1. Introduction
Mycoplasmas, the tiniest self-replicating
prokaryotes devoid of a cell wall, possess an extremely small
genome ranging from 580-2,200 kb (1,2).
While >200 species of this pathogen have been recognized in
animals, arthropods, humans and plants, only a select few have been
confirmed to induce diseases in humans. Of note, Mycoplasma
pneumoniae (M. pneumoniae), Mycoplasma pirum,
Mycoplasma genitalium, Mycoplasma hominis,
Mycoplasma fermentans, Mycoplasma penetrans and
Ureaplasma urealyticum stand out as prominent pathogenic
Mycoplasmas linked to diseases affecting the respiratory and
urogenital systems in both humans and animals (3).
Of these pathogenic strains, M. pneumoniae
stands out as the most prevalent and extensively studied species.
It significantly contributes to chronic respiratory tract illnesses
and pneumonia in humans, with children and adolescents being
particularly vulnerable. Although M. pneumoniae infections
typically tend to be self-limiting and mild, they can progress to
severe or even life-threatening conditions in certain individuals.
M. pneumoniae has been attributed to up to 40% of
community-acquired pneumonia cases among children >5 years old,
where lower respiratory tract infections pose a significant risk of
morbidity and mortality in this demographic (1,4).
It is considered that M. pneumoniae
infections are associated with bronchial asthma and chronic lung
diseases (5). Apart from
triggering severe conditions in the lower respiratory tract and
less severe in the upper respiratory tract, M. pneumoniae
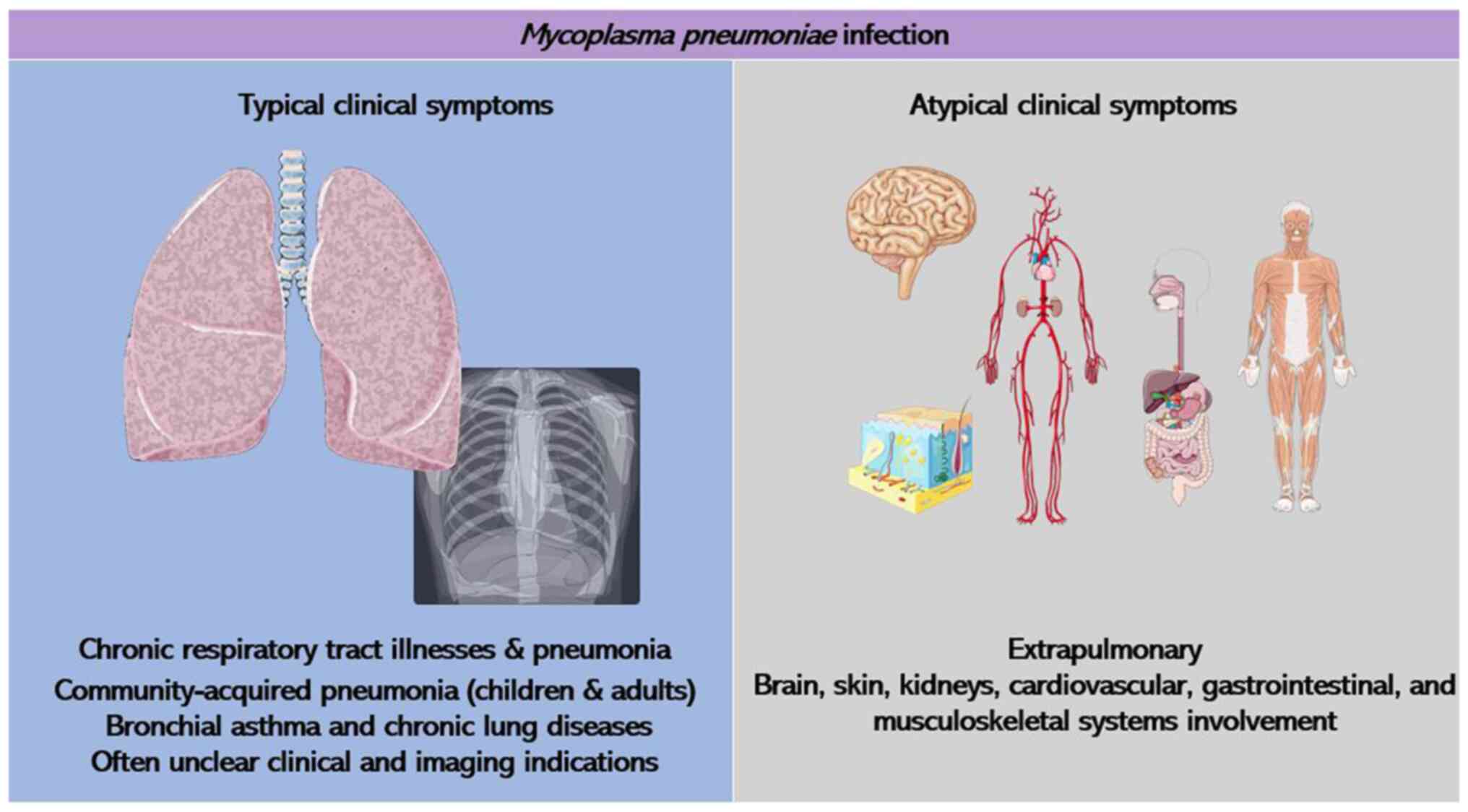
can lead to various other conditions and events after infection.
These complications manifest in diverse areas, such as the skin,
gastrointestinal system, kidneys, heart, musculoskeletal, central
nervous and circulatory system, leading to atypical clinical
manifestations (Fig. 1). Notably,
central nervous system complications are the most frequent
extrapulmonary issues arising from M. pneumoniae infection,
occasionally posing life-threatening risks. It has been highlighted
that due to the unusual symptoms and that lack of clear clinical
and imaging indications in the early stages of M. pneumoniae
infection, it is often underestimated (6). Rapid culture techniques utilizing
throat swabs, polymerase chain reaction (PCR), serology and other
laboratory diagnostic methods, including rapid antigen tests,
constitute the primary means of laboratory diagnosis (7).
Extrapulmonary effects frequently occur without
pneumonia, and both intrapulmonary and extrapulmonary complications
operate through separate pathological mechanisms (8). The indirect immune-mediated damage of
the immune system, vascular blockages brought on by vasculitis or
thrombosis and direct harm from invasion or locally induced
inflammatory cytokines are the potential causes of extrapulmonary
manifestations due to M. pneumoniae. On the other hand,
intrapulmonary infection mechanisms involve adhesion, invasion,
nutrient depletion, immune and inflammatory response and toxin
release (9). It is important to
note that these mechanisms are not mutually exclusive but can
co-exist concurrently in the body of the patient. Considering the
severity of M. pneumoniae infections, in the present review,
a summary of both intrapulmonary and extrapulmonary pathogenesis is
provided, aiming to offer valuable insight for further pathogenesis
research and treatment strategies concerning M. pneumoniae
infections. The present review provides an update of all the
pathogenetic mechanisms that have been previously mentioned in the
literature (6,9).
