Introduction
Laparoscopic surgeries, fetal monitoring systems and
a better understanding of the pharmacology of anesthetics have
increased the safety of surgeries that must be performed with
obligatory indications such as acute appendicitis, cholecystectomy
and fetal surgery during pregnancy. In the US, 1-2% of all pregnant
women undergo surgery for non-obstetric reasons (1) and ~20% undergo surgery for obstetric
reasons (2). These surgery rates
also indirectly represent the proportion of fetuses exposed to
anesthesia. In 2016, the Food and Drug Administration (FDA) issued
a statement indicating that repeated or prolonged exposure to
inhalation anesthetics during childhood and pregnancy is a
potential risk factor for adverse long-term neurocognitive outcomes
(3).
Sevoflurane (Sv) is an inhalation agent widely used
in obstetric anesthesia due to its clinical advantages. At the
receptor level, Sv increases the activity of γ-aminobutyric acid-A
and glycine receptors, while decreasing the activity of cholinergic
and N-methyl-D-aspartate (NMDA) type glutamate receptors (4,5).
However, the effects of this anesthetic agent on the developing
brain in different trimesters, at different doses and durations,
have not previously been reported, to the best of our knowledge.
Despite the widespread use of Sv, certain studies have reported
cases of neurotoxicity due to increases in neuroinflammation and
oxidative stress, decreases in neuronal transmission, changes in
lipid membrane fluidity and apoptosis of neuroprogenitor cells,
which suggests that exposure may confer long-term adverse
consequences (6-8).
A number of reports have indicated that exposure to Sv,
particularly during the early and mid-pregnancy periods, can also
reduce postnatal learning and memory capacity (9,10).
The exposure time and dose are considered the most important
determinants of the activation of damage pathways by this
anesthetic. However, it has not yet been revealed to what extent
the damage will increase as the dose and exposure time increases.
Nevertheless, perioperative pharmacological neuroprotection
requirements remain a controversial issue.
The neuroprotective efficacy of lidocaine,
thiopental, ketamine, propofol, nimodipine, glutamate, aspartate,
atorvastatin, erythropoietin and rivastigmine have previously been
assessed and reported (11-13).
Another drug with potential neuroprotective properties is magnesium
sulfate (MgSO4), which has been reported to reduce
apoptosis activation and to act as an NMDA receptor antagonist,
anti-inflammatory and antioxidant (14-17).
Zhang et al (18) reported
that MgSO4 could ameliorate isoflurane-induced
neurotoxicity by inhibiting mitochondrial dysfunction, and that it
could be used in the prevention and treatment of anesthesia
neurotoxicity. Beloosesky et al (19) reported that MgSO4 may
protect against neuroinflammation by reducing pro-inflammatory
cytokine production through an inhibition of neuronal nitric oxide
synthase and an NF-κB enhancer. The safety profile of
MgSO4, especially in obstetrics, its low cost and its
wide availability prompted the present study to explore the
neuroprotective efficacy of this agent against possible fetal
neurotoxicity caused by Sv.
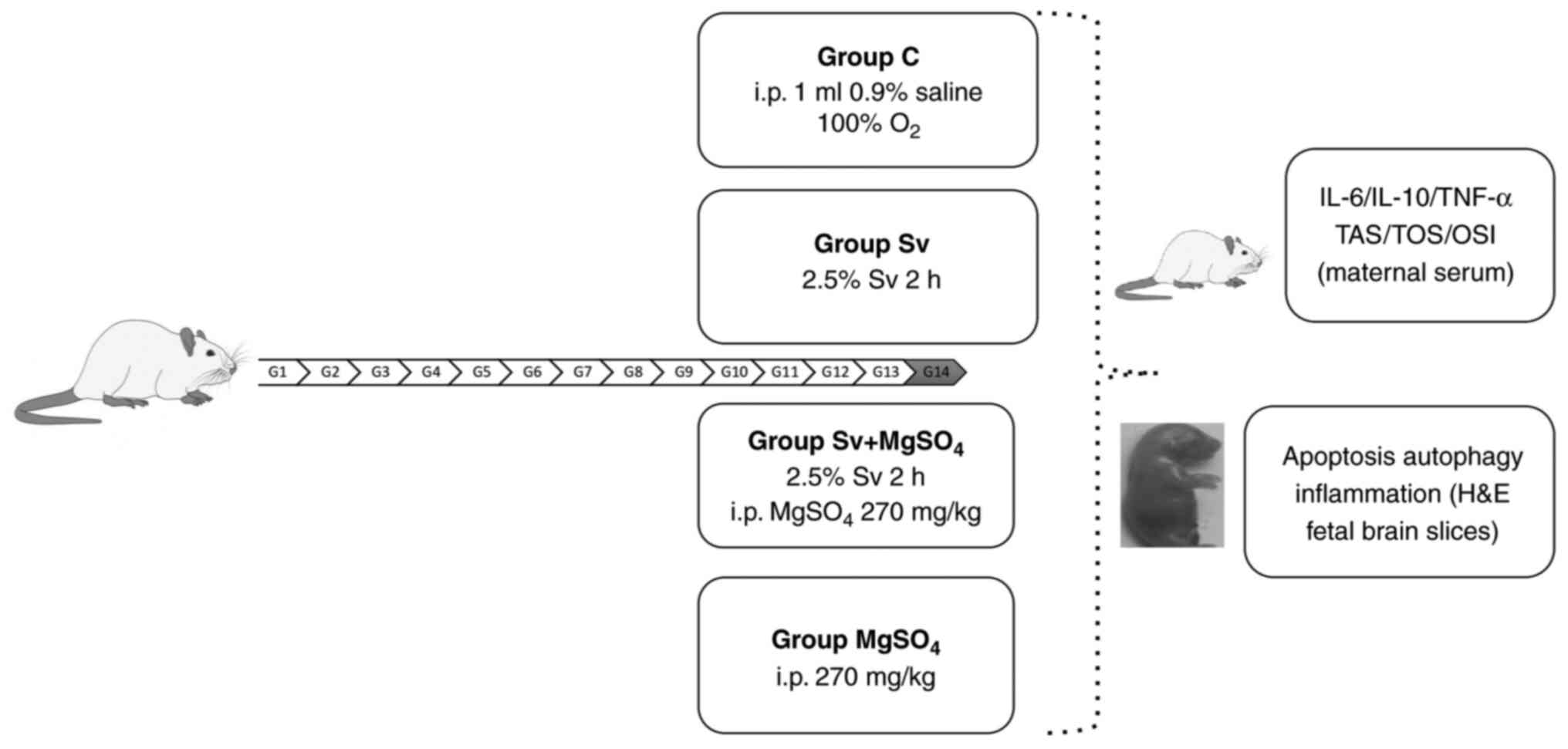
In the present study, pregnant rats in their
mid-gestational period were exposed to short-term, 1 minimum
alveolar concentration (MAC) Sv with or without prior
MgSO4 injection. The aim of the present study was to
evaluate fetal neurotoxicity by measuring changes in maternal
inflammation and oxidative stress markers, and analyzing fetal
brain histopathology.
Materials and methods
Animals and ethical approval
All procedures were approved by the Gazi University
Ethics Committee for Experimental Animals (Ankara, Turkey; approval
no. G.U.ET-20-0499). The present study was carried out at Gazi
University Faculty of Medicine between September and October 2020.
All procedures were performed according to accepted standards in
the Guide for the Care and Use of Laboratory Animals (20) and the Animal Research: Reporting of
in vivo Experiments guidelines (21). The present study included 24 female
Wistar albino rats (age, 3-4 months; weight, 200-300 g) supplied by
the Gazi University Experimental Animals Research Center. The rats
were kept in standard housing conditions, at a temperature range of
20-21˚C, an average humidity of 55±5% and with a 12-h light/dark
cycle. Food and water were available ad libitum. The rats'
pregnancy process was performed and controlled by veterinarians at
the research center.
