Introduction
An epidemiological study suggested that residential
emissions, presumed to contain carbonaceous particles as the most
toxic ingredients, globally influence premature mortality (1). In cities, diesel exhaust is a source
of particulate matter (PM) from traffic, which constitutes a large
proportion of urban dust. Various respiratory conditions, ischemic
heart disease and cancer are potentially associated with long-term
exposure to PM from traffic (2).
PM2.5 with an aerodynamic diameter of ≤2.5 µm generates
reactive oxygen species (ROS), increases the secretion of
proinflammatory cytokines, and induces matrix metalloproteinases
(MMPs), leading to senescence in both keratinocytes and dermal
fibroblasts (3-6).
The transcription factor nuclear factor erythroid
2-related factor 2 (NRF2) plays an important role in maintaining
redox balance by preventing the oxidation of macromolecules such as
DNA, lipids and proteins. It does this by increasing the levels of
cellular antioxidant enzymes, including superoxide dismutase (SOD),
catalase (CAT), glutathione peroxidase (GPX) and heme oxygenase 1
(HO-1) (7). NRF2 can serve as a
protective target, inhibiting PM2.5-induced redox
imbalance and inflammation. Recent studies have shown that natural
compounds activate NRF2 signaling to protect cells from
PM2.5-induced damage (8,9).
Astaxanthin (ATX), a naturally occurring carotenoid
dye that can be extracted from algae, yeast, shrimp and other
organisms, exhibits significant antioxidant activity (10). Therefore, ATX is considered a
potential biological compound for treating inflammation, aging and
cardiovascular diseases (11). ATX
stimulates the NRF2 signaling pathway to enhance cellular
antioxidant and anti-inflammatory capabilities, which have
neuroprotective, anti-tumorigenic, antidiabetic and
hepatoprotective effects (12).
Additionally, ATX depletes ROS, thereby preventing skin photoaging
(13). However, only a limited
number of studies have investigated the effects of ATX on
PM2.5-induced skin senescence. In the present study, the
response of the antioxidant system and senescence were examined in
HaCaT cells exposed to PM2.5, as well as the
anti-senescence mechanism of ATX.
Materials and methods
Preparation of ATX and
PM2.5
ATX (cat. no. SML0982; Sigma-Aldrich; Merck KGaA)
was dissolved in dimethyl sulfoxide (DMSO). PM2.5 (NIST
PM; cat. no. SRM 1650b; Sigma-Aldrich; Merck KGaA) was dispersed in
DMSO to prepare a stock solution (25 mg/ml). The 50 µg/ml of
PM2.5 was selected as the optimal concentration based on
our previous research (4).
Cell culture
HaCaT (cat. no. 300493; CLS Cell Lines Service GmbH)
cells were seeded in Dulbecco's modified Eagle's medium (Thermo
Fisher Scientific, Inc.) supplemented with 10% heat-inactivated
fetal calf serum (Thermo Fisher Scientific, Inc.) and 1%
antibiotic-antimycotic solution in 5% CO2 at 37˚C.
Cell viability
Cells were cultured in a 24-well plate with ATX (1,
2.5, 5, 7.5 and 10 µΜ) and/or PM2.5 for 48 h at 37˚C.
Subsequently, 100 µl
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (cat.
no. 475989; Sigma-Aldrich; Merck KGaA) was added to each well to
form an insoluble purple formazan by the action of mitochondrial
reductase in live cells at 37˚C for 4 h, which was dissolved in 600
µl DMSO. The solution was then transferred to a 96-well plate and
observed using a scanning multi-well spectrophotometer at 540
nm.
ROS detection
2',7'-Dichlorodihydrofluorescein diacetate
(H2DCFDA) (cat. no. D6883; Sigma-Aldrich; Merck KGaA), a
cell-permeant ROS probe, was used to measure intracellular ROS
content in HaCaT cells. Cells were cultured with ATX (1, 2.5, 5 and
7.5 µΜ) or a ROS scavenger (1 mM N-acetyl cysteine, NAC) (cat. no.
A9165; Sigma-Aldrich; Merck KGaA) and then exposed to 1 mM
H2O2 or 50 µg/ml PM2.5. After
staining with H2DCFDA, fluorescence in the cells was
detected individually using fluorescence spectrometer (Promega
Corporation) and BD LSR II flow cytometer with FACSDIVA software
version 6.0 (Becton, Dickinson and Company). Similarly, siControl
and siNRF2 cells were cultured with ATX or NAC and exposed to
PM2.5. ROS levels were measured using a confocal
microscope (Olympus Corporation).
Western blot analysis
Cell lysis was performed using the PRO-PREP™ protein
extraction solution (cat. no. 17081; Intron Biotechnology, Inc.) or
the NE-PER™ nuclear and cytoplasmic extraction reagents (cat. no.
78833; Thermo Fisher Scientific, Inc.). The protein concentration
was determined using a BCA assay kit (cat. no. 23225; Thermo Fisher
Scientific, Inc.). Subsequently, 40 µg cell lysates were separated
by electrophoresis on a 10 or 12% SDS-polyacrylamide gel and were
transferred onto PVDF membranes. The membranes were subjected to
blocking in 3% bovine serum albumin (Bovogen Biologicals Pty Ltd.)
for 1 h at 20˚C with agitation, incubation with primary antibodies
(1:1,000) for 2 h at 20˚C, and incubation with HRP-conjugated
secondary antibodies (1:5,000; anti-rabbit, cat. no. ab6721 and
anti-mouse, cat. no. ab205719; Abcam) for 2 h at 20˚C. The
membranes were then washed with 1X TBS-0.1% Tween-20 (cat. no.
9997; Cell Signaling Technology, Inc.). Subsequently, the membranes
with the targeted proteins were exposed to an enhanced
chemiluminescence reagent (Cytiva) and the corresponding bands were
visualized using an autoradiography film. The following primary
antibodies were used: NRF2 (cat. no. sc-722), CAT (cat. no.
sc-271803), GPX1/2 (cat. no. sc-133160), cyclin dependent kinase
inhibitor 2A (p16) (cat. no. sc-1661), HO-1 (cat. no. sc-390991)
and actin (cat. no. sc-8432) were purchased from Santa Cruz
Biotechnology, Inc. Phospho-H2A histone family member X (H2A.X;
cat. no. 2577), H2A.X (cat. no. 2595), c-Fos (cat. no. 2250), jun
proto-oncogene, activator protein-1 (AP-1) transcription factor
subunit (c-Jun; cat. no. 9165), phospho-c-Jun (cat. no. 91952) were
obtained from Cell Signaling Technology, Inc. Phospho-NRF2 (cat.
no. ab76026), interleukin (IL)-1β (cat. no. ab315084), MMP-2 (cat.
no. ab92536), MMP-9 (cat. no. ab76003) and TATA-binding protein
(TBP) (cat. no. ab818) were purchased from Abcam. Cu/Zn SOD (cat.
no. ADI-SOD-100) was purchased from Enzo Life Sciences, Inc.