2. Mechanisms of intrapulmonary
infection
The process by which M. pneumoniae causes
infection is intricate. In its initial phase, M. pneumoniae
attaches to the bronchial epithelium using specialized terminal
structures, triggering alterations in the metabolism and structure
of the infected cells. Simultaneously, it invades these host cells,
depletes essential nutrients and releases various components, such
as community-acquired respiratory distress syndrome (CARDS) toxin,
hydrogen peroxide and superoxide radicals, resulting in direct
damage. Alongside these actions, M. pneumoniae components,
including hydrogen sulfide (H2S), alanine and
pyruvate-producing enzymes (HapE), lipids, lipoproteins and
glycolipids, stimulate the production of cytokines, initiating
inflammation and causing indirect damage. Furthermore, M.
pneumoniae employs mechanisms to evade the host immune system,
potentially prolonging its survival within the body and leading to
more severe clinical manifestations (10). A summary of the mechanisms is
illustrated in Fig. 2.
 | Figure 2A summary of the various
pathophysiological mechanisms of intrapulmonary Mycoplasma
infection. Please refer to main text for more details. Parts of
this image derived from the free medical site http://smart.servier.com/ (accessed on December 15,
2023) by Servier, licenced under a Creative Commons Attribution 3.0
unported licence. CARDS, community-acquired respiratory distress
syndrome; IFN-γ, interferon-γ; TNF-α, tumor necrosis factor-α; IL,
interleukin; HapE, hydrogen sulfide, alanine and pyruvate producing
enzymes; H2S, hydrogen sulfide; KATP, ATP-sensitive
K+; IbpM, immunoglobulin-binding protein of
Mycoplasma; Ig, immunoglobulin; NETs, neutrophil
extracellular traps; EF-Tu, elongation factor thermal unstable
Tu. |
Proteins associated with adhesion
Adhesion serves as the primary factor crucial for
the pathogenicity of M. pneumoniae, relying on a specialized
polarized terminal attachment organelle. Several pathogenic effects
hinge on this initial step. The pathogen engages with the host
respiratory epithelium, binding to the bronchial ciliary epithelium
and triggering metabolic and structural alterations within the
affected cells. This interaction rearranges the cytoskeleton,
leading to the depletion of nutrients within the host cells
(6). These alterations in
intracellular metabolism are characterized by a simultaneous
decrease in the uptake rate of certain host cell components, such
as orotic acid and amino acids, along with a noticeable inhibition
of ribonucleic acid and protein synthesis. These metabolic
alterations contribute to the proliferation of the pathogen within
cells, culminating in ciliary stagnation, cell death and the
orchestration of various factors resulting in respiratory symptoms
in humans (6). M.
pneumoniae firmly attaches to host epithelial cells using its
unique attachment organelle, purportedly aiding in cell division,
cytoadherence and movement across the host cell surface (11). Sialylated and sulfated
oligosaccharides are the primary receptors that M.
pneumoniae recognizes (12).
The type and density of these host receptors
significantly impact the adhesion and movement of M.
pneumoniae, subsequently influencing the pathogenic mechanism
and infection outcomes (13). The
attachment organelle, situated at a cellular extremity, encompasses
nap-like surface formations and an internal core. It coordinates a
sophisticated adhesion process through interactions with the
intricate network of the cytoskeleton and surface adhesion proteins
(14).
The nap-like structure primarily comprises P1
adhesin, P30, P40 and P90. The internal core, crucial for forming
the attachment organelle, is subdivided into three components: A
terminal button, paired plates and a bowl (wheel) complex
positioned at the leading edge. Key proteins within this core
include high-molecular-weight proteins (HMW1, HMW2, HMW3), P65,
P200, phosphomannomutase, mpn387, Lon protease, P41 and P24, among
others (15).
The membrane protein P1 serves as the primary
cellular adhesin and is localized on the cell surface, exhibiting
sensitivity to trypsin (16). Upon
the contact of M. pneumoniae with a target cell, the
scattered P1 precursor proteins within cell membranes swiftly
migrate to the terminal organelle. Here, the leader peptide on the
amino terminal undergoes hydrolysis, transforming into a mature P1
protein that binds to the host receptor (9). It is crucial to note that for
adhesion, the P1 adhesin must be correctly positioned on the
terminal organelle. A previous study confirmed that, apart from its
role in mediating adhesion between M. pneumoniae and host
receptors, the P1 adhesin contributes to the gliding movement on
host cell surfaces (16).
Additionally, according to the same study, the significance of P1
adhesin in the cytokine response that M. pneumoniae causes
in mast cells. The interaction between M. pneumoniae and
sialylated residues on mast cell surfaces triggers mast cell
activation, leading to inflammatory damage. Antibodies targeting
the highly immunogenic carboxyl terminus of P1 are believed to
diminish M. pneumoniae's adherence to non-biological and
host cells (16).
The P30 protein exhibits a specific level of
sequence similarity with distinct domains of the P1 protein, both
serving as primary proteins governing adherence. Positioned at the
tip of the attachment organelle, the P30 adhesin holds a
significant role in transmitting cellular signals from the interior
to the exterior. These signals trigger crucial processes in
cytoadherence and movement, orchestrating the assembly of the P1
adhesin complex and fostering binding interactions between P1 and
host receptors (15). A previous
study conducted by Romero-Arroyo et al (17) confirmed the pivotal role of P30 in
cellular development. The absence of P30 results in irregular
Mycoplasma morphology, characterized by oval or leaf-like
cells with ill-defined apical structures. Nevertheless, the
introduction of P30 mutants and the wild-type P30 alleles can
restore their typical morphology.
Protein surface exposure verification confirms P116
as a crucial cell adhesin. Anti-P116 antibodies prevent M.
pneumoniae attachment to HEp-2 cells independently of P1.
Moreover, P116 stands out as a significant immunogenic antigen
within M. pneumoniae (6).
The complete P116-C-terminal protein level has been instrumental in
the serological diagnosis of M. pneumoniae. Furthermore, the
study by Tabassum et al (18) suggested that a 27-kDa N-terminal
segment of the P116 protein exhibits potential for the diagnosis of
M. pneumoniae infection serologically (18).
Protein P65 showcases an intimate spatial and
functional correlation with P30. The analysis of the fluorescent
fusion proteins, P65 and P30, expressed in growing
Mycoplasma cultures, indicates their concurrent positioning
in developing terminal organelles. The suggested role of P65
involves facilitating a close attachment between the terminal
button and the front side of the membrane by potentially
interacting with the internal structural domain of P30(15). Adhesins P40 and P90, derived from
mpn142 cleavage, collectively form a transmembrane adhesion complex
along with protein P1(16).
The p1 gene, which encodes the P1 protein, serves as
a target for detecting M. pneumoniae through reverse
transcription-quantitative PCR and for conducting genotyping
(19,20). Although the impact of
genotype-specific antibodies on re-infections by different M.
pneumoniae genotypes remains uncertain, genotyping is essential
for molecular epidemiological studies and vaccine development
(21). The P1 protein exhibits
high immunogenicity and antigenic specificity (22,23)
distinguishing its epitopes from those of other bacterial species.
Inoculating Bagg albino (BALB/c) mice with a DNA vaccine encoding
amino acids 1125-1359 of the M. pneumoniae P1 protein
C-terminal region (P1C) via intramuscular or intranasal routes led
to observable protection against M. pneumoniae infection.
This protection was associated with elevated levels of IgG (IgG1,
IgG2a, and IgG2b isotypes) and cytokines [(interferon-γ (IFN-γ) and
interleukin (IL)-4] (24).
P30 shares similar importance with P1 as an
immunogenic factor (25). M.
pneumoniae mutants lacking the P30-encoding gene are
non-infectious and incapable of adhering to host cells (26), suggesting P30 as a promising
candidate for a clinical vaccine. Szczepanek et al (27) constructed P30 cytadhesin mutant
that does not cause virus infection so that they could test how
well it works as a live-attenuated vaccine candidate in mice.
However, this live-attenuated vaccine caused serious problems in
BALB/c mice, which were likely caused by T helper type 17 (Th17)
cell responses. Previous studies have highlighted the significant
role of Th17 cells in antimicrobial immune responses and autoimmune
diseases in mouse models (28,29).
On the other hand, a vaccine based on recombinant P30 adhesin
proved to provide immune protection. It was established by Hausner
et al (30), who joined
protein P30 (amino acids 17 to 274) with the C-terminal of P1
adhesin (amino acids 1287-1518 of P1). When administered to guinea
pigs, this recombinant vaccine caused protective IgA to be released
in their respiratory tracts. This suggested that it may be possible
to create vaccines that can protect individuals from becoming
infected with M. pneumoniae.
The P116 protein is a 116-kDA protein that comprises
1,030 amino acids. In the study by Svenstrup et al (31), protein P116 was shown to be
surface-exposed and an essential protein involved in adhesion, as
anti-P116 antibody was found to prevent the attachment of M.
pneumoniae to the HEp-2 cells independently of P1.
Additionally, serum from M. pneumoniae-infected patients has
been found to contain antibodies specifically reactive with
P116(32).
