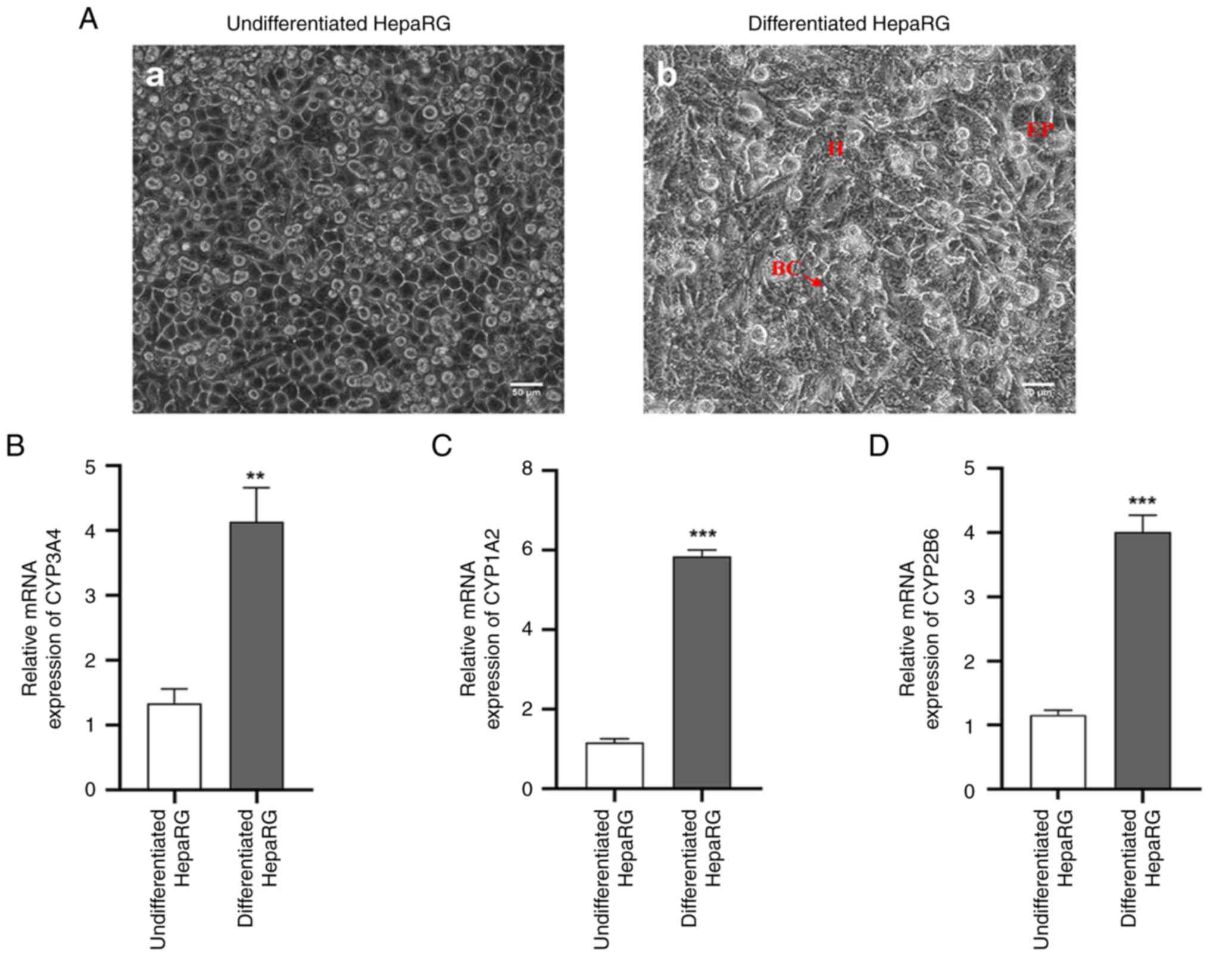
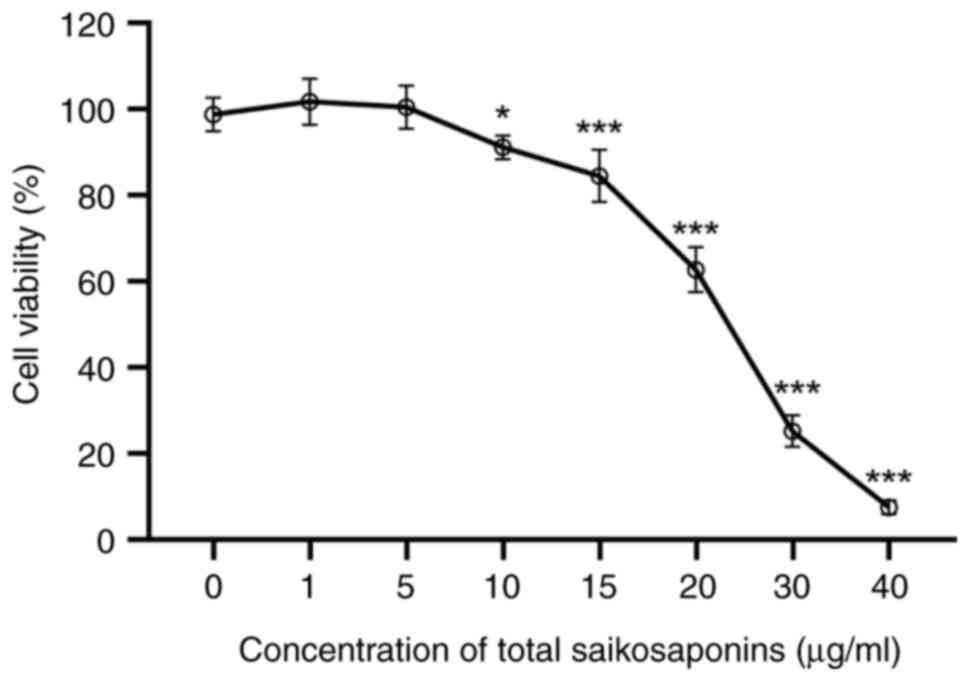
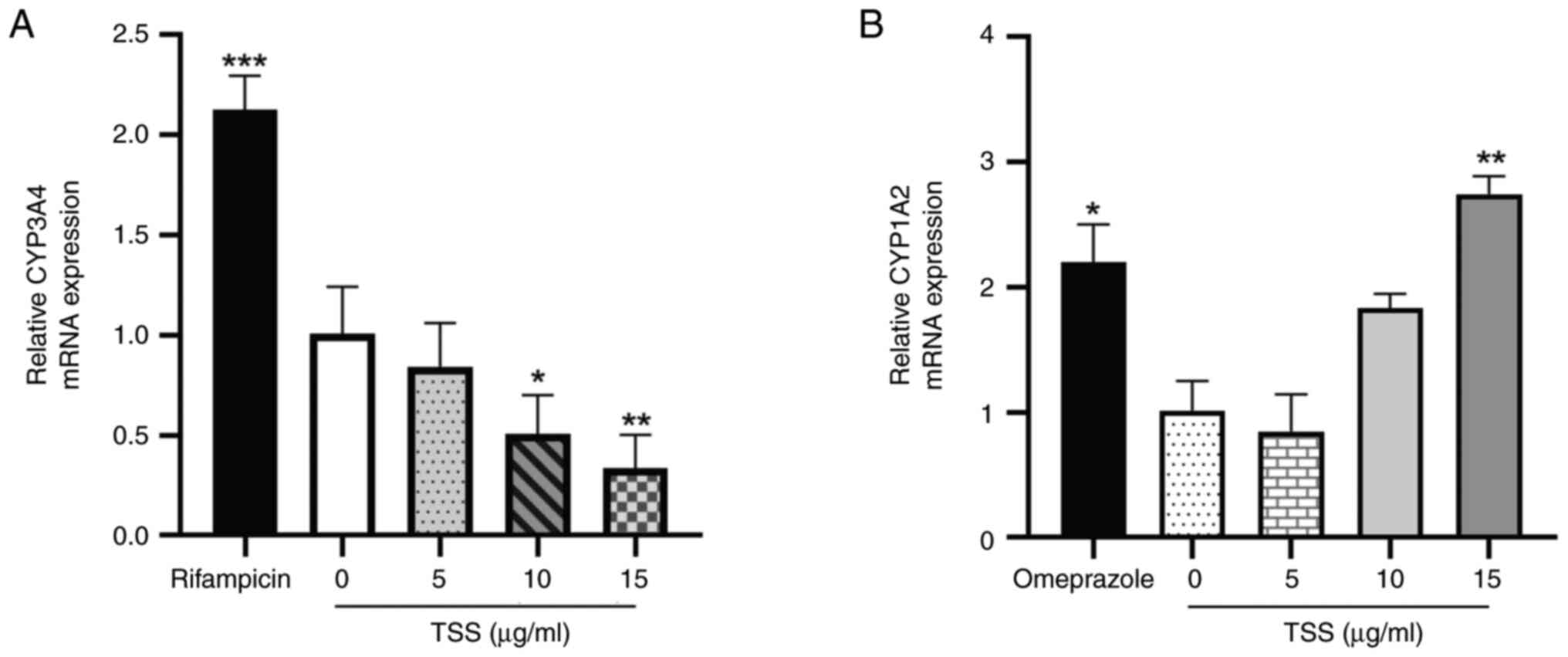
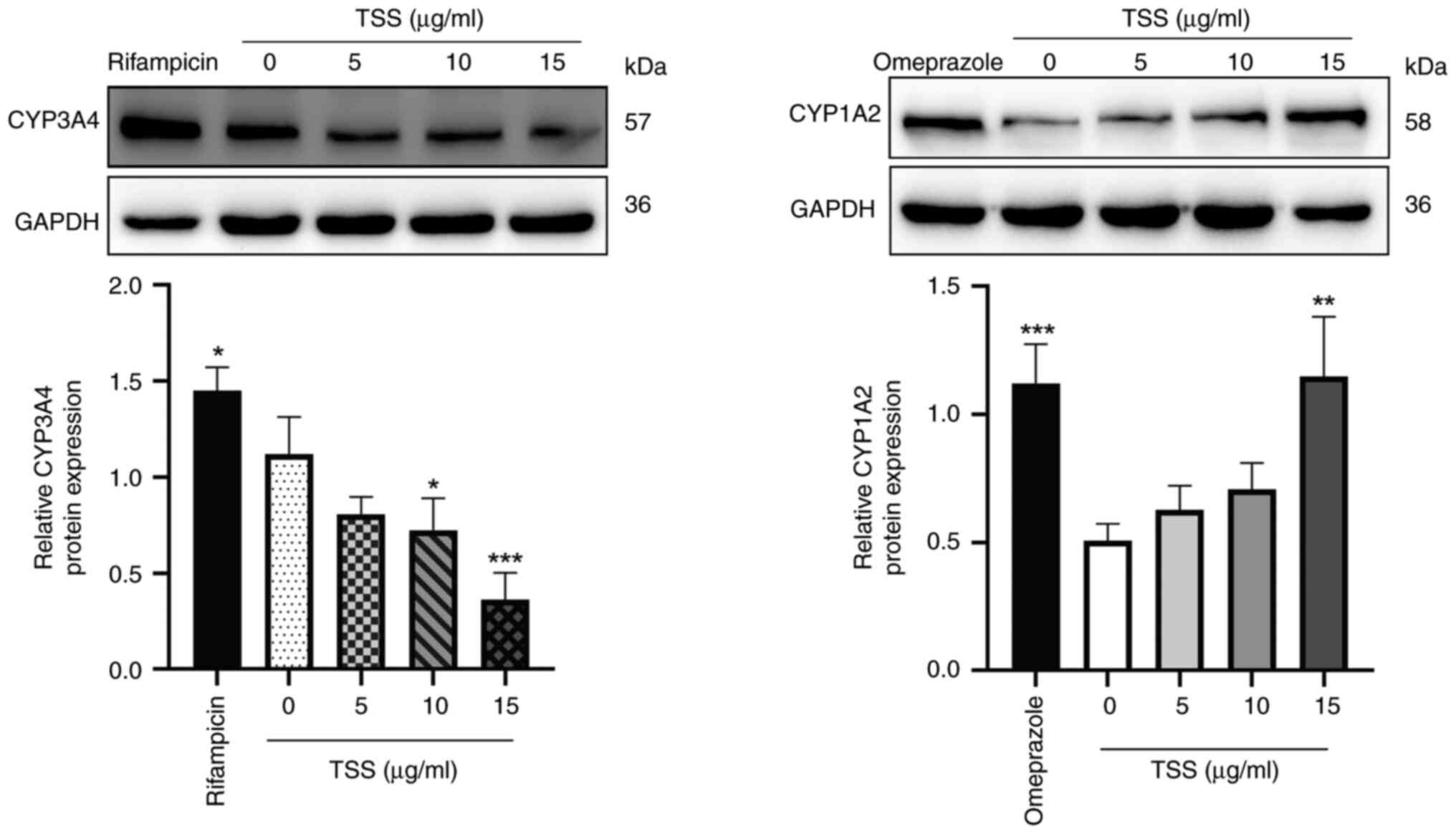
|
1
|
Yuan B, Yang R, Ma YS, Zhou S, Zhang XD
and Liu Y: A systematic review of the active saikosaponins and
extracts isolated from Radix Bupleuri and their applications. Pharm
Biol. 55:620–635. 2017.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Zhang X, Liu Z, Chen S, Li H, Dong L and
Fu X: A new discovery: Total Bupleurum saponin extracts can inhibit
the proliferation and induce apoptosis of colon cancer cells by
regulating the PI3K/Akt/mTOR pathway. J Ethnopharmacol.
283(114742)2022.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Wang H and Liu X: Research progress on
Bupleurum marginatum var. stenophyllum. World Latest Med Info.
19:110–113. 2019.(In Chinese).
