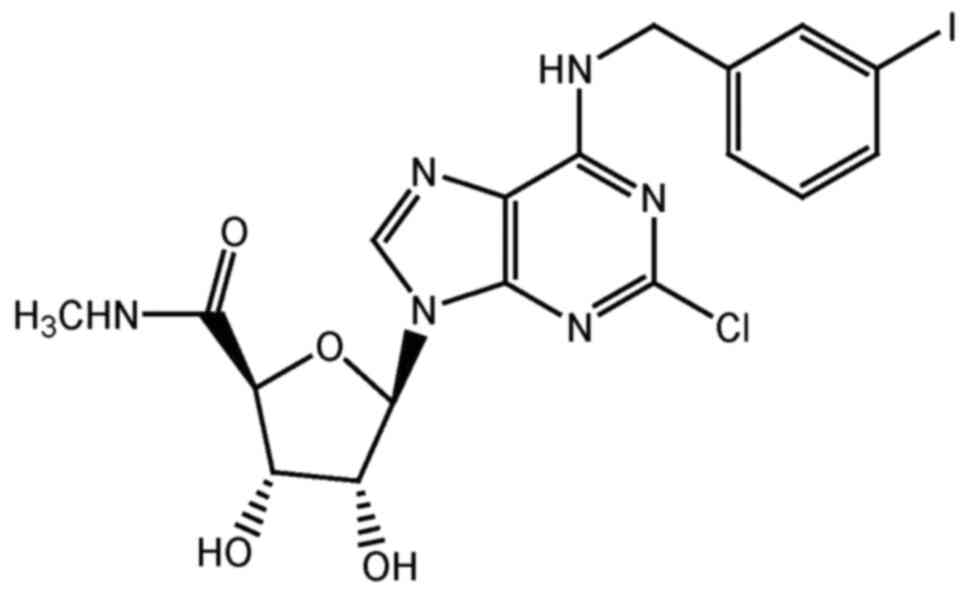
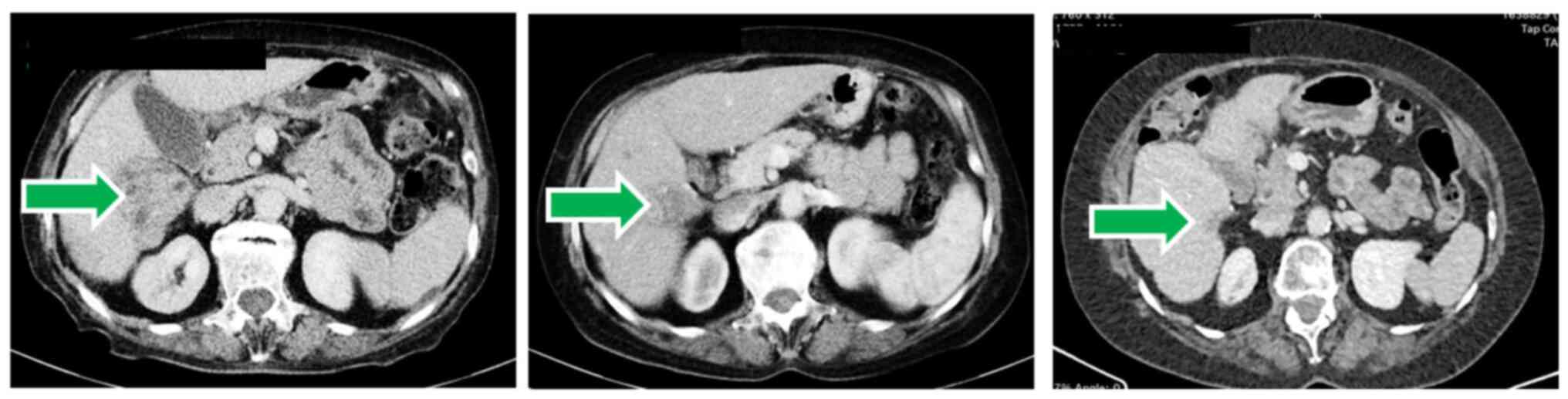
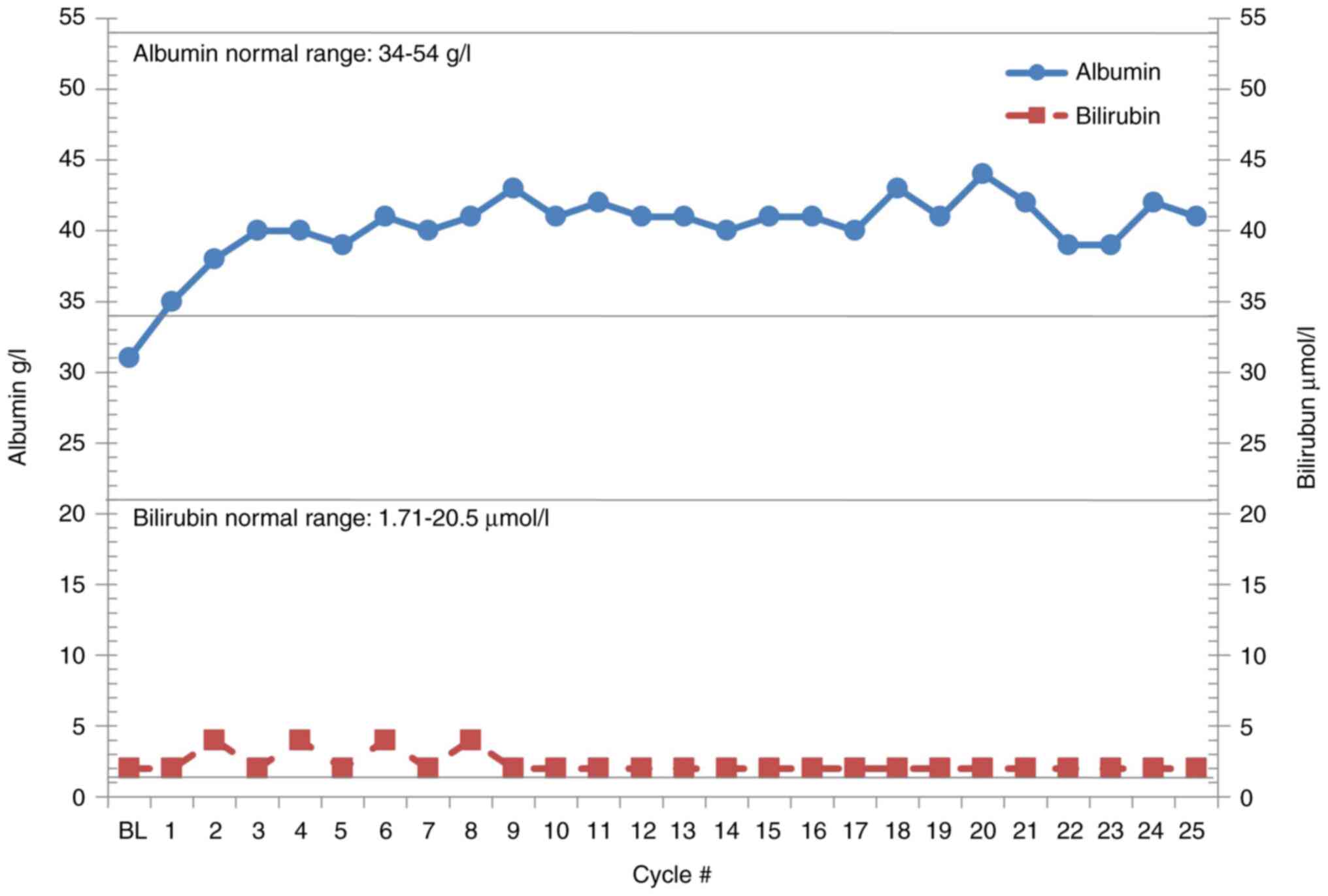
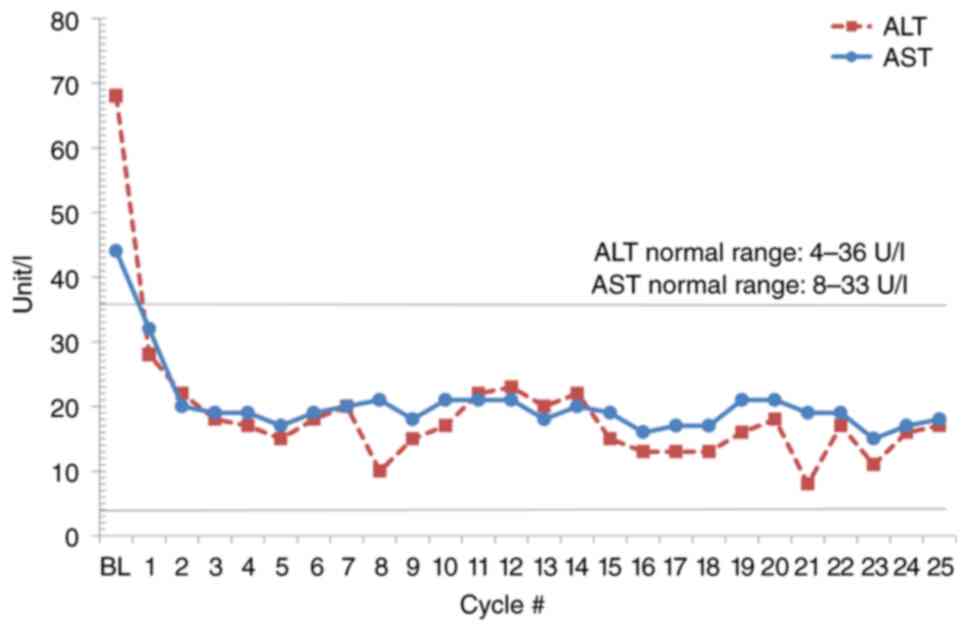
|
1
|
Sung H, Ferlay J, Siegel RL, Laversanne M,
Soerjomataram I, Jemal A and Bray F: Global cancer statistics 2020:
GLOBOCAN estimates of incidence and mortality worldwide for 36
cancers in 185 countries. CA Cancer J Clin. 71:209–249.
