1. Introduction
Desmopressin is a vasopressin (AVP) receptor 2 (V2)
agonist, first synthesised in Prague in the late 1960s by Zaoral
et al (1). At the time, the
objective was to develop an analogue of the native vasopressin
hormone that could be used in the treatment of central diabetes
insipidus, avoiding the, mainly V1-mediated, side effects of
vasopressin treatment relating to its pressor effects, while
maintaining or improving upon its antidiuretic effects. Among
several analogues produced and evaluated, desmopressin
(1-deamino-8-D-arginine vasopressin) proved to be a triumph in
achieving the required efficacy and tolerability profile: ‘Rarely
in pharmacology is an agent produced that so specifically enhances
the desired effect and simultaneously decreases the side effect’
(2).
To this day, desmopressin is used widely for central
diabetes insipidus and in other conditions requiring antidiuretic
treatment, including nocturnal enuresis (bedwetting), idiopathic
nocturnal polyuria and nocturia (nocturnal voiding) (3).
In the 1970s, a new indication came to light when
intravenous administration of high doses of desmopressin was found
to raise levels of the clotting factor Factor VIII in healthy
volunteers and in patients with mild-to-moderate haemophilia A and
von Willebrand's disease (VWD). These are both inherited bleeding
disorders characterised by low levels of Factor VIII or von
Willebrand factor (a carrier for Factor VIII), leading to impaired
blood clotting (4,5). Desmopressin became, and remains, a
valuable addition to the treatment armamentarium for these
conditions, helping to prevent bleeding episodes via intravenous or
intranasal administration (6).
While these uses of desmopressin are well
established, there has also been a significant number of reports of
clinical benefit in other diverse clinical areas, including
distinct but related areas within urology and haematology, as well
as completely different disciplines such as oncology and
psychiatry/cognition.
In this review, we explore potential for further
clinical applications of current and possible future forms of oral
desmopressin. We focus on oral formulations, rather than
intravenous, subcutaneous or intranasal, because they have the
advantages of being easily administered in the home or outpatient
setting, are more child friendly, and data suggest that side
effects are reduced with oral formulations compared with intranasal
ones (7). Because the available
oral formulations [tablet and orally disintegrating tablet (ODT)]
are used at bioequivalent doses, we refer to them collectively as
oral desmopressin. The ODT dissolves under the tongue in a few
seconds, whereas the tablet is swallowed.
Desmopressin dosing, formulations and
target tissues
The widespread distribution of V2 receptors in the
human body (Fig. 1) is itself
suggestive that a V2 agonist is likely to have diverse
physiological and clinical effects. However, there are two
important questions that need to be considered when determining the
potential for oral desmopressin to be beneficial in relevant
clinical conditions. First, is desmopressin able to reach and/or
affect each of the target organs of interest when administered
orally? Second, can desmopressin be administered orally at a dose
which is both effective and has an acceptable safety profile.
Existing oral formulations of desmopressin have low
bioavailability. It is currently available as a tablet and as an
orally disintegrating (ODT)-see Table
I for an overview of dose comparisons across formulations. The
ODT has greater bioavailability than the tablet, enabling lower
dosing. The ODT formulation has a maximum daily dose of 240 µg for
enuresis, while in adults with idiopathic nocturnal polyuria
(nocturia) the maximum daily dose is lower and sex-specific at 25
µg for women and 50 µg for men (3). Doses differ in other indications. All
formulations, however, demonstrate increased risk of side effects
with increasing dose, particularly in older age, when hyponatraemia
becomes more likely (8). For
current indications requiring higher doses, administration is
limited to single or few dosages that are used for specific one-off
events, such as surgery or trauma in patients with mild to moderate
haemophilia A and VWD.
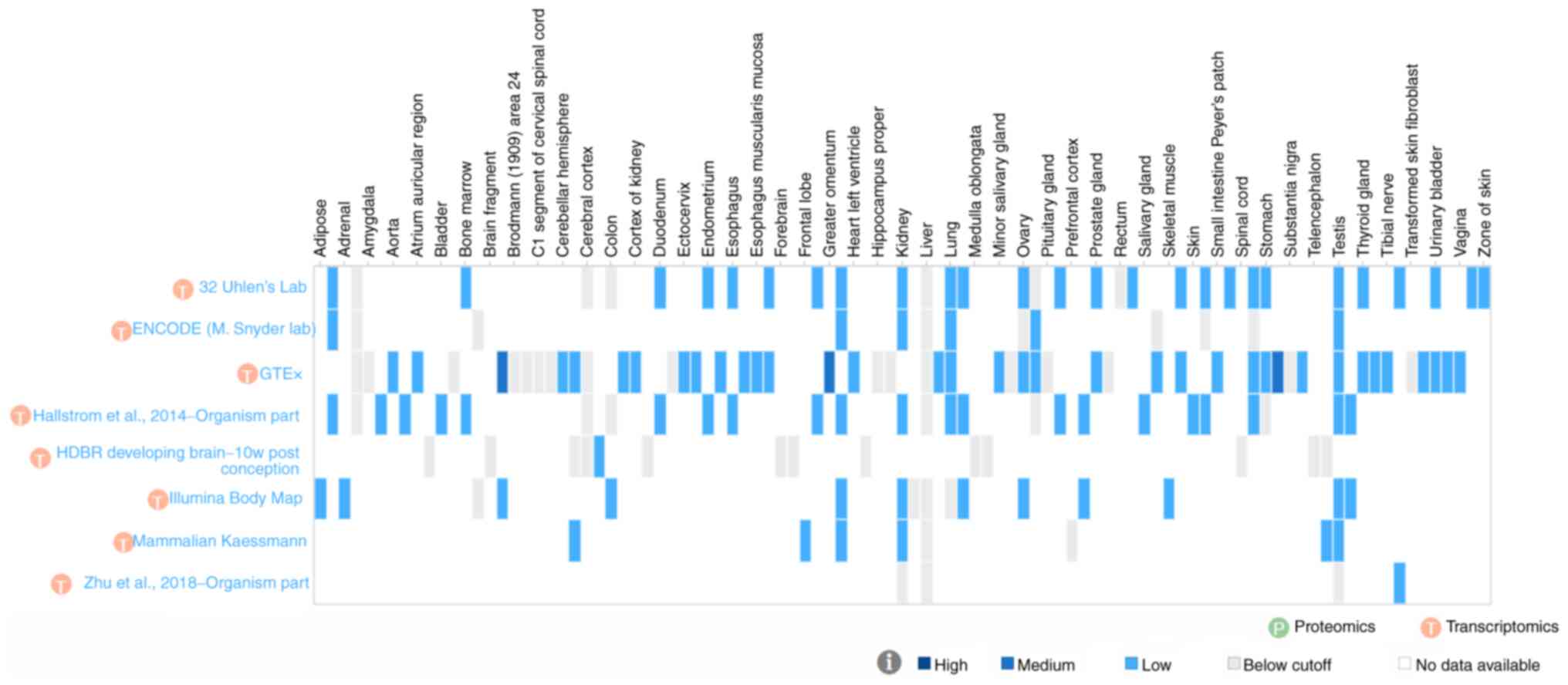 | Figure 1Sites tested for arginine vasopressin
receptor 2 expression in human tissue. The figure shows alternate
site labels only. The full listing of sites is as follows
(asterisks denote detection of vasopressin receptor 2 transcript in
at least one study) (113):
Adipose*, adipose tissue*,
adrenal*, adrenal gland, amygdala, animal
ovary*, aorta*, appendix*, atrium
auricular region*, basal ganglion (developing),
bladder*, blood, bone marrow*, brain, brain
fragment (developing), breast*, Brodmann (1909) area 24,
Brodmann (1909) area 9, C1 segment of cervical spinal cord, caudate
nucleus, cerebellar hemisphere*, cerebellum*,
cerebral cortex, choroid plexus (developing)*,
colon*, coronary artery*, cortex of
kidney*, diencephalon, duodenum*,
Epstein-Barr virus-transformed lymphocyte, ectocervix*,
endocervix*, endometrium*, esophagogastric
junction*, esophagus*, esophagus
mucosa*, esophagus muscularis mucosa*,
fallopian tube*, forebrain (developing), forebrain and
midbrain (developing), frontal lobe*, gall
bladder*, greater omentum*,
heart*, heart left ventricle*, hindbrain
(developing), hippocampus proper, hypothalamus, kidney*,
leukocyte, liver, lower leg skin*, lung*,
lymph node*, medulla oblongata (developing), midbrain
(developing), minor salivary gland*, nucleus accumbens,
ovary*, pancreas*, pituitary gland,
placenta*, prefrontal cortex, prostate*,
prostate gland*, putamen, rectum, saliva-secreting
gland*, salivary gland*, sigmoid
colon*, skeletal muscle*, skeletal muscle
tissue*, skin*, small intestine*,
small intestine Peyer's patch*, smooth muscle
tissue*, spinal cord (developing), spleen*,
stomach*, subcutaneous adipose tissue*,
substantia nigra, suprapubic skin*, telencephalon
(developing), temporal lobe (developing), testis*,
thyroid*, thyroid gland*, tibial
artery*, tibial nerve*, tonsil*,
transformed skin fibroblast, transverse colon*, urinary
bladder*, uterus*, vagina*,
vermiform appendix*, and zone of skin*.
ENCODE, The Encyclopedia of DNA Elements; GTEx, Genotype-Tissue
Expression; HDBR, Human Developmental Biology Resource; w,
weeks. |
 | Table IDose comparison of different
formulations of desmopressin. |
Table I
Dose comparison of different
formulations of desmopressin.
| Parameter | IV injection
(solution) | Intranasal
spray | Tablet | ODT |
|---|
| Bioavailability, %
(SD or 95% CI) | N/A | 6.00(±2.29) | 0.16(±0.17) | 0.25 (95% CI,
0.21-0.31) |
| Equivalent
dosesa, µg | N/A | 2.5 | 100 | 60 |
| | <0.5 | 5 | 200 | 120 |
| | <1 | 10 | 400 | 240 |
Pharmacokinetic data indicate that the desmopressin
tablet and ODT tablet formulations have a linear dose relationship
as follows: 60, 120, 240 and 360 µg ODT correspond to 0.1, 0.2, 0.4
and 0.6 mg tablet, respectively. Regarding bioequivalence of the
two oral formulations, all regulatory approvals were based upon
bioequivalence studies which showed that lower dosing of the ODT is
required due to its higher bioavailability (0.25 vs. 0.16%, as
shown in Table I). In addition, it
has been proposed that there are some clinically relevant
differences between the two oral formulations in terms of their
PK/PD profile, such as more predictable dosing (9) and lower food interaction with the ODT
(10,11).
A conversion factor between intravenous and oral
desmopressin has not been defined; however, the bioavailability of
oral desmopressin is approximately 100 times lower than that of IV
desmopressin (12). Thus, the IV
equipotent dose to 200 µg oral desmopressin would be around 2 µg
(13). Each formulation delivers
the adequate dose for the approved indications.
We have selected a subset of target organs/tissues
for discussion (Table II) based
on V2 agonism effects that are well-recognised and understood (in
blood and kidney), or on studies in the literature suggesting that
desmopressin may be of interest in these areas (ureter, CNS,
oncology).
 | Table IISelected V2 receptor locations and
functions of V2 agonist at each location. |
Table II
Selected V2 receptor locations and
functions of V2 agonist at each location.
| Therapy area | Organ | V2 agonist
function |
|---|
| Bleeding
disordersa,
bleeding controlb | Blooda | Factor
VIIIa, von
Willebrand factora |
| Renal
colicb | Ureterb, Kidneya |
Antidiuresisa, other? (such as reduction of
intra-ureteral mean pressureb or inhibition of smooth muscle
fibre contractionb) |
|
Depressionb, memoryb | CNSb |
Cognitionb, memoryb, social behaviourb, diurnal rhythmsb |
|
Oncologyb |
Variousb | Different
mechanisms proposedb |
2. Methods
Systematic literature searches of PubMed-indexed
literature were conducted using the Silvi.ai software
(https://www.silvi.ai/) in each of the potential
new areas of interest identified: bleeding control, renal colic,
CNS and oncology. Details of the searches are provided in Table III.
 | Table IIILiterature search parameters for
PubMed-indexed publications. |
Table III
Literature search parameters for
PubMed-indexed publications.
