Introduction
Glioblastoma (GBM), a primary disease that severely
endangers human health, is considered as a major disease that
requires urgent global attention. GBM, as the most common prevalent
primary malignant tumor, accounts for ~48% of all primary malignant
tumors of the central nervous system and 57% of all gliomas
(1,2). Chemotherapy is considered as the most
common treatment strategy for malignant tumors (3). It has been reported that doxorubicin
(DOX), an anthracycline antibiotic, can inhibit the growth of
several solid tumors and hematological malignancies and it is
considered as one of the most effective antitumor drugs currently
available (4). However, DOX has
numerous disadvantages, including cardiotoxicity, liver toxicity
and multidrug resistance (5-7).
Therefore, the development of strategies that enhance targeting and
minimize toxicity is of great importance for the clinical
application of DOX.
Epidermal growth factor receptor (EGFR), an
essential regulator of cellular growth in normal epithelial
tissues, is a member of the cell surface receptor family, also
known as human epidermal growth factor receptor 1 or ErbB1
(8,9). The members of the EGFR family play
crucial roles in malignant cell transformation, drug resistance and
metastasis of tumor stem cells and other tumor cells (10,11).
For example, a previous study demonstrated that the signaling
transduction of ErbB receptor tyrosine kinase could promote
neuroblastoma growth (12).
Notably, several mutations in the EGFR gene have been detected,
with epidermal growth factor receptor variant III (EGFRvIII) being
the most common one (13).
Emerging evidence has suggested that EGFRvIII, a constitutively
active mutant, can promote cell mitosis and support growth
signaling, while it is involved in the development of several human
epithelial malignancies (14).
Other studies show that EGFRvIII was overexpressed in 80% of all
human malignancies, including glioblastoma, lung cancer and gastric
cancer, but not in normal tissues (15-19).
Therefore, EGFRvIII could be a potential factor for the targeted
therapy of GBM.
Antibody-drug conjugates (ADCs), which are novel
antibody drugs, are composed of antibodies, cytotoxic drugs and
their linkers (20). ADCs can
precisely deliver potent cytotoxic drugs into malignant cells by
binding to specific antigens on their surface (21). After internalization, ADCs are
lysed, thus releasing cytotoxic drugs transferred by lysosomes to
selectively kill tumor cells (22). It has been reported that ADCs can
minimize the concentration of cytotoxic drugs in normal tissues and
organs, while increasing their concentration in malignant tumors,
eventually achieving high efficiency and low toxicity (23). Our research group had previously
generated a single-chain antibody (scFv) using genetic engineering,
called PD0721 scFv, which could target EGFRvIII (24,25).
Specifically, the nucleic acid sequence of PD0721 scFv, targeting
anti-EGFRvIII, was ligated with the pET-22b(+) plasmid to create
the PD0721-pET recombinant plasmid, which was subsequently
transformed into Escherichia coli. The monoclonal antibody
was successfully obtained by inducing the mixture at an inducer
concentration of 0.6 µM for 12 h at 15˚C until the OD600 of the
bacterial solution reached ~0.6. The above findings could aid in
the development of high-efficiency and low-toxicity ADCs targeting
the EGFRvIII antigen.
In the present study, PD0721 scFv was conjugated
with DOX to generate a PD0721-DOX ADC through an amine-aldehyde
condensation reaction. The drug-to-antibody ratio (DAR) of PD0721
scFv and DOX was calculated by ultraviolet and visible
spectrophotometry (UV-Vis). To evaluate the in vitro
targeting efficacy and anti-GBM activity of the PD0721-DOX ADC,
cytotoxicity assays, immunofluorescence, laser-scanning confocal
microscopy and flow cytometric assays were performed. Given the
difference between EGFRvIII-expressing DK-MG cells and U-87MG ATCC
cells that do not express EGFRvIII (26,27),
the present study aimed to investigate the targeting potential and
cytotoxicity of PD0721-DOX ADC in the above two cell lines.
Materials and methods
Conjugation of PD0721 scFv with
DOX
To obtain oxidized dextran T-10 (Dex T-10), 200 mg
Dex T-10 was mixed with 2 ml of NaIO4 (0.35 mmol/l,
Xilong Chemical Co., Ltd.; cat no. 0408011), stirred in a flask at
150 rpm for 20 h in the dark, followed by freeze-drying.
Subsequently, 10 mg DOX (Beijing Solarbio Science & Technology
Co., Ltd.; cat no. D8740) was diluted into 2 ml ultrapure water and
the mixture was then supplemented with 30 mg oxidized Dex T-10,
followed by stirring at 150 rpm for 20 h in the dark. Then, to
obtain the PD0721-DOX ADC, the oxidized Dex T-10/DOX mixture was
supplemented with 5 mg PD0721 scFv, followed again by stirring at
150 rpm for 20 h at 4˚C in the dark. Subsequently, 100 µl
NaBH4 (0.13 mmol; Anhui Senrise Technology Co., Ltd.;
cat no. EB180088) was added into the above mixture, which was then
mixed at 150 rpm for 2 h at 37˚C in the dark. The final product was
then subjected to ultrafiltration using a Millipore 10 k molecular
weight ultrafiltration tube (MilliporeSigma).
Subsequently, the sample solution was added to a
cuvette and through UV-Vis to obtain the UV absorption spectra
(250-600 nm) and maximum absorption wavelength of Dex T-10,
Oxidized Dex T-10, PD0721 scFv, DOX and PD0721-DOX ADCs, and
compare the spectral differences.
