Introduction
Squamous cell papilloma within the oral cavity is a
localized benign hyperplasia of the oral epithelium that typically
presents as a verrucous lesion (1). Microscopically, the outer layer
manifests as an infolded epithelium, distinguished by features
including acanthosis and hyperkeratosis, while the inner layer
comprises a fibrovascular core containing well-developed blood
vessels (2). Its etiology is
predominantly attributed to human papillomavirus (HPV) infection
(3). In addition, squamous cell
papilloma can also manifest in the nasal cavity, external auditory
canal, pharynx and esophagus (1,4,5).
Since symptoms of squamous cell papilloma typically lack
specificity, clinical presentation frequently occur during later
stages of this condition, causing patients with this disease
primarily seeking medical attention due to symptoms arising from
obstructive effects.
Although typically benign, squamous cell papilloma
have the potential for malignant transformation and recurrence
(6). The primary mode of treatment
involves surgical intervention, yielding favorable prognoses
(7). However, whilst squamous cell
papilloma mostly affect the palate, cheek, lip and tongue (8), occurrences within the mandible are
seldom reported. The present case, to our knowledge, marks the
inaugural documentation of such an instance in the literature,
though previous incidences may have existed but were not formally
recorded. The imaging characteristics of this squamous papilloma
case involving the jaw closely resemble those of malignant jaw
tumors. Furthermore, a history of recurrent anti-inflammatory
treatment failure may heighten the risk of misdiagnosis and
oversight. Consequently, the present report documented a case of
squamous cell papilloma involving the mandible at the First
Affiliated Hospital of Sun Yat-sen University (Guangzhou, China) in
January 2023. The present report aimed to enhance the comprehension
of this condition and to provide a reference for healthcare
practitioners. The literature review performed in the present study
encompassed databases including PubMed (https://pubmed.ncbi.nlm.nih.gov/), Google Scholar
(https://scholar.google.com/) and
relevant medical journals accessed between 1970 and 2023. The key
words ‘squamous cell papilloma’, ‘mandible’, ‘oral cavity’ and