Other proteins
The role of accessory proteins is crucial for the
proper assembly of the attachment organelle. The identification of
the sialic acid binding site is attributed to P40/P90 rather than
P1. Variations in the genetic domains of P1, P40 and P90 surfaces
contribute to the variability of clinical symptoms, offering new
avenues for developing vaccines against M. pneumoniae
infections (33). The functions of
HMW1, HMW2 and HMW3 proteins encompass the structure and stability
of attachment organelles, adherence, gliding, proper positioning of
adhesins and the maintenance of cell morphology. Although protein
P200 was initially considered to serve as an additional structural
element in cytoadherence, it appears to play a more essential role
in motility rather than adherence. It is closely linked to biofilms
and cell maturation (34).
Additionally, the P41/P24 proteins play a crucial role in anchoring
terminal organelles to the cell body, exerting a considerable
influence on the assembly and development of the attachment
organelle of M. pneumoniae.
Direct damage
Unlike inflammatory or immune-related injuries
triggered by M. pneumoniae infection, direct damage denotes
the harm inflicted by M. pneumoniae directly onto host
cells. This form of damage encompasses nutrient depletion,
intracellular positioning, toxin discharge, oxidative harm and the
initiation of apoptosis (35).
Nutrition depletion and intracellular
localization
M. pneumoniae depends on host cells to
acquire vital nutrients due to its compact genome and restricted
ability to synthesize compounds. Interaction between the M.
pneumoniae cell membrane and the host cell membrane facilitates
the transfer of crucial compounds necessary for its growth and
proliferation (1). Moreover, there
is a hypothesis suggesting that M. pneumoniae obtains
nutrients, including glucose, amino acids and cholesterol by
inserting microtubules into host cells (36).
The recently decoded genomic makeup of M.
pneumoniae strongly indicates a unique and limited genetic
evolution, resembling that of other intracellular bacteria. This
implies the potential specialization of this pathogen as a highly
adapted parasitic bacterium in respiratory tissue cells. It offers
initial evidence supporting the idea of the invasion of M.
pneumoniae in host cells. Some experimental data indicate that
M. pneumoniae may possess the ability to permeate host cells
intracellularly, potentially for acquiring nutrients (8).
Role of CARDS toxin
The CARDS toxin shares substantial sequence
similarities with the S1 subunit of pertussis toxin, causing
clinical manifestations similar to Bordetella pertussis.
This toxin, which is a distinctive adenosine diphosphate
ribosylating and vacuolating toxin encoded by M. pneumoniae
mpn372, requires disulfide bonds for maintenance (37). A recent study demonstrated that
CARDS toxin swiftly binds to surfactant protein-A receptors on host
target cells and enters immediately through a clathrin-mediated
pathway in a dose- and time-dependent manner. Once internalized,
CARDS toxin undergoes retrograde transportation from the endosome
via the Golgi complex to the endoplasmic reticulum. This retrograde
transport aids in toxin processing and is crucial for inducing
vacuole formation (37). Creating
an acidic setting within host cell vesicles is deemed essential for
managing the processing, movement and transfer of the CARDS toxin
of M. pneumoniae. Adjusting the acidic conditions within
host cells could present fresh opportunities to shield these cells
from vacuolation induced by the M. pneumoniae CARDS toxin
(38).
Effects of oxidative stress
Upon adhering to host cells, M. pneumoniae
penetrates these cells with microtubules, releasing hydrogen
peroxide and superoxide radicals. These compounds, combined with
the endogenously generated toxic oxygen molecules of the host cell,
create oxidative stress within the respiratory tract epithelial
cells. In addition, M. pneumoniae lacks superoxide dismutase
and catalase, allowing the radicals it produces to hinder the
catalase activity of the host cell. Consequently, there is a
reduced breakdown of peroxides, heightening the vulnerability of
host cells to oxygen molecule-induced harm (35).
It has been reported that hydrogen peroxide produced
by M. pneumoniae can regulate infected cell detachment,
aiding in the persistence of bacterial infection (10). Since it is a wall-less bacterium in
the Mycoplasma genus, M. pneumoniae primarily depends
on glycerol derived from animal or human host phospholipids for
carbon and energy (39).
L-α-glycerophosphate oxidase (GlpO), an enzyme present on the
surface, plays a pivotal role in glycerol metabolism, leading to
hydrogen peroxide production, thereby influencing the M.
pneumoniae pathogenesis (40).
GlpO, despite its potential as a vaccine antigen, might not incite
a protective immune response (25). Additionally, histidine
phosphocarrier protein kinase (HPrK), a crucial regulator of carbon
metabolism in various Gram-positive bacteria, is among the nine
regulatory proteins encoded by the M. pneumoniae genome. The
activation of HPrK by glycerol results in peroxide production,
inducing oxidative stress. This stress leads to changes in
respiratory epithelial cells, such as cilia loss, reduced oxygen
utilization, vacuolar degeneration, lower glucose intake, amino
acid absorption and macromolecular synthesis (41).
Inflammatory response
Lung macrophages hold considerable influence in
managing M. pneumoniae infection. They identify M.
pneumoniae via toll-like receptor (TLR)2, initiating the
myeloid differentiation primary response gene 88 (MyD88)-nuclear
factor κB (NF-κB) signaling cascade and engulfing the bacteria
through phagocytosis. MyD88, an essential signaling adapter
molecule downstream of TLR, plays a pivotal role in orchestrating
the lung macrophage reaction to M. pneumoniae. However, the
activation of the NF-κB pathway concurrently can provoke intense
inflammation, prompting apoptosis in macrophages, monocytes and
lymphocytes, consequently compromising immune functionality
(42).
Components released by M. pneumoniae,
including metabolites and toxins, can serve as pro-inflammatory
molecules, triggering an inflammatory response. The cytokines
released due to M. pneumoniae infection, such as IFN-γ,
tumor necrosis factor-α (TNF-α) and ILs (IL-β, IL-2, IL-4, IL-5,
IL-6, IL-8, IL-10 and IL-18) are linked to the exacerbation of
asthma. Variations in cytokines may represent one of the pathogenic
mechanisms of M. pneumoniae infection (43).
The HapE, present in M. pneumoniae, functions
as a potential virulence factor by generating H2S, which
harms blood cells (5). This
H2S production by HapE prompts phagocytes to release
pro-inflammatory factors, leading to increased expression of
several inflammatory mediators and cytokines that intensify
inflammatory reactions, ultimately causing tissue damage (44). Moreover, HapE contributes to
inflammatory responses through ATP-sensitive K+ (KATP)
channels (6). An enhanced
H2S production is achieved by breaking down cysteine
within the KATP channel complex. This increase in H2S
levels alters cellular excitability, affecting ion channel function
and intensifying inflammation.
It is hypothesized that lipids bind to TLR4, acting
as potential ligands. This interaction triggers macrophage
autophagy, activating the NOD-, LRR- and pyrin domain-containing
protein 3 (NLRP3) inflammasomes and NF-κB pathway by stimulating
reactive oxygen species (ROS) production. Eventually, this process
leads to the release of pro-inflammatory cytokines (45). When TLR2 and TLR6/TLR1 bind to
lipoproteins, they promote the production of cytokines and
mediators by immune cells (45).
Another study demonstrated that when lipid-associated membrane
proteins (LAMPs) were administered to BALB/c mice, they caused lung
lesions that were consistent with the aggravation of the disease.
The removal of lipid components from LAMPs prior to vaccination
could eradicate these symptoms (46). As regards CARDS toxin, in the
natural immune response, CARDS activates NLRP3-related
inflammasomes, thereby controlling caspase-1 activation, promoting
the release of IL-1β and IL-18, and inducing inflammatory cell
death alongside stress-related conditions. Furthermore, CARDS toxin
has been shown to increase IL-6 and TNF-α expression in a
dose-dependent manner (14,47).
In cases of refractory M. pneumoniae pneumonia (RMPP), CARDS
toxin levels have a positive association with TNF-α, rendering it a
potential diagnostic biomarker for distinguishing RMPP from
non-RMPP cases (48).