|
|
4
|
Ashour ML and Wink M: Genus Bupleurum: A
review of its phytochemistry, pharmacology and modes of action. J
Pharm Pharmacol. 63:305–321. 2011.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Liu YF, Luo WT, Tang TT, Tang C and Xiao
YP: A meta-analysis of clinical efficacy of the Dachaihu decoction
plus hypoglycemic medicine on type 2 diabetes mellitus. Clin J Chin
Med. 14(5)2022.(In Chinese).
|
|
6
|
Li HX, Yu Q, Wang S, Li HQ, Wu Q, Chen Al
and Diao LM: Meta-analysis of efficacy and safety of Chaihushugan
decoction combined with antiepileptic drugs in treatment of
epilepsy. Chin Arch Tradit Chin Med. 8:1673–7717. 2020.(In
Chinese).
|
|
7
|
Zhu MJ, Cai MQ, Yang HP, Wang L and Zhao
WX: Meta-analysis of Xiaochaihu decoction combined with entecavir
in the treatment of chronic viral hepatitis B. Western J Trad Chin
Med. 34:62–66. 2021.(In Chinese).
|
|
8
|
Zhou Y and Chu XM: Effects of oral
administration of minor Radix Buplenri granule on whole blood
cyclosporin A concentration in kidney transplantation patients.
Chin J Hosp Pharm. 19(713)1999.(In Chinese).
|
|
9
|
Lewis DF: P450 structures and oxidative
metabolism of xenobiotics. Pharmacogenomics. 4:387–395.
2003.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Faber MS, Jetter A and Fuhr U: Assessment
of CYP1A2 activity in clinical practice: Why, how, and when? Basic
Clin Pharmacol Toxicol. 97:125–134. 2005.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Goutelle S, Bourguignon L, Bleyzac N,
Berry J, Clavel-Grabit F and Tod M: In vivo quantitative prediction
of the effect of gene polymorphisms and drug interactions on drug
exposure for CYP2C19 substrates. AAPS J. 15:415–426.
2013.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Zanger UM and Schwab M: Cytochrome P450
enzymes in drug metabolism: regulation of gene expression, enzyme
activities, and impact of genetic variation. Pharmacol Ther.
138:103–141. 2013.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Chen Y, Zeng L, Wang Y, Tolleson WH, Knox
B, Chen S, Ren Z, Guo L, Mei N, Qian F, et al: The expression,
induction and pharmacological activity of CYP1A2 are
post-transcriptionally regulated by microRNA hsa-miR-132-5p.
Biochem Pharmacol. 145:178–191. 2017.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Gripon P, Rumin S, Urban S, Le Seyec J,
Glaise D, Cannie I, Guyomard C, Lucas J, Trepo C and
Guguen-Guillouzo C: Infection of a human hepatoma cell line by
hepatitis B virus. Proc Natl Acad Sci USA. 99:15655–15660.
