Introduction
Gestational hypertension (GH) is a unique condition
that affects pregnant women, which predominantly occurs after 20
weeks of pregnancy and 2 weeks after delivery. Some patients with
GH also exhibit proteinuria or edema, and severe cases are
associated with headaches, blurred vision, upper abdominal pain,
convulsion and coma (1-3).
A pregnant woman is considered to have hypertension when their
systolic blood pressure (SBP) exceeds 140 mmHg or their diastolic
blood pressure (DBP) exceeds 90 mmHg. Being overweight,
pre-pregnancy hypertension, pregnancy with multiple births, chronic
diseases and/or poor diet (high salt and fat) are all risk factors
for GH (4,5). GH not only harms the mother, but can
also affect the growth and development of the fetus. Hypofunction
of the placenta caused by GH can lead to various complications,
such as intrauterine growth retardation, stillbirth, premature
delivery or asphyxia, and can damage the organs of newborns to
varying degrees (6,7). The fetal nervous system can also be
affected, resulting in adverse long-term cognitive outcomes in
infants (8). Therefore, if
hypertension occurs during pregnancy, pregnant women and their
families should pay attention to it and actively receive prenatal
examinations, in order to ensure timely detection of the disease
and reasonable treatment to minimize harm (9).
GH should be treated actively, whether hypertension
is present before or after pregnancy. It is generally considered
that if DBP exceeds 12.0 kPa (90 mmHg), in two measurements of the
same arm taken >6 h apart, it should be treated in a timely
manner. If blood pressure is >21.3/14.6 kPa (160/110 mmHg), this
is also an absolute indication for the use of antihypertensive
drugs even without clinical symptoms (10-13).
Clinical treatment measures generally include a low-salt diet,
ensuring a balance between work and rest, elimination of mental
over-stress and adequate sleep. The aforementioned general measures
can restore the blood pressure of patients with mild hypertension
to normal. If the aforementioned measures are ineffective, stepped
care can be given according to blood pressure level (14,15).
Calcium channel blockers, such as nifedipine, nicardipine and
diltiazem, can be applied in the first and second trimesters of
pregnancy, but should not be used ~2 weeks before labor. Since
calcium channel blockers can inhibit the contractile force of the
uterine smooth muscle and affect the progress of labor, they are
not suitable for patients in labor. In developed countries,
methyldopa (tablets, central sympathoinhibitor) is the first choice
of treatment for GH syndrome, and is recognized as the safest and
most effective therapeutic drug (16-18).
At present, no fetal toxicity has been found in response to
methyldopa, and it is the only drug that has been proven to be safe
after long-term follow-up into childhood. The only disadvantage is
that it has a strong sedative effect, which limits its use
(19). Sodium nitroprusside is a
powerful vasodilator, which is only suitable for pregnant women in
hypertensive crisis for which other antihypertensive drugs are
ineffective, and the prenatal application of this drug should not
exceed 4 h (20). Labetalol is an
α-adrenoceptor blocker, as well as a β-adrenoceptor blocker, which
is utilized to treat hypertension. Its principle is to block the
adrenoceptor, slow down ventricular rhythm and reduce peripheral
vascular resistance (21).
Notably, no antihypertensive drugs are completely safe for GH, but
allowing blood pressure to rise is considered more dangerous. Thus,
for the clinical treatment of GH, antihypertensive drugs targeted
at the conditions of different patients should be selected on the
basis of fully weighing advantages and disadvantages (22).
A careful decision should be made regarding the
clinical medication of patients with GH, because the effects and
side effects of drugs differ. Magnesium sulfate (MgSO4)
is the most commonly used drug for treating GH and preventing
severe eclampsia (23). The
clinical effects and toxicity of MgSO4 are related to
its concentration in the plasma. The disappearance of the patellar
reflex in mothers at plasma concentrations between 3.5-5 mmol/l
gives the first warning of impending toxicity. With careful
management and monitoring of MgSO4, maternal toxicity is
very rare (24). The use of
multiple drugs is a common strategy for clinical treatment, such as
MgSO4 + nifedipine (25). To identify an effective treatment
option, a randomized controlled study of MgSO4 in
combination with Labetalol for the treatment of GH was performed.
Subsequently, inflammatory factors, hemorheology, pregnancy outcome
and perinatal complications of the two groups were assessed, and
the effect of MgSO4 + labetalol on the clinical
treatment of patients with GH was further discussed. The present
study may provide reference for the clinical treatment of GH.
Methods and materials
Research objects
A total of 100 patients with GH, who were registered
in the Department of Obstetrics and Gynecology, Taicang TCM
Hospital Affiliated to Nanjing University of Chinese Medicine
between June 2020 and June 2022, were recruited to the present
study. The present study adhered to The Declaration of Helsinki,
and was approved and implemented by the Ethics Committee of Taicang
Hospital of TCM (approval no. 2022026). All of the patients
voluntarily participated and signed informed consent forms prior to
the study. During the intervention, the patients' right of privacy
and confidentiality was respected, and all information obtained
from the patients was kept strictly confidential.
The inclusion criteria were as follows: i) The
patients could provide complete clinical data; ii) they were 18-36
years old; iii) they were conscious and were able to communicate
normally; iv) they had not received any drug treatment; v) they had
a natural pregnancy; and vi) only a single fetus was observed.
The exclusion criteria were as follows: i) Patients
had severe cardiac, liver or renal insufficiency; ii) they were
severely malnourished; iii) they dropped out of the experiment and
did not complete the treatment process; and iv) they had recently
suffered from an infection.
Experimental instruments and
reagents
The experimental instruments used were as follows:
DHM-30D automatic height and weight scale (Zhengzhou Dingheng
Electronic Technology Co., Ltd.), diving mercury sphygmomanometer
(Yuwell Medical Equipment & Supply Co., Ltd.) and automatic
hemorheology analyzer SH210A (Shanghai Langyi Medical Equipment
Co., Ltd.).
The experimental reagents included: 5%
MgSO4 (Shandong Pingsen Biotechnology Co., Ltd.), 10%
glucose solution (Guizhou Tiandi Pharmaceutical Co., Ltd.), 25%
MgSO4 (Shandong Pingsen Biotech Co., Ltd.), 5% glucose
solution (Guizhou Tiandi Pharmaceutical Co., Ltd.), labetalol
(Shanghai Acebright Pharmaceuticals Group Co., Ltd.) and ELISA kits
for HMGB1 (cat. no. ml085468), homocysteine (Hcy; cat. no.
ml092715) and CysC (cat. no. ml003222) all from Shanghai
Enzyme-linked Biotechnology Co., Ltd.