| Area | Date range | Search string | Inclusion
criteria | Exclusion
criteria |
|---|
| Bleeding
control |
1/1/2012-1/9/2022 | (‘Deamino Arginine
Vasopressin’[Mesh] OR desmopressin[tw]) AND (‘Blood
Transfusion’[Mesh] OR ‘blood transfusion’[tw] OR ‘Epistaxis’[Mesh]
OR ‘nose bleed*’[tw] OR ‘Menorrhagia’[Mesh] OR ‘excessive menstrual
bleeding’[tw] OR (‘tranexamic acid’[Mesh] AND
(‘2012’[Date-Publication]: ‘3000’[Date - Publication])) OR ‘General
Surgery’[Mesh] OR (‘surger*’[tw] AND (‘2012’ [Date - Publication]:
‘3000’[Date-Publication])) OR (‘preop*’[tw] AND
(‘2012’[Date-Publication]: ‘3000’[Date-Publication]))) NOT
‘bleeding disorder’[tw] NOT ‘Blood Coagulation
Disorders’[Mesh] | Human patients;
vasopressin or desmopressin; English; any type of primary
study | Outside scope; case
report; MA/SLR; not English language; not about bleeding; not about
desmopressin; not human subjects; secondary data |
| Renal colic | No start date-
1/9/2022 | (‘Deamino Arginine
Vasopressin’[MeSH Terms] OR ‘desmopressin’[Text Word]) AND
(‘Central Nervous System’[MeSH Terms] OR ‘Learning’[MeSH Terms] OR
‘Depression’[MeSH Terms] OR ‘Electroconvulsive Therapy’[MeSH Terms]
OR ‘sexual dysfunctions, psychological’[MeSH Terms] OR ‘Social
Behavior’[MeSH Terms] OR ‘Attention Deficit Disorder with
Hyperactivity’[MeSH Terms]) | Human patients;
vasopressin or desmopressin; English; any type of primary
study | Animal study; not
vasopressin; outside scope; no article available; not English
language; not a study; SLR or MA |
| Central nervous
system | No start date-
1/9/2022 | (‘Deamino Arginine
Vasopressin’[MeSH Terms] OR ‘desmopressin’[Text Word]) AND
(‘Central Nervous System’[MeSH Terms] OR ‘Learning’[MeSH Terms] OR
‘Depression’[MeSH Terms] OR ‘Electroconvulsive Therapy’[MeSH Terms]
OR ‘sexual dysfunctions, psychological’[MeSH Terms] OR ‘Social
Behavior’[MeSH Terms] OR ‘Attention Deficit Disorder with
Hyperactivity’[MeSH Terms]) | Human patients;
vasopressin or desmopressin; English; cognitive outcome variable;
any type of primary study | Animal study; no
abstract; not about desmopressin; outside scope; literature
review/MA; not English language |
| Oncology | No start date-
1/9/2022 | (‘Deamino Arginine
Vasopressin’[Mesh] OR desmopressin[tw]) AND (‘Medical
Oncology’[Mesh] OR ‘oncology’[tw] OR ‘Neoplasms’[Mesh] OR
‘cancer’[tw] OR ‘Breast Neoplasms’[Mesh] OR ‘breast cancer’[tw] OR
‘Prostatic Neoplasms’[Mesh] OR ‘prostate cancer’[tw] OR ‘Lung
Neoplasms’[Mesh] OR ‘lung cancer’[tw] OR ‘Colorectal
Neoplasms’[Mesh] OR ‘colorectal cancer’[tw] OR ‘Urinary Bladder
Neoplasms’[Mesh]) | Human patients;
vasopressin or desmopressin; English; any type of primary
study | Not human subjects;
not vasopressin; outside scope; desmopressin not treating cancer
disease; human cell culture; review or MA |
Relevant studies in humans, published in English,
were included and a table of publications compiled for each area. A
small number of additional relevant studies were found during
PubMed searches/citation chasing. Summary tables of relevant
studies, their characteristics and findings were developed to
summarise the literature in therapeutic areas where oral
desmopressin use may be feasible, and studies were categorised
according to whether findings were supportive of the use of
desmopressin or not. Although the literature searches were
systematic, the literature cited in this report is not exhaustive
due to the wide remit of interest and the fact that some
publications could not be accessed without payment-articles were
paid for only in the renal colic and CNS searches as these
retrieved fewer results. This review therefore provides a broad
overview of the evidence across multiple clinical areas. For
bleeding disorders, publications specifically of relevance to
low-dose desmopressin were sought, since there is a huge body of
literature on the approved use of high dose
(intravenous/subcutaneous) desmopressin in bleeding disorders and
summarising this literature was deemed out of scope because our
interest is specifically in oral desmopressin and possible new uses
of these formulations. Similarly, for oncology, the doses used in
human studies have been above those achievable with oral
formulations and only a brief overview of the literature is
given.
3. Results of literature review and
discussion of findings
Desmopressin and bleeding
disorders
Desmopressin (IV, subcutaneous or intranasal) is
indicated for use in bleeding disorders (VWD and mild-to-moderate
haemophilia type A), with a long history of clinical usage and many
publications confirming its efficacy (6,14).
Although IV desmopressin is recommended before surgery or for
treating severe haemorrhages because very consistent responses are
required in these situations, subcutaneous desmopressin can be
self-administered and can therefore be used at home to prevent or
treat minor bleeding episodes and in women with VWD who have
excessive bleeding at menstruation (15). In bleeding disorders, desmopressin
is used at high doses (0.3-0.4 µg/kg body weight IV) to increase
Factor VIII:C and Factor VIII:Ag in patients with mild to moderate
haemophilia A or VWD who are undergoing surgery or following trauma
(16). In order to achieve a
bioequivalent dose in an average adult, approximately 5,000-7,000
µg desmopressin ODT would be needed.
Intranasal administration is also indicated for
patients with these bleeding disorders (17) when they are undergoing surgery,
following trauma or for other bleeding episodes such as menorrhagia
and epistaxis (nosebleeds). The intranasal spray may be used (300
µg) half an hour before surgery or at bleeding. Using
bioequivalence data (Table I),
approximately 7,200 µg oral desmopressin ODT would be required to
achieve the same dosing as recommended for adults using the
intranasal spray (i.e. 10 µg intranasal=240 µg ODT, so 300 µg
intranasal=7,200 µg ODT). Currently, the maximum strength of oral
ODT is 240 ug, meaning that the use of oral desmopressin in these
indications may not be practical. However, there is currently a
recall and temporary halt in production of the nasal spray, so
there is perhaps a place for oral administration to substitute for
nasal administration, as an alternative user-friendly formulation.
Some studies suggest that lower absolute doses of desmopressin in
children could still achieve efficacy. A study by Akin (18) demonstrated that a lower dose of
desmopressin (0.15 µg subcutaneous) was effective in increasing
Factor VIII, VWF:RCo and VWF:Ag levels in children with type 1 VWD,
and wider use of desmopressin, especially in developing countries,
was recommended. In a retrospective study, half dose desmopressin
(0.15 µg/kg IV) was also found to be effective in adult bleeding
disorder patients undergoing low to moderate risk invasive
procedures (19). There are
ongoing studies looking at the feasibility of
pharmacokinetic-guided dosing of desmopressin since there is
considerable pharmacokinetic variability (20,21).
There is also variation in clinical response to desmopressin, which
was recently demonstrated to be affected by VWF genetic
variants (22). These factors
suggest that there may be a subset of patients who could benefit
from low doses of desmopressin achievable via oral administration,
but further studies would be needed in this area.
A systematic review in 2012 noted that desmopressin
has been used successfully for prevention of bleeding during
pregnancy and postpartum haemorrhage in people with bleeding
disorders (23). Most of the
studies used 0.3 µg/kg IV infusion (166 cases), while 12 and 20 µg
IV desmopressin were used in one case each. Intranasal desmopressin
was used in two studies (33 cases) at a dose of 300 µg. Intranasal
desmopressin (300 µg for two days) also effectively reduced
menstrual blood loss and improved quality of life in patients with
abnormal laboratory haemostasis, although the effect of tranexamic
acid was greater (24). There may
be a role for desmopressin in long-term prophylaxis in patients
with vWD, although Factor VIII/von Willebrand factor concentrate is
generally the preferred option here (25). It should also be noted that
patients with bleeding disorders may develop tolerance to long-term
use of desmopressin, and that there are important safety
considerations for the long-term use of high-dose desmopressin.
Additional studies-noted during general literature
searches-mentioned the use of high dose (0.3-0.4 IV µg/kg in most
studies) desmopressin in other bleeding disorders including
platelet dysfunction during antiplatelet therapy (26), Hermansky-Pudlak syndrome (27,28),
and unclassified bleeding disorders (29,30),
but in most cases there was a lack of randomised, controlled
trials, and no indication that lower, oral dosing would be
effective.
Summary: oral desmopressin in bleeding
disorders
An oral formulation of desmopressin would be a more
child-friendly formulation for prophylactic use (e.g. before dental
surgery) or treatment of bleeding episodes if adequate dosing could
be achieved in this patient population. In general, however,
bleeding disorders require high doses of desmopressin (0.3 µg/kg IV
or 300 µg intranasal) in order for the haematological effects of
the drug to be observed. Some studies suggest that half dose (0.15
µg/kg) intravenous desmopressin is still effective, but for oral
desmopressin to achieve equivalence, doses would still need to be
high.
Desmopressin and bleeding control in
patients without a bleeding disorder
In the 1980s, Lawrence Czer's group at the
Cedars-Sinai Medical Center in Los Angeles published research
indicating that desmopressin reduced bleeding time and improved
reoperation rates in patients with mediastinal haemorrhage after
cardiopulmonary bypass (31). It
was hypothesised that this was due to release of Factor VIII,
increase in von Willebrand's factor (an established effect of
desmopressin) and improvement in platelet adhesion. Since then,
several studies have evaluated desmopressin's potential use in
various areas of bleeding control, in patients who do not have a
specific bleeding disorder.
A systematic literature review was carried out for
studies investigating the use of desmopressin for bleeding control
(excluding patients with bleeding disorders-covered in the previous
section). Twenty-one studies of interest for which full-text
articles were freely available were identified (see supplementary
Table SI)-due to an error in the
PubMed filtering system, 10 pre-2012 articles were retrieved
despite the search string stipulating articles should be from 2012
onwards and these were removed from the final selection. Another
five studies were identified through other PubMed searches and
citation chasing and were also included in summary Table IV. Studies involved the use of
desmopressin for cardiac surgery, endoscopic sinus
surgery/rhinoplasty, renal surgery, gastrointestinal surgery and
intracerebral haemorrhage.
 | Table IVStudies of desmopressin in bleeding
control. |
Table IV
Studies of desmopressin in bleeding
control.
| A, Cardiac
surgery |
|---|
| First author/s,
year | Supports dDAVP
use? | Title | No. | Results | Dose | Formulation | (Refs.) |
|---|
| Altun et al,
2017 | No benefit | Emergency coronary
bypass surgery in patients under the influence of dual antiplatelet
therapy: effects of tranexamic acid and desmopressin acetate. | 54 | dDAVP and control
groups had greater duration of closure times, postoperative amounts
of drainage, volumes of erythrocyte suspension/plasma, cost of
blood products, length of intubation, length of stay in intensive
care and time to discharge. dDAVP had no significant effect on
bleeding control and delayed haemostatic efficacy of tranexamic
acid. | 0.3 µg/kg | IV | (115) |
| Bignami et
al, 2016 | No benefit | Desmopressin after
cardiac surgery in bleeding patients. A multicenter randomized
trial. | 135 | No significant
difference vs. placebo in terms of number of patients requiring red
blood cell transfusion (37/68 vs. 33/67) and no difference in blood
loss, mechanical ventilation, intensive care unit stay or
mortality. | 0.3 µg/kg | IV | (116) |
| Jahangirifard et
al, 2017 | Benefit | Effect of
Desmopressin on the Amount of Bleeding and Transfusion Requirements
in Patients Undergoing Heart Transplant Surgery. | 48 | Mean chest tube
drainage at 24 h lower in desmopressin group than the control
group. Packed red blood cells less frequently transfused in
desmopressin group vs. control group. | 0.3 µg/kg | IV | (117) |
| Jin and Ji,
2015 | Unclear or
inconsistent benefit | Effect of
desmopressin on platelet aggregation and blood loss in patients
undergoing valvular heart surgery. | 102 | Blood loss at 6 h
after surgery was reduced in dDAVP group vs. control group. No
significant differences in blood loss at 24 h after surgery.