DOX and PD0721 scFv concentration
assessment
UV-Vis was employed to determine the concentration
of PD0721 scFv and DOX. Therefore, 20 µg/ml DOX and 1 mg/ml bovine
serum albumin (BSA; Beijing Solarbio Science & Technology Co.,
Ltd.; cat no. A8020) stock solutions were prepared in ultrapure
water. The above stock solutions were used to prepare the DOX and
PD0721 scFv standard solutions (0-20 µg/ml and 0-1 mg/ml,
respectively) in ultrapure water. The concentration-absorbance
standard curves of DOX and BSA were constructed by measuring their
absorbance at wavelengths of 480, 280 and 280 nm, respectively. The
above curves were utilized to determine the concentrations of
PD0721 scFv and DOX in the PD0721-DOX ADC. The PD0721 scFv/DOX
molar ratio was calculated using the following formula: DAR=(DOX
concentration/DOX molar mass)/(PD0721 scFv concentration/PD0721
scFv molar mass).
Cell culture
DK-MG cells were purchased from the Otwo Biotech
(Shenzhen) Inc. (cat no. HTX3018). U-87MG ATCC cells (glioblastoma
of unknown origin) were purchased from the Procell Life Science
& Technology Co., Ltd. (cat no. CL-0238). To verify the
identity of U-87MG ATCC cells, short tandem repeat profiling was
performed (PC-H2023072421). The cells were cultured in DMEM
supplemented with 10% fetal bovine serum (Thermo Fisher Scientific
Inc.; cat no. 10099-141C) at 37˚C in a 5% CO2
environment. Experiments were performed using cells grown for four
passages.
Cytotoxicity assay
DK-MG and U-87MG ATCC cells were seeded into 96-well
plates at a density of 1.5x105 cells/well and cultured
to the logarithmic growth phase. PD0721-DOX ADC, DOX and PD0721
scFv were lyophilized to produce a powder, weighed using an
electronic balance at a precision of 1:100,000 and dissolved in PBS
to achieve the corresponding concentration. Subsequently, cells
were treated with PD0721-DOX ADC, DOX, or PD0721 scFv at varying
concentrations (0, 5, 10 and 20 µg/ml). Following incubation for 24
h, cell viability was assessed using a Cell Counting Kit-8 assay
(Dojindo Laboratories, Inc.; cat no. CK04).
Immunofluorescence staining
DK-MG and U-87MG ATCC cells were seeded onto glass
coverslips at a density of 1.0x105 cells/coverslip.
Following attachment, cells were treated with 20 µg/ml PD0721 scFv
or PD0721-DOX ADC for 2 h. Subsequently, cells were fixed with 4%
paraformaldehyde (Absin Bioscience Inc.; cat no. abs9179) for 15
min in room temperature, followed by permeabilization with 0.1%
Triton X-100 (Beijing Solarbio Science & Technology Co., Ltd.;
cat no. T8200) for 15 min. The cells were then incubated with 2%
BSA (Beijing Solarbio Science & Technology Co., Ltd.; cat no.
A8020) at 37˚C for 30 min, followed by incubation with FITC-H&L
labelled goat anti-human IgG (Abcam; cat no. ab6854) in the dark at
37˚C for 1 h and DAPI (1:500; Absin Bioscience Inc.; cat no.
abs47047616) for 5 min in room temperature. The cells were observed
and images were captured with confocal laser microscope (x630
magnification; ZEN system software 3.0; Zeiss AG) and analyzed
region-wide.
Process of drug entry into cells
DK-MG and U-87MG ATCC cells were seeded on glass
coverslips at a density of 1.0x105 cells/coverslip. Upon
attachment, cells were treated with 20 µg/ml PD0721-DOX ADC or DOX
incubated for 1, 3 and 6 h. Subsequently, the cells were observed
and images were captured using confocal laser microscope (x200
magnification; ZEN system software 3.0; Zeiss AG) and analyzed
region-wide.
Flow cytometry
DK-MG and U-87MG ATCC cells were seeded into 6-well
plates at a density of 5.0x105 cells/well. After
reaching logarithmic-growth phase, cells were treated with 20 µg/ml
PD0721-DOX ADC, DOX, or PD0721 scFv for 24 h. Subsequently, to
distinguish between alive and dead cells, the cells were stained
with Annexin V-APC and 7-ADD (Annexin V-APC/7-AAD Apoptosis
Analysis Kit, Absin Bioscience Inc., cat no. abs50008) for 10 and 5
min, respectively, at room temperature and away from light.
Finally, cell apoptosis was analyzed using a BD Accuri C6 Plus flow
cytometer (BD Biosciences) and the data were assessed using FlowJo
10.6 software (FlowJo LLC). Apoptotic rate was calculated as the
percentage of early + late apoptotic cells relative to the
control.
Statistical analysis
Data analysis was performed using SPSS 26.0 software
(IBM Corp.) and GraphPad Prism 8 software (GraphPad; Dotmatics).
The results are expressed as the mean ± standard deviation and are
representative of three independent experiments. Differences
between two groups were analyzed using Student's t-test and
correlation analysis was performed using Pearson's correlation
analysis. P<0.05 was considered to indicate a statistically
significant difference.