Therapeutic methods
The patients in both groups were given routine basic
treatment, including sedation, oxygen inhalation and sodium
restriction. The patients' breathing, blood pressure and heart rate
were closely monitored, and timely treatment was carried out in
case of abnormal conditions of the mothers and infants.
In the Ctrl group, patients were administered
MgSO4. Briefly, 15 ml 5% MgSO4 and 20 ml 10%
glucose solution were mixed and administered via an intravenous
drip (1-2 g/h MgSO4) and, 50 ml 25% MgSO4 and
800 ml 5% glucose solution were mixed and administered via an
intravenous drip (1-2 g/h MgSO4) later the same day,
this was repeated daily, with a 1-week course of treatment.
In the Expt group, in addition to the drug
administered to the Ctrl group, the patients were given an
intravenous drip of 100 mg labetalol mixed with 250 ml 5% glucose
solution. After the patients' blood pressure was reduced to the
expected value (DBP, 90 mmHg) and stabilized, oral labetalol 100 mg
was administrated, three times a day until delivery (26).
Determination of basic parameters of
patients
First, patients were asked to remove their hats and
shoes, and their height and weight were determined according to the
unified side-face standard. During the measurement, the patients
were requested to stand in the center of the pedal of the DHM-30D
automatic height and weight scale, and to maintain a standing
position with eyes level ahead, arms down and heels together for 5
sec. Subsequently, the patients were allowed to rest for ~15 min,
and their SBP and DBP were measured using the diving mercury
sphygmomanometer. Notably, the blood pressure of both upper arms
was measured during the first measurement.
In addition, overnight-fasting venous blood was
collected from the patients in two groups before and after
treatment in the morning, and the supernatant was obtained after
centrifugation at 1,500 x g at 4˚C for 15 min. The inflammatory
factor indicators, high mobility group box-1 protein (HMGB1),
homocysteine (Hcy) and serum cystatin C (CysC), were detected by
ELISA according to manufacturer's protocols. The automatic
hemorheological analyzer SH210A was used to detect hemorheological
indicators, including whole blood viscosity (WBV), plasma viscosity
(PV), and hematocrit (HCT), of the two groups before and after
treatment. The pregnancy outcomes in the two groups, including
cesarean section, spontaneous vaginal delivery and postpartum
hemorrhage, were followed up. Perinatal complications, including
fetal intrauterine distress (FIUD), placental abruption, neonatal
asphyxia, premature birth and neonatal death, were also
recorded.
Statistical analysis
The research data were analyzed using SPSS 19.0 (IBM
Corp.), with measurement data presented as the mean ± standard
deviation and enumeration data as value (%). The patient's basic
information was analyzed using unpaired t-tests. Comparison of
pregnancy outcomes was analyzed using the χ2 test,
whereas comparison of perinatal complications was analyzed using
the Fisher's exact test. A mixed ANOVA was performed in every
indicator between Expt and Ctrl groups, pre and post treatment.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Study design and patient
enrollment
A total of 100 patients with GH were randomly
divided into the Expt group and Ctrl group (n=50 cases/group). In
the Expt group, the age of patients was 21-40 years, the mean age
was 26.78±5.54 years, the gestational age was 31-38 weeks, and the
average gestational age was 35.17±2.05 weeks. There were 32
primiparas and 18 multiparas. In the Ctrl group, the age, average
age, gestational age and average gestational age were 20-38 years,
25.59±6.14 years, 30-37 and 36.28±2.24 weeks, respectively. There
were 30 primiparas and 20 multiparas in the Ctrl group. There was
no significant difference in age, gestational age and pregnancy
history between the Expt group and the Ctrl group (P>0.05),
indicating comparability between the groups (Table I).
 | Table IBaseline data of the Expt and Ctrl
groups. |
Table I
Baseline data of the Expt and Ctrl
groups.
| Characteristic | Expt group | Ctrl group | P-value |
|---|
| Age, years | 26.78±5.54 | 25.59±6.14 | >0.05 |
| Gestational age,
weeks | 35.17±2.05 | 36.28±2.24 | >0.05 |
| Pregnancy
history | | | >0.05 |
|
Primiparas | 32 | 30 | |
|
Multiparas | 18 | 20 | |
Comparison of blood pressure values
between groups before and after treatment
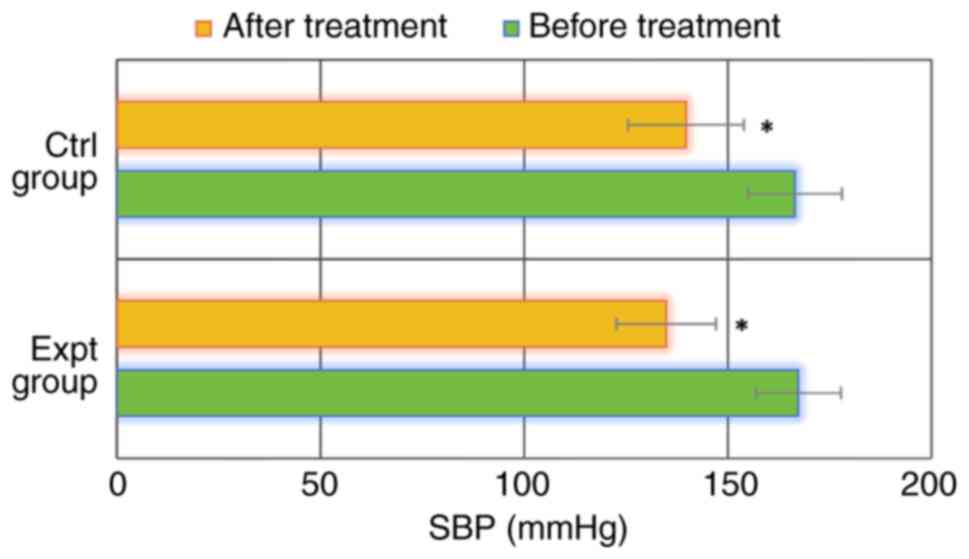
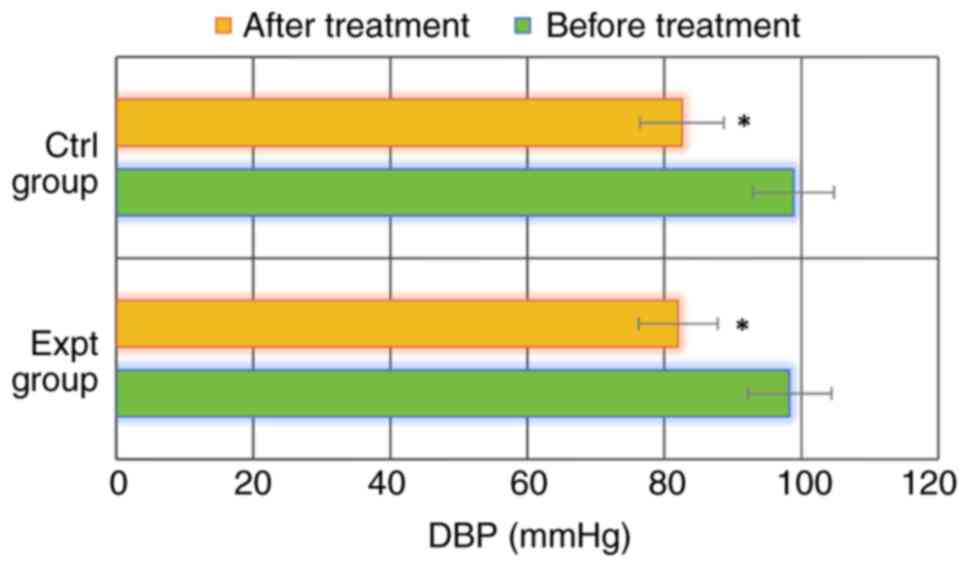
As shown in Figs. 1
and 2, SBP and DBP were
167.29±10.45 and 98.28±6.14 mmHg, respectively, in the Expt group
before treatment. SBP and DBP after treatment reached 134.81±12.25
and 82.03±5.77 mmHg, respectively. In the Ctrl group, SBP and DBP
were 166.51±11.53 and 98.84±5.93 mmHg before treatment, and
139.72±14.22 and 82.58±6.12 mmHg after treatment, respectively. SBP
and DBP in the Expt group before treatment were not significantly
different from those in the Ctrl group (P>0.05). In addition,
SBP and DBP in the Expt group after treatment were also not
significantly different from those in the Ctrl group (P>0.05).