Postoperative incidence of fresh frozen plasma transfusion
decreased in dDAVP group. There were no differences in red blood
cell and platelet transfusion rate between the two groups. | 0.3 µg/kg | IV | (118) |
| Mirmansoori et
al, 2016 | No benefit | The Effect of
Desmopressin on the Amount of Bleeding in Patients Undergoing
Coronary Artery Bypass Graft Surgery with a Cardiopulmonary Bypass
Pump After Taking Anti-Platelet Medicine. | 100 | IN desmopressin
could not reduce the amount of blood loss after coronary artery
bypass graft. Desmopressin did not have a significant effect on
coagulation status. | 40 µg | IN | (119) |
| B, Renal
surgery |
| First author/s,
year | Supports dDAVP
use? | Title | No. | Results | Dose | Formulation | (Refs.) |
| Athavale et
al, 2019 | Unclear or
inconsistent benefit | Desmopressin and
bleeding risk after percutaneous kidney biopsy. | 269 | Administration of
desmopressin to patients with serum creatinine ≥1.8
mg/dla decreased
bleeding risk (P=0.09) but increased bleeding risk when serum
creatinine was <1.8 mg/dl (P<0.001). dDAVP should be reserved
for patients undergoing percutaneous kidney biopsy who are at high
risk for bleeding. | 0.3 µg/kg; 30 min
prior to biopsy | IV | (34) |
| Cheong et
al, 2021 | No benefit | No effect of
desmopressin administration before kidney biopsy on the risk of
major post-biopsy bleeding. | 3,018 | Blood transfusions
more frequent in the desmopressin group; no differences in the
incidence of renal artery embolization, blood transfusion and total
major bleeding events. | 0.3 µg/kg | IV | (120) |
| Leclerc et
al, 2020 | Unclear or
inconsistent benefit | Use of Desmopressin
Prior to Kidney Biopsy in Patients With High Bleeding Risk. | 413 | Despite higher
bleeding risk before biopsy, patients using desmopressin had a
similar likelihood of symptomatic haematomas and a lower need for
urgent radiologic studies compared with those not receiving
desmopressin (retrospective cohort study). | 0.3 µg/kg | IV | (121) |
| Radhakrishnan et
al, 2014 | Unclear or
inconsistent benefit | Pre-procedure
desmopressin acetate to reduce bleeding in renal failure: does it
really work? | 43 | No significant
difference was found in bleeding rates between the two groups
overall. There was a trend toward benefit in patients with GFR
<15 ml/min/1.73 m2a | 0.3 µg/kg 30-60 min
prior to the procedure | IV | (33) |
| C, Sinus
surgery/rhinoplasty |
| First author/s,
year | Supports dDAVP
use? | Title | No. | Results | Dose | Formulation | (Refs.) |
| Akbarpour et
al, 2022 | Benefit | Effect of
desmopressin on bleeding during endoscopic sinus surgery: A
randomized clinical trial. | 120 | Intranasal
desmopressin at a dose of 40 µg 1 h before surgery could reduce
bleeding and improve the quality of the surgical field. | 20 or 40 µg 60 min
prior to anaesthesia | IN | (35) |
| Haddady-Abianeh
et al, 2019 | Benefit | The Hemostatic
Effect of Desmopressin on Bleeding as a Nasal Spray in Open
Septorhinoplasty. | 30 | The Boezaart score,
satisfaction scores, bleeding volume and upper eyelid ecchymosis in
the group receiving desmopressin were improved compared with those
in the control group. | Two puffs of spray
in each nostril; ‘Minirin with bioavailabi- lity of 8 µg’ | IN | (36) |
| Jahanshahi et
al, 2019 | Benefit | Effect of local
desmopressin administration on intraoperative blood loss and
quality of the surgical field during functional endoscopic sinus
surgery in patients with chronic rhinosinusitis: a triple-blinded
clinical trial. | 90 | Blood loss was
lower in the desmopressin group. Surgeons were more satisfied with
the surgical field in the desmopressin group than the control group
at all registered time points during the surgery procedure
(measured at 15, 30, 60 and 90 min). | 10 µg in each side
of nasal cavity 30 min before the surgery | IN | (37) |
| Safaeian et
al, 2021 | Benefit | Desmopressin nasal
spray reduces blood loss and improves the quality of the surgical
field during functional endoscopic sinus surgery. | 60 | Blood loss in the
dDAVP group was lower than that in the placebo group. In more than
half of patients on dDAVP, no suctioning was required 1 h after the
beginning of surgery, whereas 70% of patients in the placebo group
had bleeding scores >1 and thus required suctioning. | A nasal spray of 20
µg | IN | (38) |
| Shao et al,
2015 | Benefit | Effect of
desmopressin administration on intraoperative blood loss and
quality of the surgical field during functional endoscopic sinus
surgery: a randomized, clinical trial. | 90 | Effect of
desmopressin on blood loss and quality of the surgical field.
Patients in the desmopressin group showed lower requirements for IV
reminfentanil and esmolol than controls. Duration of surgery was
shorter in the desmopressin group (not significant). | dDAVP 0.3
µg/kg | IV | (39) |
| D, Gastrointestinal
surgery |
| First author/s,
year | Supports dDAVP
use? | Title | No. | Results | Dose | Formulation | (Refs.) |
| Wang et al,
2020 | Benefit | Desmopressin
acetate decreases blood loss in patients with massive hemorrhage
undergoing gastrointestinal surgery. | 48 | At 24 h after the
surgery, the decrease in haemoglobin in the DDAVP group was lower
than that in the normal saline group. Platelet function in the
dDAVP group was higher than that in the normal saline group at 24
h. | dDAVP 0.3 µg/kg for
30 min once a day after the surgery | IV | (122) |
| E, Intracerebral
haemorrhage under antiplatelet treatment |
| First author/s,
year | Supports dDAVP
use? | Title | No. | Results | Dose | Formulation | (Refs.) |
| Mengel et
al, 2020 | No benefit | Early
Administration of Desmopressin and Platelet Transfusion for
Reducing Hematoma Expansion in Patients With Acute Anti- platelet
Therapy Associated Intracerebral Hemorrhage. | 140 | No between-group
differences in total intracerebral haematoma expansion and
intraventricular haematoma expansion were noted. | 0.4 µg/kg +
platelet transfusion (2 U) within 60 min of intracerebral
haemorrhage | IV | (41) |
Cardiac surgery and renal surgery
All except one study of cardiac or renal surgery
used the intravenous formulation of desmopressin. Overall, studies
in cardiac surgery reported inconsistent findings but generally did
not show significant benefit with desmopressin. However, a
literature review reported that there are certain subgroups that
may benefit from desmopressin use, including patients with
demonstrable pre- or perioperative platelet dysfunction as
determined by TEG analysis or platelet function assays, those who
have received preoperative aspirin within 7 days of surgery, and
patients with cardiopulmonary bypass times in excess of 140 min
(32). However, further studies
are required.
Similar findings were reported in renal surgery
(kidney biopsy), with certain subpopulations of patients (serum
creatinine ≥1.8 mg/dl and GFR <15 ml/min/1.73 m2)
more likely to experience benefit with desmopressin (33,34).
Endoscopic sinus surgery/rhinoplasty. There
were five studies of desmopressin in endoscopic sinus surgery or
rhinoplasty (35-39)-4
used intranasal desmopressin prior to surgery (20, or 20 and 40
µg), and all found significant benefit with desmopressin, in terms
of blood loss and quality of the surgical field. In a randomised
trial of low-dose (20 µg) intranasal desmopressin, high-dose (40
µg) intranasal desmopressin and placebo, only the high dose was
found to significantly reduce volume of blood loss compared with
placebo, as well as doubling the odds of having a good surgical
field (35).
Overall, these results suggest that this may be a
promising area for future use of the drug at dose levels that could
potentially be achieved with oral formulations, although the impact
on efficacy of moving from the intranasal formulation to an oral
formulation would need to be investigated.
Intracerebral haemorrhage. Neurocritical Care
guidelines recommend consideration of desmopressin in
antiplatelet-associated intracranial haemorrhage (40). However, the one study in this
therapeutic area identified in our search reported no significant
benefit with desmopressin (0.4 µg/kg IV) in patients on
anti-platelet therapy (41). A
recent metanalysis also concluded that the available literature
does not support the routine use of desmopressin in the setting of
antiplatelet-associated intracerebral haemorrhage (42).
In contrast, a review by Andersen et al
(26) found that desmopressin
improved bleeding time and increased platelet aggregation in
patients with intracerebral or subarachnoid haemorrhage while
receiving antiplatelet therapy, as well as in non-cardiac surgery
patients and in healthy adults and animals exposed to antiplatelet
therapy. There were also some observational data to suggest that
desmopressin could reduce haematoma expansion in patients with
intracerebral haemorrhage or traumatic brain injury. Nevertheless,
the authors considered that randomised controlled trials in these
areas are still needed.
In terms of oral desmopressin formulations, as the
focus of this review, it is likely that oral administration would
not only be unsuited to this kind of acute care setting but also
would be unable to achieve equivalent dosing to the intravenous
formulation.
Summary: oral desmopressin in bleeding
control. Most studies of desmopressin in bleeding control
identified in this review used the intravenous formulation at high
doses that are unlikely to be achieved with oral formulations. In
cardiac and renal surgery, results are inconsistent in any
case.
However, the use of oral desmopressin as a
preventative measure before endoscopic sinus surgery or rhinoplasty
may be feasible given that there have been a number of studies
reporting significant benefits for bleeding and for the surgical
field with intranasal desmopressin. Results cannot necessarily be
extrapolated from localised intranasal delivery to oral
administration, however, and studies in this area would be
needed.
Desmopressin in renal colic
Renal colic is a common urological condition, with a
lifetime risk of around 12% in men and 6% in women (43), although estimates vary across
studies and geographic regions. It refers to severe pain resulting
from the presence of a stone in the urinary system causing acute
obstruction, ureteric dilatation, tensile stretch and spasmodic
activity (44-46).
The ureter releases prostaglandins in response to the obstruction,
rendering nociceptors sensitive to stimuli such as bradykinins that
induce pain and other visceral responses such as nausea (47). Prostaglandins also cause increased
renal blood flow and down-regulation of the antidiuretic hormone,
arginine vasopressin, as well as the contraction of ureteral smooth
muscle (47).
In uncomplicated cases of stones up to 10 mm, a
conservative approach may be taken, with observation for
spontaneous passage for around 4-6 weeks (45). Medical expulsive therapy (MET) is
commonly used to increase stone passage rate, decrease time to
passage and decrease pain. MET aims to increase ureteral diameter
via relaxation of the smooth muscle, and agents including
alpha-blockers, calcium channel blockers and prednisolone have been
investigated-however, the evidence base for these is limited and
some have significant side effects (48-51).
MET using alpha-blockers is recommended by the European Association
for Urology (EAU) as a potential treatment option for distal
ureteral stones >5 mm (51).
Analgesia in renal colic seeks to relax the ureteric
smooth muscle and decrease flow within the urinary tract
(decreasing the diuretic effect). Non-steroidal anti-inflammatory
drugs (NSAIDs) inhibit prostaglandin synthesis, are first-line
therapy recommended by the EAU and are considered superior to
opioids (51). However, they are
also associated with unwanted side effects such as gastrointestinal
bleeding and renal failure (52).
Since the 1990s, a number of reports have been
published documenting an analgesic effect of desmopressin, either
used alone or in combination with other therapies, in renal colic
patients. A nationwide registry study in Denmark confirmed that
desmopressin is prescribed in addition to opioids or NSAIDs, and in
some cases as monotherapy, to treat renal colic (47).
The mechanism of pain relief with desmopressin has
not been comprehensively investigated but is thought to be a result
of one or more of the following possible processes (47): reduction in intra-ureteral mean
pressure inside the excretory tract, while kidney blood perfusion
is maintained; combating the downregulation of AVP that is brought
about by prostaglandin release, encouraging antidiuresis via V2
receptors in the kidney; inhibition of smooth muscle fibre
contraction (53); release of
ß-endorphin from the hypothalamus in response to desmopressin
administration leading to central analgesic effects (54), although this has not been proven,
and-as discussed in the section of this review on CNS effects-it is
unclear whether desmopressin can cross the blood-brain barrier.