2002.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Wang Z, Luo X, Anene-Nzelu C, Yu Y, Hong
X, Singh NH, Xia L, Liu S and Yu H: HepaRG culture in tethered
spheroids as an in vitro three-dimensional model for drug safety
screening. J Appl Toxicol. 35:909–917. 2015.PubMed/NCBI View
Article : Google Scholar
|
|
16
|
Andersson TB, Kanebratt KP and Kenna JG:
The HepaRG cell line: A unique in vitro tool for understanding drug
metabolism and toxicology in human. Expert Opin Drug Metab Toxicol.
8:909–920. 2012.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Li HF, Sun SS, Zhang T and Tang FS:
Research progress on the application of HepaRG cells in drug
metabolism. Herald Med. 38:1038–1043. 2019.(In Chinese).
|
|
18
|
Li H, Tang Y, Wang Y, Wei W, Yin C and
Tang F: Effects of saikosaponin D on CYP1A2 and CYP2D6 in HepaRG
cells. Drug Des Devel Ther. 14:5251–5258. 2020.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Li H, Tang Y, Wei W, Yin C and Tang F:
Effects of saikosaponin-D on CYP3A4 in HepaRG cell and
protein-ligand docking study. Basic Clin Pharmacol Toxicol.
128:661–668. 2021.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Liu QX, Tan L, Bai YJ, Liang H and Zhao
YY: A survey of the studies on saponins from Bupleurum in past 10
years. Zhongguo Zhong Yao Za Zhi. 27(7-11.45)2002.PubMed/NCBI(In Chinese).
|
|
21
|
Huang W, Lv Z and Sun R: Research
development on chemical compositions in Bupleurum chinense related
with efficacy and toxicity. Chin J Pharmacovigil. 10:545–548.
2013.(In Chinese).
|
|
22
|
Wang YZ, Zhang XN, Zhang Y, Duan CC and
Zhang JY: Study on metabolic profiling of total saponins of
Bupleurum chinense DC in Rats. J Zunyi Med Uni. 46:21–29. 2023.(In
Chinese).
|
|
23
|
Yu P, Qiu H, Wang M, Tian Y, Zhang Z and
Song R: In vitro metabolism study of saikosaponin D and its
derivatives in rat liver microsomes. Xenobiotica. 47:11–19.
2017.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Liu G, Tian Y, Li G, Xu L, Song R and
Zhang ZJ: Metabolism of saikosaponin A in rats: Diverse oxidations
on the aglycone moiety in liver and intestine in addition to
hydrolysis of glycosidic bonds. Drug Metab Dispos. 41:622–633.
2013.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Aninat C, Piton A, Glaise D, Le
Charpentier T, Langouët S, Morel F, Guguen-Guillouzo C and
Guillouzo A: Expression of cytochromes P450, conjugating enzymes
and nuclear receptors in human hepatoma HepaRG cells. Drug Metab
Dispos. 34:75–83. 2006.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta C (T)) method. Methods. 25:402–408. 2001.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Mar PL, Gopinathannair R, Gengler BE,
Chung MK, Perez A, Dukes J, Ezekowitz MD, Lakkireddy D, Lip GYH,
Miletello M, et al: Drug interactions affecting oral anticoagulant
use. Circ Arrhythm Electrophysiol. 15(e007956)2022.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Hakkola J, Hukkanen J, Turpeinen M and
Pelkonen O: Inhibition and induction of CYP enzymes in humans: An
update. Arch Toxicol. 94:3671–3722. 2020.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Huang SM, Temple R, Throckmorton DC and
Lesko LJ: Drug interaction studies: Study design, data analysis,
and implications for dosing and labeling. Clin Pharmacol Ther.
81:298–304. 2007.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Song FX, Wang R and Yuan YF: Application
progress in cytochrome P450 enzymes and research methods of drug
metabolism. Med Recapitulate. 23:665–669. 2017.(In Chinese).
|
|
31
|
Wang YH, Qi JF and Lin M: Effects of total
saikosaponins on intestinal first-pass effect and CYP3A, CYP2E1.