Exposure and treatment
On day 14 of pregnancy, the pregnant rats were
randomly assigned and equally divided (n=6) into groups: Control
(C), Sv, MgSO4 and Sv + MgSO4 groups. All
surgical procedures were performed under general anesthesia. An
intraperitoneal (i.p.) injection comprising 50 mg/kg ketamine
hydrochloride (500 mg/10 ml Ketalar®; Parke-Davis;
Pfizer, Inc.) and 10 mg/kg xylazine hydrochloride
(Alfazyne® 2%; Alfasan International B.V.) was
administered for anesthesia. The depth of anesthesia was evaluated
using the tail-pinch test. Anesthesia was performed in a
transparent plastic box.
The rats in Group C were administered O2
for 2 h on day 14 of pregnancy. The Sv group were administered 2.5%
Sv (250 ml; Abbott Laboratories) with 2 l/min O2 for 2 h
on day 14 of pregnancy. The aforementioned concentration of Sv was
selected as it corresponded to 1.1 MAC in rats and had been used in
previous neurotoxicity studies (10,22).
The rats in the MgSO4 group were injected i.p. with 270
mg/kg MgSO4 (15% ampoule; Biofarma Pharmaceuticals) on
day 14 of pregnancy. The dosage of 270 mg/kg MgSO4 was
selected as it had been reported to show neuroprotective activity
in previous studies (14,15,23,24).
O2 at 2 l/min was administered to these rats for 2 h in
the plastic box, starting 30 min after the injection. The Sv +
MgSO4 group were injected with the same dose of i.p.
MgSO4. After waiting for 30 min, the rats were exposed
to 2.5% Sv with 2 l/min O2 for 2 h.
A previous study reported that the same dose of Sv
did not change blood pressure and blood gas values; therefore,
blood pressure monitoring was not performed in the present study
(25). After performing the
intervention and exposure steps in each treatment group, a
laparotomy was performed under anesthesia in all rats and fetuses
were removed.
Pregnant rats and fetuses were sacrificed by taking
intracardiac blood under anesthesia. The numbers and weights of
fetuses were recorded. The fetal brains were removed by craniotomy.
Blood samples (5-10 ml) were taken from the pregnant rats for
biochemical analysis. The maternal blood samples were placed in
tubes without additives, left to stand upright for 30 min at room
temperature and then serum samples were obtained by centrifugation
at 1,500 x g for 10 min at 21-23˚C. The resulting serum samples
were placed in Eppendorf tubes, frozen at -80˚C and used for
subsequent biochemical analysis. The maternal serum samples were
used for the determination of inflammatory and oxidative markers.
Biochemical analysis could not be performed on the fetal blood, as
the samples of intracardiac blood taken from the fetuses for
sacrifice were too small for analysis. Brain tissues from 3 rat
pups per group were fixed in a 10% formaldehyde solution at room
temperature for 48 h. Subsequently, automated routine paraffin
tissue processing was performed, followed by staining of 4-micron
sections with hematoxylin and eosin. The fetal brain tissues were
used for subsequent histopathological analysis of apoptosis,
autophagy and neuroinflammation. The timeline of the experiment is
shown in Fig. 1.
Biochemical evaluations
The IL-6, IL-10 and TNF-α markers in maternal serum
were analyzed using ELISA kits (cat. nos. E-EL-R0015, E-EL-R0016
and E-EL-R2856, respectively; Elabscience Biotechnology, Inc.)
according to the sandwich-ELISA principle. The micro-ELISA plates
provided in the aforementioned kits were pre-coated with antibodies
specific for rat IL-6, IL-10 and TNF-α. Standards or samples were
added to the micro-ELISA plate wells and conjugated with the
specific antibodies. The rat marker concentrations in the samples
were calculated by comparing the optical density value of the
samples with those of the prepared standard curve.
The total antioxidant status (TAS) and total oxidant
status (TOS) levels in maternal serum were measured using kits
(cat. nos. RL0017 and RL0024, respectively; Rel Assay Diagnostics).
The serum concentrations of these parameters were assessed
following the manufacturer's instructions. TOS levels were
expressed as µmol H2O2 and TAS levels as mmol
Trolox equivalent/l. TOS measurement was performed according to the
iron ion-o-dianisidine complex assay. Oxidants present in serum
oxidize the ferrous ion-o-dianisidine complex to iron ion. The
oxidation is enhanced by glycerol molecules, which are abundant in
the reaction medium. Ferric ions, in an acidic environment with
xylenol, form an orange-colored complex whose intensity is
proportional to the total amount of oxidant molecules present in
the sample (26,27).
The ratio of TOS to TAS is considered the oxidative
stress index (OSI). The OSI value was calculated according to the
following formula: OSI=TOS (µmol H2O2
equivalent/l)/TAS (µmol Trolox equivalent/l).
Histopathological evaluation
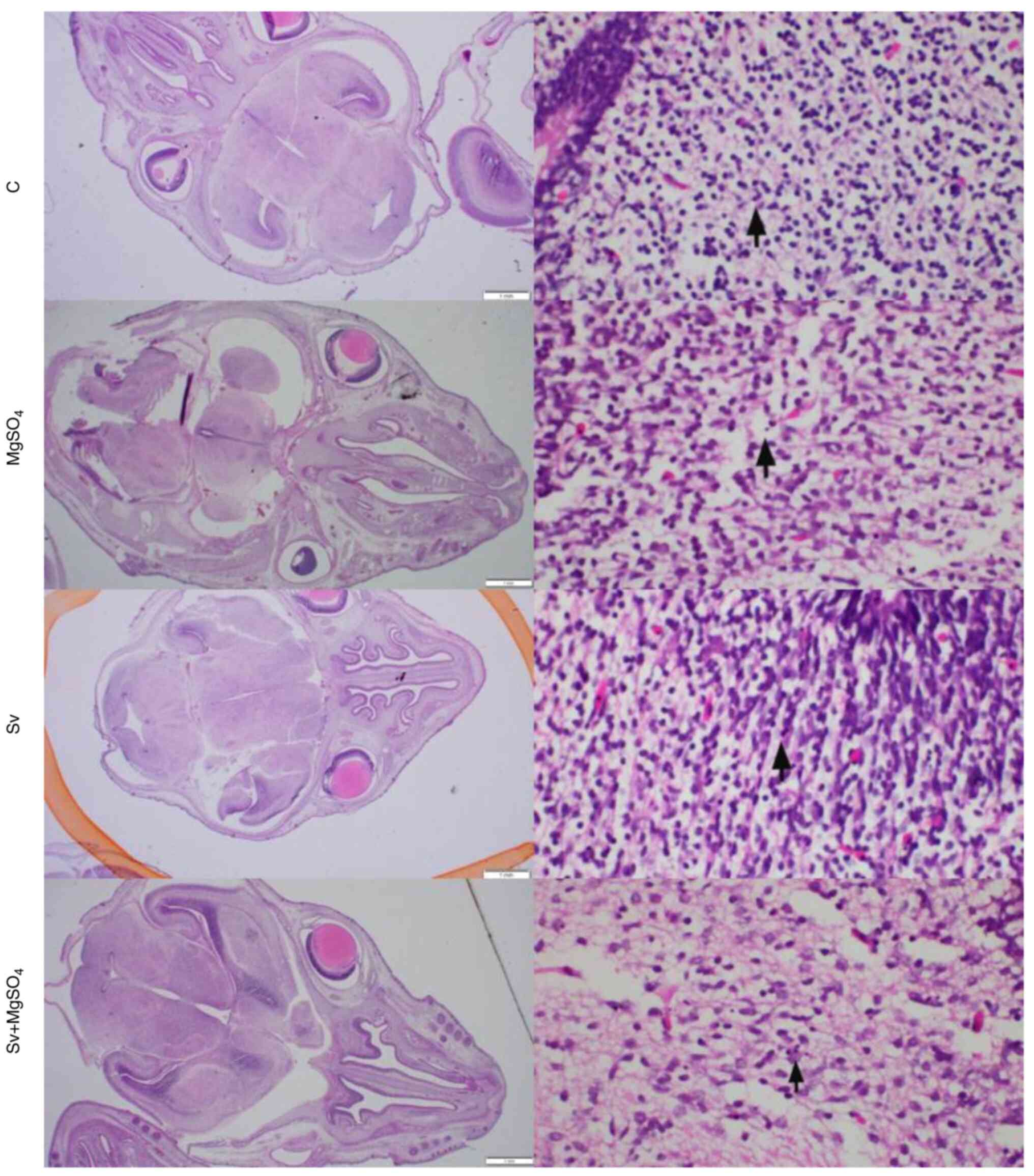
Fetal brain tissues were evaluated for apoptosis,
autophagy and inflammation. All incubations were performed at room
temperature. After fixation, the macroscopically visible damaged
area was viewed identified in fetal brains sliced into 2-mm
sagittal sections. Subsequently, the slice with the widest section
surface was loaded into a cassette. Following automated routine
paraffin tissue processing with alcohol and xylene solutions, the
tissue was embedded in paraffin blocks. For further analysis in the
pathology laboratory, sections of 4 µm were cut, deparaffinized and
stained with hematoxylin and eosin. The brain samples were imaged
using an optical microscope (magnification, x400). Apoptotic cells,
identified by shrunken condensed cytoplasm and hyperchromatic
nuclei, were examined to evaluate neuronal damage. Cellularity was
also assessed to detect inflammatory infiltration. The number of
apoptotic cells was quantified in 1 mm² areas on each slide, and
the highest ratio was recorded for each group. Microscopic images
of brain tissue samples from each rat were captured.