Studies of desmopressin in renal
colic
Our systematic literature search for studies
investigating the use of desmopressin in renal colic identified 13
relevant studies, including 12 interventional studies. Findings are
summarised in Table V, with
further study details included in supplementary Table SII.
 | Table VComparators and summary of study
findings in renal colic. |
Table V
Comparators and summary of study
findings in renal colic.
| First author/s,
year | Supports dDAVP
use? | Comparator | No. | Dose | Summary of
findings | (Refs.) |
|---|
| Arhami Dolatabadi
et al, 2017 | No benefit | IV ketorolac | 40 | 40 µg IN | IN desmopressin was
less effective than IV ketorolac. | (61) |
| Kumar et al,
2011 | No benefit | IM diclofenac | 72 | 40 µg IN | None of the
patients on IN desmopressin achieved satisfactory pain relief after
30 min and desmopressin did not enhance the effect of
diclofenac. | (64) |
| Lopes et al,
2001 | Unclear or
inconsistent benefit | | 61 | 40 µg IN | IN desmopressin, IM
diclofenac and combination therapy equally effective at 10 and 20
min; however, at 30 min, there was a slight increase in pain level
with desmopressin monotherapy. | (65) |
| Masoumi et
al, 2014 | Benefit | | 120 | 40 µg IN | Combined IN
desmopressin and IM diclofenac was more effective than IM
diclofenac alone. Difference started 15 min after drug
administration and was maintained at 60 min. | (58) |
| Roshani et
al, 2010 | Benefit | Diclofenac
suppository | 150 | 40 µg IN | Combined IN
desmopressin plus diclofenac sodium suppository resulted in prompt
pain relief with decreases in pain scores after 15 and 30 min
compared with diclofenac monotherapy. | (57) |
| Jalili et
al, 2019 | No benefit | Indomethacin
suppository | 124 | 40 µg IN | No differences in
pain reduction between the indomethacin suppository group and the
combined IN desmopressin and indomethacin group | (52) |
| Hazhir et
al, 2010 | Unclear or
inconsistent benefit | IM tramadol | 90 | 40 µg IN | IM tramadol or
combined IN desmopressin and tramadol showed no significant
differences; however, the number of subjects needing supplementary
pethidine was lower with desmopressin monotherapy. | (55) |
| Shirazi et
al, 2015 | Unclear or
inconsistent benefit | IM tramadol and
indomethacin suppository | 120 | 40 µg IN | Pain decreased in
all monotherapy groups (including 40 µg IN desmopressin) but was
lowest in those receiving tramadol. | (60) |
| Kheirollahi et
al, 2010 | Benefit | IM hyoscine
N-butylbromide | 116 | 20 µg IN | IM hyoscine
N-butylbromide was more effective in combination with IN
desmopressin. | (56) |
| Ghafouri et
al, 2020 | Unclear or
inconsistent benefit | IV paracetamol | 240 | 40 µg IN | IN desmopressin had
similar efficacy but a faster effect. | (62) |
| Keshvari Shirvani
et al, 2015 | No benefit | IM morphine | 81 | 60 µg ODT | No benefit in
adding desmopressin ODT. | (59) |
| Pricop et
al, 2016 | Benefit | IM ketorolac | 249 | 120 µg ODT, 60 µg
ODT | 120 µg desmopressin
ODT decreased absolute pain intensity more than ketorolac alone; 60
µg desmopressin and ketorolac in combination was more efficient in
decreasing absolute pain intensity than ketorolac monotherapy or
desmopressin (60 µg) monotherapy. | (63) |
The majority of studies were conducted in Iran
(52,55-62).
Two studies used the ODT formulation at 60 µg (59) or 60 and 120 µg (63), while all other interventional
studies used the intranasal formulation at doses ranging from 20 µg
in one study (56) to 40 µg in all
others. Some studies investigated desmopressin monotherapy
(60-62,64)
but most looked at combination therapy.
Overall, mixed findings are reported regarding the
efficacy of desmopressin compared with a range of comparators for
pain relief in renal colic, although most studies have reported it
to have at least some beneficial effects. Moreover, desmopressin
has advantages over many of its comparators in terms of ease of
administration and tolerability that mean that it could be useful,
especially in combination therapy and for ambulatory pain relief.
Some studies have noted a variability in response to intranasal
desmopressin across patients (65); oral formulations, which have more
reliable dosing, may produce more consistent and/or rapid effects.
One study has already demonstrated significantly greater pain
relief with 120 µg ODT compared with ketorolac (63), and the number of dropouts due to
pain escalation was significantly lower in all groups receiving
desmopressin (combination therapy or monotherapy at 60/120 µg). It
is also of note that several studies only followed patients for a
short period (e.g. 30 min) (64,65),
although desmopressin may not reach peak effectiveness until one
hour after administration with some formulations (52).
A systematic review and meta-analysis published in
2016 found that most studies were low quality but suggested that
desmopressin can be used as an adjuvant therapy in renal colic
management in combination with opioids (66). Another recent meta-analysis
concluded that desmopressin has lower pain reduction properties
than comparators (67). However,
it should be noted that some patients do not tolerate first-line
therapies well, and as such, effective alternatives are needed. We
propose that larger, high-quality studies that follow patient
response for a sufficient duration would be beneficial in settling
the current confusion and inconsistency surrounding the use of
desmopressin in renal colic.
Summary: oral desmopressin in renal colic.
Oral desmopressin (60-120 µg ODT) may be useful in renal colic but
further, high quality studies are needed to confirm the efficacy of
desmopressin in this indication. Oral desmopressin could
potentially be used as monotherapy (63) or in combination with NSAIDs-the
Pricop study showed mild but statistically significant additive
analgesic effects after 30 min of follow-up when ketorolac was used
in combination with the lower dose of desmopressin (60 µg ODT)
(63), suggesting possible
supplementary beneficial effects of this drug combination.
Desmopressin and the central nervous
system
Vasopressin, and its sister hormone oxytocin (which
differs by only two amino acids), are neuropeptides. Both have been
implicated in the modulation of social behaviour and the stress
response (68-71).
Since the 1970s, there have been a number of
sporadic reports suggesting an effect of desmopressin on different
functions of the brain or central nervous system (CNS), including
memory, learning and attention. It has also been reported that
there may be a central mode of action for desmopressin in its
established role as an effective treatment for enuresis: an
improvement in short-term memory in children with enuresis treated
with desmopressin has been reported (72), and pre-pulse inhibition of startle
has been found to be impaired in children with enuresis but
restored to normal levels by treatment with desmopressin (73). Similarly, benefits of desmopressin
treatment on memory function in patients with diabetes insipidus
have been reported (74).
Vasopressin receptors in the CNS
There are three vasopressin receptor subtypes: V1a
(primarily responsible for vasoconstriction), V1b (primary involved
in activation of the hypothalamic-pituitary-adrenal axis) and V2
(primarily responsible for antidiuresis via action in the
kidney).
The action of vasopressin in the brain has
predominantly been attributed to V1a receptors which are expressed
at higher levels in several areas of the brain (75), and have been associated with
pair-bonding, aggression and stress management in animal studies
(76). V2 receptors are largely
excluded from discussions of CNS effects of vasopressin (68,77)
because they are considered not to be expressed at high enough
levels in the brain. This may limit the relevance of desmopressin,
a V2-selective agonist, for effects on the CNS.
However, V2 receptor expression has been reported in
human cerebellum (78) and in rat
cerebellum (79), as well as in
other areas of the developing rat brain (80). The cerebellum is mainly known for
its role in motor control/coordination, but may also be involved in
other functions including cognition, emotion (81) and working memory (82).
It is also possible that there is some limited
residual effect of desmopressin on V1 receptors in the brain-this
would require specific investigation, however. It is thought that
desmopressin may be able to activate V1b receptors in certain
conditions, such as in people with ACTH-dependent Cushing's disease
(83)-this interaction may result
from upregulation of V1b receptors (or the aberrant expression of
type 2 receptors by neoplastic ACTH-producing cells) (84). Some support for the theory that
desmopressin may act centrally also comes from case studies of
patients with nephrogenic diabetes insipidus and nocturnal enuresis
caused by genetic mutations that prevent the kidney being
responsive to AVP (and therefore desmopressin). In a number of
reports, these patients have nevertheless experienced improvements
in their bedwetting, or transition from bedwetting to nocturia,
with desmopressin treatment (85,86).
It has therefore been proposed that desmopressin may be able to
impact enuresis through effects on arousal or other processes,
acting via central rather than renal AVP receptors and possibly via
V1 rather than V2 receptors.
Can desmopressin reach the CNS? In general,
it is thought that desmopressin is unable to cross the blood-brain
(or blood-CSF) barrier when administered intravenously (87-89).
However, a mechanism of bidirectional saturable transport across
the blood-brain barrier for vasopressin has been demonstrated and,
from this, it was concluded that a saturable system exists for
brain to blood transport of AVP and some structurally similar
peptides (90).
One way to bypass the blood-brain barrier is using
intranasal administration to deliver drugs to the brain via the
olfactory and trigeminal nerve pathways (91). Indeed, intranasal administration of
antidiabetic peptides has been demonstrated to allow drug delivery
directly to the brain in animals (92) and humans (93), with a view to therapeutic
application in Alzheimer's disease (94).
There may be other routes by which desmopressin
could exert CNS effects, for example by acting from the periphery
to alter gene expression in the brain (95), binding to receptors in the
periphery that feed back to the CNS, altering permeability of the
blood-brain barrier to other substances (96,97),
or by the formation of active fragments that can cross the
blood-brain barrier following peripheral administration (98-101).
In support of the ability of desmopressin to affect brain function,
an inhibitory effect of intravenous desmopressin on hypothalamic
dopamine function in humans has been reported (102)-it was unclear whether this was a
direct or indirect effect.
Studies of desmopressin in the CNS. A
systematic literature search was performed to identify studies that
investigated effects of desmopressin on the CNS in humans.
Forty-one were identified, and 16 were obtained as full-text
articles. Details of these are presented in the comprehensive table
in supplementary Table SIII. A
small number of additional studies of interest were identified
through review of abstracts, citation chasing or more focused
literature searches-these are also included in Table VI which provides a brief overview
of 23 studies.
 | Table VISummary of selected studies of
interest related to desmopressin and the CNS. |
Table VI
Summary of selected studies of
interest related to desmopressin and the CNS.
| A, Learning |
|---|
| First author/s,
year | Supports dDAVP
use? | Title | No. | Results | Dose and
formulation | (Refs.) |
|---|
| Anderson et
al, 1979 | Benefit | Passive avoidance
learning in Lesch- Nyhan disease: effect of 1-desamino-
8-arginine-vasopressin. | 3 | Ability to learn
was repeatedly and consistently improved by dDAVP. | 40 ‘units’ IN | (123) |
| Beckwith et al,
1982 | Benefit | Vasopressin analog
(DDAVP) facilitates concept learning in human males. | 54 | The group treated
with dDAVP solved all visual discrimination problems faster than
the placebo or no treatment control groups; no effect on visual
memory. | 60 µg IN | (124) |
| Eisenberg et
al, 1984 | No benefit | The effect of
vasopressin treatment on learning in Down’s syndrome. | 9 | Word list learning
showed no improvement with drug vs. placebo. Visual verbal paired
associated learning task showed trend in favour of active
drug. | 40 µg/day IN for 10
days | (125) |
| B, Memory |
| First author/s,
year | Supports dDAVP
use? | Title | No. | Results | Dose and
formulation | (Refs.) |
| Beckwith et
al, 1987 | Benefit | Vasopressin
analogue (DDAVP) facilitates recall of narrative prose. | 40 | dDAVP associated
with improved recall of idea units vs. placebo. | 60 µg IN | (126) |
| Beckwith et
al, 1995 | No benefit | Failure of
posttrial administration of vasopressin analogue (DDAVP) to
influence memory in healthy, young, male volunteers. | 45 | dDAVP after the
learning trial had no subsequent effect on recall for prose
passages 24 h after treatment. | 60 µg IN | (127) |
| Beckwith et
al, 1990 | Unclear or
inconsistent benefit | Dose-dependent
effects of DDAVP on memory in healthy young adult males: a
preliminary study. | 70 | 60 µg dDAVP not
significantly different from other doses in terms of free recall
but enhanced cued recall when compared with 5 or 15 µg. | 60, 30, 15, 5 or 0
µg IN | (128) |
| Eisenhofer et
al, 1985 | No benefit | No improvement in
ethanol-induced memory deficits after administration of a
vasopressin analog. | 26 | No differences
between placebo and dDAVP for effects of ethanol on memory scores.