Notably, SBP and DBP after treatment were significantly lower than
those before treatment in both groups (P<0.05). These data
indicated that both SBP and DBP were significantly reduced in
patients with GH treated with MgSO4, regardless of
whether they were also treated with labetalol or not.
Comparison of inflammatory factor
indicators between groups before and after treatment
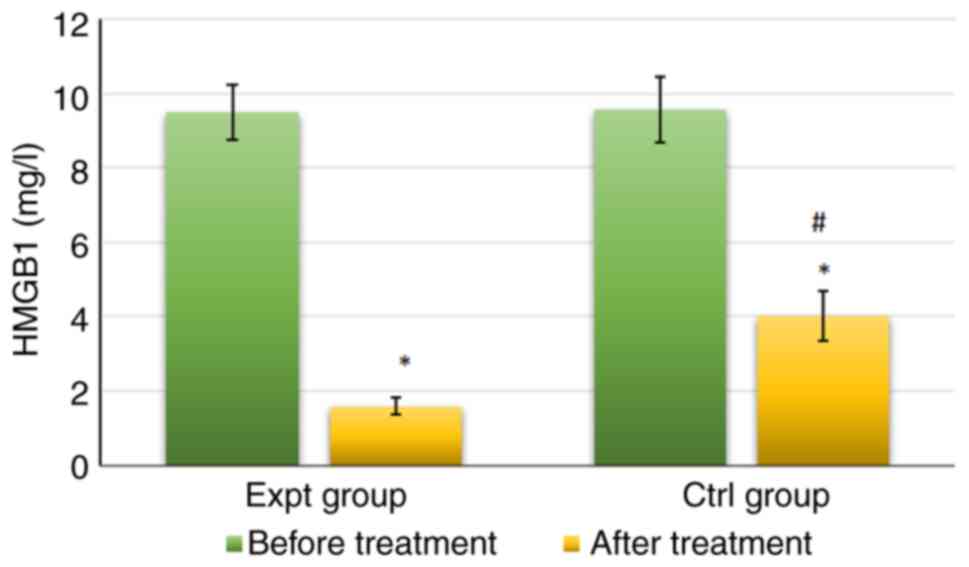
As shown in Fig. 3,
the levels of HMGB1in the Expt group were 9.48±0.75 mg/l before
treatment and 1.58±0.23 mg/l after treatment. The levels of HMGB1
in the Ctrl group were 9.57±0.88 mg/l before treatment and
4.02±0.68 mg/l after treatment. No significant difference was
observed in HMGB1 levels between the Expt and Ctrl groups before
treatment (P>0.05). By contrast, HMGB1 levels were significantly
lower in the Expt group than that in Ctrl group after treatment
(P<0.05). HMGB1 levels after treatment were also significantly
lower than those before treatment in both groups (P<0.05).
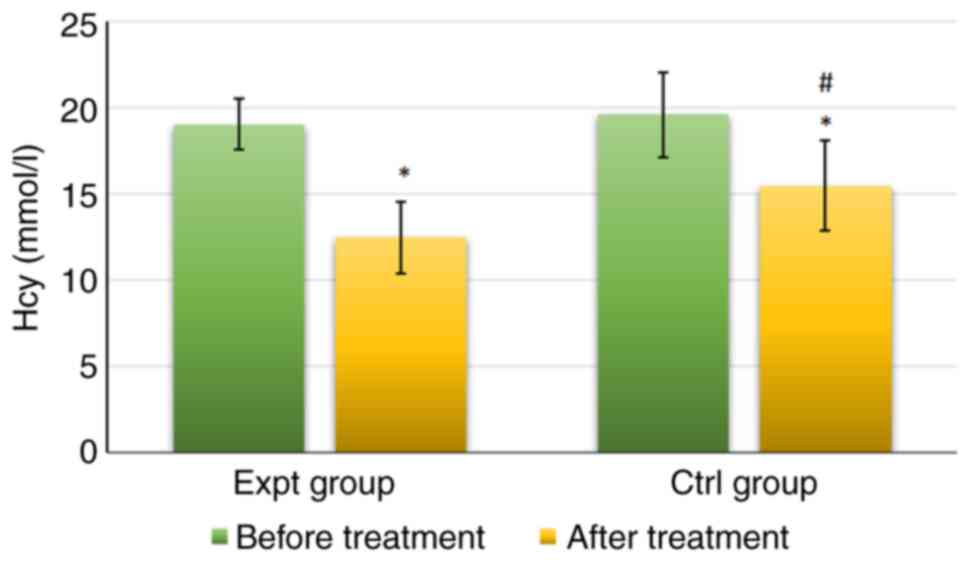
As shown in Fig. 4,
Hcy levels in the Expt group reached 19.02±1.48 mmol/l before
treatment and 12.48±2.08 mmol/l after treatment. Hcy levels in the
Ctrl group were 19.58±2.47 mmol/l before treatment and 15.44±2.61
mmol/l after treatment. There was no significant difference in Hcy
levels between the groups before treatment (P>0.05). However,
Hcy levels were significantly lower in the Expt group than those in
the Ctrl group after treatment (P<0.05). Furthermore, Hcy levels
after treatment were significantly lower than those before
treatment in both groups (P<0.05).