It has been suggested that nucleases in parasitic
Mollicutes bacteria have a notable contribution to host pathology
by catalyzing reactions that allow M. pneumoniae to extract
nucleic acids for survival in the host. The lipoprotein encoded by
M. pneumoniae, known as mpn133, operates as a
calcium-dependent nuclease responsible for the degradation of DNA
and RNA. This action leads to programmed cell death, inflammatory
cell infiltration and tissue damage (5,6).
While glycolipids and capsules are considered possible virulence
factors, their specific pathogenetic mechanisms remain ambiguous
and need further exploration (6).
Although glycolipid and capsule are potential
contributors to virulence, their specific mechanisms for promoting
illness remain unclear and require further exploration (6). Histone deacetylase 5, a participant
in inflammation control, could potentially boost inflammatory
reactions triggered by M. pneumoniae in macrophages by
activating NF-κB (49).
Immune evasion
The mpn400 protein, also known as the
immunoglobulin-binding protein of Mycoplasma (IbpM),
strongly binds to various host-produced immunoglobulins (IgM, IgA
and IgG). A previous study suggested that strains of M.
pneumoniae lacking IbpM exhibit a slight impairment in
cytotoxicity, thereby indicating the significance of IbpM as a
virulence factor (26).
M. pneumoniae is presumed to invade cells and
tissues, residing within cells to evade immune cell phagocytosis
and antibiotic effects, leading to prolonged survival in the host.
However, the precise mechanisms enabling M. pneumoniae to
evade intracellular host defenses remain unknown, necessitating
further exploration of the pathways involved in intracellular
survival. Intracellular invasion likely contributes to M.
pneumoniae evasion strategies, prolonged host incubation, and
the establishment of chronic infection (36).
Although M. pneumoniae genomes are relatively
small, they contain a notable portion, ~8%, consisting of dispersed
repetitive elements. It has been demonstrated that these elements
play a role in generating antigenic variation through homologous
recombination among specific repetitive genomic components
(50). It has been suggested that
these repetitive sequences act as a reservoir for generating
antigenic variation, particularly in P1 adhesin genes critical for
M. pneumoniae adherence and motility (51). Mutations and rearrangements in
M. pneumoniae surface antigens result in insufficient
protective antibodies in hosts post-infection, leading to recurrent
M. pneumoniae infections.
ROS constitute a part of the non-specific immune
defense of the host against invading microorganisms, produced by
nicotinamide adenine dinucleotide phosphate oxidase (52). ROS play a dual role: They function
as direct antimicrobial agents by targeting nucleic acids,
carbohydrates, lipids and proteins within M. pneumoniae
cells, leading to substantial impairment to these biological
components. Simultaneously, ROS serve as essential signals for
innate immune signaling, prompting the immune system to combat
pathogens. Consequently, M. pneumoniae has evolved
mechanisms with which to combat this oxidative challenge. In M.
pneumoniae, mpn668 encodes a protective antioxidant enzyme,
according to a recent study (53).
Potentially reducing the oxidative damage, the host causes, this
enzyme degrades hydroperoxide. Additionally, following M.
pneumoniae infection, neutrophils accumulate rapidly at the
infection site through chemokine chemotaxis. They become highly
phagocytic, leading to the formation of neutrophil extracellular
traps (NETs) and releasing various bactericidal substances,
effectively eliminating pathogens. M. pneumoniae derives
extracellular nucleases which can degrade NETs. Notably, the
magnesium-dependent nuclease encoded by M. pneumoniae mpn491
serves as a significant extracellular nuclease, enhancing the
survival rate of the pathogen and aiding in the evasion of the
immune response of the host by degrading NETs, thereby causing
additional harm to the host (53).
Factor H is a negative regulator of the complement
system of the host, preventing unintended complement activation. C3
convertase cleaves the complement component C3 into C3b, a key
effector molecule that further activates the complement system. A
previous study demonstrated that M. hyopneumoniae binds
Factor H via elongation factor thermal unstable Tu (EF-Tu),
reducing C3 deposition on the surface of M. hyopneumoniae
and effectively halting further complement activation. Several
mycoplasmas, including M. pneumoniae, exploit EF-Tu to
hijack Factor H, mimicking host molecules to evade complement
attack. Additionally, EF-Tu reinforces adherence between
mycoplasmas and tracheal epithelial cells (54).
Infection by M. pneumoniae disrupts both
innate and adaptive immunity in the host. Some researchers have
observed no significant increases in IgG, IgM and IgA
immunoglobulins over a 1-year period in M.
pneumoniae-infected patients, signaling immune system
impairment due to the infection. During the acute stage, the levels
of C3 and C4 noticeably increased; however, at a later stage,
immune suppression induced by M. pneumoniae led to a return
of C3 and C4 to normal or even lower levels. This infection prompts
the respiratory tract to release pro-inflammatory cytokines and
chemokines, activating diverse immune cells. This can lead to the
overactivity of T-cells, promoting their apoptosis. Additionally,
the adhesins and metabolites of M. pneumoniae can damage
respiratory epithelial cells and lymphocytes, causing reduced
activity and accelerated apoptosis of lymphocytes. CD4+
function reduction contributes to an imbalance in immune function,
disrupting antigen presentation, B-cell maturation and relative
antibody production. Disruptions in humoral and cellular immunity,
and the dysregulation of innate immune system brought on by M.
pneumoniae exacerbate respiratory system damage (1).
3. Mechanisms of extrapulmonary
manifestations
Beyond typical respiratory symptoms, M.
pneumoniae can induce various extrapulmonary complications.
Notably, these manifestations sometimes occur independently of
pneumonia or even respiratory symptoms. These complications can
potentially affect all bodily systems and organs. Evidence of M.
pneumoniae infection alongside central nervous system
involvement supports the notion that M. pneumoniae can
disseminate to distant organs through blood transmission, causing
disease (55,56). The underlying mechanisms of
extrapulmonary effects of M. pneumoniae are summarized in
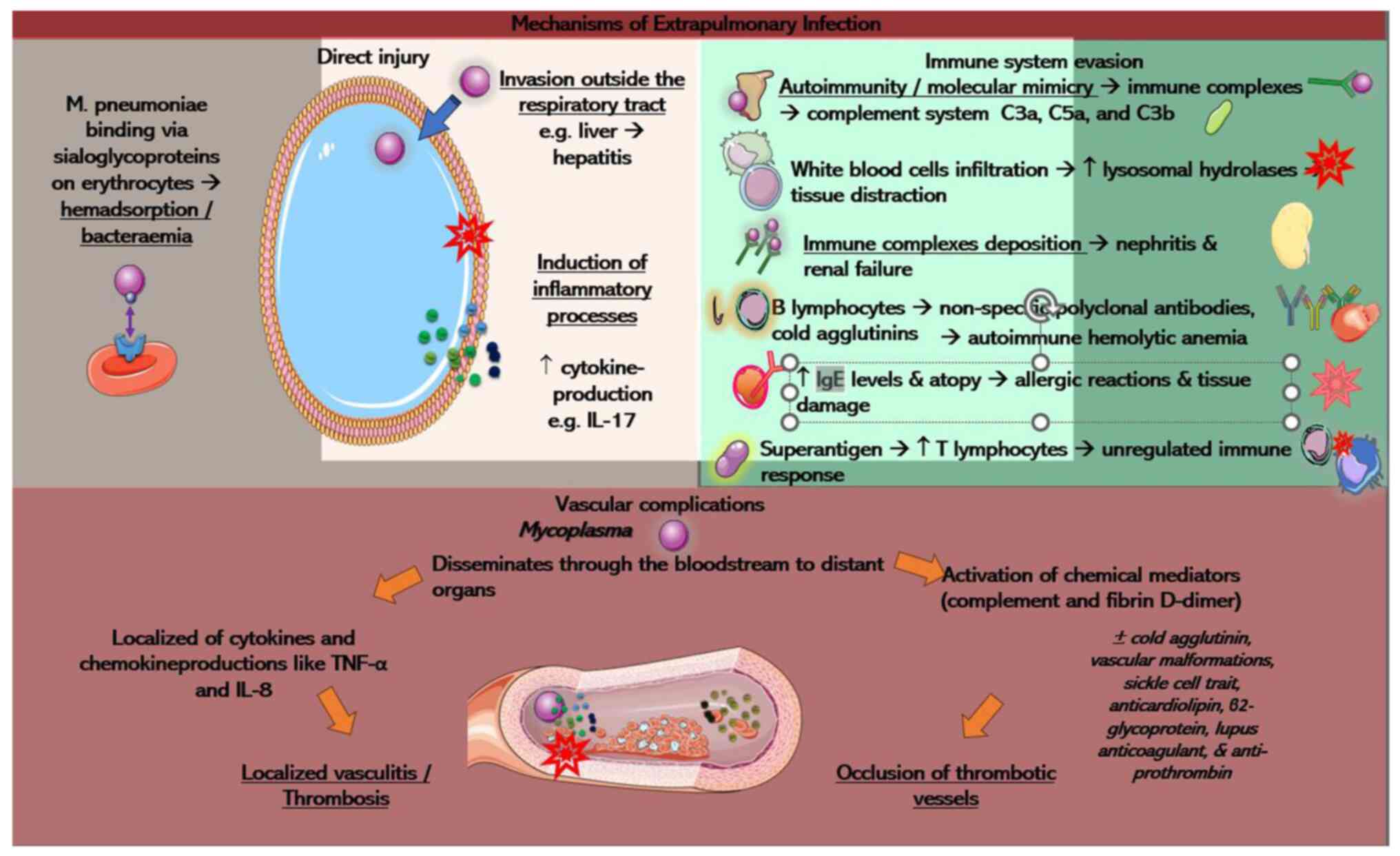
Fig. 3.