Males given dDAVP had, at 3 h after ethanol, more errors for the
Benton visual retention test than controls. | 50 µg IN | (129) |
| Guard et al,
1986 | No benefit | Effects of
vasopressin and desmopressin on memory. | 40 | No significant
change in scores between treated groups and controls. | 20 µg IN | (130) |
| Jenkins et
al, 1982 | No benefit | Effect of
desmopressin on normal and impaired memory. | 18 | None of the
patients showed significant improvement with dDAVP for any of the
test procedures. | 40 µg IN four times
a day for 2 weeks (1 week in patients with amnesia) | (131) |
| Millar et
al, 1987 | Benefit | Vasopressin and
memory: improve- ment in normal short-term recall and reduction of
alcohol-induced amnesia. | 36 | 40 µg dDAVP could
facilitate human short-term memory processes and reduce
alcohol-induced amnesia. No benefit for semantic retrieval or
reaction time. | 40 µg IN | (132) |
| Müller et
al, 2001 | Benefit | The effect of
desmopressin on short- term memory in children with primary
nocturnal enuresis. | 40 | Children with
primary nocturnal enuresis treated with desmopressin exhibited
improvement of short- term memory. | 20 µg IN | (72) |
| Nebes et al,
1984 | Benefit | The effect of
vasopressin on memory in the healthy elderly. | 48 | dDAVP reduced
response time for short- and long- term episodic memory (but did
not affect semantic memory or simple response time). | 60 µg IN (10 µg
b.i.d. on day 1, 20 µg b.i.d. on day 2 and 30 µg b.i.d. on days
3-8). | (133) |
| Till and Beckwith,
1985 | Unclear or
inconsistent benefit | Sentence memory
affected by vasopressin analog (DDAVP) in cross-over
experiment. | 42 | dDAVP may
facilitate memory, particularly retrieval processes. The effect was
clearest for individuals treated with dDAVP during the first test
session (vs. placebo). After 1 week, treatment groups showed little
or no difference in recall. | 60 µg IN | (134) |
| Weingartner et
al, 1981 | Benefit | Effects of
vasopressin on human memory functions. | 18 | Cognitively
unimpaired and impaired adults treated with dDAVP for a period of
several days learned information more effectively, as measured by
the completeness, organization and consistency (reliability) of
recall. dDAVP also appeared to partially reverse the retrograde
amnesia that follows electroconvulsive treatment. | 30-60 µg IN | (135) |
| C, Memory following
ECT |
| First author/s,
year | Supports dDAVP
use? | Title | No. | Results | Dose and
formulation | (Refs.) |
| Abdollahian et
al, 2004 | Benefit | Effects of
desmopressin (DDAVP) on memory impairment following
electroconvulsive therapy (ECT). | 50 | Increase in memory
scores with dDAVP and difference between dDAVP and placebo. | 60 µg IN | (136) |
| Lerer et al,
1983 | No benefit | Effect of
vasopressin on memory following electroconvulsive therapy. | 9 | No effect of
dDAVP. | 25 µg IN | (137) |
| D, Reaction
time/memory |
| First author/s,
year | Supports dDAVP
use? | Title | No. | Results | Dose and
formulation | (Refs.) |
| Beckwith et
al, 1983 | Unclear or
inconsistent benefit | Vasopressin analog
influences the performance of males on a reaction time task. | 15 | dDAVP improved
attentional processes in the second but not the first test session
using the Sternberg Item Recognition Task, indicating an
interaction between dDAVP and prior experience of the task. dDAVP
did not influence memory. | 60 µg IN | (138) |
| E, Attention |
| First author/s,
year | Supports dDAVP
use? | Title | No. | Results | Dose and
formulation | (Refs.) |
| Jennings et
al, 1986 | Benefit | Vasopressin peptide
(DDAVP) may narrow the focus of attention in normal elderly. | 15 | dDAVP increased
proportion of attention allocated to primary task. | 600 µg/day IN (0.3
mg twice a day) | (139) |
| F, Language |
| First author/s,
year | Supports dDAVP
use? | Title | No. | Results | Dose and
formulation | (Refs.) |
| Tsikunov and
Belokoskova, 2007 | Benefit | Psychophysiological
analysis of the influence of vasopressin on speech in patients with
post-stroke aphasias. | 26 | Speech improvement
was noted in 88% of cases following administration of dDAVP vs.
placebo. | Dose of 0.1 µg IN
for 1.5-2 months and total dosage of 4 µg. | (140) |
| G, Cognitive
function/affective disorder |
| First author/s,
year | Supports dDAVP
use? | Title | No. | Results | Dose and
formulation | (Refs.) |
| Gold et al,
1979 | Benefit | Effects of
1-desamo-8-D-arginine vasopressin on behaviour and cognition in
primary affective. disorder | 4 | A total of 3 out of
4 patients showed improvement in cognitive function during dDAVP
treatment. A total of 2 out of 4 patients also exhibited elevation
of mood and amelioration of other affective symptoms. | 60-160 mg IN for
3-7 weeks [NB: dosage as described but may be µg] | (141) |
| H,
Schizophrenia |
| First author/s,
year | Supports dDAVP
use? | Title | No. | Results | Dose and
formulation | (Refs.) |
| Brambilla et
al, 1986 | Benefit | Neuropeptide
therapies in chronic schizophrenia: TRH and vasopressin
administration. | 23 | Both dDAVP and TRH
improved negative symptoms. Memory was improved in 9/13 patients on
dDAVP vs. 10/10 patients on TRH. | 4 µg every other,
day IM | (103) |
| Brambilla et
al, 1989 | Benefit | Vasopressin (DDAVP)
therapy in chronic schizophrenia: effects on negative symptoms and
memory. | 10 | dDAVP induced
improvement of negative symptomatology and a trend toward
improvement of short- to medium-term memory. | 4 µg/day IM | (104) |
| Hosseini et
al, 2014 | Benefit | Intranasal
desmopressin as an adjunct to risperidone for negative symptoms of
schizophrenia: a randomized, double-blind, placebo-controlled,
clinical trial. | 40 | dDAVP-treated
patients showed significantly greater improvement in the negative
symptoms (P=0.001) as well as the PANSS total and general
psychopathology subscale scores (P=0.005 and P=0.003; respectively)
compared with the placebo group. | 20 µg IN | (142) |
All studies had relatively small sample sizes.
Desmopressin was administered intranasally in all
studies except two that used intramuscular injection in
schizophrenia (103,104). In general, studies with
intranasal desmopressin used doses ranging from 20-60 µg/day,
although there were some outside this range.
Variable effects of desmopressin on different
aspects of CNS function were reported. In Table VI, studies are grouped according
to outcome of interest: learning, memory, memory after
electro-convulsive therapy (ECT), reaction time, language,
cognitive function/affective disorder and schizophrenia. Overall,
14 studies showed a clear benefit of desmopressin for some or all
of the chosen endpoints (Table
VI), while a further three showed unclear or inconsistent
benefit. Memory improvements were seen more often in short-term
rather than long-term memory.
Summary: oral desmopressin in the CNS.
Further basic research is needed in this area before any
conclusions can be drawn regarding effects of desmopressin on the
CNS. Although a number of studies suggest effects of desmopressin
in areas such as learning, memory or clinical symptoms in certain
patient populations, studies are often small and of low quality.
Furthermore, there are several outstanding questions regarding the
overall concept of using desmopressin to target the CNS. These
relate primarily to two major issues: the ability of desmopressin
to penetrate the blood-brain barrier or act on the CNS from the
periphery, and the localisation of desmopressin-responsive
receptors in the brain. Given that studies of CNS effects to date
have used the intranasal formulation of desmopressin-a delivery
mode which is believed to be able to bypass the blood-brain barrier
in certain cases-the extrapolation of findings from these studies
to oral desmopressin cannot be made without further studies
specifically of oral formulations.
Desmopressin in oncology
Vasopressin receptors are present in some cancer
cells, including human lung, breast, pancreatic, colorectal, and
gastrointestinal tumours (105).
It has been suggested that V1 receptors are associated with
cellular proliferation, but that the V2 receptor is associated with
anti-proliferative effects (106). Desmopressin, as a selective
agonist for the V2 receptor, shows anti-tumour properties in
breast, colorectal, lung and prostate cancer models (105).
Mechanisms of action/early
results
Stimulation of vascular V2 receptors leads to acute
release of haemostatic factors into the bloodstream, including
Factor VIII, tissue-type plasminogen activator and von Willebrand
factor (VWF) (107). VWF is
involved in several biological processes such as coagulation (as
discussed above), vascular normalisation, cancer cell apoptosis and
metastatic resistance. Animal models suggest that efficacy of
docetaxel in castration-resistant prostate cancer is enhanced when
used in combination with desmopressin (108). Phase II trials in humans also
show some encouraging results with the use of desmopressin,
including a drop in circulating tumour cells in breast cancer
patients (109) and a reduction
in tumour vascular perfusion in rectal cancer patients with
bleeding (110). In animal
studies, infusion of desmopressin during surgery appears to inhibit
perioperative metastatic events and may impede micro-metastases
that occurred before surgery (109,111).
However, high doses of desmopressin have been used
in human trials of desmopressin for cancer treatment: two 1 µg/kg
intravenous doses (one just before surgery, one 24 h after surgery)
in one study (109) and a maximum
tolerated dose of 2x0.5 µg/kg/12 h in another (110).
The likelihood of the oral formulations of
desmopressin providing adequate dosing for use in oncological
treatment is therefore low. As such, we did not perform an
extensive review of the literature on this topic, and intend to
carry out a comprehensive review of the use of desmopressin (all
formulations) in this therapeutic area as a separate exercise.
4. Conclusion
The use of desmopressin has been explored in a
multitude of diverse areas since it was first synthesised (1). Since many of these have not been
formally investigated for the purpose of regulatory approval,
further studies and RCTs will be needed before any new indications
can be recommended. Renal colic is perhaps one of the most
promising areas with viable mechanism(s) of action and a reasonably
supportive literature on desmopressin use. More studies are
required to confirm benefits with oral formulations of desmopressin
at standard doses, in monotherapy or combination therapy. Doses at
the higher end of the normal range have yielded the most promising
results, but dose-finding studies are needed, and the ideal
protocol and management of up-titration are yet to be
determined.
In bleeding control, most studies of desmopressin
that were identified used the intravenous formulation at high doses
that are unlikely to be achieved with oral formulations. In cardiac
and renal surgery, results are inconsistent but the use of oral
desmopressin as a preventative measure before endoscopic sinus
surgery or rhinoplasty may be feasible-however, the relevance of
intranasal vs oral administration must be investigated.
Desmopressin has established efficacy in bleeding disorders but
high doses (0.15-0.3 µg/kg IV) are required. Achieving this level
of dosing with oral formulations would be challenging.
Further basic research is needed in the CNS (e.g.
location of desmopressin-responsive receptors, relevance of
blood-brain barrier) before viable uses for oral desmopressin can
be properly explored.
Desmopressin has demonstrated intriguing anti-cancer
effects in a number of studies, many of which are
animal/preclinical. In the small number of human trials, however,
high doses of desmopressin have been used (0.5-1 µg/kg IV), again
meaning that use of oral formulations is unlikely to be
practical.
There are other areas of research that are in their
infancy, that may yet prove to be important avenues of
investigation for oral forms of desmopressin, such as the bladder
and bladder contractility (112),
patients with spinal cord injury, and older adults with renal
impairment. These are not discussed in this manuscript as there are
not yet sufficient studies to gauge the potential role of
desmopressin. However, the very fact that we are continuing to
learn about credible uses of a drug that was first developed over
50 years ago is testament to the complexity of the human body and
the untold possibilities of established, as well as novel,
medicines.
Supplementary Material
Bleeding control studies
Renal colic studies.
CNS studies.
Acknowledgements
The authors would like to thank Dr Caroline Loat for
providing medical writing support.
Funding
Funding: Medical writing was supported by The Dr Frederik
Paulsen Chair at Ghent University, sponsored by Ferring
Pharmaceuticals (grant no. A20/TT/1014).
Availability of data and materials
Not applicable.
Authors' contributions
KE, THL, GBK, SR, JPW, JVW, AEK, LD, FH, AFS, JPN
and KVJ conceived and designed the study. THL performed the
literature searches. KE, THL, KVJ and JPN interpreted the data and
drafted the manuscript. Data authentication is not applicable. All
authors critically reviewed the manuscript, and have read and
approved the final version of the manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
KE has received grants and honoraria to his
institution from Ferring, Medtronic, Astellas and Idorsia. THL has
worked as a consultant for Ferring Pharmaceuticals. JVW has
participated in advisory boards and safety boards and has received
speaker fees from Alexion, Ferring, Astellas and Alnylam. FH has
received speaker fees from Astellas. JPN is a former full-time
employee of Ferring Pharmaceuticals. KVJ is a full-time employee of
Ferring Pharmaceuticals. The other authors declare that they have
no competing interests.
References
|
1
|
Zaoral M, Kolc J and Šorm F: Amino acids
and peptides. LXXI. Synthesis of
1-deamino-8-D-γ-aminobutyrine-vasopressin,
1-deamino-8-D-lysine-vasopressin, and
1-deamino-8-D-arginine-vasopressin. Collect Czech Chem Commun.
32:1250–1257. 1967.
|
|
2
|
Robinson AG: DDAVP in the treatment of
central diabetes insipidus. N Engl J Med. 294:507–511.
1976.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Desmopressin | Drugs | BNF content
published by NICE. https://bnf.nice.org.uk/drugs/desmopressin/. Accessed
March 22, 2023.
|
|
4
|
Cash JD: DDAVP and factor VIII: A tale
from Edinburgh. J Thromb Haemost. 1:619–621. 2003.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Mannucci PM, Ruggeri ZM, Pareti FI and
Capitanio A: 1-Deamino-8-d-arginine vasopressin: A new
pharmacological approach to the management of haemophilia and von
Willebrands' diseases. Lancet. 1:869–872. 1977.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Mannucci PM: Use of desmopressin in the
treatment of hemophilia A: Towards a golden jubilee. Haematologica.
103:379–381. 2018.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Robson WLM, Leung AKC and Norgaard JP: The
comparative safety of oral versus intranasal desmopressin for the
treatment of children with nocturnal enuresis. J Urol. 178:24–30.
2007.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Juul KV, Malmberg A, van der Meulen E,
Walle JV and Nørgaard JP: Low-dose desmopressin combined with serum
sodium monitoring can prevent clinically significant hyponatraemia
in patients treated for Nocturia. BJU Int. 119:776–784.