Protein bands were analyzed using ImageJ version 1.48V (National
Institutes of Health).
Detection of 8-oxoguanine DNA
glycosylase (8-oxoG)
The avidin-tetra-methyl-rhodamine isothiocyanate
(TRITC) conjugate (cat. no. A7169; Sigma-Aldrich; Merck KGaA)
exhibited highly specific binding to oxidized nucleosides 8-oxoG
(14). The cells were stained with
avidin-TRITC dye for 30 min at 37˚C and observed under a confocal
microscope.
Cell cycle analysis
Cells were cultured with ATX or/and PM2.5
treatment in 6-well plates at 37˚C for 24 h, after which, they were
fixed with 70% ethanol for 1 h at 4˚C, and stained with propidium
iodide (cat. no. P4864; Sigma-Aldrich; Merck KGaA) and RNase A
(1:1,000; cat. no. 12091-021; Thermo Fisher Scientific, Inc.) at
37˚C for 1 h. Cellular DNA content was detected using FACSCalibur
flow cytometer with CellQuest pro software 4.02 (Becton, Dickinson
and Company) for cell cycle analysis.
β-Galactosidase staining assay
Senescence-associated β-galactosidase (SA-β-Gal)
expressed in senescent cells was detected using a cellular
senescence detection kit (SPiDER-β-Gal) (cat. no. SG03; Dojindo
Laboratories, Inc.). Images and histograms were obtained using flow
cytometry and confocal microscopy, respectively.
Transient transfection of small
interfering RNA (siRNA)
Lipofectamine® RNAiMax (cat. no.
13778075; Thermo Fisher Scientific, Inc.) was used to transfect 20
nM siRNA against NRF2 (siNRF2 RNA) (cat. no. sc-37030; Santa Cruz
Biotechnology, Inc.) or negative control (siControl RNA) (cat. no.
sc-37007; Santa Cruz Biotechnology, Inc.) into cells. The siRNA
sequences were as follows: Control siRNA sense,
5'-CACAGGGUAAGGAACUCGUCUCUCA-3' and antisense,
5'-UGAGAGACGAGUUCCUUACCCUGUG-3'; and NRF2 siRNA sense,
5'-GCAUGCUACGUGAUGAAGAtt-3' and antisense,
5'-UCUUCAUCACGUAGCAUGCtt-3'. After incubation for 24 h at 37˚C, the
transfected cells were processed for ROS detection and
β-galactosidase staining assay.
Statistical analysis
All the values of measurements are expressed as the
mean ± standard deviation. The results were analyzed for pairwise
differences using one-way analysis of variance followed by Tukey's
post hoc test. P<0.05 was considered to indicate a statistically
significant difference. Statistical analysis was performed using
SigmaStat v3.5 (Systat Software Inc.).
Results
ATX scavenges ROS generated from
PM2.5
HaCaT cells were cultured with ATX (0, 1, 2.5, 5,
7.5 and 10 µM) for 48 h at 37˚C. Cell viability assay results
revealed that ATX had no cytotoxicity at a concentration <7.5 µM
(Fig. 1A). The ROS scavenging
effects of ATX were then examined. Cells were pretreated with ATX
(1, 2.5, 5 and 7.5 µM) or NAC (1 mM). The production of
H2O2-induced intracellular ROS was inhibited
significantly by ATX or NAC (Fig.
1B). In addition, ATX inhibited ROS generation from
PM2.5 (Fig. 1C).
According to the results, 7.5 µM ATX was selected as the optimal
concentration in further experiments.
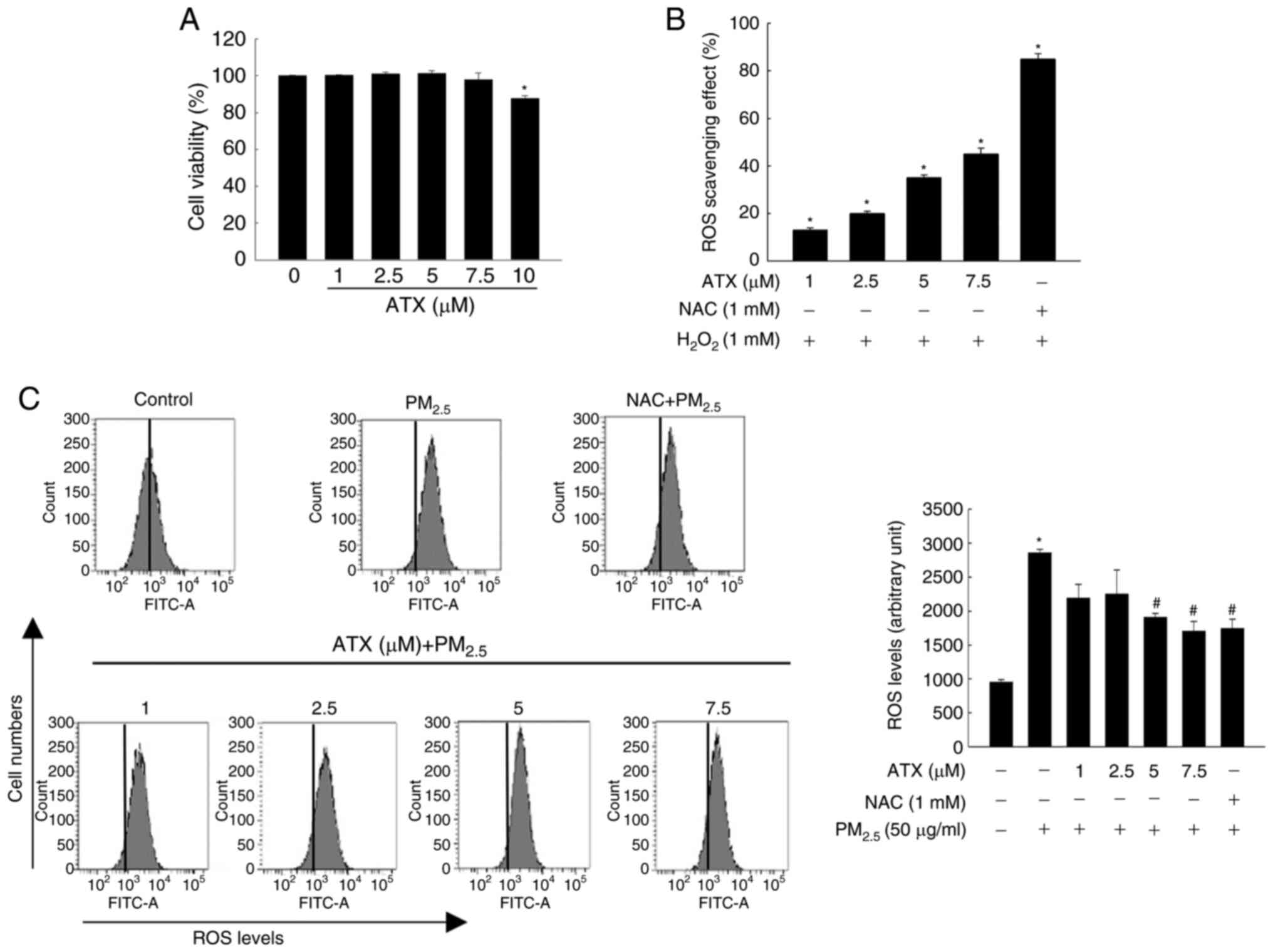 | Figure 1Inhibitory effect of ATX on
H2O2- or PM2.5-induced
intracellular ROS. (A) Viability of HaCaT cells cultured with 1,
2.5, 5, 7.5 and 10 µM ATX was detected using the MTT assay.