‘human papillomavirus’, ‘inflammatory’, ‘PTHrP’, ‘TGF-β’ and
‘chronic stress’, were used in various combinations. The search was
limited to publications available in English and Chinese. Relevant
articles, case reports and systematic reviews detailing the
clinical characteristics, diagnosis and management of squamous cell
papilloma within the jaw region were included for analysis.
Case report
Medical history and clinical
presentation
In January 2023, a 49-year-old male was admitted to
the First Affiliated Hospital of Sun Yat-sen University (Guangzhou,
China) with a chief complaint of repeated swelling and pain
persisting for 6 months following the extraction of an impacted
left mandibular tooth. The patient had a previous history of
recurrent pericoronitis associated with the left lower posterior
tooth 1 year prior to the current presentation. This recurrent
condition resulted in episodes of left buccal facial swelling and
pain that were seemingly unrelated to any apparent cause, which
prevented his mouth from opening adequately. These symptoms
manifested ~8 months after extraction of the affected tooth. The
patient reported a smoking history spanning three decades, with a
daily consumption of one pack of cigarettes. The patient denied any
history of previous viral infections, similar lesions in other
regions of the body or a family history of similar diseases.
Following examination, mild swelling and pain were observed on the
left cheek, with no notable abnormalities in the passive mandibular
opening or pattens of mandibular opening. Within the oral cavity, a
number of red polypoid masses of ~0.5x0.5 cm each were identified
within the extraction site of the left lower third molar area.
These masses were devoid of significant tenderness or bleeding.
Laboratory tests conducted on blood samples encompassed a complete
blood count, erythrocyte sedimentation rate, C-reactive protein, as
well as specific investigations for infectious or autoimmune
factors, such as serum amyloid A, immunoglobulin (Ig)A, IgM, IgG,
complement 3 (C3) and C4. Results for all tests were within normal
ranges.
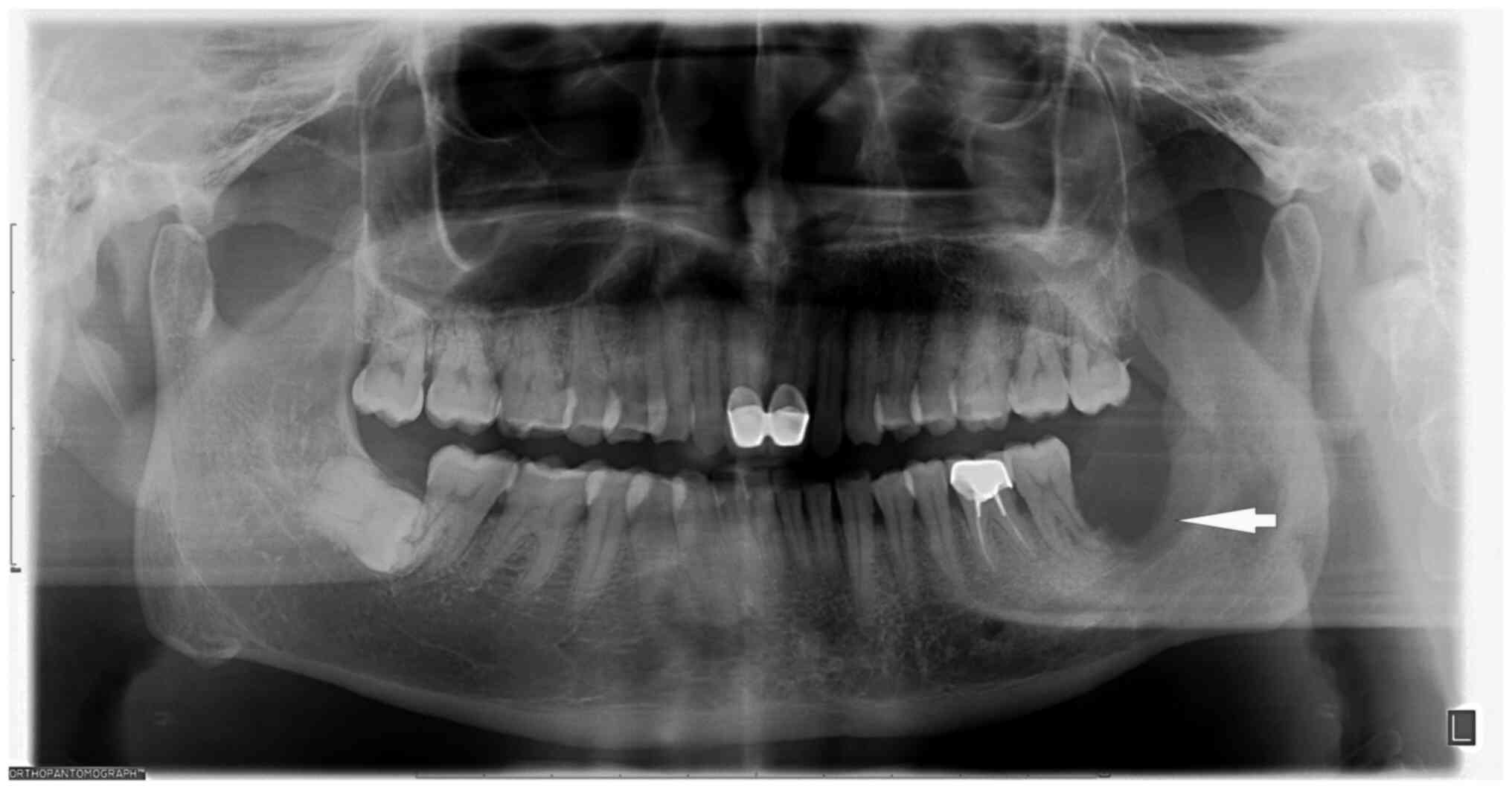
Radiographic assessments were based on reports from
two or three independent radiologists. Panoramic radiographs
revealed that the lower left third molar was missing, with