Statistical analysis
Statistical analysis was performed using SPSS
software (version 15.0; SPSS Inc.). Descriptive statistics were
presented as the median (minimum-maximum). Pregnant rat and fetal
rat weights, the number of fetal rats taken from each pregnant rat,
and maternal serum IL-6, IL-10, TNF-α, TAS, TOS and OSI values were
evaluated. The Kruskal-Wallis test was used for analysis of all
median values between groups. Parameters that were found to be
statistically significant according to the Kruskal-Wallis test were
evaluated using a post-hoc test (non-parametric Dunn Bonferroni) to
determine between which two groups there was a significant
difference. P<0.05 was considered to indicate a statistically
significant difference.
Results
Effect of midgestational Sv exposure
on rat weights
No statistically significant differences in the
weights of pregnant rats in the four groups were detected after
exposure to Sv (P=0.695; Table I).
The numbers and weights of the fetuses taken from each pregnant rat
were not significantly different when compared between the
treatment groups (P=0.619; Table
I).
 | Table IWeights of pregnant rats and weights
and numbers of fetuses in the treatment groups. |
Table I
Weights of pregnant rats and weights
and numbers of fetuses in the treatment groups.
| Parameter | Control group
(n=6) | MgSO4
group (n=6) | Sv group (n=6) | Sv +
MgSO4 group (n=6) | P-value |
|---|
| Weight of pregnant
rats, g | 260.87
(247.52-295.51) | 252.34
(240.91-284.24) | 260.19
(239.19-280.13) | 262.08
(250.13-290.64) | 0.695 |
| Fetuses, n | 7.5 (3.0-11.0) | 6.0 (3.0-10.0) | 7.0 (3.0-9.0) | 7.0 (4.0-10.0) | 0.906 |
| Weight of fetuses,
mg | 140.78
(130.42-151.98) | 145.78
(137.65-150.62) | 141.38
(139.45-147.43) | 142.45
(138.25-146.52) | 0.619 |
Effects of midgestational Sv exposure
on inflammatory markers
TNF-α, IL-6 and IL-10 levels in maternal serum were
evaluated to investigate the potential induction of inflammation as
a result of exposure to Sv. TNF-α levels were significantly
different when compared between the groups. (P=0.044; Table II). The median TNF-α level of the
Sv-treated group was significantly higher when compared with the
control group (P=0.027; Table
III). No significant differences were demonstrated in other
pairwise comparisons of the other inflammatory marker levels
between the treatment groups.
 | Table IIComparison of inflammation marker
levels between treatment groups of rats. |
Table II
Comparison of inflammation marker
levels between treatment groups of rats.
| Marker | Control group
(n=6) | MgSO4
group (n=6) | Sv group (n=6) | Sv +
MgSO4 group (n=6) | P-value |
|---|
| TNF-α, pg/ml | 71.94
(58.96-111.32) | 87.35
(63.95-263.46) | 159.64
(96.37-261.35) | 95.07
(63.12-206.79) | 0.044 |
| IL-6, ng/l | 2.69
(2.39-3.48) | 2.87
(2.38-3.23) | 2.88
(2.70-3.53) | 2.85
(2.47-3.72) | 0.492 |
| IL-10, pg/ml | 129.61
(66.88-176.90) | 160.44
(100.36-215.70) | 138.22
(100.36-220.71) | 203.08
(79.30-274.56) | 0.534 |
 | Table IIIComparison of TNF-α levels between
treatment groups of rats. |
Table III
Comparison of TNF-α levels between
treatment groups of rats.
| Comparison | Test statistic | Standard error | Standard test
statistic | P-value | Adjusted
P-value |
|---|
| C vs.
MgSO4 | -5.417 | 4.082 | -1.327 | 0.184 | >0.999 |
| C vs. Sv +
MgSO4 | -6.000 | 4.082 | -1.470 | 0.142 | 0.849 |
| C vs. Sv | -11.583 | 4.082 | -2.838 | 0.005 | 0.027 |
| Sv +
MgSO4 vs. MgSO4 | -0.583 | 4.082 | -1.43 | 0.886 | >0.999 |
| MgSO4
vs. Sv | -6.167 | 4.082 | -1.511 | 0.131 | 0.785 |
| Sv vs. Sv +
MgSO4 | 5.583 | 4.082 | 1.368 | 0.171 | >0.999 |
Effects of midgestational Sv exposure
on oxidative stress markers
The oxidant-antioxidant stress markers TAS, TOS and
OSI were evaluated in serum samples from pregnant rats. These
results demonstrated no significant differences between the groups
(TAS, P=0.153; TOS, P=0.256; and OSI, P=0.258; Table IV).
 | Table IVComparison of oxidative stress
markers between treatment groups of rats. |
Table IV
Comparison of oxidative stress
markers between treatment groups of rats.
| Marker | Control group
(n=6) | MgSO4
group (n=6) | Sv group (n=6) | Sv +
MgSO4 group (n=6) | P-value |
|---|
| TAS, mmol/l | 1.60
(1.45-1.81) | 1.64
(1.42-3.15) | 1.78
(1.63-2.35) | 1.57
(1.47-180.00) | 0.153 |
| TOS, µmol/l | 4.99
(3.65-14.44) | 7.26
(2.38-19.77) | 9.49
(5.97-67.95) | 11.43
(3.28-25.73) | 0.256 |
| OSI | 0.30
(0.24-0.79) | 0.49
(0.09-1.10) | 0.55
(0.35-2.89) | 0.75
(0.21-1.42) | 0.258 |
Histopathological evaluation
The morphology of apoptotic cells can be described
as showing cytoplasmic constriction, eosinophilic condensation and
nuclear hyperchromasia (28). The
number of apoptotic cells per 1 mm2 was determined by
examining cortical regions following the initial observation of
apoptosis and counting apoptotic cells in consecutive
high-magnification fields (Table
V). A 1-mm2 section corresponded to ~4
high-magnification fields of view (each 0.26 mm2). Brain
sections of the different treatment groups were examined to search
for macroscopic areas showing necrosis, gliosis and inflammation
that could be associated with inflammation, oxidative stress or
apoptosis. The histopathological images were similar between the
groups and no evidence, such as inflammation, pathological
apoptosis and autophagy, was found to suggest anesthesia-induced
damage. Although apoptosis was particularly concentrated in the
periventricular adjacent white matter areas in all examples, the
extent of damage was considered to be within the limits of
physiological apoptosis normally seen during embryonic development
(29) (Fig. 2). When the number of apoptotic
cells was compared between treatment groups, the group with the
highest median value was the Sv + MgSO4 group, followed
by the MgSO4, Sv and C groups, respectively. However,
the differences in the number of apoptotic cells between the groups
were not statistically significant (P=0.114; Table V).
 | Table VComparison of number of apoptotic
cells in fetal brain tissues between treatment groups of rats. |
Table V
Comparison of number of apoptotic
cells in fetal brain tissues between treatment groups of rats.
| Parameter | Control group
(n=6) | MgSO4
group (n=6) | Sv group (n=6) | Sv +
MgSO4 group (n=6) | P-value |
|---|
| Apoptotic cells,
n | 1.0 (1.0-3.0) | 4.0 (2.0-11.0) | 2.5 (2.0-4.0) | 4.5 (3.0-7.0) | 0.114 |
Discussion
Medical developments such as laparoscopy and
intrauterine fetal monitoring have enabled the performance of
emergency interventional procedures during pregnancy in a safer
manner. However, surgical procedures have increased the rate of
exposure of the fetal brain to anesthesia (30). The net effect of anesthetic drugs
on the fetal brain is a long-debated issue and previous studies
have provided conflicting results (10,31,32).
