechanical properties
of the jawbone in SD rats exposed to fluoride at different stages.
Environ Occup Med. 40:95–100. 2023.
|
|
9
|
Yang S, Liu C, Ding Q, Yang H and Jia Y:
Comparative study on fluoride accumulation in hard tissue of rats
with chronic drinking-water-borne fluorosis. J Environ Occup Med.
39:174–178. 2022.
|
|
10
|
Liu Y, Li CX, Nie J, Mi CB and Li YM:
Interactions between orthodontic treatment and gingival tissue.
Chin J Dent Res. 26:11–18. 2023.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Dey Bhowmik A, Das T and Chattopadhyay A:
Chronic exposure to environmentally relevant concentration of
fluoride impairs osteoblast's collagen synthesis and matrix
mineralization: Involvement of epigenetic regulation in skeletal
fluorosis. Environ Res. 236(116845)2023.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Miranda GHN, Gomes BAQ, Bittencourt LO,
Aragão WAB, Nogueira LS, Dionizio AS, Buzalaf MAR, Monteiro MC and
Lima RR: Chronic exposure to sodium fluoride triggers oxidative
biochemistry misbalance in mice: Effects on peripheral blood
circulation. Oxid Med Cell Longev. 2018(8379123)2018.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Wu CX, Wang YH, Li Y, Guan ZZ and Qi XL:
Changes of DNA repair gene methylation in blood of chronic
fluorosis patients and rats. J Trace Elem Med Biol. 50:223–228.
2018.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Huang Y, Sun M, Li F, Li H and Jiang Z:
Preliminary study of mechanisms of fluoride-induced suppression of
nitric oxide synthesis in human umbilical vein endothelial cells.
Biol Trace Elem Res. 185:311–315. 2018.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Noguchi T, Kitaura H, Marahleh A, Ohori F,
Nara Y, Pramusita A, Kinjo R, Ma J, Kanou K and Mizoguchi I: Tumor
necrosis factor-α enhances the expression of vascular endothelial
growth factor in a mouse orthodontic tooth movement model. J Dent
Sci. 17:415–420. 2022.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Lu JM, Zhang ZZ, Ma X, Fang SF and Qin XH:
Repression of microRNA-21 inhibits retinal vascular endothelial
cell growth and angiogenesis via PTEN dependent-PI3K/Akt/VEGF
signaling pathway in diabetic retinopathy. Exp Eye Res.
190(107886)2020.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Li C, Zhou XY, Yang SR, Li ZW and Jia Y:
Mechanism and cross-talk of signaling pathways associated with bone
damage in fluorosis. J Environ Occup Med. 38:794–800. 2021.
|
|
19
|
Tao X, Liu K, Li W, Zhao S, Liu C, Dai Q,
Dong T, Wei P, Duan J, Wang J and Xi M: Saponin of Aralia
taibaiensis promotes angiogenesis through VEGF/VEGFR2 signaling
pathway in cerebral ischemic mice. J Ethnopharmacol.
317(116771)2023.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Chen T, Chen H, Fu Y, Liu X, Huang H, Li Z
and Li S: The eNOS-induced leonurine's new role in improving the
survival of random skin flap. Int Immunopharmacol.
124(111037)2023.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Jeon HH, Teixeira H and Tsai A:
Mechanistic insight into orthodontic tooth movement based on animal
studies: A critical review. J Clin Med. 10(1733)2021.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Li Y, Zhan Q, Bao M, Yi J and Li Y:
Biomechanical and biological responses of periodontium in
orthodontic tooth movement: Up-date in a new decade. Int J Oral
Sci. 13(20)2021.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Jiang Y, Guan Y, Lan Y, Chen S, Li T, Zou
S, Hu Z and Ye Q: Mechanosensitive piezo1 in periodontal ligament
cells promotes alveolar bone remodeling during orthodontic tooth
movement. Front Physiol. 12(767136)2021.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Wang Y, Lu YH, Tang C, Xue M, Li XY, Chang
YP, Cheng Y, Li T, Yu XC, Sun B, et al: Calcium dobesilate restores
autophagy by inhibiting the VEGF/PI3K/AKT/mTOR signaling pathway.
Front Pharmacol. 10(886)2019.PubMed/NCBI View Article : Google Scholar
|