2017.PubMed/NCBI View Article : Google Scholar
|
|
9
|
De Bruyne P, De Guchtenaere A, Van
Herzeele C, Raes A, Dehoorne J, Hoebeke P, Van Laecke E and Walle
JV: Pharmacokinetics of desmopressin administered as tablet and
oral lyophilisate formulation in children with monosymptomatic
nocturnal enuresis. Eur J Pediatr. 173:223–228. 2014.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Dossche L, Michelet R, De Bruyne P, Van
Herzeele C, Gasthuys E, Rittig S, Vermeulen A and Walle JV:
Desmopressin oral lyophilisate in young children: New insights in
pharmacokinetics and pharmacodynamics. Arch Dis Child. 106:597–602.
2021.PubMed/NCBI View Article : Google Scholar
|
|
11
|
De Guchtenaere A, Van Herzeele C, Raes A,
Dehoorne J, Hoebeke P, Van Laecke E and Walle JV: Oral lyophylizate
formulation of desmopressin: Superior pharmacodynamics compared to
tablet due to low food interaction. J Urol. 185:2308–2313.
2011.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Fjellestad-Paulsen A, Höglund P, Lundin S
and Paulsen O: Pharmacokinetics of 1-deamino-8-D-arginine
vasopressin after various routes of administration in healthy
volunteers. Clin Endocrinol (Oxf). 38:177–182. 1993.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Rembratt A, Graugaard-Jensen C,
Senderovitz T, Norgaard JP and Djurhuus JC: Pharmacokinetics and
pharmacodynamics of desmopressin administered orally versus
intravenously at daytime versus night-time in healthy men aged
55-70 years. Eur J Clin Pharmacol. 60:397–402. 2004.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Mohinani A, Patel S, Tan V, Kartika T,
Olson S, DeLoughery TG and Shatzel J: Desmopressin as a hemostatic
and blood sparing agent in bleeding disorders. Eur J Haematol.
110:470–479. 2023.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Mannucci PM: Desmopressin (DDAVP) in the
treatment of bleeding disorders, Revised edition, 2012.
|
|
16
|
Electronic Medicines Compendium:
DDAVP/Desmopressin Injection-Summary of Product Characteristics
(SmPC)-(emc). https://www.medicines.org.uk/emc/product/5447/smpc#gref.
Last accessed July 12, 2023.
|
|
17
|
Electronic medicines compendium: Octim
Nasal Spray-Summary of Product Characteristics (SmPC) - (emc).
https://www.medicines.org.uk/emc/product/89/smpc#gref.
Last accessed 12 July, 2023.
|
|
18
|
Akin M: Response to low-dose desmopressin
by a subcutaneous route in children with type 1 von Willebrand
disease. Hematology. 18:115–118. 2013.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Furqan F, Sham R and Kouides P: Efficacy
and safety of half-dose desmopressin for bleeding prophylaxis in
bleeding disorder patients undergoing predominantly low to moderate
risk invasive procedures. Am J Hematol. 95:E285–E287.
2020.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Preijers T, Schütte LM, Kruip MJHA,
Cnossen MH, Leebeek FWG, van Hest RM and Mathôt RAA: Strategies for
individualized dosing of clotting factor concentrates and
desmopressin in hemophilia A and B. Ther Drug Monit. 41:192–212.
2019.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Preijers T, Schütte LM, Kruip MJHA,
Cnossen MH, Leebeek FWG, van Hest RM and Mathôt RAA: Population
pharmacokinetics of clotting factor concentrates and desmopressin
in hemophilia. Clin Pharmacokinet. 60:1–16. 2021.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Atiq F, Heijdra J, Snijders F, Boender J,
Kempers E, van Heerde WL, Maas DPMSM, Krouwel S, Schoormans SC, de
Meris J, et al: Desmopressin response depends on the presence and
type of genetic variants in patients with type 1 and type 2 von
Willebrand disease. Blood Adv. 6:5317–5326. 2022.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Trigg DE, Stergiotou I, Peitsidis P and
Kadir RA: A systematic review: The use of desmopressin for
treatment and prophylaxis of bleeding disorders in pregnancy.
Haemophilia. 18:25–33. 2012.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Kouides PA, Byams VR, Philipp CS, Stein
SF, Heit JA, Lukes AS, Skerrette NI, Dowling NF, Evatt BL, Miller
CH, et al: Multisite management study of menorrhagia with abnormal
laboratory haemostasis: A prospective crossover study of intranasal
desmopressin and oral tranexamic acid. Br J Haematol. 145:212–220.
2009.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Saccullo G and Makris M: Prophylaxis in
von Willebrand disease: Coming of age? Semin Thromb Hemost.
42:498–506. 2016.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Andersen LK, Hvas AM and Hvas CL: Effect
of desmopressin on platelet dysfunction during antiplatelet
therapy: A systematic review. Neurocrit Care. 34:1026–1046.
2021.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Benvenuto L, Qayum S, Kim H, Robbins H,
Shah L, Dimango A, Magda G, Grewal H, Lemaitre P, Stanifer BP, et
al: Lung transplantation for pulmonary fibrosis associated with
hermansky-pudlak syndrome. A single-center experience. Transplant
Direct. 8(e1303)2022.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Merideth MA, Introne WJ, Wang JA, O'Brien
KJ, Huizing M and Gochuico BR: Genetic variants associated with
Hermansky-Pudlak syndrome. Platelets. 31:544–547. 2020.PubMed/NCBI View Article : Google Scholar
|
|
29
|
MacDonald S, Wright A, Beuche F, Downes K,
Besser M, Symington E, Kelly A and Thomas W: Characterization of a
large cohort of patients with unclassified bleeding disorder
clinical features, management of haemostatic challenges and use of
global haemostatic assessment with proposed recommendations for
diagnosis and treatment. Int J Lab Hematol. 42:116–125.
2020.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Obaji S, Alikhan R, Rayment R, Carter P,
Macartney N and Collins P: Unclassified bleeding disorders: Outcome
of haemostatic challenges following tranexamic acid and/or
desmopressin. Haemophilia. 22:285–291. 2016.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Czer LS, Bateman TM, Gray RJ, Raymond M,
Stewart ME, Lee S, Goldfinger D, Chaux A and Matloff JM: Treatment
of severe platelet dysfunction and hemorrhage after cardiopulmonary
bypass: Reduction in blood product usage with desmopressin. J Am
Coll Cardiol. 9:1139–1147. 1987.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Wademan BH and Galvin SD: Desmopressin for
reducing postoperative blood loss and transfusion requirements
following cardiac surgery in adults. Interact Cardiovasc Thorac
Surg. 18:360–370. 2014.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Radhakrishnan S, Chanchlani R, Connolly B
and Langlois V: Pre-procedure desmopressin acetate to reduce
bleeding in renal failure: Does it really work? Nephron Clin Pract.
128:45–48. 2014.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Athavale A, Kulkarni H, Arslan CD and Hart
P: Desmopressin and bleeding risk after percutaneous kidney biopsy.
BMC Nephrol. 20(413)2019.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Akbarpour M, Jalali MM, Akbari M, Haddadi
S and Fani G: Effect of desmopressin on bleeding during endoscopic
sinus surgery: A randomized clinical trial. Laryngoscope Investig
Otolaryngol. 7:920–927. 2022.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Haddady-Abianeh S, Rajabpour AA,
Sanatkarfar M, Farahvash MR, Khorasani G and Molaei H: The
hemostatic effect of desmopressin on bleeding as a nasal spray in
open septorhinoplasty. Aesthetic Plast Surg. 43:1603–1606.
2019.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Jahanshahi J, Tayebi E, Hashemian F,
Bakhshaei MH, Ahmadi MS and Rabiei MA: Effect of local desmopressin
administration on intraoperative blood loss and quality of the
surgical field during functional endoscopic sinus surgery in
patients with chronic rhinosinusitis: A triple-blinded clinical
trial. Eur Arch Otorhinolaryngol. 276:1995–1999. 2019.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Safaeian R, Hassani V, Ghandi A and
Mohseni M: Desmopressin nasal spray reduces blood loss and improves
the quality of the surgical field during functional endoscopic
sinus surgery. J Anaesthesiol Clin Pharmacol. 37:261–265.
2021.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Shao H, Kuang LT, Hou WJ and Zhang T:
Effect of desmopressin administration on intraoperative blood loss
and quality of the surgical field during functional endoscopic
sinus surgery: A randomized, clinical trial. BMC Anesthesiol.
15(53)2015.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Frontera JA, Lewin JJ III, Rabinstein AA,
Aisiku IP, Alexandrov AW, Cook AM, Del Zoppo GJ, Kumar M, Peerschke
EI, Stiefel MF, et al: Guideline for reversal of antithrombotics in
intracranial hemorrhage: Executive summary. A statement for
healthcare professionals from the neurocritical care society and
the society of critical care medicine. Crit Care Med. 44:2251–2257.
2016.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Mengel A, Stefanou MI, Hadaschik KA, Wolf
M, Stadler V, Poli K, Lindig T, Ernemann U, Grimm F, Tatagiba M, et
al: Early administration of Desmopressin and platelet transfusion
for reducing hematoma expansion in patients with acute antiplatelet
therapy associated Intracerebral Hemorrhage. Crit Care Med.
48:1009–1017. 2020.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Loggini A, El Ammar F, Darzi AJ, Mansour
A, Kramer CL, Goldenberg FD and Lazaridis C: Effect of desmopressin
on hematoma expansion in antiplatelet-associated intracerebral
hemorrhage: A systematic review and meta-analysis. J Clin Neurosci.
86:116–121. 2021.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Bultitude M and Rees J: Management of
renal colic. BMJ. 345(e5499)2012.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Golzari SE, Soleimanpour H, Rahmani F,
Mehr NZ, Safari S, Heshmat Y and Bakhtavar HE: Therapeutic
approaches for renal colic in the emergency department: A review
article. Anesth Pain Med. 4(e16222)2014.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Kapila V, Kapila AK, Tailly T, Rappe B,
Juul KV and Everaert K: The analgesic action of desmopressin in
renal colic. Acta Clin Belg. 72:179–185. 2017.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Patti L and Leslie SW: Acute renal colic.
StatPearls Publishing, 2022.
|
|
47
|
Juul KV, Schroeder MK, Rittig S and
Nørgaard JP: Desmopressin as an adjuvant to opioids or NSAIDs in
treatment of renal colic: A nationwide register-based study.
Pharmacoepidemiol Drug Saf. 24:1155–1160. 2015.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Amer T, Osman B, Johnstone A, Mariappan M,
Gupta A, Brattis N, Jones G, Somani BK, Keeley FX Jr and
Aboumarzouk OM: Medical expulsive therapy for ureteric stones:
Analysing the evidence from systematic reviews and meta-analysis of
powered double-blinded randomised controlled trials. Arab J Urol.
15:83–93. 2017.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Campschroer T, Zhu X, Vernooij RW and Lock
MT: Alpha-blockers as medical expulsive therapy for ureteral
stones. Cochrane Database Syst Rev. 5(CD08509)2018.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Lim I, Sellers DJ and Chess-Williams R:
Current and emerging pharmacological targets for medical expulsive
therapy. Basic Clin Pharmacol Toxicol. 130 (Suppl 1):S16–S22.
2022.PubMed/NCBI View Article : Google Scholar
|
|
51
|
European Association of Urology (EAU): EAU
Guidelines on Urolithiasis. EAU, Arnhem, 2024. https://d56bochluxqnz.cloudfront.net/documents/full-guideline/EAU-Guidelines-on-Urolithiasis-2024.pdf.
|
|
52
|
Jalili M, Shirani F, Entezari P,
Hedayatshodeh M, Baigi V and Mirfazaelian H:
Desmopressin/indomethacin combination efficacy and safety in renal
colic pain management: A randomized placebo controlled trial. Am J
Emerg Med. 37:1009–1012. 2019.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Kimoto Y and Constantinou CE: Effects of
[1-desamino-8-D-arginine]vasopressin and papaverine on rabbit renal
pelvis. Eur J Pharmacol. 175:359–362. 1990.PubMed/NCBI View Article : Google Scholar
|
|
54
|
el-Sherif AE, Salem M, Yahia H,
al-Sharkawy WA and al-Sayrafi M: Treatment of renal colic by
desmopressin intranasal spray and diclofenac sodium. J Urol.
153:1395–1398. 1995.PubMed/NCBI
|
|
55
|
Hazhir S, Badr YAA and Darabi JN:
Comparison of intranasal desmopressin and intramuscular tramadol
versus pethidine in patients with renal colic. Urol J. 7:148–151.
2010.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Kheirollahi AR, Tehrani M and Bashashati
M: A comparison of the effect of intranasal desmopressin and
intramuscular hyoscine N-butyl bromide combination with
intramuscular hyoscine N-butyl bromide alone in acute renal colic.
J Res Med Sci. 15:214–218. 2010.PubMed/NCBI
|
|
57
|
Roshani A, Falahatkar S, Khosropanah I,
Roshan ZA, Zarkami T, Palizkar M, Emadi SA, Akbarpour M and Khaki
N: Assessment of clinical efficacy of intranasal desmopressin spray
and diclofenac sodium suppository in treatment of renal colic
versus diclofenac sodium alone. Urology. 75:540–542.