In 2016, the FDA reported that repeated or prolonged use of general
anesthetic and sedation drugs in children aged <3 years, or in
pregnant women in the third trimester, may negatively affect
children's brain development (3).
The first step in reducing fetal risk under
anesthesia is the timing of the surgical procedure. Currently, the
second trimester is accepted as the most appropriate time for
mandatory surgeries (33). The
present study aimed to contribute to the ongoing debate by focusing
on the acute damage that could potentially occur to the fetus
following exposure to anesthesia in the second trimester. Day 14 of
rat pregnancy (G14) was selected, as it is equivalent in terms of
fetal brain development to the second trimester development that
occurs during a human pregnancy (34,35).
Other risk-reducing steps include limiting the
exposure time, frequency and drug dose. Sv, as the most frequently
preferred volatile anesthetic agent for pregnant women (36), was used at a concentration of 2.5%
in the present study. The possible neurotoxic effects of Sv have
been reported in a number of studies (8,37),
but its actual neurotoxic potential, if present, has not yet been
reported, to the best of our knowledge. The 2.5% concentration of
Sv is equivalent to 1.1 MAC in rats (4). A time frame of 2 h was chosen, as
current recommendations are that surgeries should be performed in
as short a time frame as possible to reduce exposure. A previous
study reported that a 2-h anesthetic exposure was sufficient to
trigger neurotoxicity (38). The
present study was designed considering current recommendations for
clinical approach and operation times, unlike other previously
published studies that aimed to induce neurotoxicity using high Sv
doses, long exposure times and repeated applications (39,40).
In the present study, Sv did not cause neurotoxicity
in the fetal rat brain. Similar previous studies also support this
result. For example, Wu et al (32) reported no neurotoxicity in second
trimester pregnant rats after a 2-h exposure to Sv and emphasized
the importance of repeated exposures in the development of
neurotoxicity. Furthermore, Lee et al (31) reported that neither single nor
multiple exposures to Sv caused any long-term behavioral disorders
and that they did not affect long-term synaptic plasticity. By
contrast, learning disabilities and neurotoxicity in offspring rats
were reported by Zheng et al (10) following exposure to 2.5% Sv for 2 h
and by Hirotsu et al (39)
following exposure to 1-2% Sv for 3 h. The methods used by the
aforementioned studies were similar to those used in the present
study; therefore, the differences in results may be due to the
species choice (mouse or rat), anesthesia protocol (exact
gestational age, choice of anesthetic agent, dose and duration),
sensitivity of the biochemical tests and the chosen exposure time
point (fetal or neonatal). In the present study, the histopathology
of the fetal brain and the inflammatory and oxidative stress
markers in maternal blood samples were assessed for the fetal
neurotoxicity evaluation. Maternal cytokines and radicals can reach
the fetal brain through the placenta and move across the immature
fetal blood-brain barrier, thereby producing proinflammatory
cytokines that activate fetal microglia and impair neuronal
development (41).
Neuroinflammation is a pathological condition that
can lead to cognitive impairment (42). Previous studies have reported an
association between elevated cytokines in maternal serum in the
second and third trimesters of pregnancy and an increased risk of
neurodevelopmental disorders (34,39,43).
Possible mechanisms that could be responsible for this association
are that the increased cytokines in the maternal blood reach the
brain across the immature blood-brain barrier (44) or that Sv directly activates NF-κB
to increase levels of proinflammatory cytokines in the fetal brain
(37). In the present study, only
the maternal serum levels of TNF-α demonstrated a significant
increase, but this increase did not appear to cause fetal brain
damage. In another previous study investigating the effects of
maternal inflammation on the fetal brain, L-6, IL-1β, IL-10, TNF-α,
TNF-α levels were the first to increase among the cytokines tested
(44). The effects of TNF-α on
cognitive function, disease occurrence and underlying disease have
been investigated previously (45). Under pathological conditions,
microglia secrete TNF-α, an important component of
neuroinflammation. TNF-α may potentiate cytotoxicity through two
complementary mechanisms: Indirectly by inhibiting glutamate
transport by astrocytes and directly by increasing the localization
of ionotropic glutamate receptors on synapses (46). Acute inhibition of endogenous TNF-α
by treatment with soluble TNF-α receptors, neutralizing antibodies
or antisense blockers reduces ischemic and traumatic brain damage
in rodents, which suggests that TNF-α contributes to brain damage
(47-50).
TNF-α induces caspase-3 activation that causes apoptotic neuronal
cell death in hippocampal cultures (51). A number of studies have also
reported that TNF-α is involved in mediating microglia-induced
neuronal cell death (52) and
peripherin-induced dorsal root ganglion neuron apoptosis (53). TNF-α alone has also been reported
to induce oxidative stress that may lead to cell death (54,55).
However, an increase in TNF-α levels does not necessarily mean that
neurotoxicity has occurred. The minimum level of TNF-α that can
cause neurotoxicity is currently unknown. While a single dose of
TNF-α did not damage the memory of healthy mice, it caused acute
disturbances such as hypothermia, weight loss and inactivity in
mice with neurodegeneration. However, no neuronal death, synaptic
loss or tau hyperphosphorylation was observed in the brain tissue
(44). Similarly, a 3% Sv exposure
for 2 h did not increase IL-6 and TNF-α levels in 6-day-old rat
pups, whereas treatment with 3% Sv for 2 h daily for 3 days
increased IL-6 and TNF-α levels in the brain (37). In terms of neuroinflammation,
repeated exposure and prolonged exposure times to Sv may cause
increased inflammation. The increased levels of IL-6 and IL-10
reported in previous studies are most likely associated with higher
doses, longer durations and repeated exposures. However, more
extensive studies are needed to confirm this.
Compared with the adult brain, the developing brain
shows a higher rate of mitochondrial respiration and oxygen
consumption. However, the fetal brain has insufficient antioxidant
defenses compared with the adult brain, making it more susceptible
to the effects of oxidative stress (56,57).
Nevertheless, whether Sv affects the fetal brain oxidative balance
positively or negatively currently remains unclear. As the Sv
concentration increases, the antioxidant activity shifts to an
effect that increases oxidative stress (58). At present, the safety of using the
1 MAC Sv concentration frequently used in the clinic remains
unclear, as different studies have reported both antioxidant and
pro-oxidant effects at this dose. Zhou et al (7) reported relatively low plasma and
hippocampal malondialdehyde (MDA) levels of Sv at a subclinical
concentration of 0.3 MAC (1.3%) and reported that Sv could reduce
oxidative stress. Additionally, Allaouchiche et al (59) investigated the oxidative state of
the circulation and lungs during 1 MAC Sv anesthesia, and observed
relatively lower levels of MDA and glutathione peroxidase in plasma
and bronchoalveolar lavage fluid compared with levels during
propofol and desflurane anesthesia; therefore indicating the
antioxidant effect of Sv. Conversely, a study comparing surgeries
performed with propofol anesthesia and Sv showed that erythrocyte
protection was impaired during Sv anesthesia, which indicated that
Sv may cause oxidative stress (60). Exposure to Sv in newborn rats has
been reported to increase reactive oxygen species (ROS) levels
through mitochondrial dysfunction and overactivation of NADPH
oxidase, resulting in widespread neurodegeneration and long-term
behavioral disorders (10,57,61).
Exposure-induced intracellular accumulation of ROS has been
associated with neuronal apoptosis (62,63).
