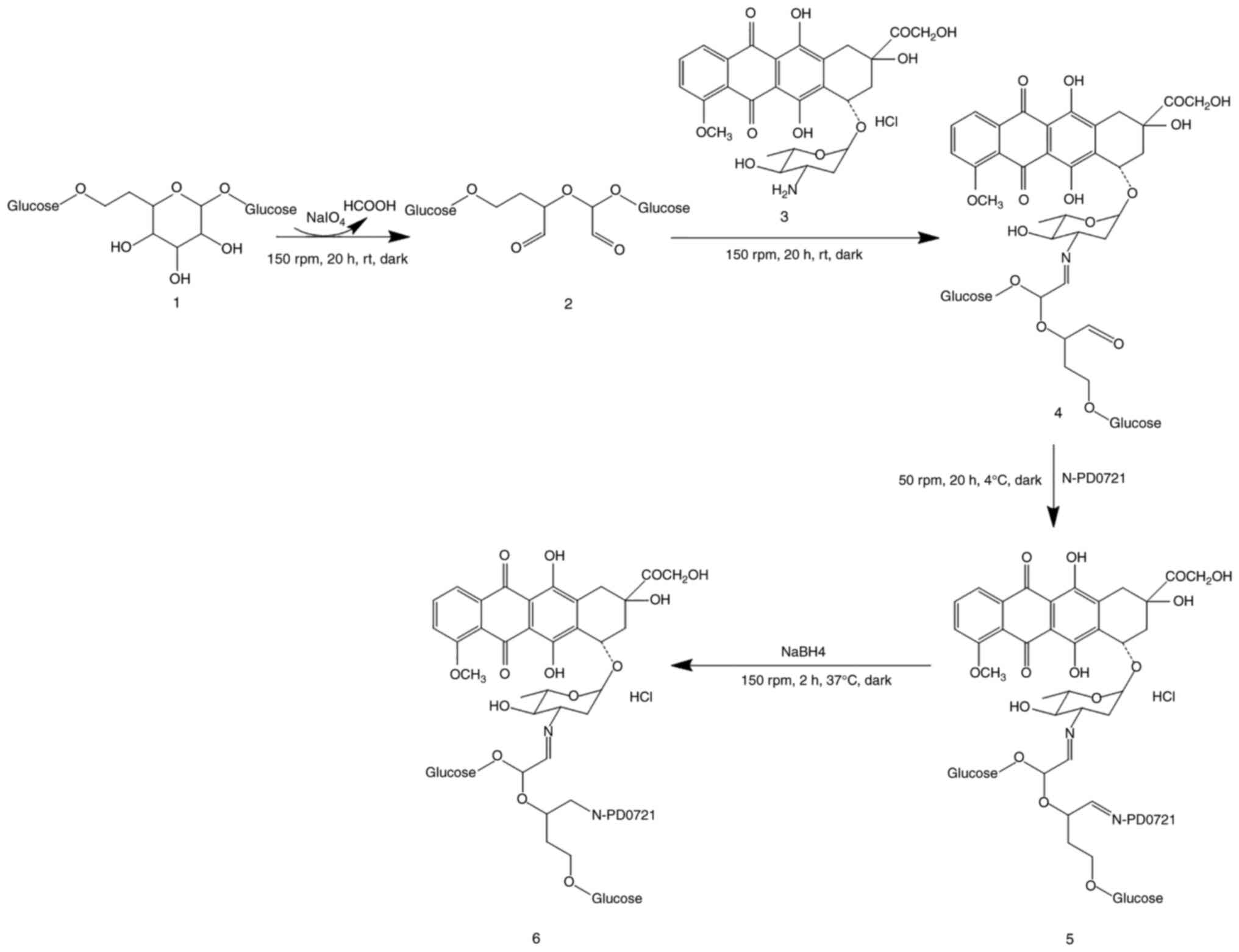
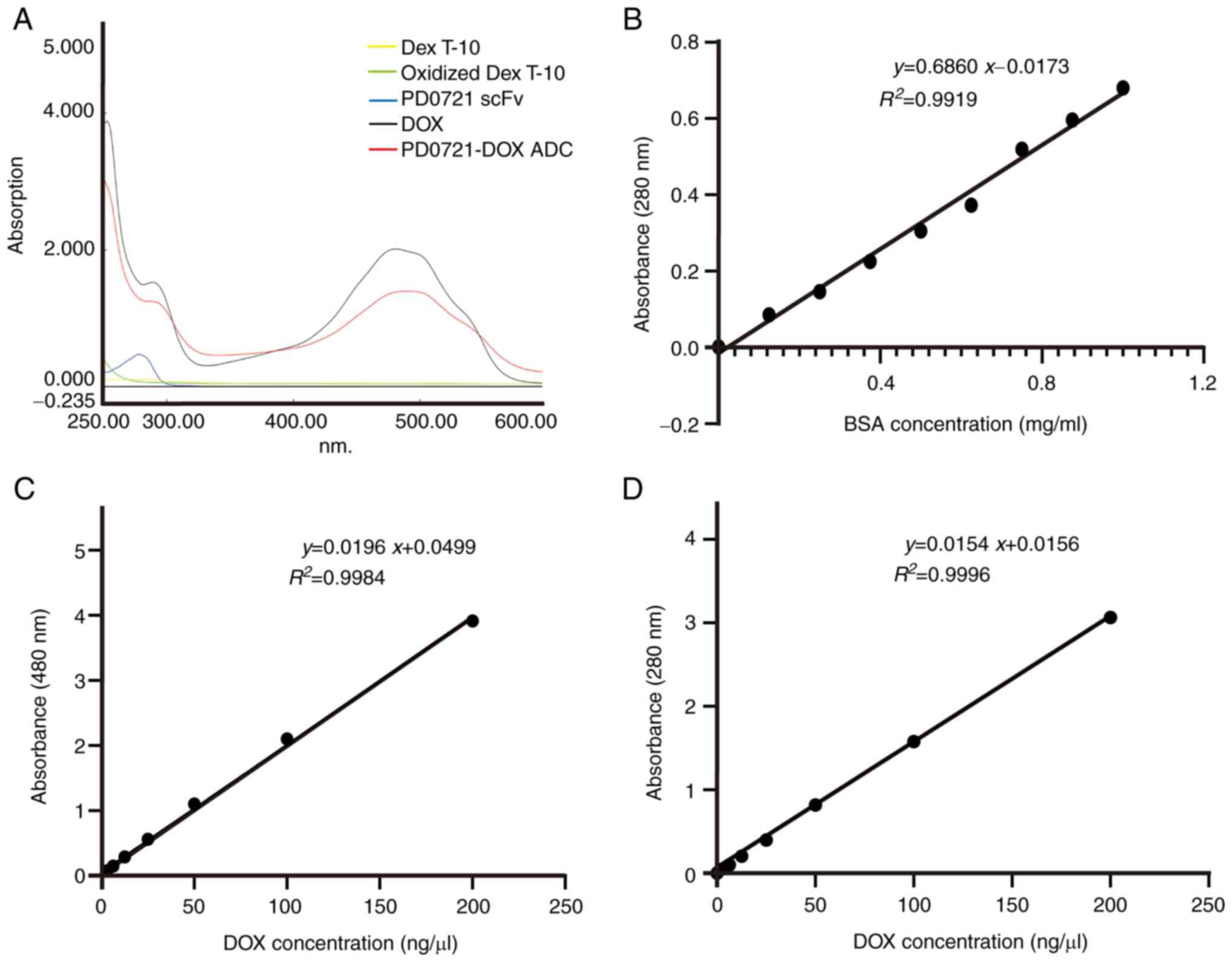
