Introduction
Letrozole, an aromatase inhibitor, is a commonly
used drug for ovulation induction in assisted reproduction
(1). It has several merits
compared with clomiphene or human menopausal gonadotropin,
including enhanced endometrial lining, improved cervical mucus,
mono-follicular development and low risk of ovarian
hyperstimulation syndrome (2-4).
Moreover, letrozole is also widely accepted by sub-fertile patients
owing to convenient oral administration and the low-cost of the
treatment (5).
However, in 2005, Novartis, a major pharmaceutical
producer of letrozole, issued a contraindication for letrozole use
to physicians worldwide soon after the disputable abstract by
Marinko Biljan in which higher risk of cardiac and locomotor
anomalies were reported for neonates born from mothers using
letrozole (6,7). In response to this controversy, a
subsequent study indicated no association between letrozole use and
increased risk of congenital anomalies (8). Despite all these findings, the
restriction on letrozole as an ovulation-inducing drug has not been
lifted yet.
Currently, in numerous developing countries such as
China and India, letrozole, mainly used as an off-label drug, has
gained great popularities among sub-fertile patients and
reproductive physicians due to the aforementioned merits (9,10).
Although previous studies have evaluated the impact of letrozole on
neonatal birth outcomes, similar studies based on the Chinese
cohort are inefficient (1,10-13).
Such a study is necessary due to genetic heterogeneity, metabolic
difference and discrepancy in protocol of ovulation induction.
Furthermore, it may possibly provide us with more evidence
concerning the safety of letrozole, and hopefully expedite the
removal of restriction on letrozole use.
In the present study, birth outcomes, congenital
anomalies and neonatal complications of singletons born to mothers
who have received letrozole for ovulation were investigated.
Neonates born to mothers who have either received other
ovulation-inducing drugs or ovulated naturally were recruited as
the control group.
Materials and methods
Study participants and design
The electronic medical archives of women who had
undergone natural cycles/ovulation-inducing cycles and intrauterine