Studies examining the effect of anesthesia exposure on oxidative
stress during pregnancy are limited. A previous study reported that
6 h of 3% Sv anesthesia in 6-day-old newborn rats caused oxidative
stress by decreasing superoxide dismutase levels and increasing MDA
levels in the rat brain (40). Liu
et al (62) reported the
same result in 7-day-old rats. However, the anesthesia exposure
time used in the aforementioned studies was not compatible with Sv
use in clinical practice, as the applied Sv concentration was
>1.1 MAC. The results reported in the present study suggested
that the application of Sv anesthesia at 1.1 MAC for 2 h did not
cause acute biochemical or pathological oxidant damage in fetal
rats.
Physiological apoptosis is responsible for the
destruction of 50-70% of neurons that develop under normal
conditions. Exposure to anesthetics at toxic levels, which may
occur during these periods when neuronal development is at the
forefront, can increase the rate of physiological apoptosis to
pathological levels (64).
Anesthesia can increase the occurrence of this physiological
process to a pathological level (8,37,65,66)
and previous studies have shown that certain areas of the brain can
be more strongly affected (67,68).
The hippocampus has been widely studied, as it is the key structure
for spatial memory and learning. However, Satomoto et al
(68) observed apoptosis in
numerous regions of the newborn rat brain immediately after
exposure to Sv. Therefore, a holistic viewpoint in
histopathological evaluation may provide more accurate results of
the damage caused by this anesthetic. However, examining tissues
that have not completed their development and whose borders are not
clear is often difficult. In the present study, the total apoptotic
cell count in the cortex was used as a measure for
histopathological evaluation, as the early gestational period (G14)
was optimal in terms of brain development. The results of the
present study did not indicate any adverse histological
pathological findings of the effects of anesthesia exposure in the
second trimester in the fetal brain. However, a previous study that
investigated the effects of 3.5% Sv for 2 h in G14 rats reported
increased apoptosis in neural stem cells and adverse effects on
behavioral tests, but these results were not observed when the
concentration of Sv was decreased to 2% (69). Although apoptosis induced by the
high dose of Sv gradually decreased as the rat fetuses progressed
further towards the neonatal period, the results of the behavioral
tests did not improve. In the present study, which was similar to
the aforementioned study in terms of exposure time point and
duration, the occurrence of apoptosis was attributed to the high
dose of Sv administered.
The current consensus is that not every apoptotic
process that may occur after exposure to anesthetics will have
long-term consequences (66).
Cognitive losses resulting from increased apoptosis may be
compensated for during development, but this process can be
unpredictable. Exposure to 3-5% Sv for 6 h in 6-day-old rats
(70) and 2.5% Sv for 4 h in
7-day-old rats did not cause neuronal loss (71), which indicates that the risk of
damage is lower when exposure is delayed. A previous study
investigated repeated exposures to 3% Sv in G14 rats, and reported
neuronal cell loss and long-term cognitive impairment accompanied
by a decrease in histone acetylation in fetal brain tissue and a
decrease in brain-derived neurotrophic factor levels (72).
These previous findings highlight the importance of
perioperative pharmacological neuroprotection, but this remains a
controversial topic. However, in patient groups where multipharmacy
is avoided, such as pregnancy and pediatrics, it would be a more
rational approach is to administer a drug with a proven safety
record that is in frequent use, rather than opting to use a new
agent. In previous years, the neuroprotective activity of
MgSO4 had been reported, which is frequently used in
preterm labor and is familiar to most obstetric anesthesiologists
(73) and to obstetric clinicians
who use it to prevent preterm labor and eclamptic seizures. A
number of studies have reported that MgSO4 protects the
central nervous system from oxidative stress and ischemic events,
and reduces neonatal learning and memory problems caused by
maternal inflammation (15,74).
However, to the best of our knowledge, the relationship between Sv
and magnesium has not previously been reported in neurons, as
previous studies have been limited to analgesia and hemodynamics.
The aim of the present study was determined by a review of the
literature, which indicated that magnesium could suppress the
damage pathways induced by Sv (14-17).
Sv induces neuroinflammation by activating the NF-κB
signaling pathway, whereas magnesium inhibits the activation of
NF-κB and reduces levels of pro-inflammatory cytokines, including
TNF-α, IL-1α and IL-6(75). Sv
increases cytosolic calcium levels, which results in abnormal
calcium release from the endoplasmic reticulum. Increased
intracellular calcium serves a role in stimulating mitochondrial
ROS production and inducing cytokine release (76). Magnesium, by contrast, is a calcium
uptake antagonist (77);
therefore, it reduces the acquisition of the neurotoxic phenotype
by microglia (78). Microglia
overproduce ROS, which serve a role in neurodegeneration (79), and Sv has also been reported to
increase ROS formation. Magnesium reduces ROS production (80). In the brain, magnesium has been
reported to reduce oxidative damage after hypoxia (81), counteracting oxidative stress
caused by maternal inflammation (79). For this reason, the present study
investigated the neuroprotective effect of MgSO4,
administered at 270 mg/kg i.p. 30 min before Sv exposure, as a
possible suppressor of Sv-induced neurotoxicity. Han et al
(14) reported that
MgSO4 at 270 mg/kg i.p. reduced oxidative stress and
inflammation after intrahepatic cholestasis in pregnant rats.
Khatib et al (15) showed
that the same dose reduced inflammation-induced fetal brain damage
in maternal late gestational inflammation. A previous study
reported that anesthetics increased cytosolic calcium levels and
elevation of cytosolic calcium was associated with increased levels
of proinflammatory cytokines, potentially through activation of the
NF-κB signaling pathway (82).
Magnesium serves a neuroprotective role by preventing the entry of
cytosolic calcium; however, Dribben et al (83) reported the occurrence of
neuroapoptosis after high-dose magnesium administration in neonatal
mice.
Magnesium has complex effects on cellular
excitability, including both stimulatory and inhibitory effects; it
has also been reported to induce neuronal apoptosis in vivo.
These two responses are considered to reflect the direct effects of
magnesium on sensitive neurons rather than secondary consequences
of systemic magnesium administration to rats (79). In clinical obstetrics,
MgSO4 administration is typically titrated to maintain
maternal serum levels at 4-8 mg/100 ml (1.6-3.3 mM) (83). These concentrations are close to
the lowest concentrations that caused the significant neuronal
toxicity reported in previous studies (83). Therefore, in the present study, the
neurotoxic potential of MgSO4 was analyzed, as well as
its potential neuroprotective activity. In the present study, no
significant difference in inflammatory and oxidative stress markers
were demonstrated between the treatment groups, except for the
increased TNF-α levels in the Sv group. The TNF-α levels in the
MgSO4-treated groups were similar compared with those in
the control group, which suggested the anti-inflammatory activity
of MgSO4. Since the IL-6, IL-10, TAS, TOS and OSI levels
were not statistically different between groups, no positive or
negative effect can be attributed to MgSO4 in terms of
these markers. However, the fact that the number of apoptotic cells
in the MgSO4-treated groups was markedly higher compared
with the other groups supports the findings reported by Dribben
et al (83). Although the
efficacy of the dose applied in the present study has been
confirmed in previous studies, lower doses should be tested in
future studies. Other injury pathways are implicated in
anesthesia-induced neurotoxicity. Autophagy, parthanatos, decreased
excitatory synapses and disruption of synaptic plasticity are other
pathways that may be affected by anesthesia and require further
investigation.
The present study had certain limitations. The first
was the lack of hemodynamic monitoring of rats during Sv exposure.
Wang et al (84) showed
that hemodynamics and blood gas parameters in rats exposed to 2 and
3.5% Sv were similar compared with the control group. Similarly, Li
et al (85) reported that
the blood pressure, heart rate, pH, arterial carbon dioxide
tension, arterial oxygen tension and arterial oxygen saturation of
Wistar Albino rats exposed to 2.6% Sv for 4 h were no different
from those of the control group. The laboratory where the
experiments were performed does not have the necessary devices for
hemodynamic monitoring. At the same time, a previous similar study
(25) showed that exposure to Sv
did not change hemodynamic parameters in Wistar rats and that the
study could be performed successfully without monitoring. These
results eliminated concerns in the present study about the possible
hemodynamic adverse effects of Sv. A second limitation of the
present study was the use of 100% O2 during anesthesia
to avoid hypoxemia. High O2 concentrations have been
reported to be harmful, but their application to every treatment
group, including the control, minimized the effect on the results
of the present study. This methodology was selected so that the
respiratory functions of the rats did not deteriorate and the rats
did not die during the experiment. In order to prevent this
limitation, the oxygen saturation of rats, partial O2
and CO2 pressures, and inlet and outlet gas levels of
gases in the closed environment should be monitored. Only in this
way can the possible toxic effects of oxygen be avoided by giving
the monitor as much oxygen as necessary.