Direct injury
Early-onset extrapulmonary manifestations may be
related to direct damage from M. pneumoniae in the
bloodstream, whereas late-onset complications may stem from
indirect causes, such as autoimmunity, vascular damage, or drug
reactions. M. pneumoniae can be found in the blood, skin and
pericardial and synovial fluid, detected through PCR and cultures.
This suggests the potential for direct damage from the
extrapulmonary pathogenesis of M. pneumoniae (35).
In cases where the immune barrier of the respiratory
tract is immature or impaired, the pathogen may bypass pneumonia
development and enter the bloodstream through weakened gaps in
damaged lung epithelial cells (57). Given that erythrocytes carry
sialoglycoproteins, M. pneumoniae can bind to these cells,
leading to hemadsorption and possibly causing systemic infection
upon infiltrating the bloodstream. The presence of
Mycoplasma bacteremia stands as a direct extrapulmonary
manifestation. Of note, two forms of direct injury are possible:
The first one involves M. pneumoniae invading outside the
respiratory tract. Instances of early-onset hepatitis, unrelated to
pneumonia, may be linked to this form of extrapulmonary
manifestation (58). While it is
hypothesized that M. pneumoniae may directly infect liver
epithelial cells, this has not been conclusively confirmed. The
second form involves inflammatory damage caused by M.
pneumoniae. In tissues rich in cytokine-producing cells, the
membrane lipoprotein of the pathogen can stimulate local cytokine
production, resulting in inflammatory tissue damage (59). A previous study emphasized the
potential impact of IL-17, a crucial immune mediator, on disease
severity and the systemic immune response, potentially influencing
extrapulmonary pathogenesis (60).
Immune-driven mechanisms
A number of studies suggested that the recognition
of M. pneumoniae by innate immune cells and the subsequent
cell activation may play a pivotal role in causing severe M.
pneumoniae complications (61). A previous study conducted by Fink
et al (62) examining serum
IgM, IgA, IgG and cerebrospinal fluid in individuals with sudden
neurological manifestations, suggested that nervous system damage
may not stem directly from M. pneumoniae invasion, but may
likely result from an immune response to the infection.
Immune-driven mechanisms play a key role in M.
pneumoniae-associated extrapulmonary diseases (MpEPDs)
(9).
Role of autoimmunity
Autoimmunity spurred by molecular mimicry entails
M. pneumoniae antigens, mimicking host cell components,
resulting in alterations in the structure of host cell membrane
antigens. This activation induces autoimmune responses, forming
immune complexes within the corresponding organs. These complexes
activate complements, generating neutrophil chemotaxis factors,
including C3a, C5a and C3b. Numerous white blood cells then
infiltrate the affected site, releasing lysosomal hydrolases that
cause destructive injuries across multiple organs. For example,
proteins including P1 and P30 located on the attachment organelles
of M. pneumoniae, exhibit significant similarity to
proteins, such as troponin, keratin, cytoskeletal proteins and
fibrinogen within the host. When antibodies are generated due to
M. pneumoniae infections, they aim at diverse host tissues,
resulting in the formation of immune complexes that lead to damage
in organs, including the liver, kidneys, brain, smooth muscle and
lungs (6). In summary,
autoimmunity significantly contributes to extrapulmonary
complications resulting from M. pneumoniae infections.
Immune complexes
In instances where M. pneumoniae infection
results in acute nephritis and renal failure, findings suggest the
presence of the M. pneumoniae genome and immune complexes
containing its antigens within the glomerulus. This discovery may
align with increased deposition of immune complexes and activation
of the complement system within the tissues. The pathological
processes of glomerulonephritis and IgA nephropathy associated with
M. pneumoniae infections are driven by the circulation of
these immune complexes (35).
The M. pneumoniae membrane antigen mimics
the red blood cell (RBC)-membrane I antigen and shares similar
components with Streptococcus pneumoniae 23 or 32 and M.
genitalium. Similar to several plants and bacteria, the
membrane glycolipids of M. pneumoniae share an antigen
present in brain and lung tissues, causing cross-reactivity. The
carboxyl end of the P1 and P30 proteins of M. pneumoniae in
its adhesive organs bears resemblance to eukaryotic cytoskeletal
proteins, such as fibrinogen, keratin and troponin. Consequently,
infection triggers autoantibody production in the brain, lungs,
RBC-membranes, lymphocytes and myocardial cells, forming immune
complexes that intensify the autoimmune response, leading to
multisystem immune damage (30,63).
Non-specific antibodies
M. pneumoniae triggers B-lymphocytes,
resulting in the production of non-specific polyclonal antibodies
not directly targeting the pathogen. The study conducted by Meyer
Sauteur et al (64)
indicated an increase in serum antibodies against both M.
pneumoniae proteins and glycolipids among infected mice and
children. The equivalent recovery of serum antibody levels in
Bruton's tyrosine kinase (Btk)-deficient and wild-type mice after
M. pneumoniae infection suggests that the pulmonary
clearance of M. pneumoniae is primarily mediated by
IgG-reactive M. pneumoniae proteins. The presence of M.
pneumoniae glycolipid-specific IgG or IgM is not crucial
(64). Of note, ~50% of M.
pneumoniae-infected patients develop cold agglutinins, a type
of IgM antibody that may persist for weeks. Cold agglutinins serve
as proof of clinical suspicions regarding M.
pneumoniae-caused primary atypical pneumonia. It has been
suggested that these agglutinins may emerge due to cross-reactive
autoantibodies between the M. pneumoniae glycolipid antigen
and the I antigen present in human erythrocytes during acute M.
pneumoniae infections. This particular antibody has the
potential to trigger autoimmune hemolytic anemia, commonly known as
cold agglutinin disease. This entity stands out as a significant
indirect manifestation outside the lungs due to M.
pneumoniae infection (65).
Increased IgE levels
Increased IgE levels and atopy denote the genetic
predisposition of the body to generate IgE antibodies when exposed
to small quantities of common environmental factors (60). Poddighe et al (66) examined 162 hospitalized children
and noted a considerable increase in the overall serum IgE level
among children affected by MpEPDs. This level was notably higher
than that found in children solely experiencing the typical
respiratory issues associated with M. pneumoniae infection,
suggesting a link between atopy and MpEPDs in children (66). Patients displaying extrapulmonary
manifestations exhibit a higher prevalence of atopy compared to
those without such manifestations, suggesting a potential
association between atopy and MpEPDs (59,66).
Elevated IgE levels are indicative of immune irregularities.