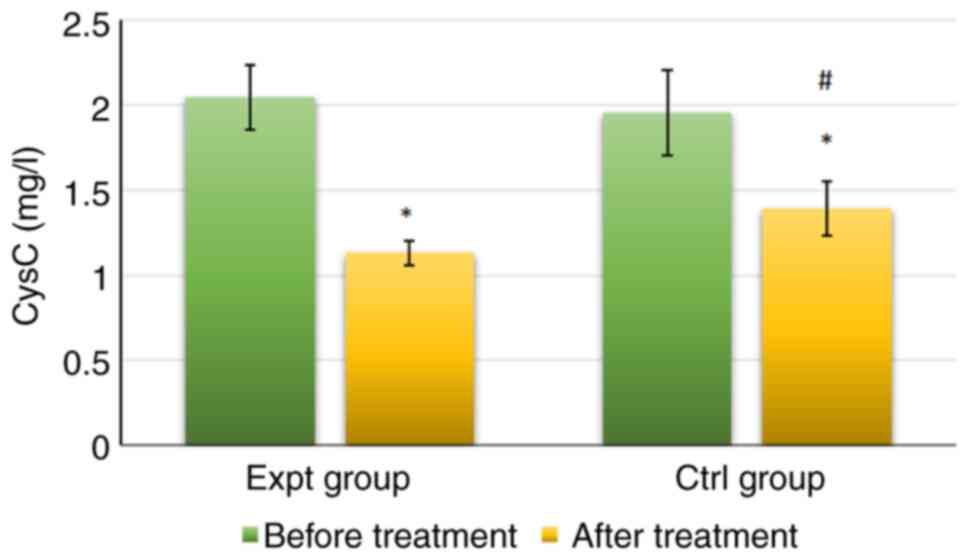
As shown in Fig. 5,
the serum CysC levels in the Expt group reached 2.04±0.19 mg/l
before treatment and 1.13±0.07 mg/l after treatment. CysC levels in
the Ctrl group reached 1.95±0.25 mg/l before treatment and
1.39±0.16 mg/l after treatment. No significant difference was
detected in CysC levels between the groups before treatment
(P>0.05). Conversely, CysC levels were significantly lower in
the Expt group than those in the Ctrl group after treatment
(P<0.05). In addition, CysC levels after treatment were
significantly lower than those before treatment in both groups
(P<0.05). Taken together, these data indicated that the addition
of labetalol was more effective in reducing the level of
inflammation in patients with GH.
Comparison of hemorheological
indicators between groups before and after treatment
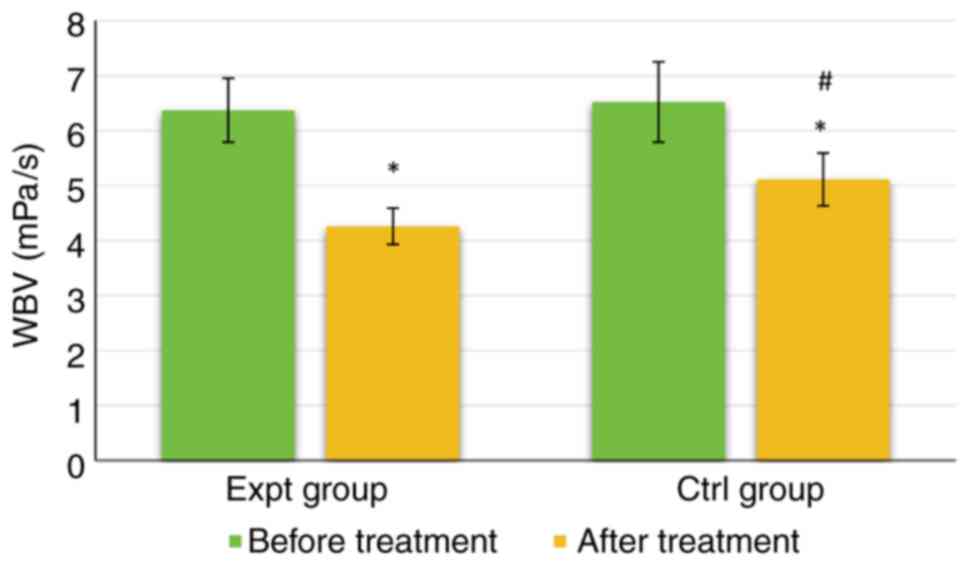
As shown in Fig. 6,
the WBV of the Expt group was 6.37±0.58 mPa/s before treatment and
4.26±0.33 mPa/s after treatment. The WBV of the Ctrl group was
6.52±0.73 mPa/s before treatment and 5.11±0.48 mPa/s after
treatment. There was no significant difference in WBV between the
groups before treatment (P>0.05). However, WBV was significantly
lower in the Expt group than that in the Ctrl group after
(P<0.05). The WBV of both groups was significantly lower after
treatment than those before (P<0.05).
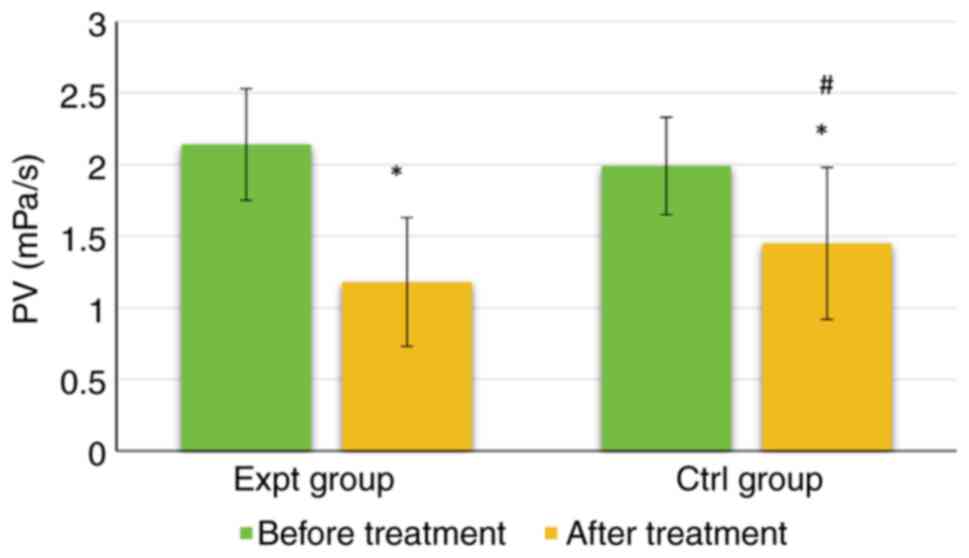
As shown in Fig. 7,
the PV of the Expt group reached 2.14±0.39 mPa/s before treatment
and 1.18±0.45 mPa/s after treatment. The PV of the Ctrl group was
1.99±0.34 mPa/s before treatment and 1.45±0.53 mPa/s after
treatment. Notably, there was no significant difference in PV
between the groups before treatment (P>0.05). However, the PV
was significantly lower in the Expt group than that in the Ctrl
group (P<0.05). Furthermore, the PV of both groups was
significantly lower after treatment than before treatment
(P<0.05).