2010.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Masoumi K, Darian AS, Forouzan A,
Barzegari H, Rahim F, Feli M, Sheidaii MF and Porozan S: The
efficacy of intranasal desmopressin as an adjuvant in the acute
renal colic pain management. Pain Res Treat.
2014(320327)2014.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Shirvani MK, Mahboub MD, Ghazi M and
Delijani A: A comparison of the effects of morphine and sublingual
desmopressin combination therapy with morphine alone in treatment
of renal colic: A controlled clinical trial. Urol J. 12:2001–2004.
2015.PubMed/NCBI
|
|
60
|
Shirazi M, Salehipour M, Afrasiabi MA and
Aminsharifi A: Analgesic effects and safety of desmopressin,
tramadol and indomethacin in patients with acute renal colic; A
randomized clinical trial. Bull Emerg Trauma. 3:41–45.
2015.PubMed/NCBI
|
|
61
|
Dolatabadi AA, Memary E, Kariman H, Gigloo
KN and Baratloo A: Intranasal desmopressin compared with
intravenous ketorolac for pain management of patients with renal
colic referring to the emergency department: A randomized clinical
trial. Anesth Pain Med. 7(e43595)2017.PubMed/NCBI View Article : Google Scholar
|
|
62
|
Ghafouri HB, Abazarian N, Yasinzadeh M and
Modirian E: Intravenous paracetamol vs intranasal desmopressin for
renal colic in the emergency department: A randomized clinical
trial. Pain Med. 21:3437–3442. 2020.PubMed/NCBI View Article : Google Scholar
|
|
63
|
Pricop C, Branisteanu DD, Orsolya M, Puia
D, Matei A and Checherita IA: Sublingual desmopressin is efficient
and safe in the therapy of lithiasic renal colic. Int Urol Nephrol.
48:183–189. 2016.PubMed/NCBI View Article : Google Scholar
|
|
64
|
Kumar S, Behera NC, Sarkar D, Prasad S,
Mandal AK and Singh SK: A comparative assessment of the clinical
efficacy of intranasal desmopressin spray and diclofenac in the
treatment of renal colic. Urol Res. 39:397–400. 2011.PubMed/NCBI View Article : Google Scholar
|
|
65
|
Lopes T, Dias JS, Marcelino J, Varela J,
Ribeiro S and Dias J: An assessment of the clinical efficacy of
intranasal desmopressin spray in the treatment of renal colic. BJU
Int. 87:322–325. 2001.PubMed/NCBI View Article : Google Scholar
|
|
66
|
Jalili M, Entezari P, Doosti-Irani A,
Masoomi R and Mirfazaelian H: Desmopressin effectiveness in renal
colic pain management: Systematic review and meta-analysis. Am J
Emerg Med. 34:1535–1541. 2016.PubMed/NCBI View Article : Google Scholar
|
|
67
|
Seghatoleslami G, Jahromi MS, Farzaneh R,
Rahsepar S, Malekshoar M, Vatankhah M, Akhavan R, Abbasi B, Akhavan
H, Abiri S, et al: Alternative medical interventions versus
conventional treatment of renal colic: An updated systematic review
and network meta-analysis. Urol J. 19:412–419. 2022.PubMed/NCBI View Article : Google Scholar
|
|
68
|
Kreek MJ, Zhou Y and Levran O: Functions
of arginine vasopressin and its receptors: Importance of human
molecular genetics studies in bidirectional translational research.
Biol Psychiatry. 70:502–503. 2011.PubMed/NCBI View Article : Google Scholar
|
|
69
|
Albers HE: The regulation of social
recognition, social communication and aggression: Vasopressin in
the social behavior neural network. Horm Behav. 61:283–292.
2012.PubMed/NCBI View Article : Google Scholar
|
|
70
|
Winter J and Jurek B: The interplay
between oxytocin and the CRF system: Regulation of the stress
response. Cell Tissue Res. 375:85–91. 2019.PubMed/NCBI View Article : Google Scholar
|
|
71
|
Marsh N, Marsh AA, Lee MR and Hurlemann R:
Oxytocin and the neurobiology of prosocial behavior.
Neuroscientist. 27:604–619. 2021.PubMed/NCBI View Article : Google Scholar
|
|
72
|
Müller D, Florkowski H, Chavez-Kattau K,
Carlsson G and Eggert P: The effect of desmopressin on short-term
memory in children with primary nocturnal enuresis. J Urol.
166:2432–2434. 2001.PubMed/NCBI
|
|
73
|
Schulz-Juergensen S, Rieger M, Schaefer J,
Neusuess A and Eggert P: Effect of 1-desamino-8-D-arginine
vasopressin on prepulse inhibition of startle supports a central
etiology of primary monosymptomatic enuresis. J Pediatr.
151:571–574. 2007.PubMed/NCBI View Article : Google Scholar
|
|
74
|
Laczi F, Valkusz Z, Lászlo FA, Wagner A,
Járdánházy T, Szász A, Szilárd J and Telegdy G: Effects of
lysine-vasopressin and 1-deamino-8-D-arginine-vasopressin on memory
in healthy individuals and diabetes insipidus patients.
Psychoneuroendocrinology. 7:185–193. 1982.PubMed/NCBI View Article : Google Scholar
|
|
75
|
EMBL-EBI: Expression Atlas-gene expression
across species and biological conditions. https://www.ebi.ac.uk/gxa/home. Last accessed 12
July, 2023.
|
|
76
|
Charles R, Sakurai T, Takahashi N, Elder
GA, Sosa MA, Young LJ and Buxbaum JD: Introduction of the human
AVPR1A gene substantially alters brain receptor expression patterns
and enhances aspects of social behavior in transgenic mice. Dis
Model Mech. 7:1013–1022. 2014.PubMed/NCBI View Article : Google Scholar
|
|
77
|
Ebstein RP, Knafo A, Mankuta D, Chew SH
and Lai PS: The contributions of oxytocin and vasopressin pathway
genes to human behavior. Horm Behav. 61:359–379. 2012.PubMed/NCBI View Article : Google Scholar
|
|
78
|
GTEX Consortium. Ardlie KG, Deluca DS,
Segrè AV, Sullivan TJ, Young T, Gelfand ET, Trowbridge C, Maller
JB, Tukiainen T, et al: The genotype-tissue expression (GTEx) pilot
analysis: Multitissue gene regulation in humans. Science.
348:648–660. 2015.PubMed/NCBI View Article : Google Scholar
|
|
79
|
Vargas KJ, Sarmiento JM, Ehrenfeld P,
Añazco CC, Villanueva CI, Carmona PL, Brenet M, Navarro J,
Müller-Esterl W and González CB: Postnatal expression of V2
vasopressin receptor splice variants in the rat cerebellum.
Differentiation. 77:377–385. 2009.PubMed/NCBI View Article : Google Scholar
|
|
80
|
Kato Y, Igarashi N, Hirasawa A, Tsujimoto
G and Kobayashi M: Distribution and developmental changes in
vasopressin V2 receptor mRNA in rat brain. Differentiation.
59:163–169. 1995.PubMed/NCBI View Article : Google Scholar
|
|
81
|
Schmahmann JD: Emotional disorders and the
cerebellum: Neurobiological substrates, neuropsychiatry, and
therapeutic implications. Handb Clin Neurol. 183:109–154.
2021.PubMed/NCBI View Article : Google Scholar
|
|
82
|
Tomlinson SP, Davis NJ, Morgan HM and
Bracewell RM: Cerebellar contributions to verbal working memory.
Cerebellum. 13:354–361. 2014.PubMed/NCBI View Article : Google Scholar
|
|
83
|
Sakai Y, Horiba N, Tozawa F, Sakai K,
Kuwayama A, Demura H and Suda T: Desmopressin stimulation test for
diagnosis of ACTH-dependent Cushing's syndrome. Endocr J.
44:687–695. 1997.PubMed/NCBI View Article : Google Scholar
|
|
84
|
Vassiliadi DA and Tsagarakis S: Diagnosis
of Endocrine Disease: The role of the desmopressin test in the
diagnosis and follow-up of Cushing's syndrome. Eur J Endocrinol.
178:R201–R214. 2018.PubMed/NCBI View Article : Google Scholar
|
|
85
|
Jonat S, Santer R, Schneppenheim R, Obser
T and Eggert P: Effect of DDAVP on nocturnal enuresis in a patient
with nephrogenic diabetes insipidus. Arch Dis Child. 81:57–59.
1999.PubMed/NCBI View Article : Google Scholar
|
|
86
|
Robben JH, Sze M, Knoers NV, Eggert P,
Deen P and Müller D: Relief of nocturnal enuresis by desmopressin
is kidney and vasopressin type 2 receptor independent. J Am Soc
Nephrol. 18:1534–1539. 2007.PubMed/NCBI View Article : Google Scholar
|
|
87
|
Stegner H, Artman HG, Leake RD and Fisher
DA: Does DDAVP (1-desamino-8-D-arginine-vasopressin) cross the
blood-CSF barrier? Neuroendocrinology. 37:262–265. 1983.PubMed/NCBI View Article : Google Scholar
|
|
88
|
Sørensen PS, Vilhardt H, Gjerris F and
Warberg J: Impermeability of the blood-cerebrospinal fluid barrier
to 1-deamino-8-D-arginine-vasopressin (DDAVP) in patients with
acquired, communicating hydrocephalus. Eur J Clin Invest.
14:435–439. 1984.PubMed/NCBI View Article : Google Scholar
|
|
89
|
Ferring Pharmaceuticals: DDAVP Tablets
0.2mg SmPC, 2012.
|
|
90
|
Banks WA, Kastin AJ, Horvath A and Michals
EA: Carrier-mediated transport of vasopressin across the
blood-brain barrier of the mouse. J Neurosci Res. 18:326–332.
1987.PubMed/NCBI View Article : Google Scholar
|
|
91
|
Su Y, Sun B, Gao X, Dong X, Fu L, Zhang Y,
Li Z, Wang Y, Jiang H and Han B: Intranasal delivery of targeted
nanoparticles loaded with miR-132 to brain for the treatment of
neurodegenerative diseases. Front Pharmacol.
11(1165)2020.PubMed/NCBI View Article : Google Scholar
|
|
92
|
Lochhead JJ, Kellohen KL, Ronaldson PT and
Davis TP: Distribution of insulin in trigeminal nerve and brain
after intranasal administration. Sci Rep. 9(2621)2019.PubMed/NCBI View Article : Google Scholar
|
|
93
|
Maeng J and Lee K: Systemic and brain
delivery of antidiabetic peptides through nasal administration
using cell-penetrating peptides. Front Pharmacol.
13(1068495)2022.PubMed/NCBI View Article : Google Scholar
|
|
94
|
Hallschmid M: Intranasal insulin for
Alzheimer's disease. CNS Drugs. 35:21–37. 2021.PubMed/NCBI View Article : Google Scholar
|
|
95
|
Mutsuga N, Shahar T, Verbalis JG, Xiang
CC, Brownstein MJ and Gainer H: Regulation of gene expression in
magnocellular neurons in rat supraoptic nucleus during sustained
hypoosmolality. Endocrinology. 146:1254–1267. 2005.PubMed/NCBI View Article : Google Scholar
|
|
96
|
Landgraf R, Hess J and Hartmann E: Effect
of oxytocin on regional 3H-orotic acid uptake in rat brain.
Endokrinologie. 70:45–52. 1977.PubMed/NCBI(In German).
|
|
97
|
Landgraf R, Hess J and Ermisch A: The
influence of vasopressin on the regional uptake of [3H] orotic acid
by rat brain. Acta Biol Med Ger. 37:655–658. 1978.PubMed/NCBI
|
|
98
|
Burbach JP, Kovács GL, de Wied D, van
Nispen JW and Greven HM: A major metabolite of arginine vasopressin
in the brain is a highly potent neuropeptide. Science.
221:1310–1312. 1983.PubMed/NCBI View Article : Google Scholar
|
|
99
|
Burbach JP, Bohus B, Kovács GL, Van Nispen
JW, Greven HM and De Wied D: Oxytocin is a precursor of potent
behaviourally active neuropeptides. Eur J Pharmacol. 94:125–131.
1983.PubMed/NCBI View Article : Google Scholar
|
|
100
|
van Bree JB, de Boer AG, Verhoef JC,
Danhof M and Breimer DD: Transport of vasopressin fragments across
the blood-brain barrier: In vitro studies using monolayer cultures
of bovine brain endothelial cells. J Pharmacol Exp Ther.
249:901–905. 1989.PubMed/NCBI
|
|
101
|
Lee MR and Jayant RD: Penetration of the
blood-brain-barrier by peripheral neuropeptides: New approaches to
enhancing transport and endogenous expression. Cell Tissue Res.