resorption of the alveolar socket extending to the root apex area
of the lower left second molar. In addition, a 0.5x1-cm low-density
shadow with an indistinct boundary was discernible in the middle
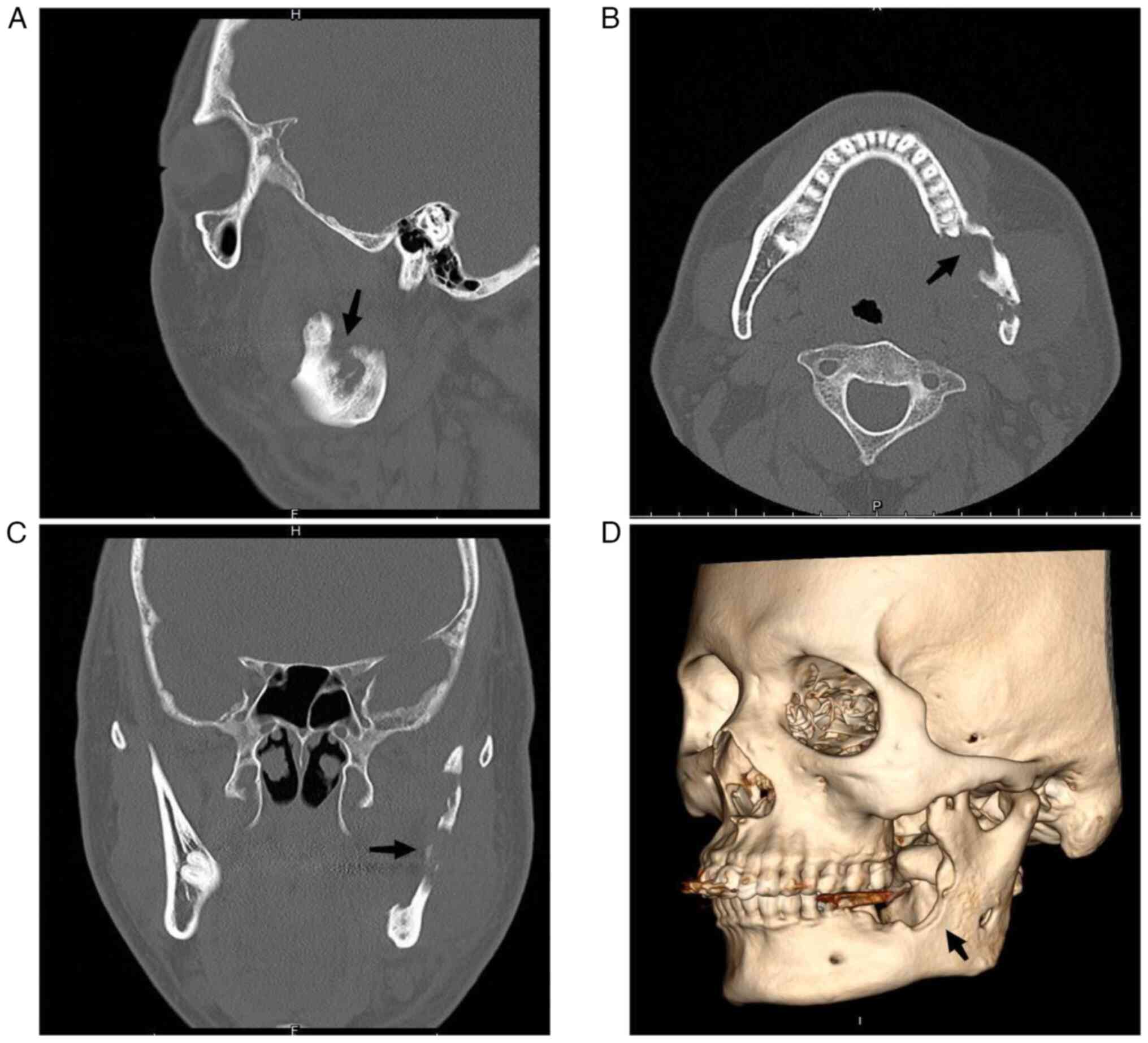
and lower portion of the left mandibular ascending branch (Fig. 1). At the sagittal plane (Fig. 2A), mandibular CT scan showed
involvement of the left mandibular coronal process and ascending
mandibular rami. Cross-sectional perimetry (Fig. 2B) revealed a local lamellar
periosteal reaction with an increase in bone mineral density in
unaffected areas. The coronal visual field (Fig. 2C) showed significant destruction of
the left ascending branch of the jaw with a soft tissue mass. A 3D
surface reconstruction (left maxillary surface, Fig. 2D) highlights the deformation of the
left mandibular coronal process, rami, and body. Given these
findings, concerns were raised regarding the possibility of an
invasive or malignant bone tumor. After admission, a preoperative
tissue biopsy was performed on the left mandibular lesion. The
collected tissue, obtained from the affected site, underwent
histopathological examination with Hematoxylin and Eosin (HE)
staining. The diagnosis of squamous cell papilloma was conclusively
confirmed based on the distinctive features observed in the stained
biopsy specimens. Consequently, it was determined that complete
resection represented the optimal course of action for the patient.
The surgical procedure was performed by an oral and maxillofacial
surgeon. A grayish-yellow mass measuring 3.0x2.0x1.5 cm was
identified in the left masseter attachment area during the
operation, which exhibited a texture akin to the tip of the nose,
tough with medium consistency, and had unclear boundaries. In
total, ~0.5 cm of the tissue surrounding the tumor was excised. No
destruction was observed on the buccal side of the left mandible.
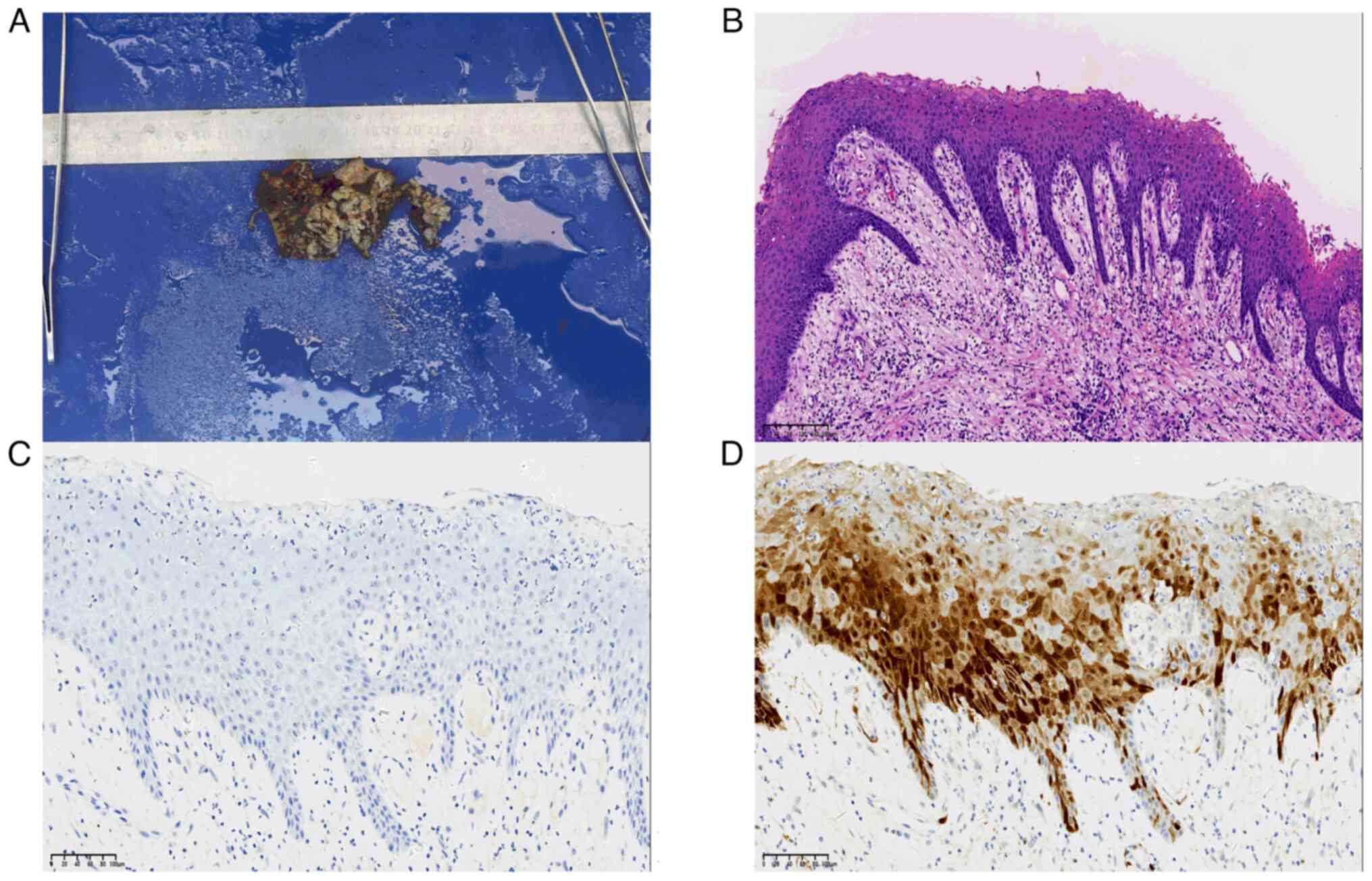
The bone surface appeared waxy and rough, involving blurred
boundaries. Numerous grayish-white papillary growths (white arrow)
were visible along the left ascending mandibular branch (Fig. 3A). A resection involving the left
mandibular body, the left ascending branch of the mandible and the
coronal process was performed to address this diseased bone.