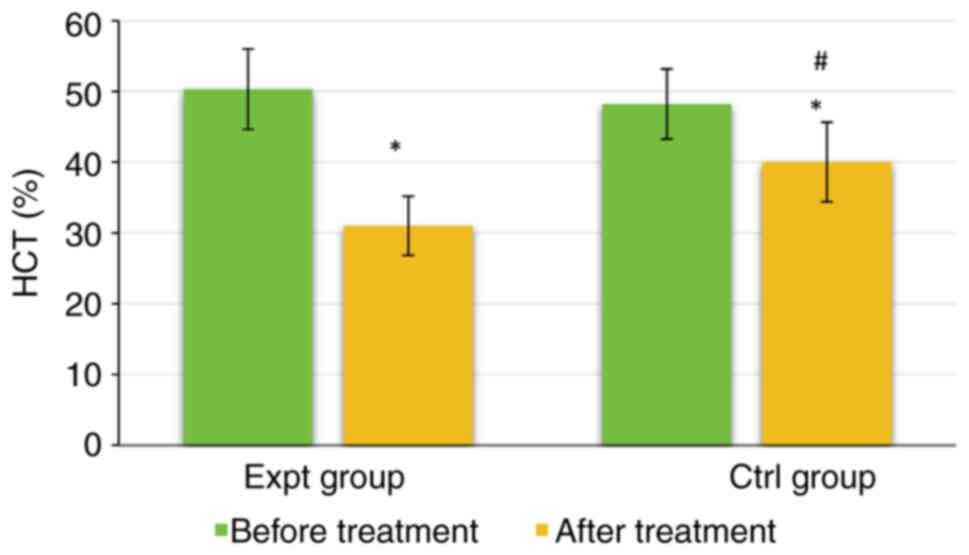
As shown in Fig. 8,
the HCT of the Expt group was 50.34±5.68% before treatment and
31.02±4.17% after treatment. The HCT of the Ctrl group was
48.22±4.95% before treatment and 40.02±5.61% after treatment. There
were no significant differences in HCT between the two groups
before treatment (P>0.05). However, the HCT of the Expt group
was significantly lower than that of the Ctrl group after treatment
(P<0.05). The HCT in both groups was significantly lower after
treatment than those before treatment (P<0.05). Taken together,
these data indicated that the addition of labetalol was more
effective in improving the hemodynamics of patients and producing
antihypertensive effects.
Comparison of pregnancy outcomes
between groups
As shown in Table
II, there were 40 cases of spontaneous vaginal delivery (80%),
8 cases of cesarean section (16%) and 2 cases of postpartum
hemorrhage (4%) in the Expt group. In the Ctrl group, there were 28
cases (56%) of spontaneous vaginal delivery, 15 cases (30%) of
cesarean section and 7 cases (14%) of postpartum hemorrhage. The
spontaneous vaginal delivery rate was significantly higher in the
Expt group than that in the Ctrl group (P<0.05). By contrast,
the cesarean section rate and postpartum hemorrhage rate of the
Expt group were significantly lower than those in the Ctrl group
(P<0.05). These data indicated that the addition of labetalol
may improve pregnancy outcome.
 | Table IIComparison of pregnancy outcomes
between groups. |
Table II
Comparison of pregnancy outcomes
between groups.
| Outcome | Expt group, n
(%) | Ctrl group, n
(%) | χ2 | P-value |
|---|
| Spontaneous
delivery | 40(80) | 28(56) | 7.026 | 0.0298 |
| Cesarean
section | 8(16) | 15(30) | | |
| Postpartum
hemorrhage | 2(4) | 7(14) | | |
Comparison of perinatal complications
between groups
As shown in Table
III, in the Expt group, there were 2 cases of FIUD (4%), 1 case
of placental abruption (2%), 1 case of neonatal asphyxia (2%), 5
cases of premature birth (10%) and 0 cases of neonatal death (0%).
In the Ctrl group, there were 4 cases of FIUD (8%), 3 cases of
placental abruption (6%), 3 cases of neonatal asphyxia (6%), 12
cases of premature birth (24%) and 1 case of death (2%). The
incidence of FIUD, placental abruption, neonatal asphyxia,
premature birth and death was significantly lower in the Expt group
than that in the Ctrl group. These data indicated that the addition
of labetalol may result in fewer cases of perinatal
complications.
 | Table IIIComparison of perinatal complications
between groups. |
Table III
Comparison of perinatal complications
between groups.
| Complications | Expt group, n
(%) | Ctrl group, n
(%) | χ2 | P-value |
|---|
| FIUD | 2(4) | 4(8) | 0.989 | >0.05 |
| Placental
abruption | 1(2) | 3(6) | | |
| Neonatal
asphyxia | 1(2) | 3(6) | | |
| Premature
birth | 5(10) | 12(24) | | |
| Neonatal death | 0 (0) | 1(2) | | |
Discussion
GH mainly refers to patients with normal blood
pressure before pregnancy, whose blood pressure rises above normal
values during or after pregnancy, with a series of related
symptoms. GH is mainly associated with increased blood pressure, as
well as edema, headache, dizziness and other phenomena in some
patients, and even convulsions in severe cases (27,28).
Long-term high blood pressure can result in serious harm to the
fetus and mother, and even prove fatal. Most patients exhibit
symptoms of systemic arteriolar spasm, which can cause
insufficiency of blood supply in the microcirculation of organs
throughout the body. In severe cases, GH can even lead to failure
and necrosis of all organs (29).
At present, effective control of blood pressure is the key to
clinical treatment of GH, and spasmolysis and pressure reduction
are the basic principles of treatment. Both labetalol and
MgSO4 are commonly used drugs for treating GH (30-33).
Therefore, the present study recruited 100 patients with GH, and
randomly split them into the Ctrl and Expt groups. The Ctrl group
was treated with MgSO4, whereas the Expt group was
administered MgSO4 + labetalol. There were no
statistically significant differences in age, gestational age and
pregnancy history between the groups (P>0.05), which indicates
that the groupings used were reasonable.