insemination (IUI) therapies in the Reproductive Center of The
First Affiliated Hospital of Shantou University Medical College
(Shantou, China) during January 1, 2016 and December 31, 2021 were
retrospectively retrieved and analyzed. The inclusion criteria were
as follows: i) Women who underwent natural or ovulation-inducing
cycles resulting in pregnancies after IUI therapies; ii) women aged
between 20 to 41 years, with a body mass index of <35
kg/m2; and iii) delivered a live singleton. Further
selection was performed according to the following exclusion
criteria: i) Spousal sperm abnormalities; ii) endometriosis; iii)
abnormalities of the uterus; iv) karyotypic abnormalities; and v)
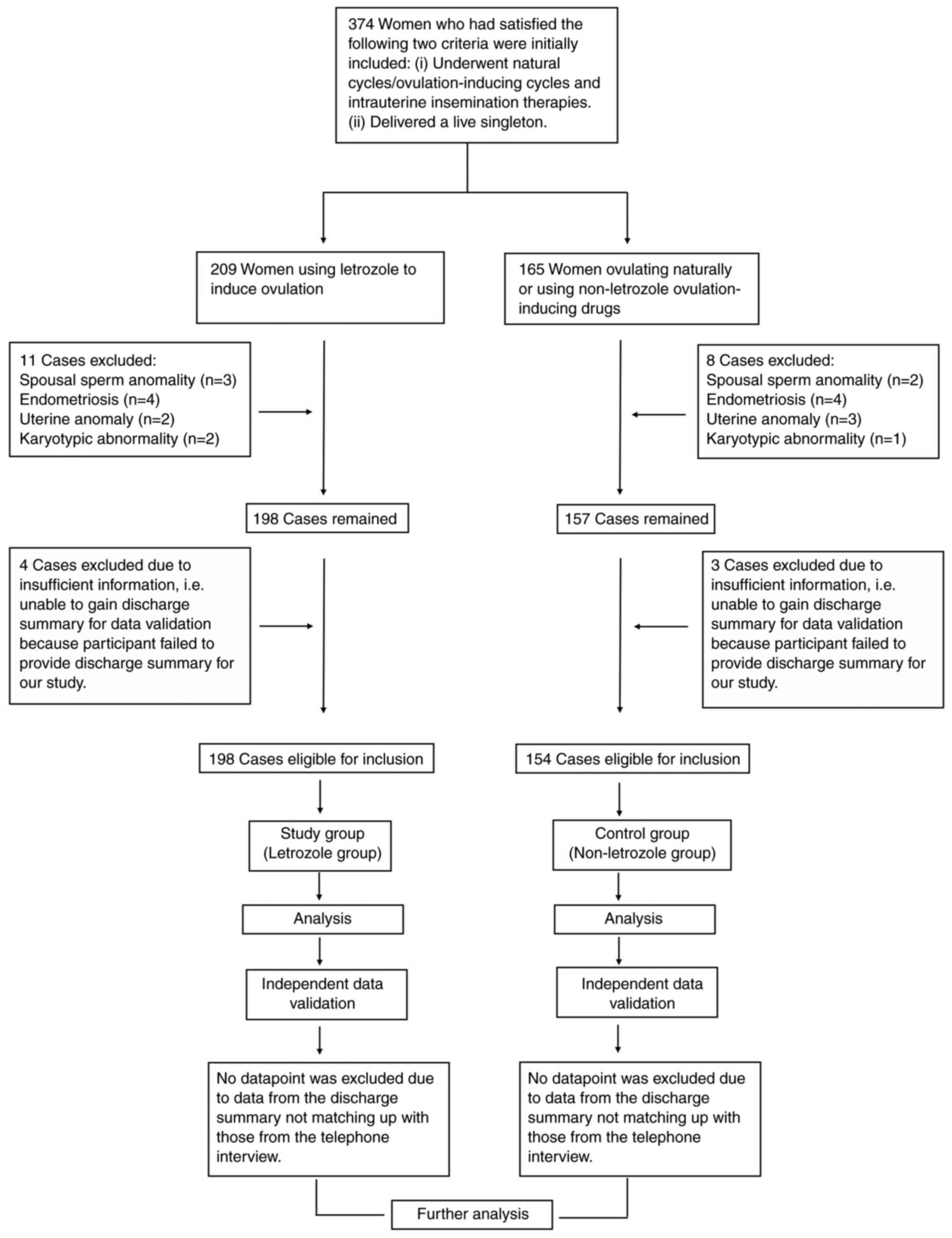
insufficient information. As illustrated in the flowchart of
Fig. 1, a total of 348 women were
finally enrolled, of which 194 women were defined as the study
group (letrozole group; using letrozole to induce ovulation) and
154 women were defined as the control group (non-letrozole group;
ovulating naturally or using non-letrozole ovulation-inducing
drugs). Before the initiation of the therapy, each woman went
through a set of standard fertility examinations in the center.
Information about neonatal birth outcomes were collected via
telephone interviews by trained senior nurses. Ethics approval was
obtained from the Institutional Ethics Committee of The First
Affiliated Hospital of Shantou University Medical College (approval
no. 2015; Shantou, China). At the early stage (such as the
recruitment stage) of the present study, all participants provided
written informed consent. The written informed consent enabled the
authors to collect data from the participants. All data collected
were treated with confidentiality and anonymity. At the late stage
of the present study, in order to acquire discharge summary for
data validation from participants who did not deliver the infant in
this hospital, additional verbal informed consent was obtained from
the participants after adequate telephone communication (refer to
subsection ‘Independent validation of the data’ for more
information). Principles of The Declaration of Helsinki (the ninth
revision, October 2013) were strictly adhered throughout the
present study.