Finally, a bone plate harvested from the medial iliac ridge of the
left side was utilized to fill the bone defect within the left
mandible. To ensure adequate fixation, the defect was secured using
titanium plates and titanium nails. In the assessment of this mass,
the primary goal was to differentiate between benign papilloma and
squamous cell carcinoma, necessitating a meticulous analysis of
cellular characteristics. The focus was in identifying any
indicators of cell atypia, heightened mitotic activity and
aggressive traits suggestive of malignancy. Furthermore, the
presence of a distinct papillary structure supports the exclusion
of squamous cell carcinoma. Clinical relevance, imaging studies and
the expertise of two independent pathologists further bolster our
ability to rule out malignancies from the list of potential
diagnoses.
For perioperative pathological examination, tissue
specimens, sliced to a thickness of 4 µm, were subjected to
fixation in 10% formalin at ambient temperature for 24 h to
maintain cellular integrity. Post-fixation, the specimens were
encased in paraffin wax. Dehydration was accomplished through a
series of graded ethanol solutions, succeeded by clearing.
Hematoxylin staining (5 min) at ambient temperature delineated
cellular nuclei, while eosin staining (2 min) at ambient
temperature conferred a pink hue to the cytoplasm and extracellular
matrix. Subsequent to a final round of dehydration and clearing,
the specimens were affixed onto glass slides. The entire procedure
was scrutinized via light microscopy to ensure standardized H&E
staining, thereby facilitating lucid visualization of tissue
morphology. The histopathological examination of the surgical
specimen yielded the following results: i) The morphology of the
left masseter muscle was consistent with a chronic abscess, with a
number of salivary glands, striated muscle and fibrous connective
tissue; ii) a large number of chronic inflammatory cells,
neutrophils and multinucleated giant cells could be seen locally in
Fig. 3B (white star); iii) the
left mandibular mass is consistent with squamous papilloma with
local ulceration, as a large number of chronic inflammatory cells
and foam cells were found in the stroma, as shown in Fig. 3B (white arrow).
Immunohistochemistry staining for HPV showed a negative result (-)
(Fig. 3C), while p16 staining
exhibited a mottled positive result (+), with pronounced positivity
primarily localized in the epithelial cells adjacent to the basal
membrane. The positive staining was observed in both the cell
nuclei and cytoplasm, presenting a heterogeneous distribution
(Fig. 3D). The immunohistochemical
staining protocol proceeded as follows: Tissue sections with a
thickness of 4 µm were fixed at room temperature using 10% formalin
for 24 h. Subsequent embedding was performed with paraffin.
Dehydration involved a series of graded ethanol solutions.
Heat-induced antigen retrieval was carried out in citrate buffer at
pH 6.0, at 95˚C for 20 min. Endogenous peroxidase activity was
quenched using 3% hydrogen peroxide for 10 min at room temperature.
Blocking was achieved with 5% bovine serum albumin for 1 h at room
temperature. Primary antibodies included anti-HPV antibody (1:20
dilution; cat. no. ab245950; Abcam) and anti-p16 antibody (1:50
dilution; cat. no. ab51243; Abcam). Secondary antibodies were
applied as follows: For HPV detection: Goat Anti-Mouse IgG H&L
(Alexa Fluor® 488; diluted at 1:500; cat. no. ab150113;
Abcam). For p16 detection: Goat anti-rabbit IgG H&L (Alexa
Fluor® 488; diluted at 1:500; cat. no. ab150077; Abcam).
Both antibodies were applied for 1 h at room temperature.
Visualization was performed using a
3,3'-diaminobenzidine reagent kit. Counterstaining involved
application of hematoxylin for 2 min at room temperature.
Immunohistochemistry (IHC) analysis was performed using a light
microscope.
During the follow-up, no signs of progression or
tumor recurrence were observed during follow-up evaluations
conducted at 1- and 4-months post-operation. The patient resumed
normal daily activities and work responsibilities, indicating a
positive outcome. The patient was discharged a week after surgery,
with surgical intervention being the sole treatment modality for
tumor management. Subsequent follow-up visits in March 2023 and
June 2023 revealed no signs of progression or tumor recurrence.
Additionally, the patient resumed normal daily activities and work
responsibilities, indicating a positive outcome.
Discussion
In the oral cavity, squamous cell papilloma
typically manifests as a papillary protrusion on the oral mucosal
surface. The precise etiology of papilloma remains elusive,
although it exhibits associations with various predisposing
factors, including tobacco consumption, alcohol misuse, chronic
oral inflammatory conditions (such as gingivitis), inappropriate
alveolar bone stimulation, HPV infection and genetic susceptibility
(9,10). In the oral cavity, squamous cell
papilloma is commonly found in the palate, cheek, lip and tongue,
where it is more commonly found in men (1). Although the most common sites of this
benign tumor have been proposed to be the soft palate, lip and
gingiva (1,11), the tongue was suggested to be the
most common site (8,10). However, squamous cell papilloma has
also been documented to occur in the trachea, nasal cavity,
sinuses, external auditory canal, esophagus and throat (12-14).