SBP and DBP of the Expt group after treatment were
not statistically different compared with those in the Ctrl group
(P>0.05); however, SBP and DBP in both groups were significantly
lower after treatment than before treatment (P<0.05). The
results of the present study are consistent with the findings of an
open-label, randomized controlled trial (34). However, recent studies have
reported the effect of labetalol on blood pressure control
(35,36), though its potency has not been
elucidated. Thus, the effectiveness of labetalol in controlling
blood pressure needs to be evaluated in a separate study.
Further comparisons of inflammatory factors
indicated that HMGB1, Hcy and serum CysC levels in the two groups
were significantly lower after treatment, and those in the Expt
group were also markedly lower than those in the Ctrl group after
treatment (P<0.05). HMGB1 is a crucial late-stage
pro-inflammatory factor, which has greater clinical significance
than tumor necrosis factor (TNF), interleukin-1 and other
early-stage immediate inflammatory factors (37). In addition, numerous studies have
identified the close connection between HMGB1 and TNF-α (38,39).
Hcy is a sulfur-containing amino acid in the human body, which is
an intermediate metabolite of methionine and cysteine. Increased
levels of Hcy can damage endothelial cells, disrupt the release and
secretion balance of vasoactive substances, and promote the
occurrence and development of hypertension (40). Serum CysC is a new endogenous
biomarker which is regulated by transforming growth
factor-β1(41). As a sensitive and
accurate indicator of early renal function damage, it also has been
reported that high serum CysC levels are associated with abnormal
cardiac diastolic properties in elderly Chinese patients with heart
failure with a preserved ejection fraction (42), CysC also has the potential of tumor
indication (43). Studies on the
regulation of the inflammatory response by labetalol are rare. Xu
et al (44,45) conducted several studies, which
reported that labetalol can improve the TNF-α-induced separation of
HTR-8/SVneo trophoblast cells from the endothelial cell network
(44); furthermore, labetalol has
been shown to reduce inflammatory factor inducible nitric oxide
synthase levels by increasing the expression of endothelial nitric
oxide synthase (45), thereby
demonstrating that CysC was a key factor in inflammation. Notably,
the present findings indicated that MgSO4 + labetalol
could effectively improve inflammatory stress in patients with GH
compared with single MgSO4 treatment.
WBV, PV and HCT of both groups were significantly
lower after treatment than those before treatment, whereas WBV, PV
and HCT after treatment were significantly lower in the Expt group
than those in the Ctrl group (P<0.05). These were similar to the
findings of Tooher et al (46), suggesting that MgSO4 +
labetalol could effectively improve the hemodynamics of patients
and produce antihypertensive effects. Labetalol is a combined α-
and β-adrenoceptor blocker, which is a non-selective antagonist of
β-adrenoceptors and a competitive antagonist of postsynaptic
α1-adrenoceptors. Labetalol acts more strongly on human
β-adrenergic receptors than on α1-adrenergic receptors; the ratio
of β-α antagonism is 3:1 and 6.9:1 after oral and intravenous
administration, respectively (47). Unlike conventional β-adrenergic
receptor blocking agents, which do not have intrinsic
sympathomimetic activity, labetalol reduces peripheral vascular
resistance and blood pressure during acute administration with
minimal effect on heart rate or cardiac output (47). The potential mechanism by which
labetalol improves blood pressure is that labetalol inhibits
sympathetic nerve excitation, reduces catechol secretion, dilates
blood vessels and reduces peripheral resistance by selectively
blocking the effects of α- and β-adrenoceptor. Therefore, the
hemodynamic indicators of patients can be improved (48-50).
Regarding pregnancy outcome, the spontaneous vaginal
delivery rate was much higher in the Expt group than that in the
Ctrl group, whereas the cesarean section rate and postpartum
hemorrhage rate were markedly lower than those in the Ctrl group
(P<0.05). These findings demonstrated that MgSO4 +
labetalol could markedly improve the pregnancy outcomes of patients
with GH. In addition, the incidences of FIUD, placental abruption,
neonatal asphyxia, premature birth and death of perinatal infants
in the Expt group were markedly lower than those in the Ctrl group
(P<0.05). This further indicated that MgSO4 +
labetalol could not only improve pregnancy outcomes of patients,
but also reduce the harm of drugs to both mothers and fetuses
(51).
In conclusion, in the present study, compared with
single MgSO4 treatment, MgSO4 + labetalol
effectively improved the inflammatory stress and hemodynamics of
patients with GH during pregnancy, and exhibited a marked
antihypertensive effect. Furthermore, MgSO4 + labetalol
could improve pregnancy outcomes and reduce perinatal
complications. However, there were some limitations in the present
study. The sample size of selected patients was quite small and all
patients were recruited from a single source. In addition, there
was no comparison of treatment effect in patients with different
conditions, such as complications of diabetes mellitus. Therefore,
more patients with GH should be included in a future study to
explore the clinical application value of MgSO4 +
labetalol. In summary, the results of the present study offered a
reference for drug treatment of patients with GH.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated and/or analysed during the
present study are available from the corresponding author upon
reasonable request.
Authors' contributions
ZG, WG and LZ conceived and designed the study. ZG,
WG and GZ performed research. YT, MW and YG analyzed data. ZG, WG
and LZ confirm the authenticity of all the raw data. MW and YG
validated data. ZG and WG wrote the paper. WG and LZ reviewed the
paper. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
The present study adhered to The Declaration of
Helsinki, and was approved by the Ethics Committee of Taicang
Hospital of TCM (approval no. 2022026). Patients provided written
informed consent.
Patient consent for publication
All participants in this study consented for
publication.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Magee LA, Brown MA, Hall DR, Gupte S,
Hennessy A, Karumanchi SA, Kenny LC, McCarthy F, Myers J, Poon LC,
et al: The 2021 International Society for the Study of Hypertension
in Pregnancy classification, diagnosis & management
recommendations for international practice. Pregnancy Hypertens.
27:148–169. 2022.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Corrigan L, O'Farrell A, Moran P and Daly
D: Hypertension in pregnancy: Prevalence, risk factors and outcomes
for women birthing in Ireland. Pregnancy Hypertens. 24:1–6.
2021.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Brown MA, Magee LA, Kenny LC, Karumanchi
SA, McCarthy FP, Saito S, Hall DR, Warren CE, Adoyi G and Ishaku S:
International Society for the Study of Hypertension in Pregnancy
(ISSHP). The hypertensive disorders of pregnancy: ISSHP
classification, diagnosis & management recommendations for
international practice. Pregnancy Hypertens. 13:291–310.