Results
Conjugation of PD0721 scFv with
DOX
Strategies based on ADC design were applied to
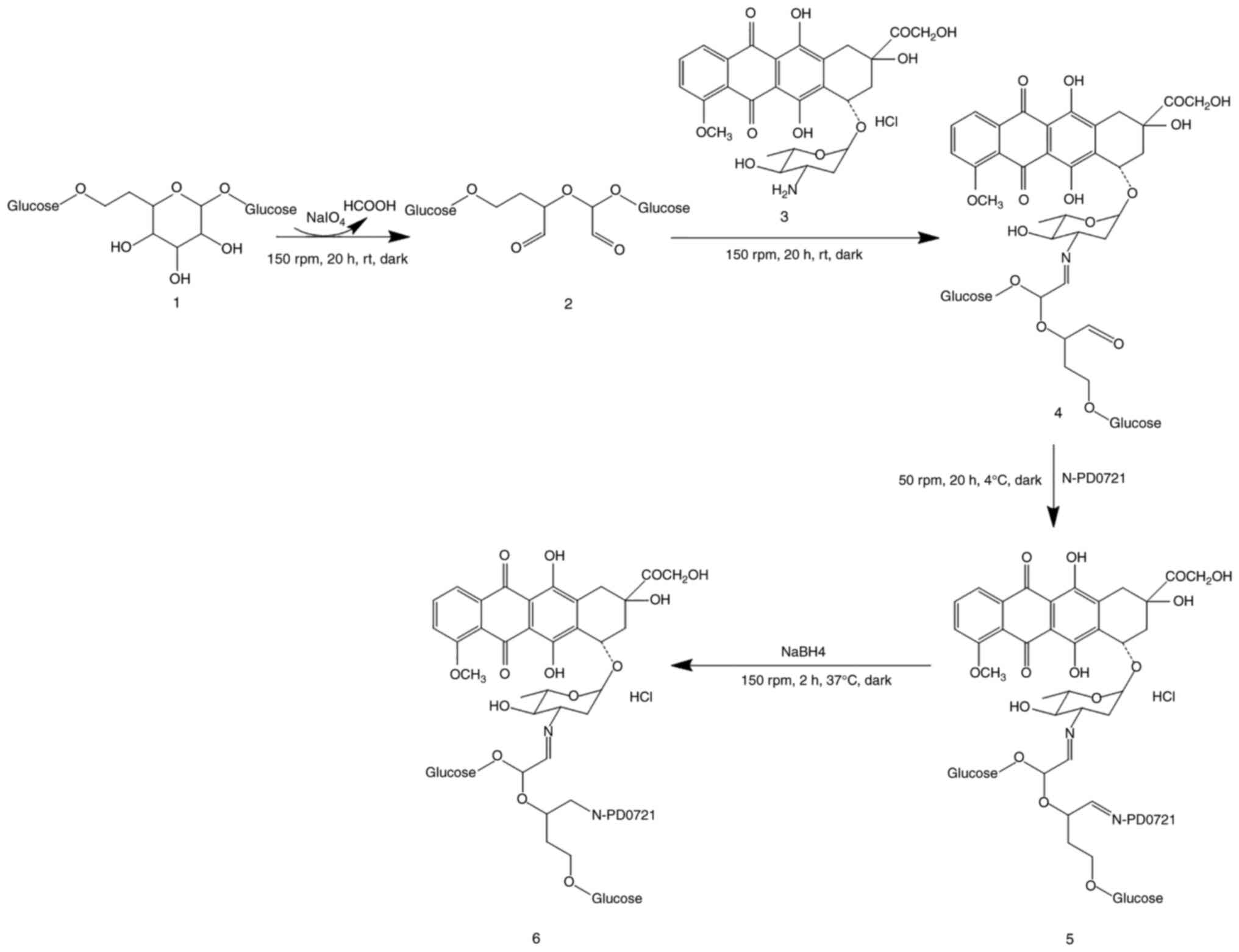
enhance DOX targeting. Therefore, compound 1 was oxidized to form
linker 2 containing dialdehyde. The two aldehyde groups of linkers
2 were connected to DOX and N-PD0721. First, the amino group of DOX
and the aldehyde group of linker 2 underwent an additional reaction
followed by dehydration to form a carbon nitrogen bond, eventually
obtaining DOX derivative 4. In addition, the aldehyde group of DOX
derivative 4 underwent the same reactions as N-PD07121 to obtain
compound 5. Finally, the target compound 6 was obtained by reducing
compound 5 with NaBH4 (Fig.
1).
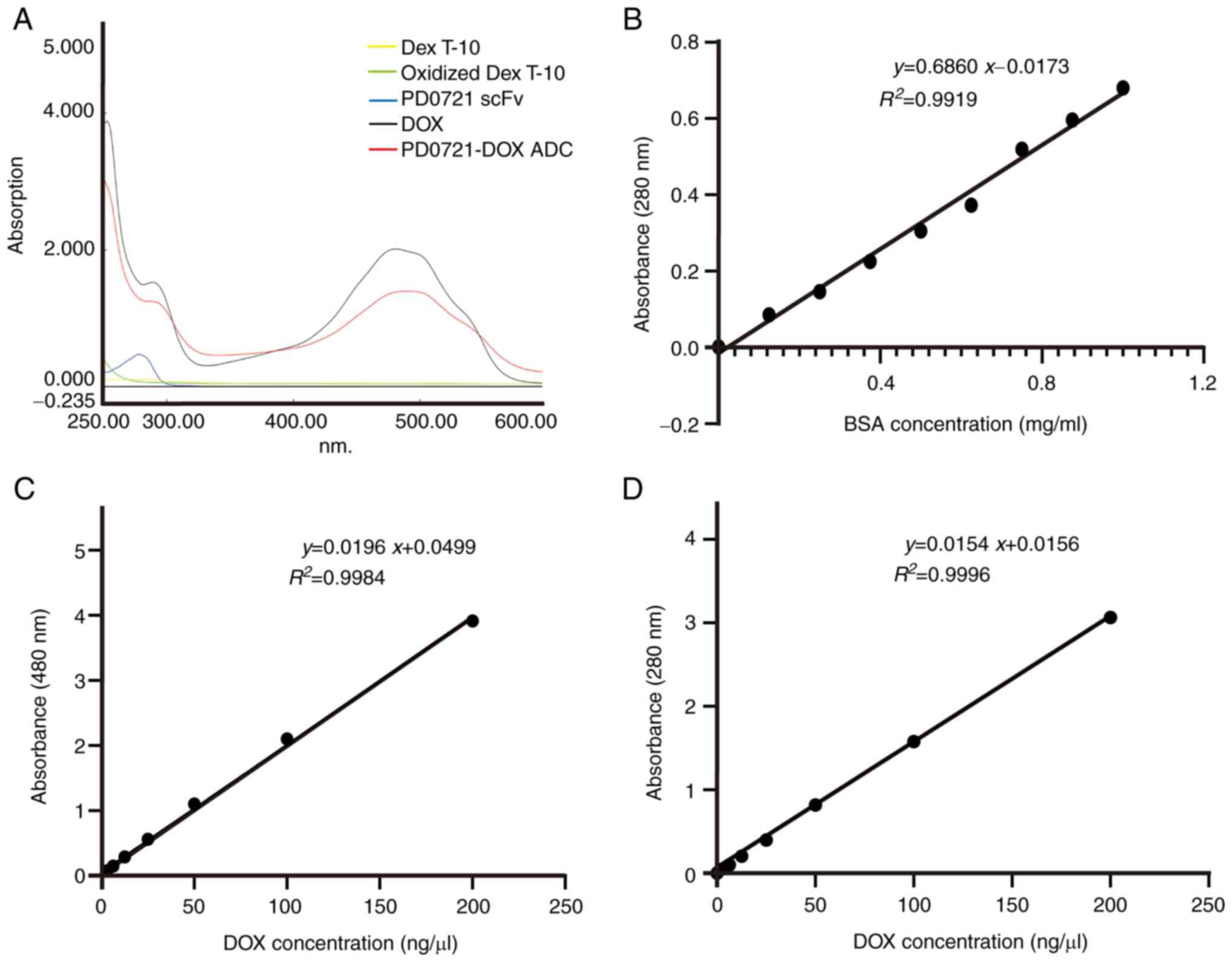
UV-Vis-mediated identification of
PD0721-DOX ADC and DAR of PD0721 scFv/DOX
The maximum absorbance of DOX was ~470 nm, while
that following DOX conjugation with PD0721 scFv (red shifted) was
~490 nm, thus indicating that PD0721 scFv and DOX were successfully
conjugated (Fig. 2A).