Self-reactive IgEs exacerbate immune-related entities, leading to
clinical symptoms such as allergic reactions (67). For instance, following M.
pneumoniae infection, the P1 protein may prompt the generation
of P1-specific IgE in individuals allergic to M. pneumoniae,
eventually causing allergic symptoms and tissue damage (68). Some individuals prone to producing
IgE may have a predisposition to developing extra-respiratory
diseases when infected by M. pneumoniae (67).
Vascular complications
Extrapulmonary manifestations are not solely linked
to the infection and autoimmunity, but also involve complications
from the vascular system. Thrombosis can occur in vessels
throughout the body, with the pulmonary vessels being the most
commonly affected sites. The primary symptom is chest pain,
followed by neurological symptoms and abdominal pain (69). A rare case of pediatric priapism
has been reported, suggesting this symptom as an exceptionally
rare, yet plausible form of vascular occlusion resulting from
infection due to M. pneumoniae (70). Extrapulmonary manifestations
associated with vascular obstruction arise from a combination of
direct and indirect mechanisms. M. pneumoniae has the
ability to disseminate through the bloodstream to distant organs,
provoking localized production of cytokines and chemokines like
TNF-α and IL-8. This local response affects the vascular wall,
potentially resulting in localized vasculitis or thrombosis without
systemic hypercoagulability (69).
The alternative form involves the activation of chemical factors,
such as complement and d-dimer, which may lead to the occlusion of
thrombotic vessels. According to the study by Liu et al
(69), some factors contributing
to thrombosis are transient, while others stem from hereditary
thrombophilia in patients experiencing thrombosis due to M.
pneumoniae infection. Moreover, specific transient elements,
such as cold agglutinin, vascular malformations, sickle cell trait
and the presence of positive antibodies such as anticardiolipin,
β2-glycoprotein, lupus anticoagulant and anti-prothrombin have been
proposed to elevate the risk of thrombosis (71,72).
These factors contribute to thrombotic vessel occlusion (69).
Superantigen
Additional factors, such as the M.
pneumoniae superantigen, may also play a role in extrapulmonary
manifestations. Superantigens derived from different bacteria have
the potential to stimulate excessive production of T-lymphocytes
and lipid-related membrane proteins, resulting in an unregulated
immune response reminiscent of the pathogenic process observed in
Kawasaki disease (73).
Immunosuppression
Infection with M. pneumoniae has been
observed to trigger immunosuppressive effects in the body, leading
to imbalances in T-cell subgroups. Research has shown that this
infection severely damages both B-cells and T-cells (74). Between 13- and 18 weeks following
M. pneumoniae infection, there is a decrease in serum IgG
levels in patients (75). Some
children infected with M. pneumoniae experience
hypogammaglobulinemia, reduced neutrophil chemoattraction,
diminished responsiveness to phytohemagglutinin phytolectin and
lowered resistance to concurrent infections with other pathogens,
including S. pneumoniae (74). These alterations suggest that M.
pneumoniae infection has the potential to induce
immunosuppression.
4. Conclusions
Recent advancements have enhanced the understanding
of the mechanisms through which M. pneumoniae triggers both
pulmonary and extrapulmonary manifestations. The underlying
mechanisms leading to manifestations beyond pulmonary involvement
include direct damage through invasion and inflammatory components,
indirect harm from the immune response of the host and vascular
blockages. The mechanisms behind intrapulmonary and extrapulmonary
pathogenesis in M. pneumoniae infection, though distinct,
are interconnected and share certain similarities. Despite the
complexity of the pathogenic mechanisms of M. pneumoniae,
the specifics remain incompletely understood, warranting further
research for a detailed comprehension of its pathogenesis.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
Not applicable.
Authors' contributions
DAS and VEG conceptualized the study. IGL, VEG, NT,
PS and DAS made a substantial contribution to data interpretation
and analysis and wrote and prepared the draft of the manuscript.
DAS and VEG analyzed the data and provided critical revisions. All
authors contributed to manuscript revision, and have read and
approved the final version of the manuscript. Data authentication
is not applicable.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
DAS is the Editor-in-Chief for the journal, but had
no personal involvement in the reviewing process, or any influence
in terms of adjudicating on the final decision, for this article.
The other authors declare that they have no competing
interests.
Use of artificial intelligence tools
During the preparation of this work, AI tool Chat
GPT was used to improve the readability and language of the
manuscript, and subsequently, the authors revised and edited the
content produced by the AI tool as necessary, taking full
responsibility for the ultimate content of the present
manuscript.
References
|
1
|
Zhang L, Lai M, Ai T, Liao H, Huang Y,
Zhang Y, Liu Y, Wang L and Hu J: Analysis of Mycoplasma
pneumoniae infection among children with respiratory tract
infections in hospital in Chengdu from 2014 to 2020. Transl
Pediatr. 10:990–997. 2021.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Bajantri B, Venkatram S and Diaz-Fuentes
G: Mycoplasma pneumoniae: A potentially severe infection. J
Clin Med Res. 10:535–544. 2018.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Lanao AE, Chakraborty RK and
Pearson-Shaver AL: Mycoplasma. Infections. In: StatPearls.
StatPearls Publishing, Treasure Island, FL, 2023. Available from:
https://www.ncbi.nlm.nih.gov/books/NBK536927/.
|
|
4
|
Kutty PK, Jain S, Taylor TH, Bramley AM,
Diaz MH, Ampofo K, Arnold SR, Williams DJ, Edwards KM, McCullers
JA, et al: Mycoplasma pneumoniae among children hospitalized
with community-acquired pneumonia. Clin Infect Dis. 68:5–12.
2019.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Waites KB, Xiao L, Liu Y, Balish MF and
Atkinson TP: Mycoplasma pneumoniae from the respiratory
tract and beyond. Clin Microbiol Rev. 30:747–809. 2017.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Jiang Z, Li S, Zhu C, Zhou R and Leung
PHM: Mycoplasma pneumoniae infections: Pathogenesis and
vaccine development. Pathogens. 10(119)2021.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Waites KB, Xiao L, Paralanov V, Viscardi
RM and Glass JI: Molecular methods for the detection of
Mycoplasma and ureaplasma infections in humans: A paper from
the 2011 William beaumont hospital symposium on molecular
pathology. J Mol Diagn. 14:437–450. 2012.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Poddighe D: Extra-pulmonary diseases
related to Mycoplasma pneumoniae in children: Recent
insights into the pathogenesis. Curr Opin Rheumatol. 30:380–387.
2018.PubMed/NCBI View Article : Google Scholar
|
|
9
|
He J, Liu M, Ye Z, Tan T, Liu X, You X,
Zeng Y and Wu Y: Insights into the pathogenesis of Mycoplasma
pneumoniae (review). Mol Med Rep. 14:4030–4036. 2016.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Yamamoto T, Kida Y and Kuwano K:
Mycoplasma pneumoniae protects infected epithelial cells
from hydrogen peroxide-induced cell detachment. Cell Microbiol.
21(e13015)2019.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Nakane D, Murata K, Kenri T, Shibayama K
and Nishizaka T: Molecular ruler of the attachment organelle in
Mycoplasma pneumoniae. PLoS Pathog.
17(e1009621)2021.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Williams CR, Chen L, Sheppard ES, Chopra
P, Locklin J, Boons GJ and Krause DC: Distinct Mycoplasma
pneumoniae interactions with sulfated and sialylated receptors.
Infect Immun. 88:e00392–20. 2020.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Williams CR, Chen L, Driver AD, Arnold EA,
Sheppard ES, Locklin J and Krause DC: Sialylated receptor setting
influences Mycoplasma pneumoniae attachment and gliding
motility. Mol Microbiol. 109:735–744. 2018.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Chaudhry R, Ghosh A and Chandolia A:
Pathogenesis of Mycoplasma pneumoniae: An update. Indian J
Med Microbiol. 34:7–16. 2016.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Miyata M and Hamaguchi T: Integrated
information and prospects for gliding mechanism of the pathogenic
bacterium Mycoplasma pneumoniae. Front Microbiol.