2018.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Tan JS, Liu NN, Guo TT, Hu S and Hua L:
Genetic predisposition to COVID-19 may increase the risk of
hypertension disorders in pregnancy: A two-sample Mendelian
randomization study. Pregnancy Hypertens. 26:17–23. 2021.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Ma XL, Zhai X, Liu JW, Xue XX, Guo SZ, Xie
H, Chen JX, Zhao HH and Wang W: Study on the biological basis of
hypertension and syndrome with liver-fire hyperactivity based on
data mining technology. World J Tradit Chin Med. 4:176–180.
2018.
|
|
6
|
Thombre Kulkarni M, Holzman C, Wasilevich
E, Luo Z, Scheid J and Allswede M: Pregnancy hypertension and its
associations with pre-pregnancy depression, anxiety,
antidepressants, and anxiolytics. Pregnancy Hypertens. 16:67–74.
2019.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Chen L, Shortreed SM, Easterling T,
Cheetham TC, Reynolds K, Avalos LA, Kamineni A, Holt V, Neugebauer
R, Akosile M, et al: Identifying hypertension in pregnancy using
electronic medical records: The importance of blood pressure
values. Pregnancy Hypertens. 19:112–118. 2020.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Ives CW, Sinkey R, Rajapreyar I, Tita ATN
and Oparil S: Preeclampsia-Pathophysiology and Clinical
Presentations: JACC State-of-the-Art Review. J Am Coll Cardiol.
76:1690–1702. 2020.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Ishikuro M, Murakami K, Yokozeki F, Onuma
T, Noda A, Ueno F, Obara T and Kuriyama S: Hypertension in
pregnancy as a possible factor for child autistic behavior at two
years old. Pregnancy Hypertens. 25:88–90. 2021.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Avorgbedor F, Silva S, McCoy TP,
Blumenthal JA, Merwin E, Seonae Y and Holditch-Davis D:
Hypertension and infant outcomes: North Carolina pregnancy risks
assessment monitoring system data. Pregnancy Hypertens. 28:189–193.
2022.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Ukah UV, De Silva DA, Payne B, Magee LA,
Hutcheon JA, Brown H, Ansermino JM, Lee T and von Dadelszen P:
Prediction of adverse maternal outcomes from pre-eclampsia and
other hypertensive disorders of pregnancy: A systematic review.
Pregnancy Hypertens. 11:115–123. 2018.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Cheu LA, Drexler K and Kominiarek MA:
Using m-Health apps to diagnose hypertension in pregnancy.
Pregnancy Hypertens. 22:99–100. 2020.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Sahni S, Palkar AV, Rochelson BL, Kępa W
and Talwar A: Pregnancy and pulmonary arterial hypertension: A
clinical conundrum. Pregnancy Hypertens. 5:157–164. 2015.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Fletcher B, Chappell LC, Lavallee L,
Wilson HM, Stevens R, Mackillop L, McManus RJ and Tucker KL:
Changes to management of hypertension in pregnancy, and attitudes
to self-management: An online survey of obstetricians, before and
following the first wave of the COVID-19 pandemic. Pregnancy
Hypertens. 26:54–61. 2021.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Du Q, Jovanović S, Tulić L, Tulić I and
Jovanović A: Pregnancy-induced hypertension is associated with
down-regulation of Kir6.1 in human myometrium. Pregnancy Hypertens.
18:96–98. 2019.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Badon SE, Dublin S, Nance N, Hedderson MM,
Neugebauer R, Easterling T, Cheetham TC, Chen L, Holt VL and Avalos
LA: Gestational weight gain and adverse pregnancy outcomes by
pre-pregnancy BMI category in women with chronic hypertension: A
cohort study. Pregnancy Hypertens. 23:27–33. 2021.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Naeh A and Ray JG: Pregnancy hypertension:
An international journal of women's cardiovascular health-Reply:
Letter to the editor. Pregnancy Hypertens. 30(96)2022.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Lu CQ, Lin J, Yuan L, Zhou JG, Liang K,
Zhong QH, Huang JH, Xu LP, Wu H, Zheng Z, et al: Pregnancy induced
hypertension and outcomes in early and moderate preterm infants.
Pregnancy Hypertens. 14:68–71. 2018.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Myerscough PR: Infant growth and
development after treatment of maternal hypertension. Lancet.
1(883)1980.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Vidyasagar S, Kumar S and Morton A:
Screening for primary aldosteronism in pregnancy. Pregnancy
Hypertens. 25:171–174. 2021.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Nash CM and Shetty N: Current state of
affairs: A study regarding diagnosis, treatment and use of home
blood pressure monitoring for hypertension in pregnancy. Pregnancy
Hypertens. 24:96–99. 2021.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Gasnier R, Valério EG, Vettorazzi J,
Martins-Costa SH, Barros EG and Ramos JG: Calcium-to-creatinine
ratio in pregnancy-induced hypertension. Pregnancy Hypertens.
2:59–64. 2012.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Lu JF and Nightingale CH: Magnesium
sulfate in eclampsia and pre-eclampsia: Pharmacokinetic principles.
Clin Pharmacokinet. 38:305–314. 2000.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Okusanya BO, Oladapo OT, Long Q,
Lumbiganon P, Carroli G, Qureshi Z, Duley L, Souza JP and
Gülmezoglu AM: Clinical pharmacokinetic properties of magnesium
sulphate in women with pre-eclampsia and eclampsia. BJOG.
123:356–366. 2016.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Wu Y, Wang DJ, Zhang Y, Zhang YX and Zhang
R: Regulation of magnesium sulfate combined with nifedipine and
labetalol on disease-related molecules in serum and placenta in the
treatment of preeclampsia. Eur Rev Med Pharmacol Sci. 24:5062–5070.
2020.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Podymow T and August P: Antihypertensive
drugs in pregnancy. Semin Nephrol. 31:70–85. 2011.PubMed/NCBI View Article : Google Scholar
|
|
27
|
DeSisto CL, Robbins CL, Ritchey MD, Ewing
AC, Ko JY and Kuklina EV: Hypertension at delivery
hospitalization-United States, 2016-2017. Pregnancy Hypertens.
26:65–68. 2021.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Nzelu D, Dumitrascu-Biris D, Hunt KF,
Cordina M and Kametas NA: Pregnancy outcomes in women with previous
gestational hypertension: A cohort study to guide counselling and
management. Pregnancy Hypertens. 12:194–200. 2018.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Mhiri R, Mvogo A, Kamga A, Yassinguezo S,
Fagla H, Dotou D and Kallel H: Epidemiology and maternal prognosis
of hypertension disorders of pregnancy in French Guiana. Pregnancy
Hypertens. 20:96–101. 2020.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Rodrigues Â, Barata C, Marques I and
Almeida MC: Diagnosis of White Coat Hypertension and pregnancy
outcomes. Pregnancy Hypertens. 14:121–124. 2018.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Akbar MIA, Adibrata MA, Aditiawarman
Aryananda RA, Angsar MD and Dekker G: Maternal and perinatal
outcome related to severity of chronic hypertension in pregnancy.