Fertility examinations prior to
therapy
The fertility examinations were as follows: i)
Visual inspection of the female reproductive organs; ii)
Serological determination of sex hormones, including estradiol,
progesterone, prolactin, testosterone, luteinizing hormone and
follicle-stimulating hormone; iii) Hysterosalpingography for tubal
patency test; iv) Gynecologic ultrasonography to detect
abnormalities of the uterus and adnexa, and, in particular, to
assess ovarian function through evaluation of the morphology and
size of the ovaries and calculation of the antral follicle count;
v) Measurement of anti-Müllerian hormone when necessary. vi)
Cervical cytology; vii) TORCH test, including toxoplasma, rubella,
cytomegalovirus and herpes; viii) Screening for human
immunodeficiency virus (HIV), hepatitis B virus (HBV), hepatitis C
virus (HCV), human papilloma virus (HPV), syphilis, chlamydia and
gonococcus and ix) karyotype analysis when necessary. Items ii),
v), vi), vii), viii) and ix) were completed in the Department of
Clinical Laboratory of The First Affiliated Hospital of Shantou
University Medical College. The rest of the examinations were
completed in the Reproductive Center of The First Affiliated
Hospital of Shantou University Medical College.
The inclusion and exclusion criteria
for vetting patients for IUI therapy
The inclusion and exclusion criteria were as
follows: i) Patients with vaginal atresia or complete transverse
vaginal septum were excluded from IUI. Patients with congenital
absence of uterus, rudimentary uterus, infantile uterus,
hydrosalpinx, endometrial polyp, ovarian neoplasm, ovarian mass and
chocolate cyst of ovary were excluded. Patients with cervical
precancerous lesion or cervical carcinoma were excluded. ii)
Patients with unilateral or bilateral tubal patency were eligible,
those with bilateral tubal obstruction were excluded. iii) Patients
with reproductive endocrinal disorder were excluded and advised to
receive additional treatments. iv) Patients with adequate ovarian
function were eligible, those with poor ovarian function were
excluded and advised to receive in vitro fertilization. v)
Patients with infertility caused by cervical factors were eligible;
patients with immune infertility or unexplained infertility were
eligible. vi) Patients with karyotypic abnormality or familial
hereditary disease were excluded. vii) Patients with acute
infection of the genitourinary system or sexually transmitted
disease were excluded. Patients in acute stage of infection of any
of the following pathogens were excluded: Toxoplasma, rubella,
cytomegalovirus, herpes, HIV, HBV, HCV and HPV. viii) Patients with
mental disorder were excluded.
Protocols for controlled ovulation
stimulation (COS) and IUI
For each patient, the initiating dose of COS was
individually tailored by a fertility physician according to age,
body mass index, history of previous ovarian response and the
present ovarian reserve. Generally, COS started 3-5 days after the
menstruation. COS and IUI were then performed as described in a
previous study by the authors (14). A total of 2 weeks after the IUI
therapy, serum test for beta-human chorionic gonadotropin (β-hCG)
was carried out to detect the existence of pregnancy. Luteal
support was conducted as stated in a previous study by the authors
(15). Live birth was defined as
the birth of a live infant of >28 weeks of gestation.
Follow-up interview
Follow-up interview was initiated two weeks after
the IUI therapy and was carried out once every two months after
that. With the help of a customized questionnaire (Table SI), a trained senior nurse
collected data concerning maternal health, pregnancy and neonatal
birth outcomes via telephone calls. Interview was terminated if one
of the following circumstances occurred: i) negative reaction in
test for serum β-hCG 2 weeks after the IUI therapy; ii)
miscarriage; and iii) delivery of a live neonate. In the present
study, all included participants were adequately followed. The
follow-up rate was 100%.
Independent validation of the
data
In the present study, participants were from four
cities (Shantou, Chaozhou, Jieyang and Shanwei) of eastern
Guangdong (China). Furthermore, women who came from the three other
cities and became pregnant after receiving treatments in The First
Affiliated Hospital of Shantou University Medical College, would
likely decide to deliver their infants in the nearby hospital of
their cities. As a result, some neonates of the present study were
born in other hospitals.
Neonates born in The First Affiliated Hospital of
Shantou University Medical College and those born in other
hospitals (81 in total, 46 in letrozole group and 35 in
non-letrozole group) were included in the present study so as to
achieve an adequate sample size for analysis. First, for all
neonates included in the current study, information about maternal
health, pregnancy and neonatal birth outcome were collected via
telephone interviews by trained senior nurses. Second, for neonates
born in our hospital, data pertaining to pregnancy outcome and
neonatal birth outcome were directly extracted from the electronic
medical records of the hospital, and were subsequently used for
verification against information collected via telephone interview.