In conclusion, 2 h exposure to Sv at a concentration
of 1.1 MAC did not cause maternal inflammation, oxidative stress or
neuronal damage in the fetal brain. The increase in TNF-α levels
may indicate that this exposure level may trigger inflammation,
although it may be too low to cause damage. The findings of the
present study suggested that short-term administration of Sv could
be used safely in the second trimester, based on the results of a
rat model. Although there appear to be potential anti-inflammatory
and antioxidant activities of MgSO4, more comprehensive
studies are required to confirm any neuroprotective benefits due to
concerns about the potential for inducing apoptosis.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
CO and BI designed the study and performed the
experiments. CO collected samples. CO, BI, GK and MAI confirm the
authenticity of all the raw data. GK performed the biochemical
assessments. MAI performed the histopathological analysis of brain
tissue. CO analyzed and interpreted data. CO and BI drafted the
manuscript. All authors have read and approved the final version of
the manuscript.
Ethics approval and consent to
participate
Ethical approval for the study was obtained from
Gazi University Experimental Animals Ethics Committee (Ankara,
Turkey; approval no. G.U.ET-20-0499).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Authors' information
Dr Cagri Ozdemir, ORCID no. 0000-0002-1266-8054;
Professor Berrin Isik, ORCID no. 0000-0002-0420-6589; Dr Gulce
Koca, ORCID no. 0000-0002-2646-1003; and Dr Mehmet Arda Inan, ORCID
no. 0000-0002-6179-2828.
References
|
1
|
Ní Mhuireachtaigh R and O'Gorman DA:
Anesthesia in pregnant patients for nonobstetric surgery. J Clin
Anesth. 18:60–66. 2006.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Osterman MJ and Martin JA: Primary
cesarean delivery rates, by state: Results from the revised birth
certificate, 2006-2012. Natl Vital Stat Rep. 63:1–11.
2014.PubMed/NCBI
|
|
3
|
United States Food and Drug
Administration. FDA drug safety communication: FDA review results
in new warnings about using general anesthetics and sedation drugs
in young children and pregnant women. Updated 2016. Accessed
Februry 10, 2017.
|
|
4
|
Krasowski MD and Harrison NL: The actions
of ether, alcohol and alkane general anaesthetics on GABAA and
glycine receptors and the effects of TM2 and TM3 mutations. Br J
Pharmacol. 129:731–743. 2000.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Eger EI, Liao M, Laster MJ, Won A,
Popovich J, Raines DE, Solt K, Dutton RC, Cobos FV and Sonner JM:
Contrasting roles of the N-methyl-D-aspartate receptor in the
production of immobilization by conventional and aromatic
anesthetics. Anesth Analg. 102:1397–1406. 2006.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Lerner RA: A hypothesis about the
endogenous analogue of general anesthesia. Proc Natl Acad Sci USA.
94:13375–13377. 1997.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Zhou ZB, Yang XY, Tang Y, Zhou X, Zhou LH
and Feng X: Subclinical concentrations of sevoflurane reduce
oxidative stress but do not prevent hippocampal apoptosis. Mol Med
Rep. 14:721–727. 2016.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Shen X, Dong Y, Xu Z, Wang H, Miao C and
Soriano SG: Selective anesthesia-induced neuroinflammation in
developing mouse brain and cognitive impairment. Anesthesiology.
118:502–515. 2013.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Cui FH, Li J, Li KZ, Xie YG and Zhao XL:
Effects of sevoflurane exposure during different stages of
pregnancy on the brain development of rat offspring. J Anesth.
35:654–662. 2021.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Zheng H, Dong Y, Xu Z, Crosby G, Culley
DJ, Zhang Y and Xie Z: Sevoflurane anesthesia in pregnant mice
induces neurotoxicity in fetal and offspring mice. Anesthesiology.
118:516–526. 2013.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Bilotta F, Gelb AW, Stazi E, Titi L,
Paoloni FP and Rosa G: Pharmacological perioperative brain
neuroprotection: A qualitative review of randomized clinical
trials. Br J Anaesth. 110: (Suppl 1):S113–S120. 2013.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Hudetz JA, Iqbal Z, Gandhi SD, Patterson
KM, Byrne AJ, Hudetz AG, Pagel PS and Warltier DC: Ketamine
attenuates post-operative cognitive dysfunction after cardiac
surgery. Acta Anaesthesiol Scand. 53:864–872. 2009.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Roach GW, Newman MF, Murkin JM, Martzke J,
Ruskin A, Li J, Guo A, Wisniewski A and Mangano DT: Multicenter
Study of Perioperative Ischemia (MsSPI). Ineffectiveness of burst
suppression therapy in mitigating perioperative cerebrovascular
dysfunction. Anesthesiology. 99:1255–1264. 1999.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Han F, Xu L, Huang Y, Chen T, Zhou T and
Yang L: Magnesium sulphate can alleviate oxidative stress and
reduce inflammatory cytokines in rat placenta of intrahepatic
cholestasis of pregnancy model. Arch Gynecol Obstet. 298:631–638.
2018.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Khatib N, Ginsberg Y, Shalom-Paz E, Dabaja
H, Gutzeit O, Zmora O, Millo Z, Ross MG and Beloosesky R: Fetal
neuroprotective mechanism of maternal magnesium sulfate for late
gestation inflammation: In a rodent model. J Matern Fetal Neonatal
Med. 33:3732–3739. 2020.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Temkin NR, Anderson GD, Winn HR,
Ellenbogen RG, Britz GW, Schuster J, Lucas T, Newell D, Nelson
Mansfield P, Machamer JE, et al: Magnesium sulfate for
neuroprotection after traumatic brain injury: A randomised
controlled trial. Lancet Neurol. 6:29–38. 2007.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Hallak M, Hotra JW and Kupsky WJ:
Magnesium sulfate protection of fetal rat brain from severe
maternal hypoxia. Obstet Gynecol. 96:124–128. 2000.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Zhang Y, Dong Y, Xu Z and Xie Z: Propofol
and magnesium attenuate isoflurane-induced caspase-3 activation via
inhibiting mitochondrial permeability transition pore. Med Gas Res.
2(20)2012.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Beloosesky R, Khatib N, Ginsberg Y,
Anabosy S, Shalom-Paz E, Dahis M, Ross MG and Weiner Z: Maternal
magnesium sulfate fetal neuroprotective effects to the fetus:
inhibition of neuronal nitric oxide synthase and nuclear factor
kappa-light-chain-enhancer of activated B cells activation in a
rodent model. Am J Obstet Gynecol. 215:382.e1–e6. 2016.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Animal Experiments Center Ethics
Committee. Legislation. Available from: https://hadmek.tarimorman.gov.tr/Sayfa/Detay/644.
Accessed November 22, 2022.
|
|
21
|
Percie du Sert N, Ahluwalia A, Alam S,
Avey MT, Baker M and Browne WJ: Reporting animal research:
Explanation and elaboration for the ARRIVE guidelines 2.0. PLoS
Biol. 18(e3000410)2020.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Song R, Ling X, Peng M, Xue Z, Cang J and
Fang F: Maternal Sevoflurane exposure causes abnormal development
of fetal prefrontal cortex and induces cognitive dysfunction in
offspring. Stem Cells Int. 2027(6158468)2017.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Sameshima H, Ota A and Ikenoue T:
Pretreatment with magnesium sulfate protects against
hypoxic-ischemic brain injury but postasphyxial treatment worsens
brain damage in seven-day-old rats. Am J Obstet Gynecol.