*P<0.05 vs. ATX-untreated cells. (B and C) Cells were
cultured with 1, 2.5, 5 and 7.5 µM ATX, 1 mM NAC, 1 mM
H2O2, or 50 µg/ml PM2.5. ROS
scavenging effects were measured (B) via fluorescence spectrometer
(*P<0.05 vs. H2O2-treated
cells) and (C) through flow cytometry after staining with
H2DCFDA (*P<0.05 vs. the
PM2.5-untreated cells; #P<0.05 vs.
PM2.5-treated cells). ATX, astaxanthin;
PM2.5, particulate matter 2.5; ROS, reactive oxygen
species; NAC, N-acetyl cysteine. |
ATX recovers the homeostasis of the
antioxidant enzymes by activating NRF2
To maintain cellular homeostasis, NRF2 plays an
important role in the regulation of oxidative stress by activating
antioxidant enzymes (SOD, CAT, GPX and HO-1) to eliminate ROS
(7). In the present study, the
active form of NRF2 in nuclear fraction and the expression levels
of antioxidant enzymes were tested. After PM2.5
treatment, the expression of phospho-NRF2 was the highest in the
first 12 h and then decreased gradually (Fig. 2A). Conversely, it increased
gradually in a time-dependent manner up to 72 h after ATX
pretreatment (Fig. 2B).
Accordingly, phospho-NRF2 levels in the nuclear fraction, which
were reduced after PM2.5 exposure for 48 h, increased
after pretreatment with ATX (Fig.
2C). The expression of Cu/Zn SOD, CAT and GPX1/2 decreased
following treatment with PM2.5 in a dose-dependent
manner; HO-1 expression was elevated at 12 and 24 h and then
decreased significantly after exposure to PM2.5
(Fig. 2D). However, the reduced
levels of Cu/Zn SOD, CAT, GPX1/2, and HO-1 following
PM2.5 exposure were increased after pretreatment with
ATX (Fig. 2E). Therefore, it was
revealed that ATX restored intracellular redox homeostasis by
activating NRF2 and its related enzymes, which were decreased by
PM2.5.
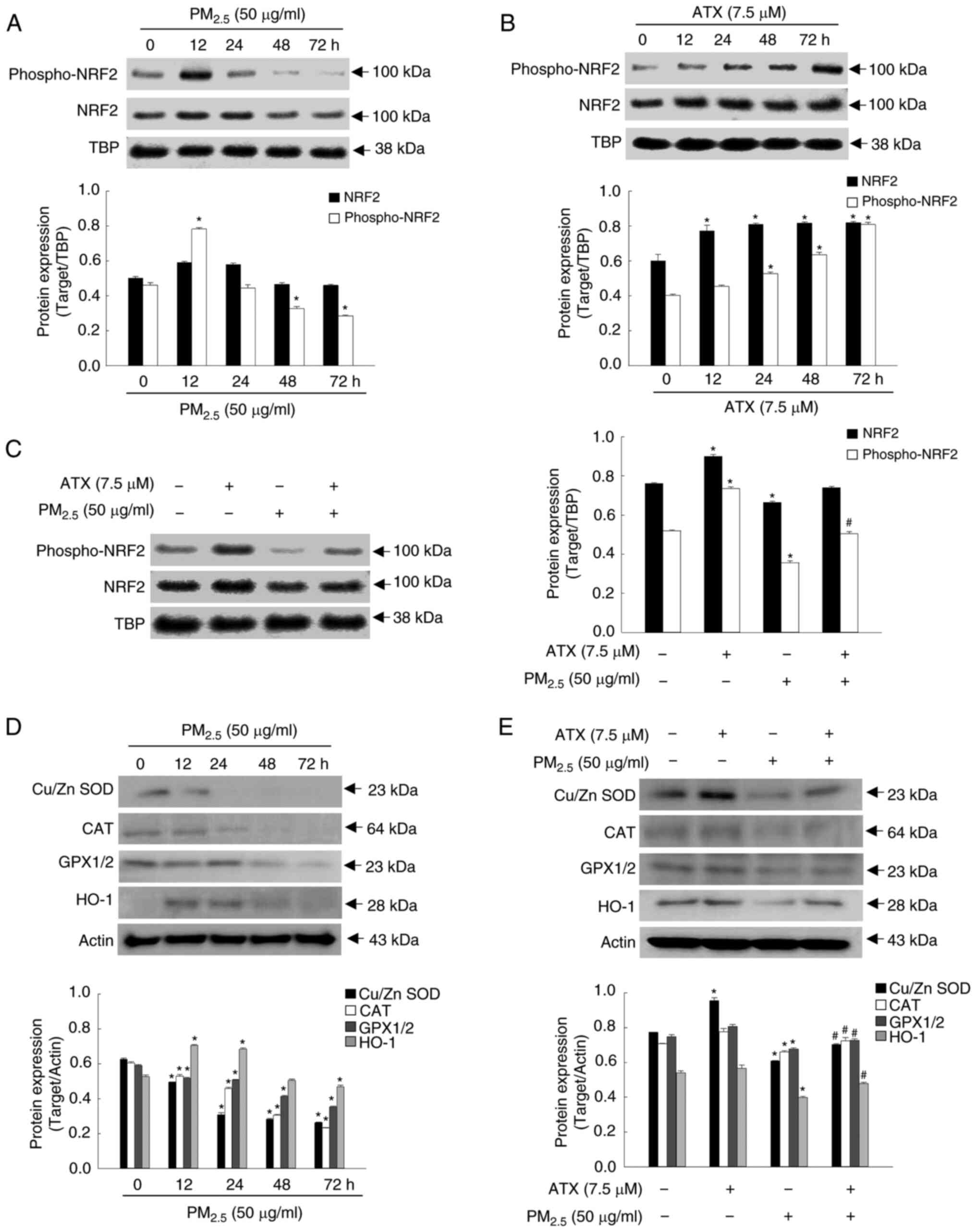 | Figure 2Recovery effect of ATX on
antioxidant-related mediator inhibited by PM2.5. (A and
B) The protein levels of phospho-NRF2 and NRF2, after (A)
PM2.5 or (B) ATX treatment for various time intervals
were detected by western blotting. TBP was used a nuclear fraction
loading control. *P<0.05 vs. PM2.5 or
ATX-untreated cells at 0 h. (C) The protein levels of phospho-NRF2
and NRF2 after treatment with ATX and/or PM2.5 were
detected by western blotting. *P<0.05 vs. ATX or
PM2.5-untreated cells; #P<0.05 vs.