For neonates born in other hospitals, similar data were acquired
from the discharge summary of the puerpera. After verbal informed
consent was obtained from the puerpera through phone call, copy of
the discharge summary was obtained from the puerpera via electronic
mail or regular mail. These collected data were later used for
verification.
For each pair of mother and neonate, the data
validation was regarded as acceptable if the following three
requirements were satisfied: i) Data from medical archives matched
up with those from telephone interviews in terms of the basic
information of the parents, including name, age, address and
contact number. ii) Data from medical archives matched up with
those from telephone interviews in terms of neonatal birth outcome,
including date of birth, hospital where the delivery occurred, sex,
gestational age, mode of delivery, weight, height and Apgar score.
Apgar scoring system was used to evaluate the condition of neonates
1 min after birth of the neonates. The numbers were determined by
observations of 5 signs (heart rate, respiratory effort, reflex
irritability, muscle tone, and color). A rating of 0, 1 or 2 was
given to each sign. Apgar score of 10 indicated a good condition
for the neonate, while Apgar score <7 indicated a poor condition
(16). iii) Data from medical
archives matched up with those from telephone interviews in terms
of pregnancy outcome, neonatal birth defect (if any) and neonatal
complication (if any). If inconformity occurred during the
verification, data acquisition was repeated to address the
inconformity, data were collected again from another round of
telephone interview and the discharge summary was obtained again
when necessary, and validation was performed again.
Neonatal birth outcomes
Preterm birth was defined as delivery of an infant
before 37 weeks of gestation. Low birth weight was defined as the
birth of an infant with birth weight <2,500 g. Macrosomia was
defined as an infant with birth weight >4,000 g. Fetal growth
restriction referred to an infant with birth weight less than the
10th percentile for gestational age. Birth defects were categorized
according to the 10th Edition of the Q-code of the International
Statistical Classification of Diseases and Related Health Problems
(17). Neonatal complications in
the present study included neonatal intensive care unit admission,
fetal growth restriction, fetal asphyxia, oligohydramnios, preterm
birth, low birth weight, macrosomia, Apgar score <7 and
congenital defects.
Statistical analysis
Data analyses were conducted with the SPSS program
(Version 20.0, IBM Corp.). Proportion data were presented as number
or percentage and were compared using Pearson's chi-squared or
Fisher's exact test. Continuous data were expressed as the mean ±
standard deviation or median (minimum-maximum) and compared using
unpaired Student's t-test or Mann-Whitney U test, accordingly,
depending on the data distribution. Spearman's rank correlation
analysis was employed to explore possible correlation between
maternal use of letrozole and neonatal birth outcomes. Logistic
regression model was constructed to calculate the contributing
strength of one specific factor associated with neonatal
complications. Missing data were addressed using the listwise
deletion method as recommended by SPSS. Statistically significant
difference was set at a two-tailed P<0.05.
Results
Baseline characteristics of women in
letrozole and non-letrozole group
First, the baseline characteristics of women using
letrozole to counterparts from women who did not receive letrozole
were compared. As shown in Table
I, women of the letrozole group were younger than those from
the non-letrozole group (P<0.001 vs. the non-letrozole group).