Subsequently, the PD0721 scFv and DOX standard curves at 280 and
480 nm were drawn (Fig. 2B and
C). The absorbance of PD0721-DOX
ADC at a wavelength of 480 nm was inserted into the regression
equation of DOX standard curve. Therefore, the analysis revealed
that DOX concentration in the PD0721-DOX ADC was 2.7 mg/ml. Since
DOX also shows interference absorption at 280 nm, the standard
curve of DOX at 280 nm was also plotted. Therefore, the regression
equation of the DOX standard curve was obtained at 280 nm (Fig. 2D). Based on the DOX concentration
of 2.7 mg/ml, the interference absorbance of DOX at a wavelength of
280 nm was 1.06. After subtracting the interference absorbance of
DOX at 280 nm from that of PD0721-DOX ADC at 280 nm, the resulting
value was then entered into the PD0721 scFv standard curve
regression equation. Therefore, the concentration of PD0721 scFv in
PD0721-DOX ADC was estimated at 13.2 mg/ml. Based on the
concentration-absorbance standard curve, the DAR of PD0721 scFv and
DOX was calculated to be 9.23:1, thus indicating that each PD0721
scFv molecule was conjugated with ~9.23 DOX molecules.
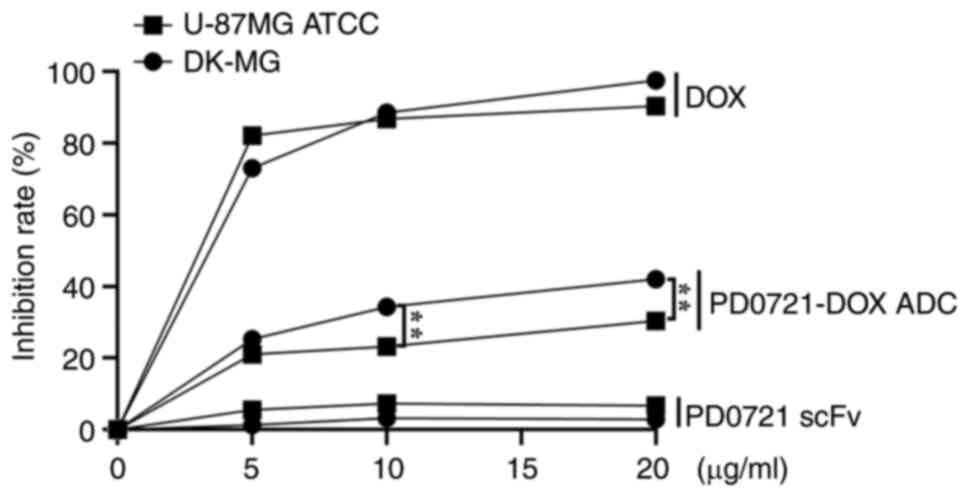
PD0721-DOX ADC promotes cytotoxicity
in DK-MG cells
To further validate the anti-GBM efficacy of
PD0721-DOX ADC, its effects on cytotoxicity of DK-MG and U-87MG
ATCC cells were investigated. PD0721-DOX ADC at 10 and 20 µg/ml
significantly inhibited DK-MG cell proliferation compared with
U-87MG ATCC cells (all P<0.01; Fig.
3). However, cells treatment with PD0721 scFv or DOX alone did
not exhibit significant differences in DK-MG and U-87MG ATCC cell
proliferation, thus indicating that the developed PD0721-DOX ADC
possessed particular targeting activity, while maintaining
toxicity. Therefore, in subsequent experiments, the most effective
dosage identified in the preliminary studies was used, a 20-µg/ml
concentration of PD0721-DOX ADC, to ensure the most optimal
experimental conditions were achieved.
PD0721-DOX ADC can specifically target
EGFRvIII
Microscopic analysis demonstrated that
EGFRvIII-expressing DK-MG cells treated with PD0721 scFv or
PD0721-DOX ADC emitted strong green fluorescence compared with the
U-87MG ATCC cells (not expressing EGFRvIII), thus verifying the
high specificity of PD0721 scFv for targeting EGFRvIII (Fig. 4). Additionally, prominent red
fluorescence was observed in DK-MG cells after treatment with
PD0721-DOX ADC, thus providing additional evidence of the
successful construction of the PD0721-DOX ADC.