PM2.5-treated cells. (D) The protein levels of Cu/Zn
SOD, CAT, GPX1/2 and HO-1 after PM2.5 treatment at
various time intervals were detected by western blotting. Actin was
used as a loading control. *P<0.05 vs.
PM2.5-untreated cells at 0 h. (E) The protein levels of
Cu/Zn SOD, CAT, GPX1/2 and HO-1 after cells were treated with ATX
and/or PM2.5 were detected by western blot analysis.
*P<0.05 vs. ATX or PM2.5-untreated cells;
#P<0.05 vs. PM2.5-treated cells. ATX,
astaxanthin; PM2.5, particulate matter 2.5; TBP,
TATA-binding protein; NRF2, nuclear factor erythroid 2-related
factor 2; SOD, superoxide dismutase; CAT, catalase; GPX1/2,
glutathione peroxidase 1/2; HO-1, heme oxygenase 1. |
ATX protects cells from
PM2.5-induced DNA damage
8-OxoG and phospho-H2A.X are two specific markers of
DNA damage and present in high levels in the PM2.5
treatment group of keratinocytes (14). According to the results, ATX showed
protective effects from PM2.5-induced nucleoside
oxidization and phospho-H2A.X expression (Fig. 3A-C). Cell cycle checkpoints monitor
DNA damage and the response to cell cycle by DNA damage is executed
by a cell cycle control mechanism (15). PM2.5 perturbed the cell
cycle, causing G0/G1 arrest, which was
reversed by ATX treatment (Fig.
3D). ATX also improved cell viability, which had been reduced
by exposure to PM2.5 (Fig.
3E). Therefore, ATX exhibited DNA protective effects, inhibited
cell cycle arrest, and promoted cell viability in
PM2.5-treated cells.
ATX inhibits the secretion of
PM2.5-induced cytokine and MMPs
Cellular senescence is caused by damaging stimuli
that contribute to an irreversible state of cell cycle arrest, in
which cytokines and MMPs are secreted (16). In addition, AP-1, which comprises
the transcription factors c-Fos and c-Jun, is highly regulated by