2021.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Rumgay H, Arnold M, Ferlay J, Lesi O,
Cabasag CJ, Vignat J, Laversanne M, McGlynn KA and Soerjomataram I:
Global burden of primary liver cancer in 2020 and predictions to
2040. J Hepatol. 77:1598–1606. 2022.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Ramadori G, Schleyer E and Armbrust T:
Safety of imatinib in patients with liver cirrhosis and
hepatocellular carcinoma. J Clin Oncol. 22:4244. 2004.
|
|
4
|
Shannon AH, Ruff SM and Pawlik TM: Expert
insights on current treatments for hepatocellular carcinoma:
Clinical and molecular approaches and bottlenecks to progress. J
Hepatocell Carcinoma. 9:1247–1261. 2022.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Tsoris A and Marlar CA: Use of the child
Pugh score in liver disease. In: StatPearls. Treasure Island (FL),
2021.
|
|
6
|
Granito A and Bolondi L: Non-transplant
therapies for patients with hepatocellular carcinoma and
Child-Pugh-Turcotte class B cirrhosis. Lancet Oncol. 18:e101–e112.
2017.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Sorafenib (package insert). Whippany, NJ:
Bayer Health Care Pharmaceuticals Inc; 2017.
|
|
8
|
Llovet JM, Ricci S, Mazzaferro V, Hilgard
P, Gane E, Blanc JF, de Oliveira AC, Santoro A, Raoul JL, Forner A,
et al: Sorafenib in advanced hepatocellular carcinoma. N Engl J
Med. 359:378–390. 2008.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Llovet JM, Di Bisceglie AM, Bruix J,
Kramer BS, Lencioni R, Zhu AX, Sherman M, Schwartz M, Lotze M,
Talwalkar J, et al: Design and endpoints of clinical trials in
hepatocellular carcinoma. J Natl Cancer Inst. 100:698–711.
2008.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Bar-Yehuda S, Stemmer SM, Madi L, Castel
D, Ochaion A, Cohen S, Barer F, Zabutti A, Perez-Liz G, Del Valle L
and Fishman P: The A3 adenosine receptor agonist CF102 induces
apoptosis of hepatocellular carcinoma via de-regulation of the Wnt
and NF-kappaB signal transduction pathways. Int J Oncol.
33:287–295. 2008.PubMed/NCBI
|
|
11
|
Cohen S, Stemmer SM, Zozulya G, Ochaion A,
Patoka R, Barer F, Bar-Yehuda S, Rath-Wolfson L, Jacobson KA and
Fishman P: CF102 an A3 adenosine receptor agonist mediates
anti-tumor and anti-inflammatory effects in the liver. J Cell
Physiol. 226:2438–2447. 2011.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Harish A, Hohana G, Fishman P, Arnon O and
Bar-Yehuda S: A3 adenosine receptor agonist potentiates natural
killer cell activity. Int J Oncol. 23:1245–1249. 2003.PubMed/NCBI
|
|
13
|
Stemmer SM, Benjaminov O, Medalia G,
Ciuraru NB, Silverman MH, Bar-Yehuda S, Fishman S, Harpaz Z,
Farbstein M, Cohen S, et al: CF102 for the treatment of
hepatocellular carcinoma: A phase I/II, open-label, dose-escalation
study. Oncologist. 18:25–26. 2013.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Stemmer SM, Manojlovic NS, Marinca MV,
Petrov P, Cherciu N, Ganea D, Ciuleanu TE, Pusca IA, Beg MS,
Purcell WT, et al: Namodenoson in advanced hepatocellular carcinoma
and Child-Pugh B cirrhosis: Randomized placebo-controlled clinical
trial. Cancers (Basel). 13(187)2021.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Fishman P, Stemmer SM, Bareket-Samish A,
Silverman MH and Kerns WD: Targeting the A3 adenosine receptor to
treat hepatocellular carcinoma: Anti-cancer and hepatoprotective
effects. Purinergic Signal. 19:513–522. 2023.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Shah RR, Morganroth J and Shah DR:
Hepatotoxicity of tyrosine kinase inhibitors: Clinical and
regulatory perspectives. Drug Saf. 36:491–503. 2013.PubMed/NCBI View Article : Google Scholar
|
|
17
|
National Library of Medicine (NLM):
Description of the ‘Namodenoson in the treatment of advanced
hepatocellular carcinoma in patients with Child-Pugh Class B7
cirrhosis (LIVERATION)’ study (NCT05201404). Clinical
Trials.gov ID, NCT05201404. NLM, Bethesda, MD, 2023. https://clinicaltrials.gov/study/NCT05201404?intr=namodenoson&rank=2&tab=table.
Accessed February 20, 2024.
|