The majority of cases are characterized by the absence of a clear
unified set of symptoms, with clinical manifestations typically
arising from secondary symptoms caused by physical obstruction.
These may include nasal congestion, stridor, hearing loss,
discomfort or difficulty swallowing and breathing disturbances such
as disruptive dyspnea (1-3).
Compared with other locations, squamous cell
papilloma of the jaw is an exceedingly rare occurrence. This benign
neoplasm typically originates from the stratified squamous
epithelial lining of the oral mucosa (15). However, the presence of residual
epithelial elements within the jaw is limited, consisting of
remnants of the dental lamina epithelium, epithelial rests of
Malassez and reduced enamel epithelium (16). These epithelial tissues may be the
origin of some common epithelial tumors in the jaw. Furthermore,
squamous cell papilloma is predominantly encountered within
systemic lacunar ducts, such as those found in the esophagus,
respiratory tract, external auditory canal, cervix and breast
ducts. It is imperative to recognize that the trabecular bone
within the jaw constitutes an extension of the cortical bone
encasing the cancellous bone, which forms a complex irregular
three-dimensional network unlike the characteristic lacunar duct
architecture found in the aforementioned organs (17). The origin of the epithelial lesion
in this case could potentially stem from the gingival epithelium or
the epithelium of the jaw.
The extended period of inflammation within the
patient's jawbone likely mediated a pivotal role in precipitating
the tumor-like metamorphosis. This may be attributed to four
underlying factors. Inflammatory induction of aberrant cell growth
is one such factor. The prolonged inflammation triggering aberrant
cell proliferation within the jawbone observed in the present case
aligns with previous studies that also emphasized the role of
inflammation in cancer development. Trinchieri (18) previously underscored the induction
of inflammation promoting cancer long before tumor formation.
Specifically, they observed that before tumor formation,
inflammation may have a role by affecting the genetic stability of
tissues, epigenetic modifications and immune responses. Various
inflammatory diseases, such as inflammatory bowel diseases, chronic
hepatitis, Helicobacter-induced gastritis or
schistostoma-induced bladder inflammation, can all heighten the
susceptibility to malignant transformation (18). Prolonged inflammation can trigger
the activation of local immune cells, leading to the release of
proinflammatory cytokines, such as IL-1, TNF and IL-6, along with
various growth factors. Among these factors are reactive oxygen
species, reactive nitrogen species and interferon-γ (19,20).
These cytokines in turn stimulate cell proliferation in the
neighboring tissues, including the epithelial cells residing within
the jawbone. This dysregulated cellular growth may cause the
inception of papilloma formation (19,20).
Close inter-cellular interplay may serve as another one of the four
factors. Previous studies on tumor-induced bone diseases found a
close interaction between tumor cells and bone cells, termed the
‘vicious cycle’ (21,22). This concept posits that tumor cells
secrete a number of factors, such as parathyroid hormone-related
protein (PTHrP), provoking osteoblasts into producing receptor
activator of nuclear factor κB ligand (RANKL), thereby promoting
osteoclast formation and bone destruction. Consequently, this form
of bone destruction leads to the release of growth factors, such as
insulin-like growth factor II and transforming growth factor β3
(TGF-β3) (23), embedded in the
bone matrix, further stimulating tumor growth and aggravating bone
destruction. Therefore, it may be hypothesized that PTHrP secretion
by the tumor cells in the papilloma may have induced RANKL
production in the osteoblasts, in turn promoting osteoclast
formation and bone degradation. Subsequent bone destruction by the
osteoclasts may then lead to the release of intraosseous growth
factors that continue to stimulate papilloma growth (24,25).
In terms of soluble factors in the tumor microenvironment,
cytokines and the surrounding matrix may also have served a role in
the present case. Specifically, the persistent inflammation
experienced by the present patient may have induced alterations in
the jawbone matrix. Such alterations may encompass changes in bone
matrix hardness and the cytokine profile, such as interleukin (IL)
and tumor necrosis factor (TNF)-α. During the inflamed state, the
dysregulated activity of TGF-β signaling may reverse the inhibition
of cell proliferation (26,27).
This reversal in turn can lift the inhibitory restrictions tumor
cells, contributing to their aberrant growth. Furthermore, TGF-β
may enhance the migratory and invasive capabilities of tumor cells
(28,29), enabling them to infiltrate
neighboring tissues, blood vessels or lymphatic vessels.
Simultaneously, TGF-β may also dampen the immune system's ability
to identify and eliminate tumors (27,29).