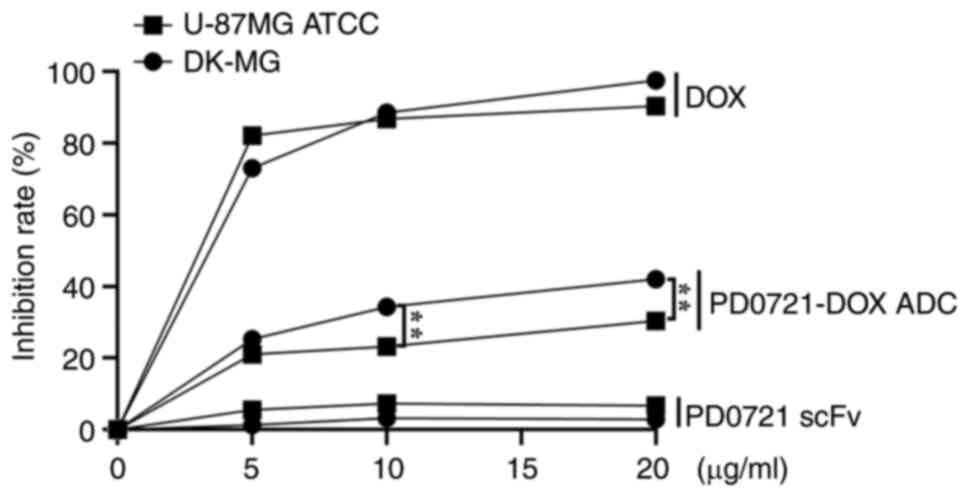
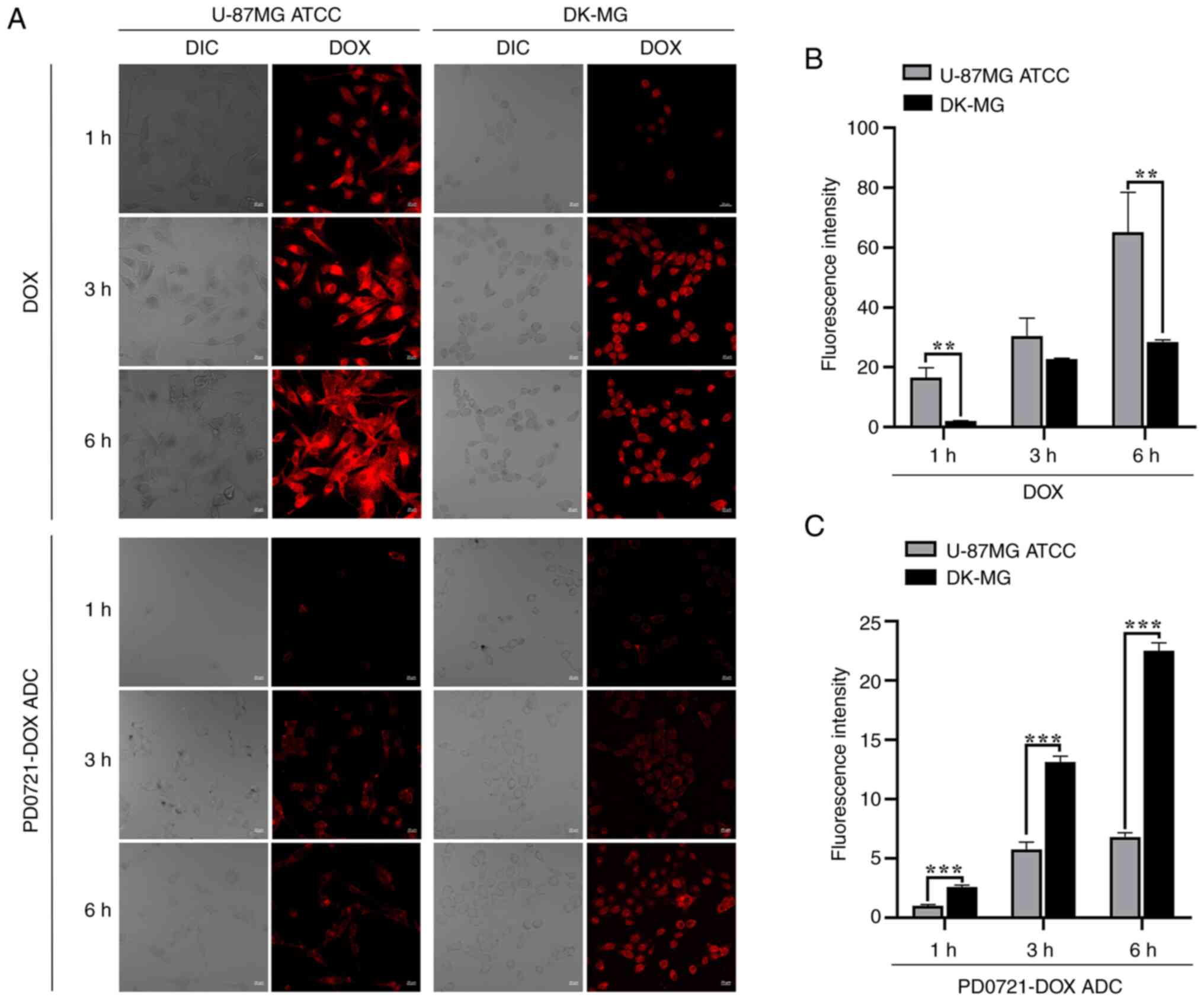
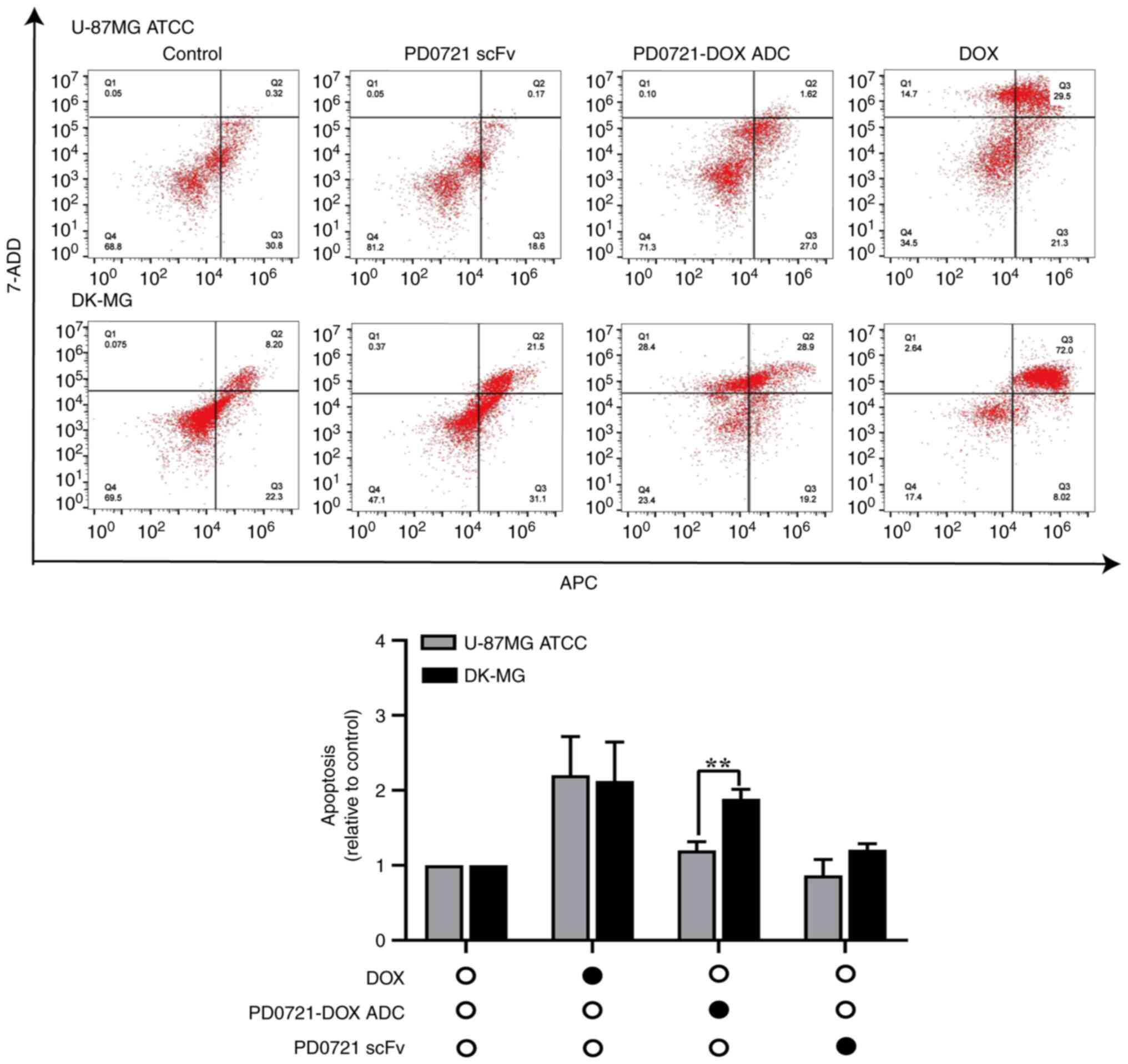
|
1
|
Tan AC, Ashley DM, López GY, Malinzak M,
Friedman HS and Khasraw M: Management of glioblastoma: State of the
art and future directions. CA Cancer J Clin. 70:299–312.