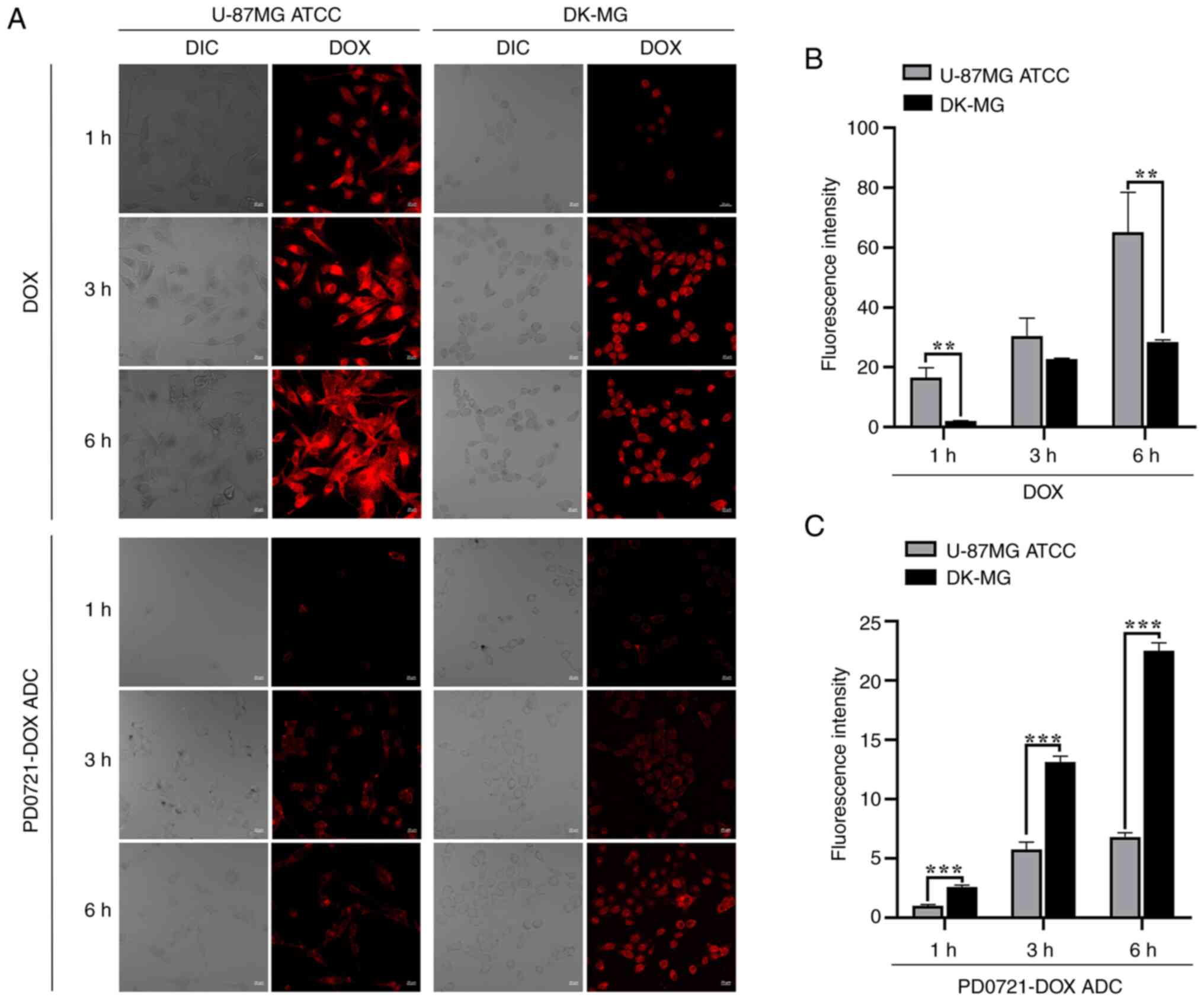
PD0721-DOX ADC is well internalized by
DK-MG cells
Microscopic observation revealed an increase in red
fluorescence in the U-87MG ATCC cell lines compared with that in
the DK-MG cells after DOX treatment (all P<0.01; Fig. 5A and B). However, a significant red
fluorescence was observed in DK-MG cells compared with U-87MG ATCC
cells following treatment with PD0721-DOX ADC, especially at 1, 3
and 6 h after treatment (all P<0.001; Fig. 5A and C), highlighting the excellent specificity
of PD0721-DOX ADC.