180:725–730. 1999.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Cho GJ, Hong HR, Hong SC, Oh MJ and Kim
HJ: The neuroprotective effect of magnesium sulfate in preterm
fetal mice. J Perinatal Med. 45:537–543. 2015.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Dong Y, Zhang G, Zhang B, Moir RD, Xia W,
Marcantonio ER, Culley DJ, Crosby G, Tanzi RE and Xie Z: The common
inhalational anesthetic sevoflurane induces apoptosis and increases
beta-amyloid protein levels. Arch Neurol. 66:620–631.
2009.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Erel O: A new automated colorimetric
method for measuring total oxidant status. Clin Biochem.
12:1103–1111. 2005.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Rubio CP and Cerón JJ: Spectrophotometric
assays for evaluation of Reactive Oxygen Species (ROS) in serum:
General concepts and applications in dogs and humans. BMC Vet Res.
17(226)2021.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Blaylock M, Engelhardt T and Bissonnette
B: Fundamentals of neuronal apoptosis relevant to pediatric
anesthesia. Paediatr Anaesth. 20:383–395. 2010.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Ikonomidou C: Triggers of apoptosis in the
immature brain. Brain Dev. 31:488–492. 2009.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Li X, Jiang X and Zhao P: Effects of
pregnancy anesthesia on fetal nervous system. Front Pharmacol.
11(523514)2021.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Lee S, Chung W, Park H, Park H, Yoon S,
Park S, Park J, Heo JY, Ju X, Yoon SH, et al: Single and multiple
sevoflurane exposures during pregnancy and offspring behavior in
mice. Paediatr Anaesth. 27:742–751. 2017.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Wu Z, Li X, Zhang Y, Tong D, Wang L and
Zhao P: Effects of Sevoflurane exposure during mid-pregnancy on
learning and memory in offspring rats: Beneficial effects of
maternal exercise. Front Cell Neurosci. 12(122)2018.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Andropoulos DB and Greene MF: Anesthesia
and developing brains-implications of the FDA warning. N Engl J
Med. 376:905–907. 2017.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Palanisamy A: Maternal anesthesia and
fetal neurodevelopment. Int J Obstet Anesth. 21:152–162.
2012.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Workman AD, Charvet CJ, Clancy B,
Darlington RB and Finlay BL: Modeling transformations of
neurodevelopmental sequences across mammalian species. J Neurosci.
33:7368–7383. 2013.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Brioni JD, Varughese S, Ahmed R and Bein
B: A clinical review of inhalation anesthesia with sevoflurane:
From early research to emerging topics. J Anesth. 5:764–778.
2017.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Zhou X, Li W, Chen X, Yang X, Zhou Z, Lu D
and Feng X: Dose-dependent effects of sevoflurane exposure during
early lifetime on apoptosis in hippocampus and neurocognitive
outcomes in Sprague-Dawley rats. Int J Physiol Pathophysiol
Pharmacol. 8:111–119. 2016.PubMed/NCBI
|
|
38
|
Wang S, Peretich K, Zhao Y, Liang G, Meng
Q and Wei H: Anesthesia-induced neurodegeneration in fetal rat
brains. Pediatr Res. 66:435–440. 2009.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Hirotsu A, Iwata Y, Tatsumi K, Miyai Y,
Matsuyama T and Tanaka T: Maternal exposure to volatile anesthetics
induces IL-6 in fetal brains and affects neuronal development. Eur
J Pharmacol. 863(172682)2019.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Zhang Y, Li M, Cui E, Zhang H, Zhu X, Zhou
J, Yan M and Sun J: Dexmedetomidine attenuates sevoflurane-induced
neurocognitive impairment through α2-adrenoceptors. Mol Med Rep.
23(38)2021.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Bergdolt L and Dunaevsky A: Brain changes
in a maternal immune activation model of neurodevelopmental brain
disorders. Prog Neurobiol. 175:1–19. 2019.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Wan Y, Xu J, Ma D, Zeng Y, Cibelli M and
Maze M: Postoperative impairment of cognitive function in rats: A
possible role for cytokine-mediated inflammation in the
hippocampus. Anesthesiology. 106:436–443. 2007.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Fan CH, Peng B and Zhang FC: The
postoperative effect of sevoflurane inhalational anesthesia on
cognitive function and inflammatory response of pediatric patients.
Eur Rev Med Pharmacol Sci. 22:3971–3975. 2018.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Ginsberg Y, Khatib N, Weiner Z and
Beloosesky R: Maternal inflammation, fetal brain implications and
suggested neuroprotection: A summary of 10 years of research in
animal models. Rambam Maimonides Med J. 8(e0028)2018.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Hennessy E, Gormley S, Lopez-Rodriguez AB,
Murray C, Murray C and Cunningham C: Systemic TNF-α produces acute
cognitive dysfunction and exaggerated sickness behavior when
superimposed upon progressive neurodegeneration. Brain Behav Immun.
59:233–244. 2017.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Pickering M, Cumiskey D and O'Connor JJ:
Actions of TNF-alpha on glutamatergic synaptic transmission in the
central nervous system. Exp Physiol. 90:663–670. 2005.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Barone FC, Arvin B, White RF, Miller A,
Webb CL, Willette RN, Lysko PG and Feuerstein GZ: Tumor necrosis
factor-alpha. A mediator of focal ischemic brain injury. Stroke.
28:1233–1244. 1997.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Mayne M, Ni W, Yan HJ, Xue M, Johnston JB,
Del Bigio MR, Peeling J and Power C: Antisense oligodeoxynucleotide
inhibition of tumor necrosis factor-alpha expression is
neuroprotective after intracerebral hemorrhage. Stroke. 32:240–248.
2001.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Nawashiro H, Martin D and Hallenbeck JM:
Inhibition of tumor necrosis factor and amelioration of brain
infarction in mice. J Cereb Blood Flow Metab. 17:229–232.
1997.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Carpentier PA, Dingman AL and Palmer TD:
Placental TNF-α signaling in illness-induced complications of
pregnancy. Am J Pathol. 6:2802–2810. 2011.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Zhao X, Bausano B, Pike BR,
Newcomb-Fernandez JK, Wang KK, Shohami E, Ringger NC, DeFord SM,
Anderson DK and Hayes RL: TNF-alpha stimulates caspase-3 activation
and apoptotic cell death in primary septo-hippocampal cultures. J
Neurosci Res. 64:121–131. 2001.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Hemmer K, Fransen L, Vanderstichele H,
Vanmechelen E and Heuschling P: An in vitro model for the study of
microglia-induced neurodegeneration: Involvement of nitric oxide
and tumor necrosis factor-alpha. Neurochem Int. 38:557–565.
2001.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Robertson J, Beaulieu JM, Doroudchi MM,
Durham HD, Julien JP and Mushynski WE: Apoptotic death of neurons
exhibiting peripherin aggregates is mediated by the proinflammatory
cytokine tumor necrosis factor-alpha. J Cell Biol. 155:217–226.
2001.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Haddad JJ: Redox regulation of
pro-inflammatory cytokines and IkappaB-alpha/NF-kappaB nuclear
translocation and activation. Biochem Biophys Res Commun.
296:847–856. 2002.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Hoek JB and Pastorino JG: Ethanol,
oxidative stress, and cytokineinduced liver cell injury. Alcohol.
27:63–68. 2002.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Wu Y, Song J, Wang Y, Wang X, Culmsee C
and Zhu C: The potential role of ferroptosis in neonatal brain
injury. Front Neurosci. 13(115)2019.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Bhat AH, Dar KB, Anees S, Zargar MA,
Masood A, Sofi MA and Ganie SA: Oxidative stress, mitochondrial
dysfunction and neurodegenerative diseases; a mechanistic insight.
Biomed Pharmacother. 74:101–110. 2015.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Xu Z and Qian B: Sevoflurane
anesthesia-mediated oxidative stress and cognitive impairment in
hippocampal neurons of old rats can be ameliorated by expression of
brain derived neurotrophic factor. Neurosci Lett.
721(134785)2020.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Allaouchiche B, Debon R, Goudable J,
Chassard D and Duflo F: Oxidative stress status during exposure to
propofol, sevoflurane and desflurane. Anesth Analg. 93:981–985.
2001.PubMed/NCBI View Article : Google Scholar
|
|
60
|
Tsuchiya M, Asada A, Kasahara E, Sato EF,
Shindo M and Inoue M: Antioxidant protection of propofol and its
recycling in erythrocyte membranes. Am J Respir Crit Care Med.