Furthermore, statistically significant difference was observed in
the history of previous IUI therapies between the two groups
(P=0.049 vs. the non-letrozole group). However, for other
characteristics listed in Table I,
data were all comparable between the two groups (all P>0.05 vs.
the non-letrozole group).
 | Table IBaseline characteristics of women in
letrozole and non-letrozole group. |
Table I
Baseline characteristics of women in
letrozole and non-letrozole group.
| Characteristics | Letrozole
(n=194) | Non-letrozole
(n=154) | P-value |
|---|
| Age (years) | 28 (20-41) | 29 (21-38) | 0.000a |
| Duration of
infertility (years) | 3 (1-10) | 3 (1-13) | 0.381 |
| Etiology of
infertility | | | |
|
Primary | 137 (70.6%) | 108 (70.1%) | 0.921 |
|
Secondary | 57 (29.4%) | 46 (29.9%) | |
| IUI cycle | | | |
|
1st
cycle | 94 (48.7%) | 94 (62.2%) | 0.049a |
|
2nd
cycle | 67 (34.7%) | 44 (29.1%) | |
|
3rd
cycle | 24 (12.4%) | 9 (5.9%) | |
|
Cycles after
3 attempts | 8 (4.1%) | 4 (2.6%) | |
| Body mass index
(kg/m2) | 21.04
(16.8-32.5) | 21.35
(13.4-31.6) | 0.544 |
| Basal estradiol
(pg/ml) | 38.0 (14-405) | 42.5 (11-125) | 0.373 |
| Basal progesterone
(ng/ml) | 0.32 (0.01-1.39) | 0.34 (0.01-1.72) | 0.325 |
| Number of mature
follicles on the trigger day | 1.0 (1-4) | 1.0 (1-4) | 0.127 |
| Endometrium thickness
on the trigger day (mm) | 10.0 (5.5-16.0) | 10.00 (7.5-15.8) | 0.792 |
Birth outcomes of singletons in
letrozole group and non-letrozole group
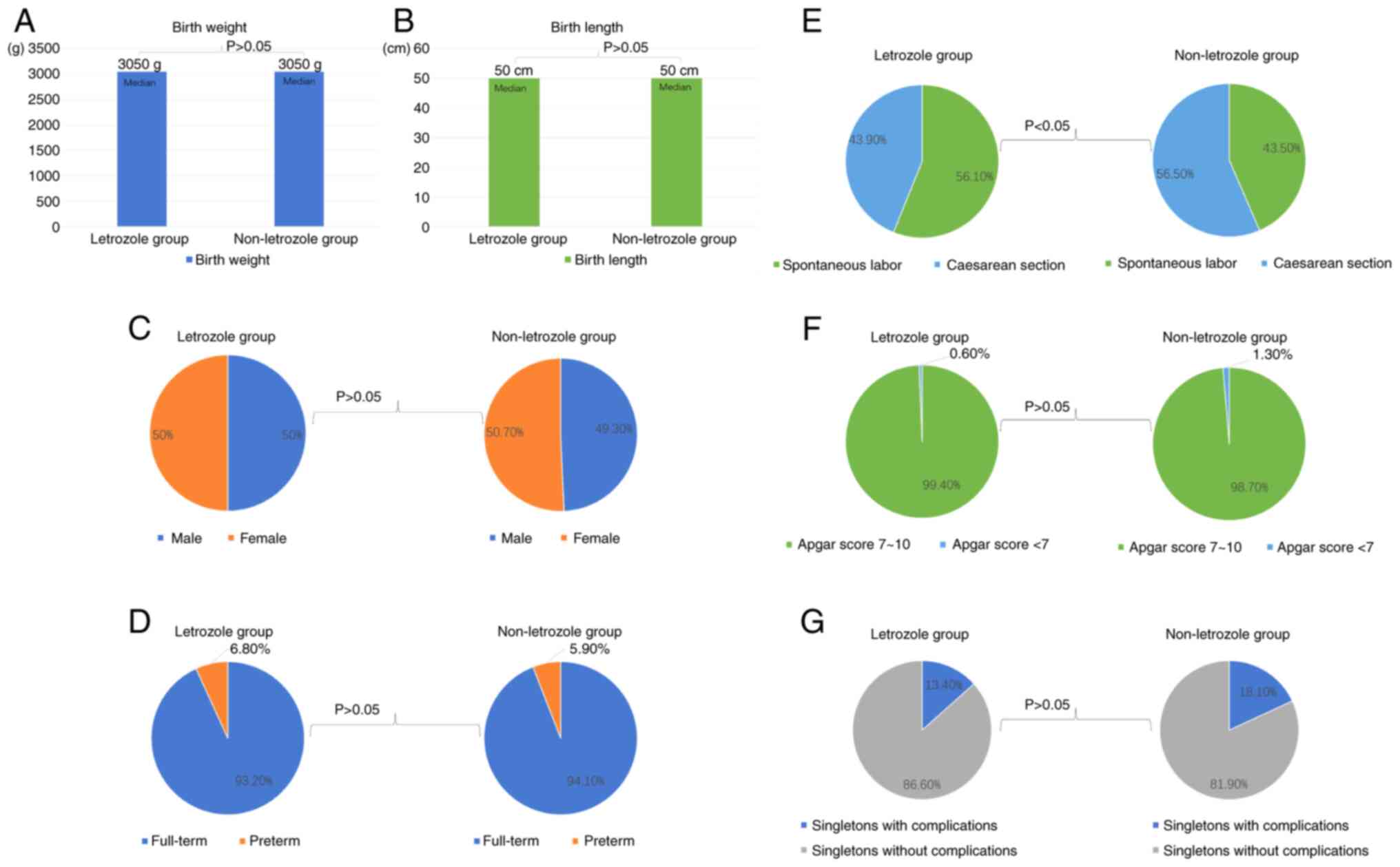
Subsequently, the birth outcomes of singletons in