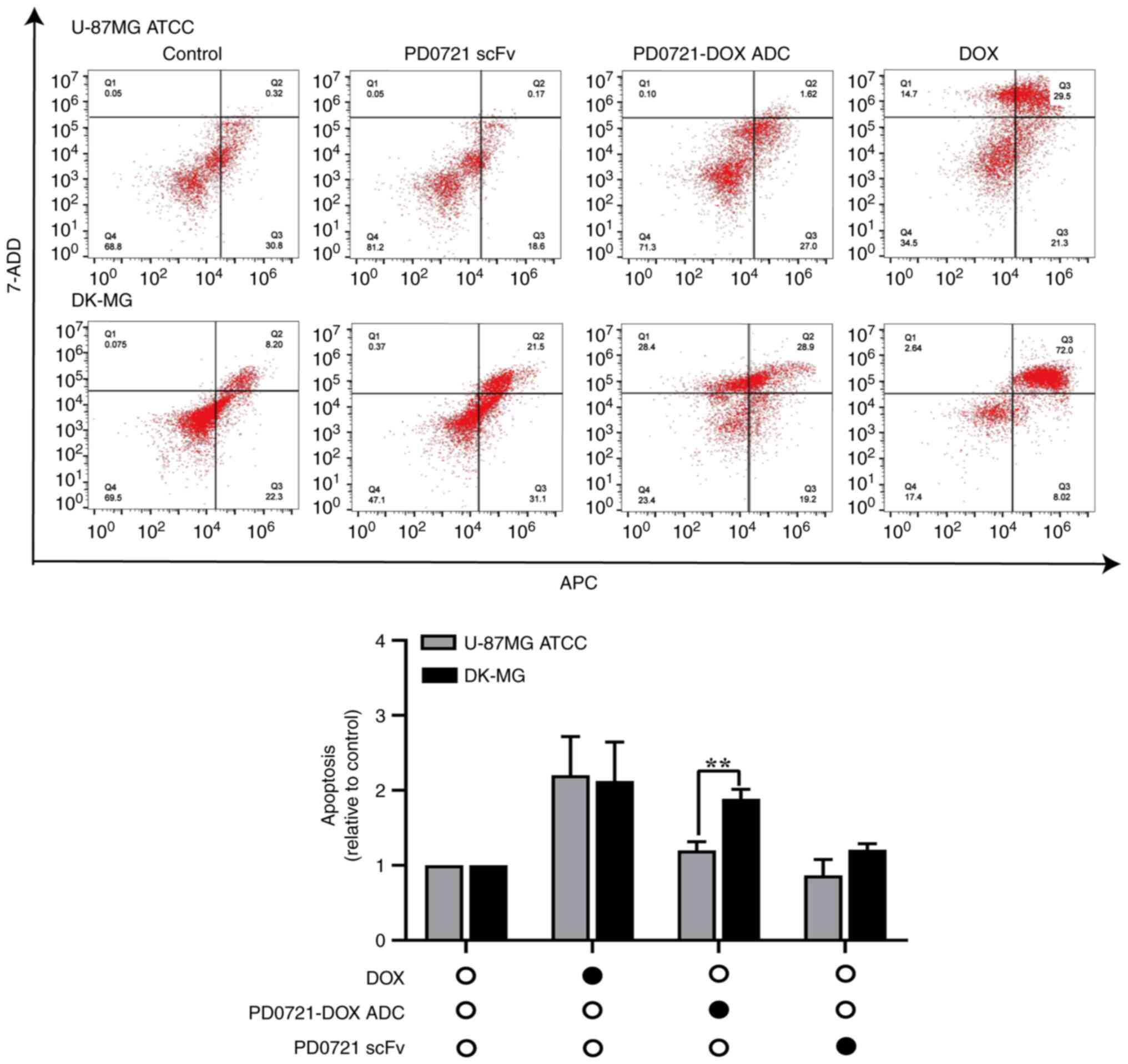
PD0721-DOX ADC promotes apoptosis in
DK-MG cells
The effect of PD0721-DOX ADC on inducing cell
apoptosis was further evaluated by flow cytometry. The results
showed that cell treatment with PD0721 scFv and DOX alone could not
cause significant differences between DK-MG and U-87MG ATCC cells.
However, PD0721-DOX ADC notably promoted the apoptosis of DK-MG
cells compared with U-87MG ATCC cells (P<0.01; Fig. 6), thus suggesting that PD0721-DOX
ADC could exert a strong anti-GBM activity. The aforementioned
findings highlighted the targeting specificity of PD0721-DOX ADC
towards EGFRvIII and its potential as an anti-GBM factor.
Discussion
Chemotherapeutic drugs commonly exhibit strong
cytotoxicity against tumor cells. However, they can also cause
damage to normal cells (28). ADCs
have the potential to enhance the efficacy of chemotherapeutic
drugs against tumor cells, while minimizing their toxicity on
normal cells (23,29). The present study aimed to describe
the construction of a PD0721-DOX ADC through the chemical coupling
of PD0721 scFv with DOX. Furthermore, it aimed to investigate the
targeting ability and apoptosis-inducing potential of PD0721-DOX
ADC in vitro in DK-MG and U-87MG ATCC cells. The PD0721-DOX
ADC was developed via using a chemical method to couple PD0721 scFv
with DOX. The in vitro experiments verified that the
PD0721-DOX ADC was efficiently internalized by GBM cells and
exhibited excellent targeting ability towards EGFRvIII-expressing
DK-MG cells. Furthermore, PD0721-DOX ADC could notably enhance
cytotoxicity and apoptosis in DK-MG cells compared with U-87MG ATCC
cells.
Given the crucial role of EGFRvIII in GBM, in a
previous study of our laboratory, novel monoclonal antibodies were
generated and their efficiency was compared with that of
commercially available antibodies (24,25).
Emerging evidence has suggested that dextran is a high-molecular
polysaccharide compound formed by interconnecting glucose through
1,6-glycosidic bonds. Due to its non-toxicity, water solubility and
good biocompatibility, dextran has been used extensively in the
biomedical and pharmaceutical fields (30). Herein, Dex T-10 was utilized as a
linker for the preparation of PD0721-DOX ADC. Following conjugation
of PD0721 scFv with DOX, the UV-Vis spectrum analysis revealed a
significant red shift in the maximum absorption, primarily
attributed to the presence of two aldehyde groups in oxidized Dex
T-10. The latter could undergo condensation with the amino groups
in DOX and PD0721 scFv, thus forming a covalent cross-linking
structure, known as the Schiff base (31). As a result, the molecular weight
and the degree of conjugation were increased. Therefore, the UV-Vis
absorption displayed a pronounced red-shift. The aforementioned
results verified the successful coupling of PD0721 scFv with DOX.
Based on UV-Vis, the DAR of PD0721 scFv and DOX was estimated to be
9.23:1.