375:287–293. 2019.PubMed/NCBI View Article : Google Scholar
|
|
102
|
Lal S, Nair NP, Isaac I, Thavundayil J and
Guyda H: Effect of some peptides on dopaminergic function in man. J
Neural Transm Suppl. 29:173–181. 1990.PubMed/NCBI View Article : Google Scholar
|
|
103
|
Brambilla F, Aguglia E, Massironi R,
Maggioni M, Grillo W, Castiglioni R, Catalano M and Drago F:
Neuropeptide therapies in chronic schizophrenia: TRH and
vasopressin administration. Neuropsychobiology. 15:114–121.
1986.PubMed/NCBI View Article : Google Scholar
|
|
104
|
Brambilla F, Bondiolotti GP, Maggioni M,
Sciascia A, Grillo W, Sanna F, Latina A and Picotti GB: Vasopressin
(DDAVP) therapy in chronic schizophrenia: Effects on negative
symptoms and memory. Neuropsychobiology. 20:113–119.
1989.PubMed/NCBI View Article : Google Scholar
|
|
105
|
Pifano M, Garona J, Capobianco CS,
Gonzalez N, Alonso DF and Ripoll GV: Peptide agonists of
vasopressin V2 receptor reduce expression of neuroendocrine markers
and tumor growth in human lung and prostate tumor cells. Front
Oncol. 7(11)2017.PubMed/NCBI View Article : Google Scholar
|
|
106
|
Keegan BP, Akerman BL, Péqueux C and North
WG: Provasopressin expression by breast cancer cells: Implications
for growth and novel treatment strategies. Breast Cancer Res Treat.
95:265–277. 2006.PubMed/NCBI View Article : Google Scholar
|
|
107
|
Sobol NT, Solernó LM, Beltrán B, Vásquez
L, Ripoll GV, Garona J and Alonso DF: Anticancer activity of
repurposed hemostatic agent desmopressin on AVPR2-expressing human
osteosarcoma. Exp Ther Med. 21(566)2021.PubMed/NCBI View Article : Google Scholar
|
|
108
|
Bass R, Roberto D, Wang DZ, Cantu FP,
Mohamadi RM, Kelley SO, Klotz L and Venkateswaran V: Combining
desmopressin and docetaxel for the treatment of
castration-resistant prostate cancer in an orthotopic model.
Anticancer Res. 39:113–118. 2019.PubMed/NCBI View Article : Google Scholar
|
|
109
|
Weinberg RS, Grecco MO, Ferro GS,
Seigelshifer DJ, Perroni NV, Terrier FJ, Sánchez-Luceros A, Maronna
E, Sánchez-Marull R, Frahm I, et al: A phase II dose-escalation
trial of perioperative desmopressin (1-desamino-8-d-arginine
vasopressin) in breast cancer patients. Springerplus.
4(428)2015.PubMed/NCBI View Article : Google Scholar
|
|
110
|
Iseas S, Roca EL, O'Connor JM, Eleta M,
Sanchez-Luceros A, Di Leo D, Tinelli M, Fara ML, Spitzer E, Demarco
IA, et al: Administration of the vasopressin analog desmopressin
for the management of bleeding in rectal cancer patients: Results
of a phase I/II trial. Invest New Drugs. 38:1580–1587.
2020.PubMed/NCBI View Article : Google Scholar
|
|
111
|
Hermo GA, Torres P, Ripoll GV, Scursoni
AM, Gomez DE, Alonso DF and Gobello C: Perioperative desmopressin
prolongs survival in surgically treated bitches with mammary gland
tumours: A pilot study. Vet J. 178:103–108. 2008.PubMed/NCBI View Article : Google Scholar
|
|
112
|
Ikeda Y, Zabbarova I, de Rijk M, Kanai A,
Wolf-Johnston A, Weiss JP, Jackson E and Birder L: Effects of
vasopressin receptor agonists on detrusor smooth muscle tone in
young and aged bladders: Implications for nocturia treatment.
Continence (Amst). 2022(100032)2022.PubMed/NCBI View Article : Google Scholar
|
|
113
|
Papatheodorou I, Moreno P, Manning J,
Fuentes AM, George N, Fexova S, Fonseca NA, Füllgrabe A, Green M,
Huang N, et al: Expression atlas update: From tissues to single
cells. Nucl Acids Res. 48:D77–D83. 2020.PubMed/NCBI View Article : Google Scholar
|
|
114
|
Oiso Y, Robertson GL, Nørgaard JP and Juul
KV: Clinical review: Treatment of neurohypophyseal diabetes
insipidus. J Clin Endocrinol Metab. 98:3958–3967. 2013.PubMed/NCBI View Article : Google Scholar
|
|
115
|
Altun G, Hemşinli D, Pulathan Z and
Civelek A: Emergency coronary bypass surgery in patients under the
influence of dualantiplatelet therapy: Effects of tranexamic acid
and desmopressin acetate. Turk J Med Sci. 47:doi:
10.3906/sag-1612-140. 2017.PubMed/NCBI View Article : Google Scholar
|
|
116
|
Bignami E, Cattaneo M, Crescenzi G,
Ranucci M, Guarracino F, Cariello C, Baldassarri R, Isgrò G,
Baryshnikova E, Fano G, et al: Desmopressin after cardiac surgery
in bleeding patients. A multicenter randomized trial. Acta
Anaesthesiol Scand. 60:892–900. 2016.PubMed/NCBI View Article : Google Scholar
|
|
117
|
Jahangirifard A, Razavi MR, Ahmadi ZH and
Forozeshfard M: Effect of desmopressin on the amount of bleeding
and transfusion requirements in patients undergoing heart
transplant surgery. Basic Clin Pharmacol Toxicol. 121:175–180.
2017.PubMed/NCBI View Article : Google Scholar
|
|
118
|
Jin L and Ji HW: Effect of desmopressin on
platelet aggregation and blood loss in patients undergoing valvular
heart surgery. Chin Med J. 128:644–647. 2015.PubMed/NCBI View Article : Google Scholar
|
|
119
|
Mirmansoori A, Farzi F, Sedighinejad A,
Imantalab V, Mohammadzadeh A, Roushan ZA, Tehran SZ, Nemati M and
Dehghan A: The effect of desmopressin on the amount of bleeding in
patients undergoing coronary artery bypass graft surgery with a
cardiopulmonary bypass pump after taking anti-platelet medicine.
Anesth Pain Med. 6(e39226)2016.PubMed/NCBI View Article : Google Scholar
|
|
120
|
Cheong M, Lee TY, Lee J and Kim SB: No
effect of desmopressin administration before kidney biopsy on the
risk of major post-biopsy bleeding. Nefrologia (Engl Ed).
26:S0211–S6995. 2021.PubMed/NCBI View Article : Google Scholar : (In English,
Spanish).
|
|
121
|
Leclerc S, Nadeau-Fredette AC, Elftouh N,
Lafrance JP, Pichette V and Laurin LP: Use of desmopressin prior to
kidney biopsy in patients with high bleeding risk. Kidney Int Rep.
5:1180–1187. 2020.PubMed/NCBI View Article : Google Scholar
|
|
122
|
Wang LC, Hu YF, Chen L, Xing R, Lin XF and
Kou QY: Desmopressin acetate decreases blood loss in patients with
massive hemorrhage undergoing gastrointestinal surgery. Turk J
Gastroenterol. 31:474–481. 2020.PubMed/NCBI View Article : Google Scholar
|
|
123
|
Anderson LT, David R, Bonnet K and Dancis
J: Passive avoidance learning in Lesch-Nyhan disease: Effect of
1-desamino-8-arginine-vasopressin. Life Sci. 24:905–910.
1979.PubMed/NCBI View Article : Google Scholar
|
|
124
|
Beckwith BE, Petros T, Kanaan-Beckwith S,
Couk DI, Haug RJ and Ryan C: Vasopressin analog (DDAVP) facilitates
concept learning in human males. Peptides. 3:627–630.
1982.PubMed/NCBI View Article : Google Scholar
|
|
125
|
Eisenberg J, Hamburger-Bar R and Belmaker
RH: The effect of vasopressin treatment on learning in Down's
syndrome. J Neural Transm. 60:143–147. 1984.PubMed/NCBI View Article : Google Scholar
|
|
126
|
Beckwith BE, Petros TV, Bergloff PJ and
Staebler RJ: Vasopressin analogue (DDAVP) facilitates recall of
narrative prose. Behav Neurosci. 101:429–432. 1987.PubMed/NCBI View Article : Google Scholar
|
|
127
|
Beckwith BE, Petros TV, Bergloff PJ,
Swenson RR and Paulson R: Failure of posttrial administration of
vasopressin analogue (DDAVP) to influence memory in healthy, young,
male volunteers. Peptides. 16:1327–1328. 1995.PubMed/NCBI View Article : Google Scholar
|
|
128
|
Beckwith BE, Till RE, Reno CR and Poland
RE: Dose-dependent effects of DDAVP on memory in healthy young
adult males: A preliminary study. Peptides. 11:473–476.
1990.PubMed/NCBI View Article : Google Scholar
|
|
129
|
Eisenhofer G, Lambie DG and Robinson BJ:
No improvement in ethanol-induced memory deficits after
administration of a vasopressin analog. Life Sci. 37:2499–2505.
1985.PubMed/NCBI View Article : Google Scholar
|
|
130
|
Guard O, Marchal G, Graule A, Dumas R and
D'Athis P: Effects of vasopressin and desmopressin on memory. A
double-blind study in 40 healthy volunteers. Neuropsychobiology.
15:80–83. 1986.PubMed/NCBI View Article : Google Scholar
|
|
131
|
Jenkins JS, Mather HM and Coughlan AK:
Effect of desmopressin on normal and impaired memory. J Neurol
Neurosurg Psychiatry. 45:830–831. 1982.PubMed/NCBI View Article : Google Scholar
|
|
132
|
Millar K, Jeffcoate WJ and Walder CP:
Vasopressin and memory: Improvement in normal short-term recall and
reduction of alcohol-induced amnesia. Psychol Med. 17:335–341.
1987.PubMed/NCBI View Article : Google Scholar
|
|
133
|
Nebes RD, Reynolds CF III and Horn LC: The
effect of vasopressin on memory in the healthy elderly. Psychiatry
Res. 11:49–59. 1984.PubMed/NCBI View Article : Google Scholar
|
|
134
|
Till RE and Beckwith BE: Sentence memory
affected by vasopressin analog (DDAVP) in cross-over experiment.
Peptides. 6:397–402. 1985.PubMed/NCBI View Article : Google Scholar
|
|
135
|
Weingartner H, Gold P, Ballenger JC,
Smallberg SA, Summers R, Rubinow DR, Post RM and Goodwin FK:
Effects of vasopressin on human memory functions. Science.
211:601–603. 1981.PubMed/NCBI View Article : Google Scholar
|
|
136
|
Abdollahian E, Sargolzaee MR, Hajzade M,
Mohebbi MD and Javanbakht A: Effects of desmopressin (DDAVP) on
memory impairment following electroconvulsive therapy (ECT). Acta
Neuropsychiatr. 16:130–137. 2004.PubMed/NCBI View Article : Google Scholar
|
|
137
|
Lerer B, Zabow T, Egnal N and Belmaker RH:
Effect of vasopressin on memory following electroconvulsive
therapy. Biol Psychiatry. 18:821–824. 1983.PubMed/NCBI
|
|
138
|
Beckwith BE, Couk DI and Till TS:
Vasopressin analog influences the performance of males on a
reaction time task. Peptides. 4:707–709. 1983.PubMed/NCBI View Article : Google Scholar
|
|
139
|
Jennings JR, Nebes RD and Reynolds CF III:
Vasopressin peptide (DDAVP) may narrow the focus of attention in
normal elderly. Psychiatry Res. 17:31–39. 1986.PubMed/NCBI View Article : Google Scholar
|
|
140
|
Tsikunov SG and Belokoskova SG:
Psychophysiological analysis of the influence of vasopressin on
speech in patients with post-stroke aphasias. Span J Psychol.
10:178–188. 2007.PubMed/NCBI View Article : Google Scholar
|
|
141
|
Gold PW, Weingartner H, Ballenger JC,
Goodwin FK and Post RM: Effects of 1-desamo-8-D-arginine
vasopressin on behaviour and cognition in primary affective
disorder. Lancet. 2:992–994. 1979.PubMed/NCBI View Article : Google Scholar
|
|
142
|
Hosseini SMR, Farokhnia M, Rezaei F,
Gougol A, Yekehtaz H, Iranpour N, Salehi B, Tabrizi M, Tajdini M,
Ghaleiha A and Akhondzadeh S: Intranasal desmopressin as an adjunct
to risperidone for negative symptoms of schizophrenia: A
randomized, double-blind, placebo-controlled, clinical trial. Eur
Neuropsychopharmacology. 24:846–855. 2014.PubMed/NCBI View Article : Google Scholar
|