the letrozole and the non-letrozole group were investigated. As
indicated in Table II and
Fig. 2, a significantly low
proportion of caesarean section deliveries were found in the
letrozole group (caesarean section deliveries, 43.8% of the
letrozole group vs. 56.4% of the non-letrozole group, P=0.019). For
other characteristics presented in Table II and Fig. 2, including neonatal sex, full-term
or preterm birth, birth weight, birth length, Apgar score and
neonatal complications, data were all similar between the two
groups (P>0.05 vs. the non-letrozole group).
 | Table IIBirth outcomes of singletons in
letrozole and non-letrozole group. |
Table II
Birth outcomes of singletons in
letrozole and non-letrozole group.
| Characteristics | Letrozole
(n=194) | Non-letrozole
(n=154) | P-value |
|---|
| Sex | | | |
|
Male | 97 (50%) | 76 (49.3%) | 0.904 |
|
Female | 97 (50%) | 78 (50.6%) | |
| Full-term or preterm
birth | | | |
|
Full-term | 181 (93.2%) | 145 (94.1%) | 0.744 |
|
Preterm | 13 (6.7%) | 9 (5.8%) | |
| Delivery mode | | | |
|
Spontaneous
labor | 109 (56.1%) | 67 (43.5%) | 0.019a |
|
Caesarean
section | 85 (43.8%) | 87 (56.4%) | |
| Birth weight (g) | 3,050
(2,000-4,600) | 3,050
(1,900-5,300) | 0.599 |
| Birth length
(cm) | 50 (41-55) | 50 (40-54) | 0.575 |
| Apgar score | | | |
|
7~10 | 193 (99.4%) | 152 (98.7%) | 0.586 |
|
<7 | 1 (0.5%) | 2 (1.2%) | |
| Singletons with
complicationsb | | | |
|
Yes | 26 (13.4%) | 28 (18.1%) | 0.221 |
|
No | 168 (86.5%) | 126 (81.8%) | |
Congenital anomalies of singletons in
letrozole group and non-letrozole group