UV light during photoaging and closely related to the expression of
ILs and MMPs (17). In the present
study, the protein levels of phospho-c-Jun and c-Fos were increased
significantly by PM2.5, whereas they were decreased by
pretreatment with ATX (Fig. 4A).
Cytokines, such as IL-1β, MMP-2 and MMP-9, were expressed at higher
levels in PM2.5-treated group than in the control group;
however, they were inhibited by treatment with ATX (Fig. 4B). Therefore, ATX protected
keratinocytes from PM2.5-induced senescence-associated
cytokines and MMPs.
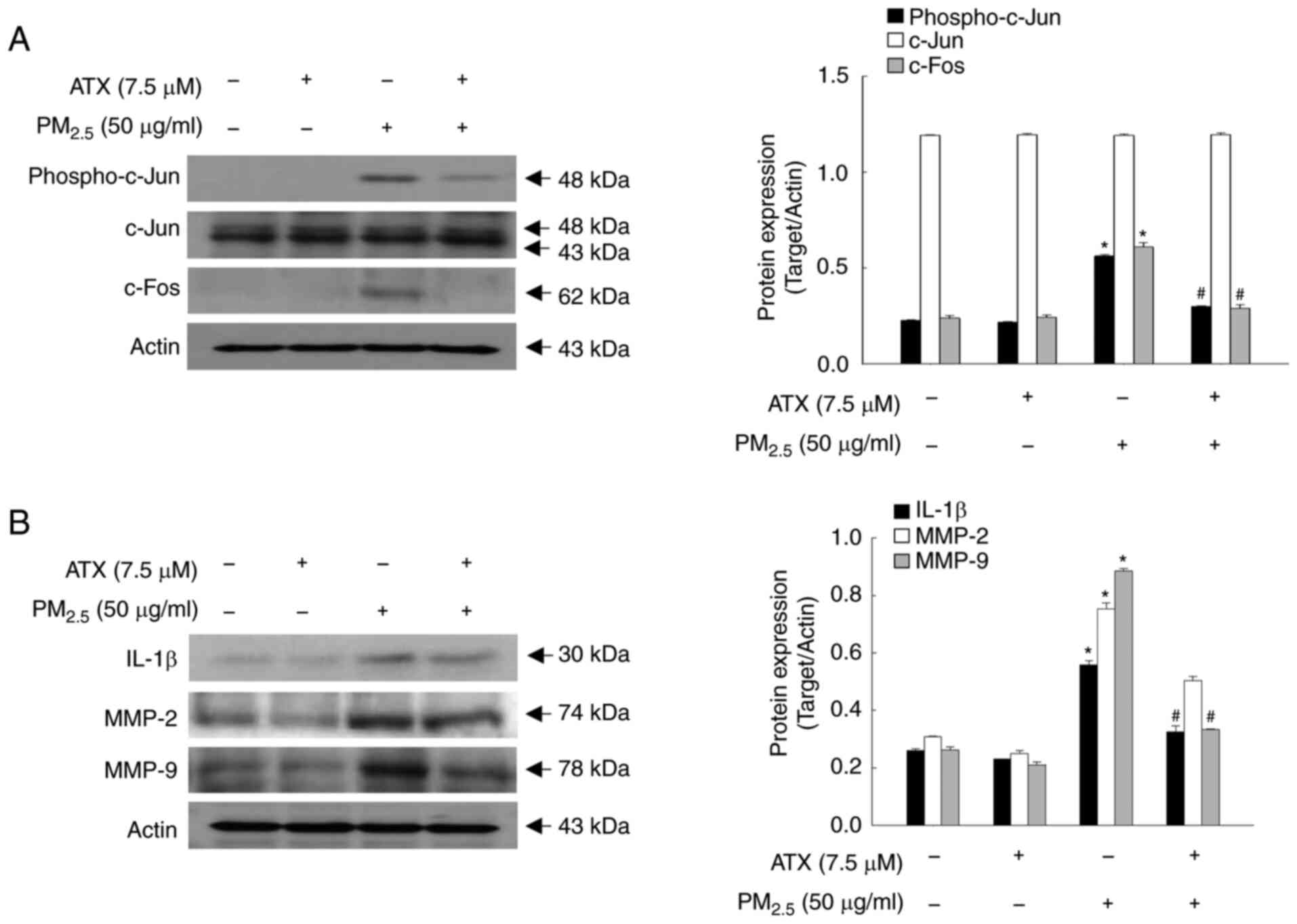 | Figure 4Inhibitory effects of ATX on
PM2.5-induced transcription factor, AP-1,
pro-inflammatory cytokines and MMPs. (A and B) Western blot assay
was performed for the detection of (A) protein levels of
phospho-c-Jun, c-Jun, c-Fos, and (B) protein levels of IL-1β, MMP-2
and MMP-9. *P<0.05 vs. ATX or
PM2.5-untreated cells; #P<0.05 vs.
PM2.5-treated cells. MMPs, matrix metalloproteinases;
c-Jun, jun proto-oncogene, AP-1 transcription factor subunit;
c-Fos, fos proto-oncogene, AP-1 transcription factor subunit. |
ATX protects cells from
PM2.5-induced senescence-associated secretory phenotype
(SASP)
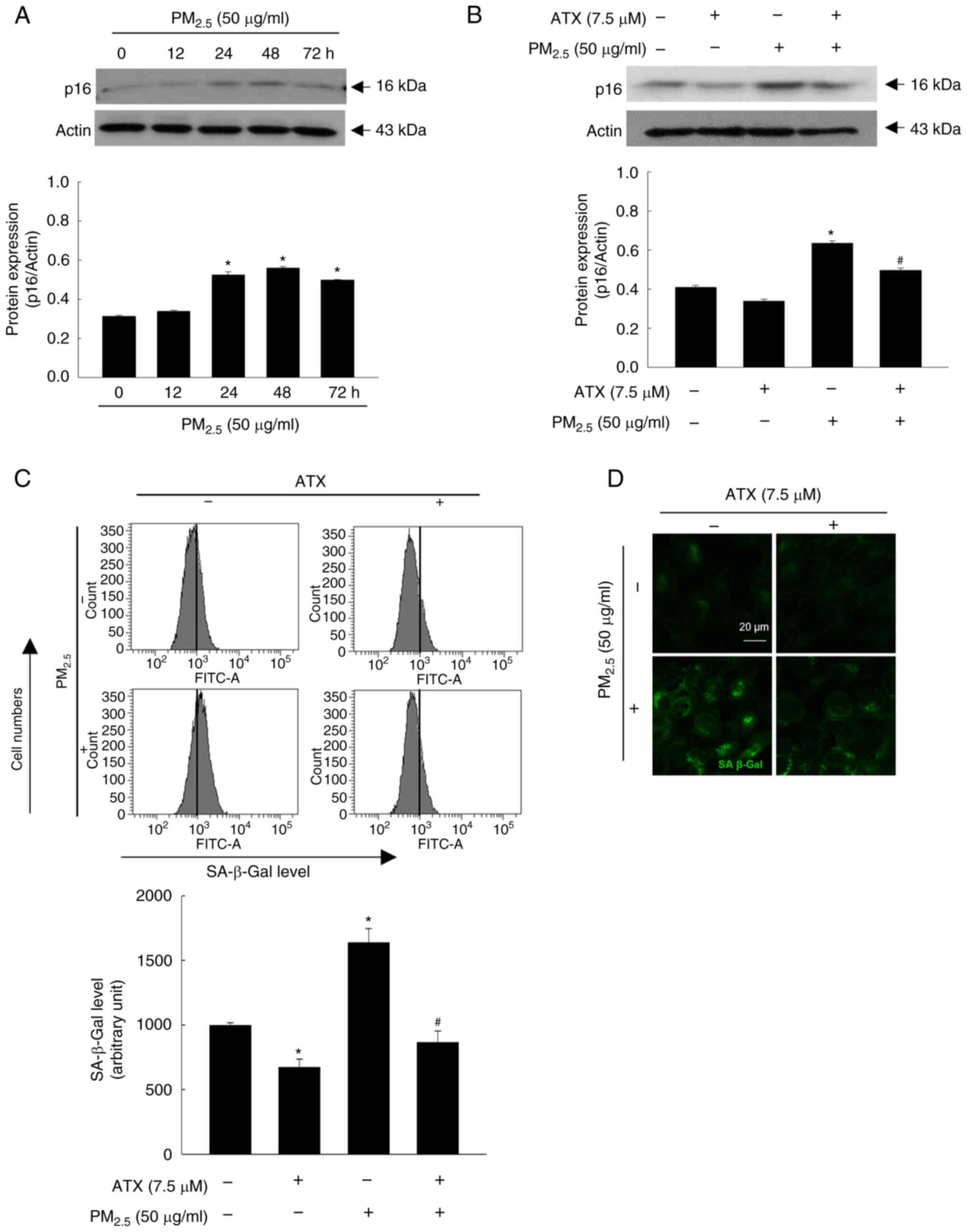
p16 and SA-β-Gal are the key markers of SASP, which
indicates the state of skin aging (18). Therefore, in the present study, p16
protein expression in keratinocytes was examined over time among
the four groups. The p16 level increased up to 48 h by
PM2.5 (Fig. 5A) and was
decreased upon pretreatment with ATX (Fig. 5B). Moreover, ATX inhibited cellular
SA-β-Gal, which was observed by flow cytometry (Fig. 5C) and confocal microscopy (Fig. 5D). Therefore, ATX protected
keratinocytes from PM2.5-induced senescence.