Chin J Clin Pharm Ther. 16:740–748. 2011.(In Chinese).
|
|
32
|
Tang YY, Lan X, Shang DC and Tang FS:
Research overview of Xiaochuhu decoction combined with chemical
drugs and their interaction. China Pharm. 30:846–850. 2019.(In
Chinese).
|
|
33
|
Xue ZK and Wei H: Comparison of
pharmacokinetics between recombinant liver microsomes with CYP3A4
and CYP3A29. Herald Med. 34:15–21. 2015.
|
|
34
|
Kang KJ, Min BH, Lee JH, Kim ER, Sung CO,
Cho JY, Seo SW and Kim JJ: Alginate hydrogel as a potential
alternative to hyaluronic acid as submucosal injection material.
Dig Dis Sci. 58:1491–1496. 2013.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Michaut A, Le Guillou D, Moreau C, Bucher
S, McGill MR, Martinais S, Gicquel T, Morel I, Robin MA, Jaeschke H
and Fromenty B: A cellular model to study drug-induced liver injury
in nonalcoholic fatty liver disease: Application to acetaminophen.
Toxicol Appl Pharmacol. 292:40–55. 2016.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Bozina N, Bradamante V and Lovri M:
Genetic polymorphism of metabolic enzymes P450 (CYP) as a
susceptibility factor for drug response, toxicity, and cancer risk.
Arh Hig Rada Toksikol. 60:217–242. 2009.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Liu ZJ, Zhang Y, Yin PH and Zhao L:
Toxicity evaluation of landfill leachate before and after SND/UF/RO
treatment on HepG2 cells. Acta Scientiae Circumstantiae.
41:660–669. 2021.(In Chinese).
|
|
38
|
Godoy P, Hewitt NJ, Albrecht U, Andersen
ME, Ansari N, Bhattacharya S, Bode JG, Bolleyn J, Borner C, Böttger
J, et al: Recent advances in 2d and 3d in vitro systems using
primary hepatocytes, alternative hepatocyte sources and
non-parenchymal liver cells and their use in investigating
mechanisms of hepatotoxicity, cell signaling and ADME. Arch
Toxicol. 87:1315–1530. 2013.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Lübberstedt M, Müller-Vieira U, Mayer M,
Biemel KM, Knöspel F, Knobeloch D, Nüssler AK, Gerlach JC and
Zeilinger K: HepaRG human hepatic cell line utility as a surrogate
for primary human hepatocytes in drug metabolism assessment in
vitro. J Pharmacol Toxicol Methods. 63:59–68. 2011.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Pernelle K, Le Guevel R, Glaise D, Stasio
CG, Le Charpentier T, Bouaita B, Corlu A and Guguen-Guillouzo C:
Automated detection of hepatotoxic compounds in human hepatocytes
using HepaRG cells and image-based analysis of mitochondrial
dysfunction with Jc-1 dye. Toxicol Appl Pharmacol. 254:256–266.
2011.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Sugiyama I, Murayama N, Kuroki A, Kota J,
Iwano S, Yamazaki H and Hirota T: Evaluation of cytochrome P450
inductions by anti-epileptic drug oxcarbazepine,
10-hydroxyoxcarbazepine, and carbamazepine using human hepatocytes
and HepaRG cells. Xenobiotica. 46:765–774. 2006.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Leite SB, Wilk-Zasadna I, Zaldivar JM,
Airola E, Reis-Fernandes MA, Mennecozzi M, Guguen-Guillouzo C,
Chesne C, Guillou C, Alves PM and Coecke S: Three-dimensional
HepaRG model as an attractive tool for toxicity testing. Toxicol
Sci. 130:106–116. 2012.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Jiang L and Wang YM: Biological
characteristics and application of HepaRG cells. Chin Hepatol.
15:380–383. 2010.(In Chinese).
|
|
44
|
Wuerger LTD, Hammer HS, Hofmann U,
Kudiabor F, Sieg H and Braeuning A: Okadaic acid influences
xenobiotic metabolism in HepaRG cells. EXCLI J. 21:1053–1065.