7(960)2016.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Widjaja M, Berry IJ, Jarocki VM, Padula
MP, Dumke R and Djordjevic SP: Cell surface processing of the P1
adhesin of Mycoplasma pneumoniae identifies novel domains
that bind host molecules. Sci Rep. 10(6384)2020.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Romero-Arroyo CE, Jordan J, Peacock SJ,
Willby MJ, Farmer MA and Krause DC: Mycoplasma pneumoniae
protein P30 is required for cytadherence and associated with proper
cell development. J Bacteriol. 181:1079–1087. 1999.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Tabassum I, Chaudhry R, Chourasia BK and
Malhotra P: Identification of an N-terminal 27 kDa fragment of
Mycoplasma pneumoniae P116 protein as specific immunogen in
M. pneumoniae infections. BMC Infect Dis. 10(350)2010.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Fan L, Li D, Zhang L, Hao C, Sun H, Shao
X, Xu J and Chen Z: Pediatric clinical features of Mycoplasma
pneumoniae infection are associated with bacterial P1 genotype.
Exp Ther Med. 14:1892–1898. 2017.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Sun H, Xue G, Yan C, Li S, Zhao H, Feng Y
and Wang L: Changes in molecular characteristics of Mycoplasma
pneumoniae in clinical specimens from children in Beijing
between 2003 and 2015. PLoS One. 12(e0170253)2017.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Xue G, Cao L, Wang L, Zhao H, Feng Y, Ma L
and Sun H: Evaluation of P1 adhesin epitopes for the serodiagnosis
of Mycoplasma pneumoniae infections. FEMS Microbiol Lett.
340:86–92. 2013.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Meng YL, Wang WM, Lv DD, An QX, Lu WH,
Wang X and Tang G: The effect of Platycodin D on the expression of
cytoadherence proteins P1 and P30 in Mycoplasma pneumoniae
models. Environ Toxicol Pharmacol. 49:188–193. 2017.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Rodman Berlot J, Krivec U, Praprotnik M,
Mrvič T, Kogoj R and Keše D: Clinical characteristics of infections
caused by Mycoplasma pneumoniae P1 genotypes in children.
Eur J Clin Microbiol Infect Dis. 37:1265–1272. 2018.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Zhu C, Wu Y, Chen S, Yu M, Zeng Y, You X,
Xiao J and Wang S: Protective immune responses in mice induced by
intramuscular and intranasal immunization with a Mycoplasma
pneumoniae P1C DNA vaccine. Can J Microbiol. 58:644–652.
2012.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Varshney AK, Chaudhry R, Kabra SK and
Malhotra P: Cloning, expression, and immunological characterization
of the P30 protein of Mycoplasma pneumoniae. Clin Vaccine
Immunol. 15:215–120. 2008.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Blötz C, Singh N, Dumke R and Stülke J:
Characterization of an immunoglobulin binding protein (IbpM) from
Mycoplasma pneumoniae. Front Microbiol.
11(685)2020.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Szczepanek SM, Majumder S, Sheppard ES,
Liao X, Rood D, Tulman ER, Wyand S, Krause DC, Silbart LK and Geary
SJ: Vaccination of BALB/c mice with an avirulent Mycoplasma
pneumoniae P30 mutant results in disease exacerbation upon
challenge with a virulent strain. Infect Immun. 80:1007–1014.
2012.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Rathore JS and Wang Y: Protective role of
Th17 cells in pulmonary infection. Vaccine. 34:1504–1514.
2016.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Yang J, Sundrud MS, Skepner J and Yamagata
T: Targeting Th17 cells in autoimmune diseases. Trends Pharmacol
Sci. 35:493–500. 2014.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Hausner M, Schamberger A, Naumann W,
Jacobs E and Dumke R: Development of protective anti-Mycoplasma
pneumoniae antibodies after immunization of guinea pigs with
the combination of a P1-P30 chimeric recombinant protein and
chitosan. Microb Pathog. 64:23–32. 2013.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Svenstrup HF, Nielsen PK, Drasbek M,
Birkelund S and Christiansen G: Adhesion and inhibition assay of
Mycoplasma genitalium and M. pneumoniae by
immunofluorescence microscopy. J Med Microbiol. 51:361–373.
2002.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Duffy MF, Walker ID and Browning GF: The
immunoreactive 116 kDa surface protein of Mycoplasma
pneumoniae is encoded in an operon. Microbiology (Reading).
143:3391–3402. 1997.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Vizarraga D, Kawamoto A, Matsumoto U,
Illanes R, Pérez-Luque R, Martín J, Mazzolini R, Bierge P, Pich OQ,
Espasa M, et al: Immunodominant proteins P1 and P40/P90 from human
pathogen Mycoplasma pneumoniae. Nat Commun.
11(5188)2020.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Feng M, Schaff AC, Cuadra Aruguete SA,
Riggs HE, Distelhorst SL and Balish MF: Development of
Mycoplasma pneumoniae biofilms in vitro and the limited role
of motility. Int J Med Microbiol. 324:324–334. 2018.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Hu J, Ye Y, Chen X, Xiong L, Xie W and Liu
P: Insight into the pathogenic mechanism of Mycoplasma
pneumoniae. Curr Microbiol. 80(14)2022.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Yang J, Hooper WC, Phillips DJ and
Talkington DF: Cytokines in Mycoplasma pneumoniae
infections. Cytokine Growth Factor Rev. 15:157–168. 2004.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Balasubramanian S, Pandranki L, Maupin S,
Ramasamy K, Taylor AB, Hart PJ, Baseman JB and Kannan TR: Disulfide
bond of Mycoplasma pneumoniae community-acquired respiratory
distress syndrome toxin is essential to maintain the
ADP-ribosylating and vacuolating activities. Cell Microbiol.
21(e13032)2019.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Ramasamy K, Balasubramanian S, Kirkpatrick
A, Szabo D, Pandranki L, Baseman JB and Kannan TR: Mycoplasma
pneumoniae CARDS toxin exploits host cell endosomal acidic pH
and vacuolar ATPase proton pump to execute its biological
activities. Sci Rep. 11(11571)2021.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Blötz C and Stülke J: Glycerol metabolism
and its implication in virulence in Mycoplasma. FEMS
Microbiol Rev. 41:640–652. 2017.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Schumacher M, Nicholson P, Stoffel MH,
Chandran S, D'Mello A, Ma L, Vashee S, Jores J and Labroussaa F:
Evidence for the cytoplasmic localization of the
L-α-glycerophosphate oxidase in members of the ‘Mycoplasma
mycoides cluster’. Front Microbiol. 10(1344)2019.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Waites KB, Balish MF and Atkinson TP: New
insights into the pathogenesis and detection of Mycoplasma
pneumoniae infections. Future Microbiol. 3:635–648.
2008.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Segovia JA, Chang TH, Winter VT, Coalson
JJ, Cagle MP, Pandranki L, Bose S, Baseman JB and Kannan TR: NLRP3
is a critical regulator of inflammation and innate immune cell
response during Mycoplasma pneumoniae infection. Infect
Immun. 86:e00548–17. 2017.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Shimizu T, Kimura Y, Kida Y, Kuwano K,
Tachibana M, Hashino M and Watarai M: Cytadherence of Mycoplasma
pneumoniae induces inflammatory responses through autophagy and
toll-like receptor 4. Infect Immun. 82:3076–3086. 2014.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Li S, Xue G, Zhao H, Feng Y, Yan C, Cui J
and Sun H: The Mycoplasma pneumoniae HapE alters the
cytokine profile and growth of human bronchial epithelial cells.
Biosci Rep. 39(BSR20182201)2019.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Luo H, He J, Qin L, Chen Y, Chen L, Li R,
Zeng Y, Zhu C, You X and Wu Y: Mycoplasma pneumoniae lipids
license TLR-4 for activation of NLRP3 inflammasome and autophagy to
evoke a proinflammatory response. Clin Exp Immunol. 203:66–79.
2021.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Mara AB, Gavitt TD, Tulman ER, Geary SJ
and Szczepanek SM: Lipid moieties of Mycoplasma pneumoniae
lipoproteins are the causative factor of vaccine-enhanced disease.
NPJ Vaccines. 5(31)2020.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Narita M: Classification of extrapulmonary
manifestations due to Mycoplasma pneumoniae Infection on the
basis of possible pathogenesis. Front Microbiol.