In establishing the dosage parameters for the
experiments, the present study initially conducted a series of
preliminary tests to ascertain the effective dose range of the
PD0721-DOX ADC. Acknowledging the complexities involved in
preparing PD0721-DOX ADC samples, a concentration range was
carefully selected, specifically 0, 5, 10 and 20 µg/ml, for
thorough investigation. Furthermore, in determining the optimal
treatment duration, the preliminary experiments revealed that the
PD0721-DOX ADC antibody gradually exhibited increased cytotoxicity
over time. Consequently, a different treatment period in was chosen
for experiments to more effectively demonstrate the cytotoxic
efficacy of PD0721-DOX ADC against cancer cells. CCK-8 assays were
carried out to assess the cytotoxicity effect of PD0721-DOX ADC on
GBM cell lines. Therefore, PD0721-DOX ADC significantly inhibited
the proliferation of DK-MG cells compared with U-87MG ATCC cells,
thus indicating a particular cytotoxic effect of PD0721-DOX ADC on
DK-MG cells.
The mechanism of action of ADC includes two main
processes, namely its specifically binding to the target and
subsequent internalization of the antibody drugs (32). Following internalization, ADCs are
transported intracellularly from the early to the late endosome and
eventually reach the lysosomal compartment, where the cytotoxic
payload is released to promote cell death. (22) In the present study, the
immunofluorescence results detected green (PD0721 scFv) and red
fluorescence (DOX) on the surface of DK-MG cells. By contrast, due
to the absence of the EGFRvIII antigen on their surface, U-87MG
ATCC cells did not exhibit noticeable green or red fluorescence,
thus indicating that PD0721-DOX ADC could directly target
EGFRvIII-expressing cells. Further, the cell internalization
results demonstrated superior and faster entry of PD0721-DOX ADC
into DK-MG cells, compared with U-87MG ATCC cells. To minimize
experimental errors, an Annexin V-APC/7-AAD apoptosis detection kit
was utilized to detect apoptosis in PD0721-DOX ADC-treated cancer
cells. The results demonstrated that PD0721-DOX ADC effectively
induced DK-MG cell apoptosis, thus supporting its potent anti-GBM
activity. Furthermore, the variations in DOX levels between the two
cell types may be attributed to various factors, such as cell type
distinctions and drug transporters, which will be the focus of
further comprehensive examination and analysis.
However, the present study had some limitations.
Firstly, in vivo experiments and in vitro
(co-cultivation of DK-MG and U-87MG ATCC cells) should be performed
to validate the findings of the current study and provide
additional insights beyond the current in vitro
verification. Although co-culture of the two types of cells was
attempted, difficulties were encountered in distinguishing them
under the microscope. Despite these challenges there will be
further exploration of strategies and methodologies employed in the
co-culturing of the two cell types. Second, the experimental
conditions should be further optimized to enhance the targeting
specificity and anti-GBM effect of PD0721-DOX ADC in DK-MG cells.
However, PD0721-DOX ADC could offer a novel therapeutic approach
for malignant tumors and lays a foundation for the clinical
application of DOX.
In summary, in the present study a PD0721-DOX ADC
delivery system was constructed through conjugating PD0721 scFv
with DOX using Dex T-10 to enhance the targeting capacity of DOX
against EGFRvIII. The results demonstrated that PD0721-DOX ADC
exhibited effective targeting against the EGFRvIII antigen, while
it could significantly promote cytotoxicity and cell apoptosis. The
findings hold potential implications for the advancement of ADC and
DOX development and the treatment of GBM in clinical practice.
Acknowledgements
Not applicable.
Funding
Funding: The present study was supported by grants from the
project of the National Natural Science Foundation of China (grant
no. 82360817), Guizhou Science and Technology Department [grant
nos. ZK(2022) key 037, (2021)5619 and (2023)006].
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
SC and TL conceived the present study. YZ and DL
were responsible for designing the study and for data
authentication. MH and HL were responsible for data analysis,
writing and original draft preparation. LZ and YW were responsible
for the assessment of all the raw data. SC and TL were responsible
for project supervision and administration. LZ and TL were
responsible for funding acquisition. MH and TL confirm the
authenticity of all the raw data. All authors have read and
approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Tan AC, Ashley DM, López GY, Malinzak M,
Friedman HS and Khasraw M: Management of glioblastoma: State of the
art and future directions. CA Cancer J Clin. 70:299–312.
2020.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Sung H, Ferlay J, Siegel RL, Laversanne M,
Soerjomataram I, Jemal A and Bray F: Global cancer statistics 2020:
GLOBOCAN estimates of incidence and mortality worldwide for 36
cancers in 185 countries. CA Cancer J Clin. 71:209–249.
2021.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Avazzadeh R, Vasheghani-Farahani E,
Soleimani M, Amanpour S and Sadeghi M: Synthesis and application of
magnetite dextran-spermine nanoparticles in breast cancer
hyperthermia. Prog Biomater. 6:75–84. 2017.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Tang L, Jiang WH, Wu L, Yu XL, He Z, Shan
WG, Fu LL, Zhang ZH and Zhao YC: TPGS2000-DOX prodrug micelles for
improving breast cancer therapy. Int J Nanomedicine. 16:7875–7890.
2021.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Yang N, Ma HY, Jiang Z, Niu LH, Zhang XS,
Liu YY, Wang YH, Cheng ST, Deng Y, Qi HY and Wang ZR: Dosing
depending on SIRT3 activity attenuates doxorubicin-induced
cardiotoxicity via elevated tolerance against mitochondrial
dysfunction and oxidative stress. Biochem Biophys Res Commun.
517:111–117. 2019.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Aljobaily N, Viereckl MJ, Hydock DS,
Aljobaily H, Wu TY, Busekrus R, Jones B, Alberson J and Han Y:
Creatine alleviates doxorubicin-induced liver damage by inhibiting
liver fibrosis, inflammation, oxidative stress and cellular
senescence. Nutrients. 13(41)2020.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Guo Y, Ding YY, Zhang T and An HL:
Sinapine reverses multi-drug resistance in MCF-7/dox cancer cells
by downregulating FGFR4/FRS2α-ERK1/2 pathway-mediated NF-κB
activation. Phytomedicine. 23:267–273. 2016.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Santos EDS, Nogueira KAB, Fernandes LCC,
Martins JRP, Reis AVF, Neto JBV, Júnior I, Pessoa C, Petrilli R and
Eloy JO: EGFR targeting for cancer therapy: Pharmacology and
immunoconjugates with drugs and nanoparticles. Int J Pharm.