165:54–60. 2002.PubMed/NCBI View Article : Google Scholar
|
|
61
|
Sun Z, Satomoto M, Adachi YU, Kinoshita H
and Makita K: Inhibiting NADPH oxidase protects against long-term
memory impairment induced by neonatal sevoflurane exposure in mice.
Br J Anaesth. 117:80–86. 2016.PubMed/NCBI View Article : Google Scholar
|
|
62
|
Liu B, Gu Y, Xiao H, Lei X, Liang W and
Zhang J: Altered metabolomic profiles may be associated with
sevoflurane-induced neurotoxicity in neonatal rats. Neurochem Res.
40:788–799. 2015.PubMed/NCBI View Article : Google Scholar
|
|
63
|
Xu G, Lu H, Dong Y, Shapoval D, Soriano
SG, Liu X, Zhang Y and Xie Z: Coenzyme Q10 reduces
sevoflurane-induced cognitive deficiency in young mice. Br J
Anaesth. 119:481–449. 2017.PubMed/NCBI View Article : Google Scholar
|
|
64
|
Oppenheim RW: Cell death during
development of the nervous system. Annu Rev Neurosci. 14:453–501.
1991.PubMed/NCBI View Article : Google Scholar
|
|
65
|
Wang Y, Li Y, Xing Q, Han XG, Dong X, Lu Y
and Zhou M: Sevoflurane anesthesia in pregnant rats negatively
affects nerve function in offspring potentially via inhibition of
the Wnt/β-catenin pathway. Mol Med Rep. 15:2753–2759.
2017.PubMed/NCBI View Article : Google Scholar
|
|
66
|
Yu Z, Wang J, Wang H, Wang J, Cui J and
Junzhang P: Effects of sevoflurane exposure during late pregnancy
on brain development and beneficial effects of enriched environment
on offspring cognition. Cell Mol Neurobiol. 40:1339–1352.
2020.PubMed/NCBI View Article : Google Scholar
|
|
67
|
Areias J, Sola C, Chastagnier Y, Pico J,
Bouquier N, Dadure C, Perroy J and Szabo V: Whole-brain
characterization of apoptosis after sevoflurane anesthesia reveals
neuronal cell death patterns in the mouse neonatal neocortex. Sci
Rep. 13(14763)2023.PubMed/NCBI View Article : Google Scholar
|
|
68
|
Satomoto M, Itoh H, Uchida A and Makita K:
Resveratrol did not prevent sevoflurane-induced neuroapoptosis in
the neonatal mice brain. Masui. 62:1184–1187. 2013.PubMed/NCBI
|
|
69
|
Wang Y, Yin S, Xue H, Yang Y, Zhang N and
Zhao P: Mid-gestational sevoflurane exposure inhibits fetal neural
stem cell proliferation and impairs postnatal learning and memory
function in a dose-dependent manner. Dev Biol. 435:185–197.
2018.PubMed/NCBI View Article : Google Scholar
|
|
70
|
Bercker S, Bert B, Bittigau P,
Felderhoff-Müser U, Bührer C, Ikonomidou C, Weise M, Kaisers UX and
Kerner T: Neurodegeneration in newborn rats following propofol and
sevoflurane anesthesia. Neurotox Res. 16:140–147. 2009.PubMed/NCBI View Article : Google Scholar
|
|
71
|
Jia M, Liu WX and Yang JJ, Xu N, Xie ZM,
Ju LS, Ji MH, Martynyuk AE and Yang JJ: Role of histone acetylation
in long-term neurobehavioral effects of neonatal Exposure to
sevoflurane in rats. Neurobiol Dis. 91:209–220. 2016.PubMed/NCBI View Article : Google Scholar
|
|
72
|
Wang SQ, Fang F, Xue ZG, Cang J and Zhang
XG: Neonatal sevoflurane anesthesia induces long-term memory
impairment and decreases hippocampal PSD-95 expression without
neuronal loss. Eur Rev Med Pharmacol Sci. 17:941–950.
2013.PubMed/NCBI
|
|
73
|
Costantine MM and Drever N: Antenatal
exposure to magnesium sulfate and neuroprotection in preterm
infants. Obstet Gynecol Clin North Am. 38:351–366. 2011.PubMed/NCBI View Article : Google Scholar
|
|
74
|
Lamhot VB, Khatib N, Ginsberg Y, Anunu R,
Richter-Levin G, Weiner Z, Ross MG, Divon MY, Hallak M and
Beloosesky R: Magnesium sulfate prevents maternal
inflammation-induced impairment of learning ability and memory in
rat offspring. Am J Obstet Gynecol. 213:851.e1–e8. 2015.PubMed/NCBI View Article : Google Scholar
|
|
75
|
Zhu X, Yao Y, Guo M, Li J, Yang P, Xu H
and Lin D: Sevoflurane increases intracellular calcium to induce
mitochondrial injury and neuroapoptosis. Toxicol Lett. 336:11–20.
2021.PubMed/NCBI View Article : Google Scholar
|
|
76
|
Gao F, Ding B, Zhou L, Gao X, Guo H and Xu
H: Magnesium sulfate provides neuroprotection in
lipopolysaccharide-activated primary microglia by inhibiting NF-κB
pathway. J Surg Res. 184:944–950. 2013.PubMed/NCBI View Article : Google Scholar
|
|
77
|
Andretta A, Schieferdecker MEM, Petterle
RR, Dos Santos Paiva E and Boguszewski CL: Relations between serum
magnesium and calcium levels and body composition and metabolic
parameters in women with fibromyalgia. Adv Rheumatol Lond Engl.
60(18)2020.PubMed/NCBI View Article : Google Scholar
|
|
78
|
Pan K and Garaschuk O: The role of
intracellular calcium-store-mediated calcium signals in in vivo
sensor and effector functions of microglia. J Physiol.
601:4203–4215. 2022.PubMed/NCBI View Article : Google Scholar
|
|
79
|
Woodburn SC, Bollinger JL and Wohleb ES:
The semantics of microglia activation: Neuroinflammation,
homeostasis, and stress. J Neuroinflamm. 18(258)2021.PubMed/NCBI View Article : Google Scholar
|
|
80
|
Khatib N, Ginsberg Y, Ben David C, Ross
MG, Vitner D, Zipori Y, Zamora O, Weiner Z and Beloosesky R:
Magnesium sulphate neuroprotection mechanism is placental mediated
by inhibition of inflammation, apoptosis and oxidative stress.
Placenta. 127:29–36. 2022.PubMed/NCBI View Article : Google Scholar
|
|
81
|
Mohammadi H, Shamshirian A, Eslami S,
Shamshirian D and Ebrahimzadeh MA: Magnesium sulfate attenuates
lethality and oxidative damage induced by different models of
hypoxia in mice. Biomed Res Int. 2020(2624734)2020.PubMed/NCBI View Article : Google Scholar
|
|
82
|
Yang H, Liang G, Hawkins BJ, Madesh M,
Pierwola A and Wei H: Inhalational anesthetics induce cell damage
by disruption of intracellular calcium homeostasis with different
potencies. Anesthesiology. 109:243–250. 2008.PubMed/NCBI View Article : Google Scholar
|
|
83
|
Dribben WH, Creeley CE, Wang HH, Smith DJ,
Farber NB and Olney JW: High dose magnesium sulfate exposure
induces apoptotic cell death in the developing neonatal mouse
brain. Neonatology. 96:23–32. 2009.PubMed/NCBI View Article : Google Scholar
|
|
84
|
Wang Y, Yin SW, Zhang N and Zhao P:
High-concentration sevoflurane exposure in mid-gestation induces
apoptosis of neural stem cells in rat offspring. Neural Regen Res.
9:1575–1584. 2018.PubMed/NCBI View Article : Google Scholar
|
|
85
|
Li D, Liu L, Li L, Li X, Huang B, Zhou C,
Zhang Z, Wang C, Dong P, Zhang X, et al: Sevoflurane induces
exaggerated and persistent cognitive decline in a type II diabetic
rat model by aggregating hippocampal inflammation. Front Pharmacol.
8(886)2017.PubMed/NCBI View Article : Google Scholar
|