2020.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Sung H, Ferlay J, Siegel RL, Laversanne M,
Soerjomataram I, Jemal A and Bray F: Global cancer statistics 2020:
GLOBOCAN estimates of incidence and mortality worldwide for 36
cancers in 185 countries. CA Cancer J Clin. 71:209–249.
2021.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Avazzadeh R, Vasheghani-Farahani E,
Soleimani M, Amanpour S and Sadeghi M: Synthesis and application of
magnetite dextran-spermine nanoparticles in breast cancer
hyperthermia. Prog Biomater. 6:75–84. 2017.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Tang L, Jiang WH, Wu L, Yu XL, He Z, Shan
WG, Fu LL, Zhang ZH and Zhao YC: TPGS2000-DOX prodrug micelles for
improving breast cancer therapy. Int J Nanomedicine. 16:7875–7890.
2021.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Yang N, Ma HY, Jiang Z, Niu LH, Zhang XS,
Liu YY, Wang YH, Cheng ST, Deng Y, Qi HY and Wang ZR: Dosing
depending on SIRT3 activity attenuates doxorubicin-induced
cardiotoxicity via elevated tolerance against mitochondrial
dysfunction and oxidative stress. Biochem Biophys Res Commun.
517:111–117. 2019.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Aljobaily N, Viereckl MJ, Hydock DS,
Aljobaily H, Wu TY, Busekrus R, Jones B, Alberson J and Han Y:
Creatine alleviates doxorubicin-induced liver damage by inhibiting
liver fibrosis, inflammation, oxidative stress and cellular
senescence. Nutrients. 13(41)2020.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Guo Y, Ding YY, Zhang T and An HL:
Sinapine reverses multi-drug resistance in MCF-7/dox cancer cells
by downregulating FGFR4/FRS2α-ERK1/2 pathway-mediated NF-κB
activation. Phytomedicine. 23:267–273. 2016.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Santos EDS, Nogueira KAB, Fernandes LCC,
Martins JRP, Reis AVF, Neto JBV, Júnior I, Pessoa C, Petrilli R and
Eloy JO: EGFR targeting for cancer therapy: Pharmacology and
immunoconjugates with drugs and nanoparticles. Int J Pharm.
592(120082)2021.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Li MF, Yang JL, Zhang LH, Tu SF, Zhou X,
Tan Z, Zhou WJ, He YJ and Li YH: A low-molecular-weight compound
exerts anticancer activity against breast and lung cancers by
disrupting EGFR/Eps8 complex formation. J Exp Clin Cancer Res.
38(211)2019.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Sigismund S, Avanzato D and Lanzetti L:
Emerging functions of the EGFR in cancer. Mol Oncol. 12:3–20.
2018.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Hampton KK and Craven RJ: Pathways driving
the endocytosis of mutant and wild-type EGFR in cancer.
Oncoscience. 1:504–512. 2014.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Richards KN, Zweidler-McKay PA, Van Roy N,
Speleman F, Trevino J, Zage PE and Hughes DP: Signaling of ERBB
receptor tyrosine kinases promotes neuroblastoma growth in vitro
and in vivo. Cancer. 116:3233–3243. 2010.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Gan HK, Cvrljevic AN and Johns TG: The
epidermal growth factor receptor variant III (EGFRvIII): Where wild
things are altered. FEBS J. 280:5350–5370. 2013.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Guo G, Gong K, Wohlfeld B, Hatanpaa KJ,
Zhao D and Habib AA: Ligand-independent EGFR signaling. Cancer Res.
75:3436–3441. 2015.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Zheng Y, Ma Y, Yue H, Liu GZ and Han SY:
EGFRvIII epigenetically regulates ARHI to promote glioma cell
proliferation and migration. Exp Mol Pathol.