2022.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Ashraf MN, Asghar MW, Rong Y, Doschak MR
and Kiang TKL: Advanced in vitro HepaRG culture systems for
xenobiotic metabolism and toxicity characterization. Eur J Drug
Metab Pharmacokinet. 44:437–458. 2019.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Hammour MM, Othman A, Aspera-Werz R, Braun
B, Weis-Klemm M, Wagner S, Nadalin S, Histing T, Ruoß M and Nüssler
AK: Optimisation of the HepaRG cell line model for drug toxicity
studies using two different cultivation conditions: Advantages and
limitations. Arch Toxicol. 96:2511–2521. 2022.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Meirinho S, Rodrigues M, Fortuna A, Falcão
A and Alves G: Study of the metabolic stability profiles of
perampanel, rufinamide and stiripentol and prediction of drug
interactions using HepaRG cells as an in vitro human model. Toxicol
In Vitro. 82(105389)2022.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Anthérieu S, Chesné C, Li R, Camus S,
Lahoz A, Picazo L, Turpeinen M, Tolonen A, Uusitalo J,
Guguen-Guillouzo C and Guillouzo A: Stable expression, activity,
and inducibility of cytochromes P450 in differentiated HepaRG
cells. Drug Metab Dispos. 38:516–525. 2010.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Xiao LX, Zhou HN and Jiao ZY: Present and
future prospects of the anti-cancer activities of saikosaponins.
Curr Cancer Drug Targets. 23:2–14. 2022.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Li J, Zou B, Cheng XY, Yang XH, Li J, Zhao
CH, Ma RX, Tian JX and Yao Y: Therapeutic effects of total
saikosaponins from Radix Bupleuri against Alzheimer’s disease.
Front Pharmacol. 13(940999)2022.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Zhou Z, Chen H, Tang X, He B, Gu L and
Feng H: Total saikosaponins attenuates depression-like behaviors
induced by chronic unpredictable mild stress in rats by regulating
the PI3K/AKT/NF-ΚappaB signaling axis. Evid Based Complement
Alternat Med. 2022(4950414)2022.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Sun X, Li X, Pan R, Xu Y, Wang Q and Song
M: Total saikosaponins of Bupleurum yinchowense reduces depressive,
anxiety-like behavior and increases synaptic proteins expression in
chronic corticosterine-treated mice. BMC Complement Altern Med.
18(117)2018.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Li X, Li X, Huang N, Liu R and Sun R: A
comprehensive review and perspectives on pharmacology and
toxicology of saikosaponins. Phytomedicine. 50:73–87.
2018.PubMed/NCBI View Article : Google Scholar
|
|
54
|
He ZH, Fan R, Zhang CH, Tang T, Cui HJ,
Liu X, Liang QH and Wang Y: Research on total saponins of Radix
Bupleuri regulating cytochrome P450 similar to Chaihushugan powder
exerting antidepressant effects. J Hunan Univ Tradit Chin Med.
39:693–698. 2019.(In Chinese).
|
|
55
|
Li H, Tang Y, Wei W, Yin C and Tang F:
Effects of Xiaochaihu decoction on the expression of cytochrome
P450s in rats. Exp Ther Med. 21(588)2021.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Kong Q, Gao N, Wang Y, Hu G, Qian J and
Chen B: Functional evaluation of cyclosporine metabolism by CYP3A4
variants and potential drug interactions. Front Pharmacol.
13(1044817)2023.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Wu B, Liu P, Gao Y and Wang Y: Effect of
water extract from traditional Chinese medicines Rehmannia
glutinosa, Scrophularia ningpoensis, Asparagus cochinchinensis and
Ophiopogon japonicas on contents of CYP450 and activities of CYP3A,
CYP2E1 and CYP1A2 in rat. Zhongguo Zhong Yao Za Zhi. 36:2710–2714.
2011.PubMed/NCBI(In Chinese).
|
|
58
|
Liu H, Narayanan R, Hoffmann M and
Surapaneni S: The uremic toxin indoxyl-3-sulfate induces CYP1A2 in
primary human hepatocytes. Drug Metab Lett. 10:195–199.
2016.PubMed/NCBI View Article : Google Scholar
|
|
59
|
FDA, (2023). Drug development and drug
interactions | table of substrates, inhibitors and inducers.
https://www.fda.gov/drugs/drug-interactions-labeling/drug-development-and-drug-interactions-table-substrates-inhibitors-and-inducers.
|