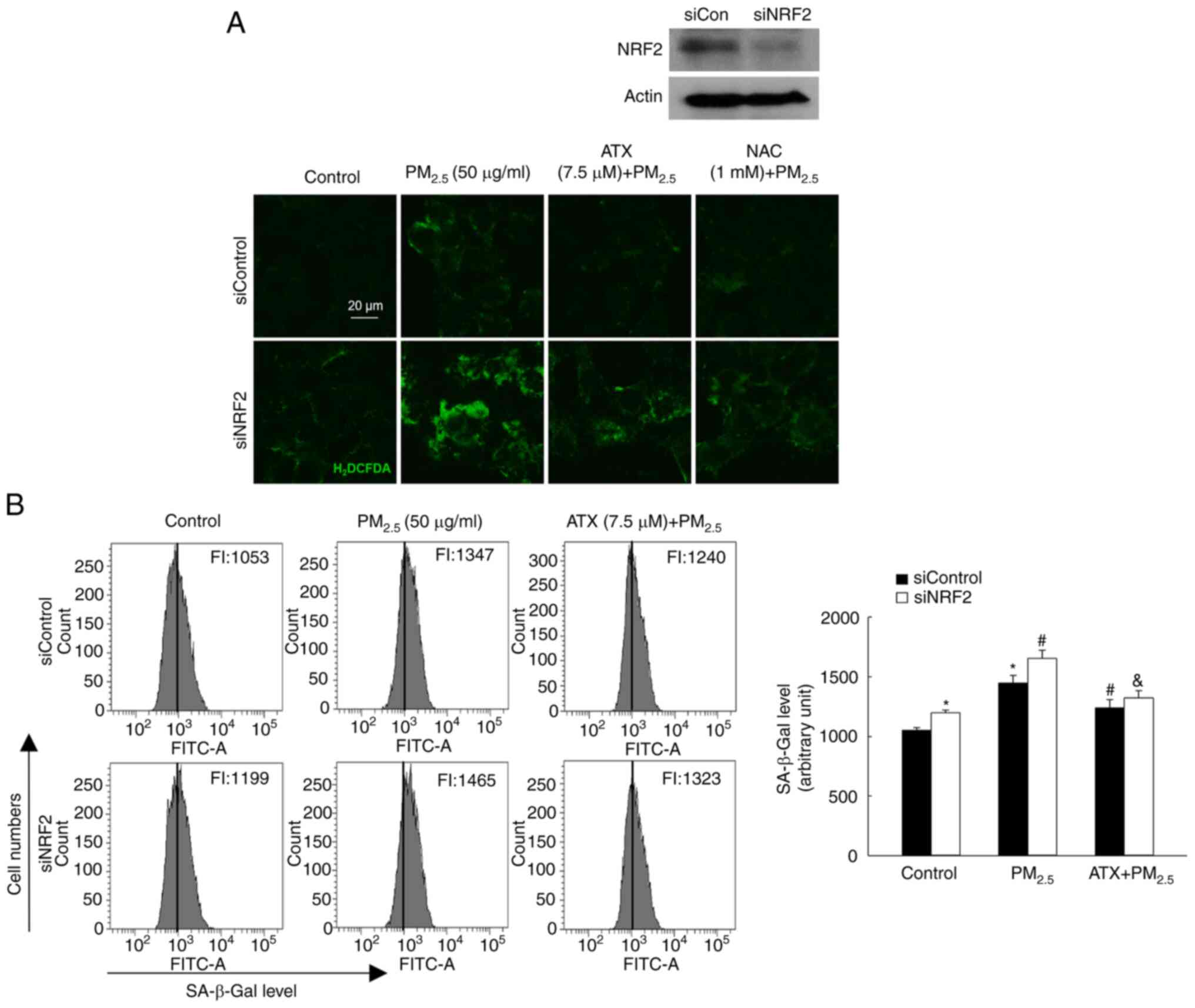
ATX attenuates
PM2.5-induced senescence by inhibiting ROS via the
NRF2
To confirm the role of NRF2 in
PM2.5-induced senescence, a siRNA was used to interfere
with NRF2 mRNA expression. After exposure to PM2.5, the
ROS levels were significantly higher in cells transfected with
siNRF2 RNA than in those transfected with siControl RNA, which was
inhibited by treatment with ATX and NAC (Fig. 6A). After exposure to
PM2.5, cells transfected with siNRF2 RNA showed higher
SA-β-Gal fluorescence than siControl RNA cells, a phenomenon that
was decreased significantly by ATX treatment (Fig. 6B). Therefore, ROS amelioration of
ATX relieved senescence induced by PM2.5 through the
NRF2 pathway.
Discussion
PM2.5 is currently a major concern, and
research is underway to understand its effects on the human body.
The human skin acts as the first barrier against environmental
stress, however PM2.5 can penetrate this barrier and
cause skin problems. Previous studies by the authors have
demonstrated that PM2.5 could penetrate skin cells and
damage the skin by inducing oxidative stress (3-5).
This leads to the destruction of cellular macromolecules and
organelles, as well as apoptotic cell death (4). It was also revealed that
PM2.5 activates the inflammatory pathway toll-like
receptor 5-NADPH oxidase 4-NFκB-IL-6 in both wild-type mice and
flaky tail mice. This suggested that PM2.5-induced
inflammation may contribute to the development and exacerbation of
atopic dermatitis (3).
Furthermore, it was revealed that PM2.5 induced skin
senescence by the aryl hydrocarbon receptor-ROS-p16 pathway via
epigenetic modification (19).
These findings suggested that ROS are key factors in the induction
of inflammation and aging by PM2.5, and a solution in
natural products was sought. Previously, various studies have
revealed that various natural compounds from marine algae can
decrease excessive ROS levels in skin cells (20,21).
In addition, agar oligosaccharide, a marine prebiotic, has
anti-aging effects via the activation of antioxidant enzymes, such
as Cu/Zn SOD and CAT, in Drosophila melanogaster (22). Moreover, oligosaccharides from
green algae have anti-aging effects by increasing CAT and GSH
levels and decreasing lipid oxidation levels in mice (23).