112(104344)2020.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Yang K, Ren X, Tao L, Wang P, Jiang H,
Shen L, Zhao Y, Cui Y, Li M and Lin S: Prognostic implications of
epidermal growth factor receptor variant III expression and nuclear
translocation in Chinese human gliomas. Chin J Cancer Res.
31:188–202. 2019.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Zhang Z, Jiang J, Wu X, Zhang M, Luo D,
Zhang R, Li S, He Y, Bian H and Chen Z: Chimeric antigen receptor T
cell targeting EGFRvIII for metastatic lung cancer therapy. Front
Med. 13:57–68. 2019.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Bagley SJ and O'Rourke DM: Clinical
investigation of CAR T cells for solid tumors: Lessons learned and
future directions. Pharmacol Ther. 205(107419)2020.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Wee P and Wang Z: Epidermal growth factor
receptor cell proliferation signaling pathways. Cancers.
9(52)2017.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Nagayama A, Ellisen LW, Chabner B and
Bardia A: Antibody-drug conjugates for the treatment of solid
tumors: Clinical experience and latest developments. Target Oncol.
12:719–739. 2017.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Sievers EL and Senter PD: Antibody-drug
conjugates in cancer therapy. Annu Rev Med. 64:15–29.
2013.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Okajima D, Yasuda S, Maejima T, Karibe T,
Sakurai K, Aida T, Toki T, Yamaguchi J, Kitamura M, Kamei R, et al:
Datopotamab deruxtecan, a novel TROP2-directed antibody-drug
conjugate, demonstrates potent antitumor activity by efficient drug
delivery to tumor cells. Mol Cancer Ther. 20:2329–2340.
2021.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Hafeez U, Parakh S, Gan HK and Scott AM:
Antibody-drug conjugates for cancer therapy. Molecules.
25(4764)2020.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Zhang YB, Wu ZX, He B, Yang C, Li YJ and
Liu T: Establishment and optimization of an ELISA for affinity
detection of single-chain antibodies to EGFRvIII. Xi Bao Yu Fen Zi
Mian Yi Xue Za Zhi. 37:73–78. 2021.PubMed/NCBI(In Chinese).
|
|
25
|
Zhang YB, Ye LF, Wu ZX, Xue WN, He B, Yang
C, Li YJ, Wang YL and Liu T: Optimization and identification of
prokaryotic expression conditions of PD0721 single-chain antibody
in vitro. J Food Sci Biotech. 40:42–49. 2021.
|
|
26
|
Struve N, Riedel M, Schulte A, Rieckmann
T, Grob TJ, Gal A, Rothkamm K, Lamszus K, Petersen C, Dikomey E and
Kriegs M: EGFRvIII does not affect radiosensitivity with or without
gefitinib treatment in glioblastoma cells. Oncotarget.
6:33867–33877. 2015.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Allen M, Bjerke M, Edlund H, Nelander S
and Westermark B: Origin of the U87MG glioma cell line: Good news
and bad news. Sci Transl Med. 8(354re3)2016.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Zraik IM and Heß-Busch Y: Management of
chemotherapy side effects and their long-term sequelae. Urologe A.
60:862–871. 2021.PubMed/NCBI View Article : Google Scholar : (In German).
|
|
29
|
Fu ZW, Li SJ, Han SF, Shi C and Zhang Y:
Antibody drug conjugate: The ‘biological missile’ for targeted
cancer therapy. Signal Transduct Target Ther. 7(93)2022.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Hu QB, Lu YJ and Luo YC: Recent advances
in dextran-based drug delivery systems: From fabrication strategies
to applications. Carbohydr Polym. 264(117999)2021.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Helal MH, Al-Mudaris ZA, Al-Douh MH, Osman
H, Wahab HA, Alnajjar BO, Abdallah HH and Abdul Majid AM:
Diaminobenzene schiff base, a novel class of DNA minor groove
binder. Int J Oncol. 41:504–510. 2012.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Marei HE, Cenciarelli C and Hasan A:
Potential of antibody-drug conjugates (ADCs) for cancer therapy.
Cancer Cell Int. 22(255)2022.PubMed/NCBI View Article : Google Scholar
|