It was also noteworthy that chronic stress and the sympathetic
nervous system may have involvements in the tumor in the present
cases, where the persistent inflammation may have activated the
sympathetic nervous system. This conjecture is consistent with the
previous findings on the role of adrenergic receptor signaling in
the bone. Pierroz et al (30) previously explored the effects of
β-adrenergic receptor deletion on bone phenotypes and response to
mechanical stimulation in mice. It was demonstrated that mice
deficient in β2-adrenergic receptors exhibited greater trabecular
bone microarchitecture, with lower degrees of bone resorption and
increased levels of bone formation as they aged. These alterations
could include increased trabecular thickness, higher trabecular
number, reduced trabecular spacing, and enhanced trabecular
connectivity. Furthermore, Liang et al (31) elucidated the impact of
β2-adrenergic receptor (β2-AR) signaling on osteocytes and its
effect on osteoclast formation. Activation of β2-AR led to
increased RANKL expression, promoting osteoclastogenesis, while
also altering the balance between RANKL and OPG. Furthermore,
neuropeptides crucial for bone regulation were inhibited by β2-AR
stimulation. These findings highlight the significant role of β2-AR
signaling in bone metabolism. Therefore, sustained inflammation can
potentially trigger the release of catecholamines to exacerbate
jawbone destruction (32). The
patient's history of smoking may have further exacerbated the
inflammatory environment, since smoking can impair the immune
functions of neutrophils and increase the release of inflammatory
factors in the body, such as TNF-α and IL-6 (33,34).
In the present case, the pathological
immunohistochemistry results indicated HPV negativity but the
presence of mottled p16 positivity. While HPV infection was not
detected, p16 is commonly employed as a surrogate marker for HPV in
pathology. However, elevated p16 expression does not definitively
indicate the presence of HPV infection. It is worth noting that it
may yield false-negative HPV results, possibly due to undetected
specific HPV subtypes, low viral loads associated with infection,
inadequate tissue sampling or the localized nature of HPV
infection. Given these considerations, quantitative HPV DNA testing
emerges as a potentially more crucial diagnostic tool. Despite the
absence of detectable HPV infection through IHC examination, it
remains pertinent to explore the potential involvement of HPV
infection in this context. Squamous cell papilloma may be
associated with HPV infection, particularly HPV subtypes 2, 4, 6,
11, 13 and 32. HPV subtypes 6 and 11 are commonly associated with
benign papillomatous lesions, including squamous cell papilloma
(35,36). However, in certain sites, such as
the throat, squamous cell papilloma associated with various
high-risk HPV subtypes, such as HPV16 and HPV18, may also pose an
increased risk of cancer development (9,37).
The expression of p16 is frequently utilized as a surrogate
indicator for high-risk HPV types. This association arises from the
correlation between HPV infection and an elevated probability of
p16-positive disease advancing to malignancy. Of note, in certain
instances of squamous cell papilloma, the presence of p16
positivity despite HPV negativity may imply alternative pathways or
factors contributing to tumor progression. During tumorigenesis,
HPV E6 and E7 proteins target the p53 and retinoblastoma proteins
for degradation, respectively. This inactivates the two most
important tumor suppressor genes in the cell, resulting in the
aberrant overexpression of cyclin p16 (33,38,39).
In addition, susceptibility of cells to HPV infection can depend on
the cell type and the local microenvironment. Certain cell types
and microenvironments are more conducive to HPV infection and
transformation. Single-layered tonsillar crypts are particularly
susceptible to cellular transformation in the head and neck region.
In the head and neck region, the epithelium lining the
single-layered tonsillar crypts is particularly susceptible to
cellular transformation, particularly following infection with HPV.
Long-term inflammation can lead to changes in epithelial
continuity, creating opportunities for pathogens such as HPV to
attach and infect cells, thereby promoting the formation of
papilloma (40,41).
The therapeutic approaches for oral squamous
papilloma typically encompass surgical excision, particularly in
cases of substantial size or suspected malignancy. Radiation
therapy and chemotherapy may occasionally be applied in cases of
malignant transformation (22).
Although early diagnosis and intervention hold paramount
importance, the papilloma detected in the jaw of the patient in the
present case exhibited striking imaging similarities to malignant
jaw tumors, as observed in mandibular CT scans. This resemblance
may be associated with the patient's prolonged pericoronal
inflammation resulting from the impacted wisdom teeth. The
repetitive inflammatory episodes resulted in multifocal bone
destruction, as indicated by areas of decreased density observed on
imaging. Concurrently, the local lamellar periosteal reactions were
indicative of the reparative response by the bone triggered by
inflammation. Benign neoplasms typically manifest as clearly
demarcated masses; contrastingly, malignant tumors exhibit a more
aggressive growth pattern, often coupled with local tissue
destruction (42-44).
The chronic inflammatory environment appears to have promoted the
development of poorly circumscribed soft tissue masses, further
complicating the differentiation between benign and malignant
tumors. This observation highlights the essential requirement for
the thorough evaluation of imaging results in patients with
persistent inflammation. In addition, comprehensive pathological
and clinical assessments are necessary to ascertain the true nature
of the tumor.
In conclusion, the present case report documented
the rare occurrence of squamous papilloma in the jawbone,
highlighting the diagnostic challenges posed by their atypical
clinical presentation. Furthermore, the scarcity of robust clinical
data on squamous cell papilloma involving the jawbone presents a
significant hindrance in establishing a standardized diagnostic and
therapeutic protocol for such cases. The present report several key
factors that can contribute to the formation of squamous papilloma
within the jaw. Specifically, the prolonged inflammation triggering
tumor transformation, inflammation-induced aberrant cell growth,
inter-cellular interplay, cytokine and matrix effects and chronic
stress-mediated sympathetic nervous system activation, are such
potential key factors that offer avenues for further exploration.
In addition, the association between HPV and head and neck
papilloma was outlined, suggesting that sustained inflammation may
heighten papilloma incidence by compromising epithelial tissue
integrity, fostering an environment conducive to HPV infection.