7(23)2016.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Li G, Fan L, Wang Y, Huang L, Wang M, Zhu
C, Hao C, Ji W, Liang H, Yan Y and Chen Z: High co-expression of
TNF-α and CARDS toxin is a good predictor for refractory
Mycoplasma pneumoniae pneumonia. Mol Med.
25(38)2019.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Zhao Y, Ma G and Yang X: HDAC5 promotes
Mycoplasma pneumoniae-induced inflammation in macrophages
through NF-κB activation. Life Sci. 221:13–19. 2019.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Hakim MS, Annisa L, Jariah ROA and Vink C:
The mechanisms underlying antigenic variation and maintenance of
genomic integrity in Mycoplasma pneumoniae and Mycoplasma
genitalium. Arch Microbiol. 203:413–429. 2021.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Kenri T, Kawakita Y, Kudo H, Matsumoto U,
Mori S, Furukawa Y, Tahara YO, Shibayama K, Hayashi Y, Arai M and
Miyata M: Production and characterization of recombinant P1 adhesin
essential for adhesion, gliding, and antigenic variation in the
human pathogenic bacterium Mycoplasma pneumoniae. Biochem
Biophys Res Commun. 508:1050–1055. 2019.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Lee D, Lal NK, Lin ZD, Ma S, Liu J, Castro
B, Toruño T, Dinesh-Kumar SP and Coaker G: Regulation of reactive
oxygen species during plant immunity through phosphorylation and
ubiquitination of RBOHD. Nat Commun. 11(1838)2020.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Chen LS, Li C, You XX, Lin YW and Wu YM:
The mpn668 gene of Mycoplasma pneumoniae encodes a novel
organic hydroperoxide resistance protein. Int J Med Microbiol.
308:776–783. 2018.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Yu Y, Wang J, Han R, Wang L, Zhang L,
Zhang AY, Xin J, Li S, Zeng Y, Shao G, et al: Mycoplasma
hyopneumoniae evades complement activation by binding to factor H
via elongation factor thermo unstable (EF-Tu). Virulence.
11:1059–1074. 2020.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Waites KB and Talkington DF: Mycoplasma
pneumoniae and its role as a human pathogen. Clin Microbiol
Rev. 17:697–728, table of contents. 2004.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Mirijello A, La Marca A, D'Errico MM,
Curci S, Vendemiale G, Grandone E and De Cosmo S: Venous
thromboembolism during Mycoplasma pneumoniae infection: Case
report and review of the literature. Eur Rev Med Pharmacol Sci.
24:10061–10068. 2020.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Choi SY, Choi YJ, Choi JH and Choi KD:
Isolated optic neuritis associated with Mycoplasma
pneumoniae infection: Report of two cases and literature
review. Neurol Sci. 38:1323–1327. 2017.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Song WJ, Kang B, Lee HP, Cho J, Lee HJ and
Choe YH: Pediatric Mycoplasma pneumoniae infection
presenting with acute cholestatic hepatitis and other
extrapulmonary manifestations in the absence of pneumonia. Pediatr
Gastroenterol Hepatol Nutr. 20:124–129. 2017.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Mitsuo N: Classifcation of extrapulmonary
manifestations due to Mycoplasma pneumoniae infection on the
basis of possible pathogenesis. Fron Microbiol.
7(23)2016.PubMed/NCBI View Article : Google Scholar
|
|
60
|
Wang Z, Sun J, Liu Y and Wang Y: Impact of
atopy on the severity and extrapulmonary manifestations of
childhood Mycoplasma pneumoniae pneumonia. J Clin Lab Anal.
33(e22887)2019.PubMed/NCBI View Article : Google Scholar
|
|
61
|
Naghib M, Hatam-Jahromi M, Niktab M,
Ahmadi R and Kariminik A: Mycoplasma pneumoniae and
toll-like receptors: A mutual avenue. Allergol Immunopathol (Madr).
46:508–513. 2018.PubMed/NCBI View Article : Google Scholar
|
|
62
|
Fink CG, Sillis M, Read SJ, Butler L and
Pike M: Neurological disease associated with Mycoplasma
pneumoniae infection. PCR evidence against a direct invasive
mechanism. Clin Mol Pathol. 48:M51–M54. 1995.PubMed/NCBI View Article : Google Scholar
|
|
63
|
Shimizu T, Kida Y and Kuwano K: A
dipalmitoylated lipoprotein from Mycoplasma pneumoniae
activates NF-kappa B through TLR1, TLR2, and TLR6. J Immunol.
175:4641–4646. 2005.PubMed/NCBI View Article : Google Scholar
|
|
64
|
Meyer Sauteur PM, de Bruijn ACJM, Graça C,
Tio-Gillen AP, Estevão SC, Hoogenboezem T, Hendriks RW, Berger C,
Jacobs BC, van Rossum AMC, et al: Antibodies to protein but not
glycolipid structures are important for host defense against
Mycoplasma pneumoniae. Infect Immun. 87:e00663–18.
2019.PubMed/NCBI View Article : Google Scholar
|
|
65
|
Widén J, Jönsson G and Karlsson U:
Mycoplasma pneumonia with severe cold agglutinin hemolysis,
thrombocytosis, leukemoid reaction and acute renal failure.
IDCases. 31(e01689)2023.PubMed/NCBI View Article : Google Scholar
|
|
66
|
Poddighe D, Comi EV, Brambilla I, Licari
A, Bruni P and Marseglia GL: Increased total serum immunoglobulin e
in children developing Mycoplasma pneumoniae-related
extra-pulmonary diseases. Iran J Allergy Asthma Immunol.
17:490–496. 2018.PubMed/NCBI
|
|
67
|
Poddighe D and Marseglia GL: Is there any
relationship between extra-pulmonary manifestations of
Mycoplasma pneumoniae infection and atopy/respiratory
allergy in children? Pediatr Rep. 8(6395)2016.PubMed/NCBI View Article : Google Scholar
|
|
68
|
Ye Q, Mao JH, Shu Q and Shang SQ:
Mycoplasma pneumoniae induces allergy by producing
P1-specific immunoglobulin E. Ann Allergy Asthma Immunol.
121:90–97. 2018.PubMed/NCBI View Article : Google Scholar
|
|
69
|
Liu J, He R, Wu R, Wang B, Xu H, Zhang Y,
Li H and Zhao S: Mycoplasma pneumoniae pneumonia associated
thrombosis at Beijing Children's hospital. BMC Infect Dis.
20(51)2020.PubMed/NCBI View Article : Google Scholar
|
|
70
|
Hirshberg SJ, Charles RS and Ettinger JB:
Pediatric priapism associated with Mycoplasma pneumoniae.
Urology. 47:745–746. 1996.PubMed/NCBI View Article : Google Scholar
|
|
71
|
Liu J and Li Y: Thrombosis associated with
Mycoplasma pneumoniae infection (Review). Exp Ther Med.
22(967)2021.PubMed/NCBI View Article : Google Scholar
|
|
72
|
Pachet A, Dumestre-Perard C, Moine M,
Marlu R, Rubio A and Bost-Bru C: Splenic infarction associated with
transient anti-prothrombin antibodies is a rare manifestation of
acute Mycoplasma pneumoniae infection. Arch Pediatr.
26:483–486. 2019.PubMed/NCBI View Article : Google Scholar
|
|
73
|
Dietz SM, van Stijn D, Burgner D, Levin M,
Kuipers IM, Hutten BA and Kuijpers TW: Dissecting Kawasaki disease:
A state-of-the-art review. Eur J Pediatr. 176:995–1009.
2017.PubMed/NCBI View Article : Google Scholar
|
|
74
|
Okoli K, Gupta A, Irani F and Kasmani R:
Immune thrombocytopenia associated with Mycoplasma
pneumoniae infection: A case report and review of literature.
Blood Coagul Fibrinolysis. 20:595–598. 2009.PubMed/NCBI View Article : Google Scholar
|
|
75
|
Rastawicki W, Rokosz N and Jagielski M:
Subclass distribution of human IgG antibodies to Mycoplasma
pneumoniae in the course of mycoplasmosis. Med Dosw Mikrobiol.
61:375–379. 2009.PubMed/NCBI(In Polish).
|