ATX, a potent antioxidant, has been demonstrated to
mitigate the physiological adverse effects of oxidative stress
during the senescence process and extend lifespan both in
vitro and in vivo (24). Furthermore, ATX has been revealed
to alleviate oxidative stress and immune impairment in rats with
galactose-induced aging by activating the NRF2/KEAP1 pathway and
suppressing the NFκB pathway (25). In the present study, it was aimed
to investigate the beneficial effects of ATX isolated from algae,
on PM2.5-induced DNA damage, cell cycle arrest and
senescence in HaCaT cells. As demonstrated in Fig. 1C, ATX pretreatment inhibited
PM2.5-induced cellular ROS generation. The data of the
present study also revealed that ATX increased the activation of
NRF2 and the expression of antioxidant-related proteins that are
downregulated by PM2.5 (Fig. 2). These results indicated that ATX
suppressed PM2.5-induced ROS generation through the
activation of the NRF2-antioxidant enzyme pathways.
PM2.5-induced oxidative stress causes DNA
damage, which leads to cell cycle arrest in skin cells (26). The data of the present study
revealed that ATX decreased base modification or breakage of DNA
damage in PM2.5-treated cells (Fig. 3A-C). Oxidative stress-induced DNA
damage is one way to induce senescence and can maintain
G1 confinement, accelerating aging under stress
(27). The results of the present
study demonstrated that PM2.5 stimulated G1
arrest. However, treatment with ATX reversed the effects (Fig. 3D). A previous study revealed that
PM2.5 induces MMPs via the AP-1 signaling pathway
through ROS generation (26). In
addition, ROS increase the secretion of pro-inflammatory cytokines
to high levels in most senescent cells (28). In the present study, it was
demonstrated that PM2.5 activated the transcription
factor of inflammatory cytokines, AP-1 (Fig. 4A), followed by the secretion of
pro-inflammatory cytokines and MMPs (Fig. 4B). However, ATX inhibited the AP-1,
cytokine and MMP secretion induced by PM2.5. DNA damage
has been considered an activator of SASP associated with cell cycle
arrest (15). IL-1β and IL-6 are
the most important SASP factors and have been detected at high
levels in senescent cells (28).
Furthermore, the expression of a senescence marker, p16, and
β-galactosidase activity were stimulated by PM2.5;
however, these decreased upon pretreatment with ATX (Fig. 5).
Several studies have reported that NRF2, a regulator
of antioxidant enzymes, plays a role in anti-aging mechanisms
(29-31).
The active form of vitamin D, 1,25(OH)2D3,
also plays a role in delaying aging. It does this by upregulating
NRF2, inhibiting oxidative stress and DNA damage, inactivating the
p53-p21 and p16-Rb signaling pathways, and inhibiting cellular
senescence and SASP (29).
Furthermore, ATX is reported to have anti-inflammatory properties
and exerts its protective effects by stimulating the NRF2 signaling
pathway (14). The results of the
present study revealed that NRF2 knockdown increased the
β-galactosidase activity induced by PM2.5; however ATX
decreased the β-galactosidase activity (Fig. 6), suggesting that ATX inhibited
PM2.5-induced senescent cells through NRF2.
In conclusion, the induction of the antioxidant
system through NRF2 upregulation by ATX resulted in inhibiting the
generation of ROS by PM2.5 and the DNA damage response,
thereby preventing cell cycle arrest. Additionally, ATX inhibited
the AP-1 signaling pathway, thereby reversing the secretion of
pro-inflammatory cytokines and MMPs, ultimately inhibiting
PM2.5-induced senescence. Notably, ATX exhibited an
anti-PM2.5-induced senescence effect and could be
utilized as a preventive agent against air pollution-triggered skin
aging.
Acknowledgements
Not applicable.
Funding
Funding: The present study was supported by (grant no.
RS-2023-00270936) the Basic Science Research Program through the
National Research Foundation of Korea (NRF), funded by the Ministry
of Education.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
KAK, AXZ and JWH conceived and designed the present
study, and wrote the main manuscript. KAK, AXZ and MJP performed
the experiments and acquired data. PDSMF and HMULH analyzed and
interpreted the data, and performed the literature searches. KAK
and JWH confirm the authenticity of the raw data. All authors have
read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Lelieveld J, Evans JS, Fnais M, Giannadaki
D and Pozzer A: The contribution of outdoor air pollution sources
to premature mortality on a global scale. Nature. 525:367–371.
2015.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Boogaard H, Patton AP, Atkinson RW, Brook
JR, Chang HH, Crouse DL, Fussell JC, Hoek G, Hoffmann B, Kappeler
R, et al: Long-term exposure to traffic-related air pollution and
selected health outcomes: A systematic review and meta-analysis.
Environ Int. 164(107262)2022.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Ryu YS, Kang KA, Piao MJ, Ahn MJ, Yi JM,
Hyun YM, Kim SH, Ko MK, Park CO and Hyun JW: Particulate matter
induces inflammatory cytokine production via activation of NFκB by
TLR5-NOX4-ROS signaling in human skin keratinocyte and mouse skin.
Redox Biol. 21(101080)2019.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Piao MJ, Ahn MJ, Kang KA, Ryu YS, Hyun YJ,
Shilnikova K, Zhen AX, Jeong JW, Choi YH, Kang HK, et al:
Particulate matter 2.5 damages skin cells by inducing oxidative
stress, subcellular organelle dysfunction, and apoptosis. Arch
Toxicol. 92:2077–2091. 2018.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Hyun YJ, Piao MJ, Kang KA, Zhen AX,
Madushan Fernando PDS, Kang HK, Ahn YS and Hyun JW: Effect of
fermented fish oil on fine particulate matter-induced skin aging.
Mar Drugs. 17(61)2019.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Reynolds WJ, Hanson PS, Critchley A,
Griffiths B, Chavan B and Birch-Machin MA: Exposing human primary
dermal fibroblasts to particulate matter induces changes associated
with skin aging. FASEB J. 34:14725–14735. 2020.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Mendonça ELSS, Xavier JA, Fragoso MBT,
Silva MO, Escodro PB, Oliveira ACM, Tucci P, Saso L and Goulart
MOF: E-stilbenes, general chemical and biological aspects,
potential pharmacological activity based on the Nrf2 pathway.
Pharmaceuticals (Basel). 17(232)2024.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Zhang LM, Lv SS, Fu SR, Wang JQ, Liang LY,
Li RQ, Zhang F and Ma YX: Procyanidins inhibit fine particulate
matter-induced vascular smooth muscle cells apoptosis via the
activation of the Nrf2 signaling pathway. Ecotoxicol Environ Saf.