592(120082)2021.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Li MF, Yang JL, Zhang LH, Tu SF, Zhou X,
Tan Z, Zhou WJ, He YJ and Li YH: A low-molecular-weight compound
exerts anticancer activity against breast and lung cancers by
disrupting EGFR/Eps8 complex formation. J Exp Clin Cancer Res.
38(211)2019.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Sigismund S, Avanzato D and Lanzetti L:
Emerging functions of the EGFR in cancer. Mol Oncol. 12:3–20.
2018.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Hampton KK and Craven RJ: Pathways driving
the endocytosis of mutant and wild-type EGFR in cancer.
Oncoscience. 1:504–512. 2014.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Richards KN, Zweidler-McKay PA, Van Roy N,
Speleman F, Trevino J, Zage PE and Hughes DP: Signaling of ERBB
receptor tyrosine kinases promotes neuroblastoma growth in vitro
and in vivo. Cancer. 116:3233–3243. 2010.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Gan HK, Cvrljevic AN and Johns TG: The
epidermal growth factor receptor variant III (EGFRvIII): Where wild
things are altered. FEBS J. 280:5350–5370. 2013.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Guo G, Gong K, Wohlfeld B, Hatanpaa KJ,
Zhao D and Habib AA: Ligand-independent EGFR signaling. Cancer Res.
75:3436–3441. 2015.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Zheng Y, Ma Y, Yue H, Liu GZ and Han SY:
EGFRvIII epigenetically regulates ARHI to promote glioma cell
proliferation and migration. Exp Mol Pathol.
112(104344)2020.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Yang K, Ren X, Tao L, Wang P, Jiang H,
Shen L, Zhao Y, Cui Y, Li M and Lin S: Prognostic implications of
epidermal growth factor receptor variant III expression and nuclear
translocation in Chinese human gliomas. Chin J Cancer Res.
31:188–202. 2019.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Zhang Z, Jiang J, Wu X, Zhang M, Luo D,
Zhang R, Li S, He Y, Bian H and Chen Z: Chimeric antigen receptor T
cell targeting EGFRvIII for metastatic lung cancer therapy. Front
Med. 13:57–68. 2019.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Bagley SJ and O'Rourke DM: Clinical
investigation of CAR T cells for solid tumors: Lessons learned and
future directions. Pharmacol Ther. 205(107419)2020.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Wee P and Wang Z: Epidermal growth factor
receptor cell proliferation signaling pathways. Cancers.
9(52)2017.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Nagayama A, Ellisen LW, Chabner B and
Bardia A: Antibody-drug conjugates for the treatment of solid
tumors: Clinical experience and latest developments. Target Oncol.
12:719–739. 2017.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Sievers EL and Senter PD: Antibody-drug
conjugates in cancer therapy. Annu Rev Med. 64:15–29.
2013.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Okajima D, Yasuda S, Maejima T, Karibe T,
Sakurai K, Aida T, Toki T, Yamaguchi J, Kitamura M, Kamei R, et al:
Datopotamab deruxtecan, a novel TROP2-directed antibody-drug
conjugate, demonstrates potent antitumor activity by efficient drug
delivery to tumor cells. Mol Cancer Ther. 20:2329–2340.
2021.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Hafeez U, Parakh S, Gan HK and Scott AM:
Antibody-drug conjugates for cancer therapy. Molecules.
25(4764)2020.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Zhang YB, Wu ZX, He B, Yang C, Li YJ and
Liu T: Establishment and optimization of an ELISA for affinity
detection of single-chain antibodies to EGFRvIII. Xi Bao Yu Fen Zi
Mian Yi Xue Za Zhi. 37:73–78. 2021.PubMed/NCBI(In Chinese).
|
|
25
|
Zhang YB, Ye LF, Wu ZX, Xue WN, He B, Yang
C, Li YJ, Wang YL and Liu T: Optimization and identification of
prokaryotic expression conditions of PD0721 single-chain antibody
in vitro. J Food Sci Biotech. 40:42–49. 2021.
|
|
26
|
Struve N, Riedel M, Schulte A, Rieckmann
T, Grob TJ, Gal A, Rothkamm K, Lamszus K, Petersen C, Dikomey E and
Kriegs M: EGFRvIII does not affect radiosensitivity with or without
gefitinib treatment in glioblastoma cells. Oncotarget.
6:33867–33877. 2015.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Allen M, Bjerke M, Edlund H, Nelander S
and Westermark B: Origin of the U87MG glioma cell line: Good news
and bad news. Sci Transl Med. 8(354re3)2016.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Zraik IM and Heß-Busch Y: Management of
chemotherapy side effects and their long-term sequelae. Urologe A.
60:862–871. 2021.PubMed/NCBI View Article : Google Scholar : (In German).
|
|
29
|
Fu ZW, Li SJ, Han SF, Shi C and Zhang Y:
Antibody drug conjugate: The ‘biological missile’ for targeted
cancer therapy. Signal Transduct Target Ther. 7(93)2022.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Hu QB, Lu YJ and Luo YC: Recent advances
in dextran-based drug delivery systems: From fabrication strategies
to applications. Carbohydr Polym. 264(117999)2021.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Helal MH, Al-Mudaris ZA, Al-Douh MH, Osman
H, Wahab HA, Alnajjar BO, Abdallah HH and Abdul Majid AM:
Diaminobenzene schiff base, a novel class of DNA minor groove
binder. Int J Oncol. 41:504–510. 2012.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Marei HE, Cenciarelli C and Hasan A:
Potential of antibody-drug conjugates (ADCs) for cancer therapy.
Cancer Cell Int. 22(255)2022.PubMed/NCBI View Article : Google Scholar
|