Pregnancy Hypertens. 16:154–160. 2019.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Bowen L, Pealing L, Tucker K, McManus RJ
and Chappell LC: Adherence with blood pressure self-monitoring in
women with pregnancy hypertension, and comparisons to clinic
readings: A secondary analysis of OPTIMUM-BP. Pregnancy Hypertens.
25:68–74. 2021.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Nguyen NTT, Kurtovic K, Mitchell C,
Evangelista M, Del Valle R, McWay Boling S and Hughes BL: Improving
treatment of severe hypertension in pregnancy and postpartum using
a hypertensive pathway. Pregnancy Hypertens. 30:1–6.
2022.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Easterling T, Mundle S, Bracken H,
Parvekar S, Mool S, Magee LA, von Dadelszen P, Shochet T and
Winikoff B: Oral antihypertensive regimens (nifedipine retard,
labetalol, and methyldopa) for management of severe hypertension in
pregnancy: An open-label, randomised controlled trial. Lancet.
394:1011–1021. 2019.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Gupta A, Nayak D, Sharma J and
Keepanasseril A: Comparing the efficacy of oral labetalol with oral
amlodipine in achieving blood pressure control in women with
postpartum hypertension: Randomized controlled trial (HIPPO
study-Hypertension In Pregnancy & Postpartum
Oral-antihypertensive therapy). J Hum Hypertens. 37:1056–1062.
2023.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Muhammad S, Usman H, Dawha YM, Yahya A,
Yekeen A and Bako B: Comparison of intravenous labetalol and
hydralazine for severe hypertension in pregnancy in Northeastern
Nigeria: A randomized controlled trial. Pregnancy Hypertens.
29:1–6. 2022.PubMed/NCBI View Article : Google Scholar
|
|
37
|
He Y, Tian J, Blizzard L, Oddy WH, Dwyer T
and Venn AJ: Associations of childhood adiposity and changes in
adiposity status from childhood to adulthood with pregnancy
hypertension. Pregnancy Hypertens. 19:218–225. 2020.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Wang Y, Zhang H, Chen Q, Jiao F, Shi C,
Pei M, Lv J, Zhang H, Wang L and Gong Z: TNF-α/HMGB1 inflammation
signalling pathway regulates pyroptosis during liver failure and
acute kidney injury. Cell Prolif. 53(e12829)2020.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Xu X, Piao HN, Aosai F, Zeng XY, Cheng JH,
Cui YX, Li J, Ma J, Piao HR, Jin X and Piao LX: Arctigenin protects
against depression by inhibiting microglial activation and
neuroinflammation via HMGB1/TLR4/NF-κB and TNF-α/TNFR1/NF-κB
pathways. Br J Pharmacol. 177:5224–5245. 2020.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Webster LM, Reed K, Myers JE, Burns A,
Gupta P, Patel P, Wiesender C, Seed PT and Nelson-Piercy C and
Nelson-Piercy C: Quantifying adherence to antihypertensive
medication for chronic hypertension during pregnancy. Pregnancy
Hypertens. 17:12–14. 2019.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Gressner AM, Lahme B, Meurer SK, Gressner
O and Weiskirchen R: Variable expression of cystatin C in cultured
trans-differentiating rat hepatic stellate cells. World J
Gastroenterol. 12:731–738. 2006.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Xu CC, Fu GX, Liu QQ and Zhong Y:
Association between cystatin C and heart failure with preserved
ejection fraction in elderly Chinese patients. Z Gerontol Geriatr.
51:92–97. 2018.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Dikovskaya MA, Russkikh GS, Loktev KV,
Johnston TP, Gevorgyan MM, Voronina NP, Chernykh VV, Trunov AN and
Korolenko TA: Cystatin C and cystatin SN as possible soluble tumor
markers in malignant uveal melanoma. Radiol Oncol. 56:83–91.
2021.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Xu B, Charlton F, Makris A and Hennessy A:
PP042. Anti-hypertensive drugs hydralazine, clonidine and labetalol
improve trophoblast integration into endothelial cellular networks
in vitro. Pregnancy Hypertens. 2(264)2012.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Xu B, Bobek G, Makris A and Hennessy A:
Antihypertensive methyldopa, labetalol, hydralazine, and clonidine
reversed tumour necrosis factor-α inhibited endothelial nitric
oxide synthase expression in endothelial-trophoblast cellular
networks. Clin Exp Pharmacol Physiol. 44:421–427. 2017.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Tooher J, Thornton C, Makris A, Korda A,
Ogle R, Horvath J and Hennessy A: PP102. Hypertension in pregnancy
and long term cardiovascular mortality outcomes. Pregnancy
Hypertens. 2(295)2012.PubMed/NCBI View Article : Google Scholar
|
|
47
|
MacCarthy EP and Bloomfield SS: Labetalol:
A review of its pharmacology, pharmacokinetics, clinical uses and
adverse effects. Pharmacotherapy. 3:193–219. 1983.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Visser VS, Hermes W, Twisk J, Franx A, van
Pampus MG, Koopmans C, Mol BWJ and de Groot CJM: Prognostic model
for chronic hypertension in women with a history of hypertensive
pregnancy disorders at term. Pregnancy Hypertens. 10:118–123.
2017.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Magee LA, Rey E, Asztalos E, Hutton E,
Singer J, Helewa M, Lee T, Logan AG, Ganzevoort W, Welch R, et al:
Management of non-severe pregnancy hypertension-A summary of the
CHIPS Trial (Control of Hypertension in Pregnancy Study) research
publications. Pregnancy Hypertens. 18:156–162. 2019.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Oogaki Y, Ozawa R, Seshima K, Shinoda R,
Torii Y, Takahashi H, Iwata H, Kuwayama T and Shirasuna K: Uncaria
tomentosa extract (AC-11) improves pregnancy hypertension together
with suppression of sFlt-1 and sEng. Pregnancy Hypertens.
26:127–132. 2021.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Hauspurg A, Countouris ME, Jeyabalan A,
Hubel CA, Roberts JM, Schwarz EB and Catov JM: Risk of hypertension
and abnormal biomarkers in the first year postpartum associated
with hypertensive disorders of pregnancy among overweight and obese
women. Pregnancy Hypertens. 15:1–6. 2019.PubMed/NCBI View Article : Google Scholar
|