223(112586)2021.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Kahremany S, Hofmann L, Eretz-Kdosha N,
Silberstein E, Gruzman A and Cohen G: SH-29 and SK-119 attenuates
air-pollution induced damage by activating Nrf2 in HaCaT cells. Int
J Environ Res Public Health. 18(12371)2021.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Han SI, Chang SH, Lee C, Jeon MS, Heo YM,
Kim S and Choi YE: Astaxanthin biosynthesis promotion with pH shock
in the green microalga, Haematococcus lacustris. Bioresour Technol.
314(123725)2020.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Kumar S, Kumar R, Diksh Kumari A and
Panwar A: Astaxanthin: A super antioxidant from microalgae and its
therapeutic potential. J Basic Microbiol. 62:1064–1082.
2022.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Ashrafizadeh M, Ahmadi Z, Yaribeygi H,
Sathyapalan T and Sahebkar A: Astaxanthin and Nrf2 signaling
pathway: A novel target for new therapeutic approaches. Mini Rev
Med Chem. 22:312–321. 2022.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Imokawa G: Intracellular signaling
mechanisms involved in the biological effects of the xanthophyll
carotenoid astaxanthin to prevent the photo-aging of the skin in a
reactive oxygen species depletion-independent manner: The key role
of mitogen and stress-activated protein kinase 1. Photochem
Photobiol. 95:480–489. 2019.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Zhen AX, Piao MJ, Hyun YJ, Kang KA,
Madushan Fernando PDS, Cho SJ, Ahn MJ and Hyun JW:
Diphlorethohydroxycarmalol attenuates fine particulate
matter-induced subcellular skin dysfunction. Mar Drugs.
17(95)2019.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Matthews HK, Bertoli C and de Bruin RAM:
Cell cycle control in cancer. Nat Rev Mol Cell Biol. 23:74–88.
2022.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Hernandez-Segura A, Nehme J and Demaria M:
Hallmarks of cellular senescence. Trends Cell Biol. 28:436–453.
2018.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Oh JH, Joo YH, Karadeniz F, Ko J and Kong
CS: Syringaresinol inhibits UVA-induced MMP-1 expression by
suppression of MAPK/AP-1 signaling in HaCaT keratinocytes and human
dermal fibroblasts. Int J Mol Sci. 21(3981)2020.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Samdavid Thanapaul RJR, Shvedova M, Shin
GH, Crouch J and Roh DS: Elevated skin senescence in young mice
causes delayed wound healing. Geroscience. 44:1871–1878.
2022.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Ryu YS, Kang KA, Piao MJ, Ahn MJ, Yi JM,
Bossis G, Hyun YM, Park CO and Hyun JW: Particulate matter-induced
senescence of skin keratinocytes involves oxidative
stress-dependent epigenetic modifications. Exp Mol Med. 51:1–14.
2019.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Zhen AX, Piao MJ, Kang KA, Fernando PD,
Herath HM, Cho SJ and Hyun JW: 3-Bromo-4,5-dihydroxybenzaldehyde
protects keratinocytes from particulate matter 2.5-induced damages.
Antioxidants (Basel). 12(1307)2023.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Wang L, Lee W, Jayawardena TU, Cha SH and
Jeon YJ: Dieckol, an algae-derived phenolic compound, suppresses
airborne particulate matter-induced skin aging by inhibiting the
expressions of pro-inflammatory cytokines and matrix
metalloproteinases through regulating NF-κB, AP-1, and MAPKs
signaling pathways. Food Chem Toxicol. 146(111823)2020.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Ma C, Yang K, Wang Y and Dai X: Anti-aging
effect of agar oligosaccharide on male Drosophila melanogaster and
its preliminary mechanism. Mar Drugs. 17(632)2019.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Liu XY, Liu D, Lin GP, Wu YJ, Gao LY, Ai
C, Huang YF, Wang MF, El-Seedi HR, Chen XH and Zhao C: Anti-ageing
and antioxidant effects of sulfate oligosaccharides from green
algae Ulva lactuca and Enteromorpha prolifera in SAMP8 mice. Int J
Biol Macromol. 139:342–351. 2019.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Sorrenti V, Davinelli S, Scapagnini G,
Willcox BJ, Allsopp RC and Willcox DC: Astaxanthin as a putative
geroprotector: Molecular basis and focus on brain aging. Mar Drugs.
18(351)2020.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Chen Z, Xiao J, Liu H, Yao K, Hou X, Cao Y
and Liu X: Astaxanthin attenuates oxidative stress and immune
impairment in D-galactose-induced aging in rats by activating the
Nrf2/Keap1 pathway and suppressing the NF-κB pathway. Food Funct.
11:8099–8111. 2020.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Herath HMUL, Piao MJ, Kang KA, Zhen AX,
Fernando PDSM, Kang HK, Yi JM and Hyun JW: Hesperidin exhibits
protective effects against PM2.5-mediated mitochondrial damage,
cell cycle arrest, and cellular senescence in human HaCaT
keratinocytes. Molecules. 27(4800)2022.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Kumari R and Jat P: Mechanisms of cellular
senescence: Cell cycle arrest and senescence associated secretory
phenotype. Front Cell Dev Biol. 9(645593)2021.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Zhou Q, Wang W, Wu J, Qiu S, Yuan S, Fu
PL, Qian QR and Xu YZ: Ubiquitin-specific protease 3 attenuates
interleukin-1β-mediated chondrocyte senescence by deacetylating
forkhead box O-3 via sirtuin-3. Bioengineered. 13:2017–2027.
2022.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Chen L, Yang R, Qiao W, Zhang W, Chen J,
Mao L, Goltzman D and Miao D: 1,25-Dihydroxyvitamin D exerts an
antiaging role by activation of Nrf2-antioxidant signaling and
inactivation of p16/p53-senescence signaling. Aging Cell.
18(e12951)2019.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Lee JJ, Ng SC, Hsu JY, Liu H, Chen CJ,
Huang CY and Kuo WW: Galangin reverses
H2O2-induced dermal fibroblast senescence via
SIRT1-PGC-1α/Nrf2 signaling. Int J Mol Sci. 23(1387)2022.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Kumar N, Reddi S, Devi S, Mada SB, Kapila
R and Kapila S: Nrf2 dependent antiaging effect of milk-derived
bioactive peptide in old fibroblasts. J Cell Biochem.
120:9677–9691. 2019.PubMed/NCBI View Article : Google Scholar
|