While HPV infection was not detected, p16 is commonly employed as a
surrogate marker for HPV in pathology. However, elevated p16
expression does not definitively indicate the presence of HPV
infection. In fact, it may yield false-negative HPV results,
possibly due to undetected specific HPV subtypes, low viral loads
associated with infection, inadequate tissue sampling, or the
localized nature of HPV infection. In light of the limitations and
knowledge gaps identified, future endeavors should focus on
elucidating the intricate molecular mechanisms underlying the
development of squamous papilloma. Prospective multicenter clinical
trials are indispensable for bridging the currently available
clinical data to deepen the understanding into the pathophysiology
of this papilloma and to facilitate the development of effective
diagnostic and therapeutic modalities.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
XZ and QH identified and selected this case. XZ took
the lead in drafting the original manuscript, while XZ and LL
collaborated extensively in the discussion section, contributing to
the analysis and interpretation of the collected data. All authors
actively participated in the critical review and finalization of
the manuscript. CC, DT and SH performed a critical literature
review and contributed to the drafting of the introduction and
discussion sections. SL and QH were involved in the patient's
clinical management. All authors have read and approved the final
version of the manuscript. QH and LL have reviewed and confirm the
authenticity of all the raw data presented in this manuscript.
Ethics approval and consent to
participate
The present case report was conducted in accordance
with the ethical standards from the 1964 Declaration of Helsinki
and its later amendments. Local ethical approval was obtained from
the Ethics Committee of the First Affiliated Hospital of Sun
Yat-sen University [Guangzhou, China; approval ID: (2023) 391].
Patient consent for publication
The patient provided written informed consent for
the publication of his data and associated images.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Jaju PP, Suvarna PV and Desai RS: Squamous
papilloma: Case report and review of literature. Int J Oral Sci.
2:222–225. 2010.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Frigerio M, Martinelli-Kläy CP and
Lombardi T: Clinical, histopathological and immunohistochemical
study of oral squamous papillomas. Acta Odontol Scand. 73:508–515.
2015.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Longworth MS and Laimins LA: Pathogenesis
of human papillomaviruses in differentiating epithelia. Microbiol
Mol Biol Rev. 68:362–372. 2004.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Carrasco MFL, Alvarado JMP, Rodríguez VPR,
Ramos VRV and Reinoso Carrasco JDC: Squamous papilloma in the oral
cavity: Case presentation and review of the literature. JDHODT.
9:2018.
|
|
5
|
Darwish G: Squamous papilloma of the soft
palate: A case report. Cureus. 11(e37423)2023.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Hui-Ying H, Zhen-Kun Y, Shuang-Ba H,
Qing-Xiang Z, Shan-Chun G and Hai-Dong Z: Distribution of HPV
infection in laryngopharyngeal squamous cell carcinoma and
laryngeal papilloma, 2017.
|
|
7
|
Yadav S: Management of oral squamous
papilloma using annona squamosa (Custard Apple) leaves: A novel
case. Cureus. 15(e34806)2023.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Sivaramakrishnan M, Aroumougam A, Kumar KS
and Premlal KR: Oral Squamous Papilloma. J Scientific Dentistry.
7:46–49. 2017.
|
|
9
|
Sabatini ME and Chiocca S: Human
papillomavirus as a driver of head and neck cancers. Br J Cancer.
122:306–314. 2020.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Oliveira AC, Cavalcanti de Lima IC, Frez
Marques VM, Alves de Araújo WH and de Campos Ferreira C: Human
papillomavirus prevalence in oral and oropharyngeal squamous cell
carcinoma in South America: A systematic review and meta-analysis.
Oncol Rev. 16(552)2022.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Aladham Y, Ahmed O and Laycock J: Squamous
cell papilloma of the oesophagus: A human papilloma virus lesion.
Cureus. 13(e19903)2021.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Jideh B, Weltman M, Wu Y and Chan CH:
Esophageal squamous papilloma lacks clear clinicopathological
associations. World J Clin Cases. 5(134)2017.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Molodtsova V, Ryabova M, Dvorakovskaya I,
Vasilyeva M and Akopov A: Recurrent respiratory papillomatosis with
lung involvement. Respir Med Case Rep. 25:323–326. 2018.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Hirai R, Makiyama K, Higuti Y, Ikeda A,
Miura M, Hasegawa H, Kinukawa N and Ikeda M: Pharyngeal squamous
cell papilloma in adult Japanese: Comparison with laryngeal
papilloma in clinical manifestations and HPV infection. Eur Arch
Otorhinolaryngol. 269:2271–2276. 2012.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Vidor EV, José Pavan AJ and Da Silva MJ:
Squamous papilloma: Treatment in dentistry. J Surg Clin Dentistry.
7:16–19. 2015.
|
|
16
|
Reichart PA and Philipsen HP: Revision of
the 1992 edition of the WHO histological typing of odontogenic
tumors. A suggestion. Mund Kiefer Gesichtschir. 7:88–93.
2003.PubMed/NCBI View Article : Google Scholar : (In German).
|
|
17
|
Nicolielo LFP, Van Dessel J, Jacobs R,
Quirino Silveira Soares M and Collaert B: Relationship between
trabecular bone architecture and early dental implant failure in
the posterior region of the mandible. Clinical Oral Implants Res.
31:153–161. 2020.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Trinchieri-2012-Cancer and inflammation an
old intuition with Rap.pdf.
|
|
19
|
Alnek K, Kisand K, Heilman K, Peet A,
Varik K and Uibo R: Increased blood levels of growth factors,
proinflammatory cytokines, and th17 cytokines in patients with
newly diagnosed type 1 diabetes. PLoS One.
10(e0142976)2015.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Krenytska D, Strubchevska K, Kozyk M, Vovk
T, Halenova T, Kot L, Raksha N, Savchuk O, Falalyeyeva T, Tsyryuk O
and Ostapchenko L: Circulating levels of inflammatory cytokines and
angiogenesis-related growth factors in patients with osteoarthritis
after COVID-19. Front Med (Lausanne). 10(1168487)2023.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Yoneda T, Sasaki A and Mundy GR:
Osteolytic bone metastasis in breast cancer. Breast Cancer Res
Treat. 32:73–84. 1994.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Buenrostro D, Mulcrone PL, Owens P and
Sterling JA: The bone microenvironment: A fertile soil for tumor
growth. Curr Osteoporos Rep. 14:151–158. 2016.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Baylink DJ, Finkelman RD and Mohan S:
Journal of bone and mineral research. J Bone Miner Res. 8
(Suppl):S565–S572. 1993.
|
|
24
|
Lv Z, Wu X, Cao W, Shen Z, Wang L, Xie F,
Zhang J, Ji T, Yan M and Chen W: Parathyroid hormone-related
protein serves as a prognostic indicator in oral squamous cell
carcinoma. J Exp Clin Cancer Res. 33(100)2014.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Mackenzie SL, Gillespie MT, Scurry JP,
Planner RS, Martin TJ and Danks JA: Parathyroid hormone-related
protein and human papillomavirus in gynecological tumors. Int J
Cancer. 56:324–330. 1994.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Batlle E and Massagué J: Transforming
Growth Factor-β signaling in immunity and cancer. Immunity.
50:924–940. 2019.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Smith SS, Chu D, Qu T, Aggleton JA and
Schneider RA: Species-specific sensitivity to TGFβ signaling and
changes to the Mmp13 promoter underlie avian jaw development and
evolution. Elife. 11(e66005)2022.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Stuelten CH and Zhang YE: Transforming
growth Factor-β: An agent of change in the tumor microenvironment.
Front Cell Dev Biol. 9(764727)2021.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Jiao K, Zhang M, Niu L, Yu S, Zhen G, Xian
L, Yu B, Yang K, Liu P, Cao X and Wang M: Overexpressed TGF-β in
subchondral bone leads to mandibular condyle degradation. J Dent
Res. 93:140–147. 2014.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Pierroz DD, Bonnet N, Bianchi EN, Bouxsein
ML, Baldock PA, Rizzoli R and Ferrari SL: Deletion of β-adrenergic
receptor 1, 2, or both leads to different bone phenotypes and
response to mechanical stimulation. J Bone Miner Res. 27:1252–1262.
2012.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Liang H, Zeng Y, Feng Y, Wu H, Gong P and
Yao Q: Selective β2-adrenoreceptor signaling regulates
osteoclastogenesis via modulating RANKL production and
neuropeptides expression in osteocytic MLO-Y4 cells. J Cell
Biochem. 120:7238–7247. 2019.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Godoy LD, Rossignoli MT, Delfino-Pereira
P, Garcia-Cairasco N and De Lima Umeoka EH: A comprehensive
overview on stress neurobiology: Basic concepts and clinical
implications. Front Behav Neurosci. 12(127)2018.PubMed/NCBI View Article : Google Scholar
|
|
33
|
von Zeidler SV, Miracca EC, Nagai MA and
Birman EG: Hypermethylation of the pl6 gene in normal oral mucosa
of smokers. Int J Mol Med. 14:807–811. 2004.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Powell SF, Vu L, Spanos WC and Pyeon D:
The key differences between human Papillomavirus-Positive and
-Negative head and neck cancers: Biological and clinical
implications. Cancers. 13(5206)2021.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Augustin JG, Lepine C, Morini A, Veyer D,
Brochard C, Mirghani H, Péré H and Badoual C: HPV detection in head
and neck squamous cell carcinomas: What is the issue? Front Oncol.
10(1751)2020.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Islam MdS, Chakraborty B and Panda CK:
Human papilloma virus (HPV) profiles in breast cancer: Future
management. Ann Transl Med. 8:650. 2020.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Castro TPPG and Filho IB: Prevalence of
human papillomavirus (HPV) in oral cavity and oropharynx. Braz J
Otorhinolaryngol. 72:272–281. 2006.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Bradley KT, Budnick SD and Logani S:
Immunohistochemical detection of p16INK4a in dysplastic lesions of
the oral cavity. Modern Pathology. 19:1310–1316. 2006.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Gologan O, Barnes EL and Hunt JL:
Potential diagnostic use of p16INK4A, a new marker that correlates
with dysplasia in oral squamoproliferative lesions. Am J Surg
Pathol. 29:792–796. 2005.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Lee SH: Intestinal permeability regulation
by tight junction: Implication on inflammatory bowel diseases.
Intest Res. 13(11)2015.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Koch S and Nusrat A: The life and death of
epithelia during inflammation: Lessons learned from the gut. Annu
Rev Pathol Mech Dis. 7:35–60. 2012.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Gersing AS, Pfeiffer D, Kopp FK, Schwaiger
BJ, Knebel C, Haller B, Noël PB, Settles M, Rummeny EJ and Woertler
K: Evaluation of MR-derived CT-like images and simulated
radiographs compared to conventional radiography in patients with
benign and malignant bone tumors. Eur Radiol. 29:13–21.
2019.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Onal T, Afacan GO, Akansel G, Arslan AS,
Anik Y, Inan N, Muezzinoglu B and Corapcioglu F: The performance of
radiographic criteria for bone malignancy when applied to computed
tomography and magnetic resonance imaging. J Med Imaging Radiat
Sci. 49:84–89. 2018.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Miller TT: Bone tumors and tumorlike
conditions: Analysis with conventional radiography. Radiology.
246:662–674. 2008.PubMed/NCBI View Article : Google Scholar
|