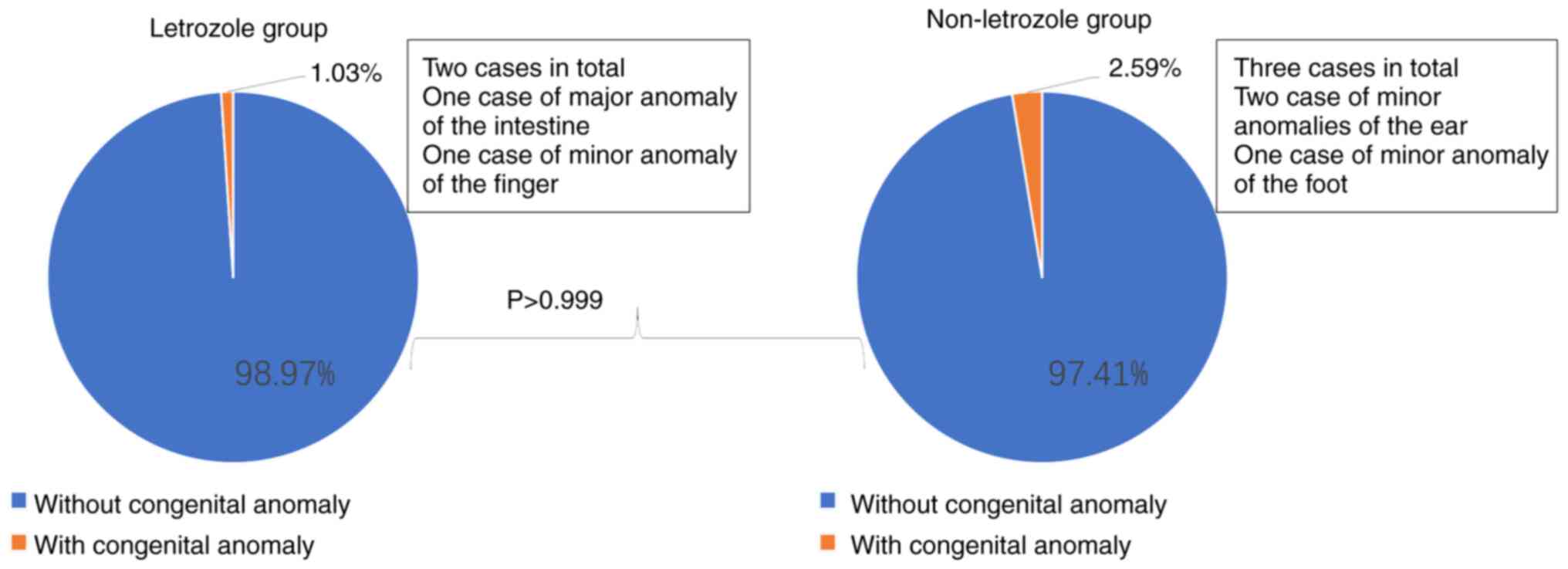
Moreover, congenital anomalies of singletons in the
letrozole group and the non-letrozole group were analyzed. Overall,
no significant difference was found between the two groups
(Table III and Fig. 3, all P>0.05 vs. the
non-letrozole group). One case of major congenital anomaly
(congenital intestinal atresia, required surgical intervention) was
reported in the letrozole group, as compared with none in the
non-letrozole group (non-significant difference, P>0.999 vs. the
non-letrozole group). In addition, 1 case of minor congenital
anomaly (finger anomaly) was discovered in the letrozole group, and
3 cases of minor congenital anomalies (1 case of ankylotia, 1 case
of undersize right ear and 1 case of strephenopodia) in the
non-letrozole group (non-significant difference, P=0.658 vs. the
non-letrozole group).
 | Table IIICongenital anomalies of singletons in
letrozole and non-letrozole group. |
Table III
Congenital anomalies of singletons in
letrozole and non-letrozole group.
| Congenital
anomalies | Letrozole
(n=194) | Non-letrozole
(n=154) | P-value |
|---|
| Major congenital
anomalies | 1 (0.51%) | 0 (0%) | >0.999 |
| Minor congenital
anomalies | 1 (0.51%) | 3 (2.59%) | 0.326 |
| Total congenital
anomalies | 2 (1.03%) | 3 (2.59%) | 0.658 |
| Brain and nervous
system | 0 (0%) | 0 (0%) | |
| Face and neck | 0 (0%) | 2 (1.29%) | 0.195 |
| Circulatory
system | 0 (0%) | 0 (0%) | |
| Digestive
system | 1 (0.51%) | 0 (0%) | >0.999 |
| Genitourinary
system | 0 (0%) | 0 (0%) | |
| Anomalies of
limbs | 1 (0.51%) | 1 (0.64%) | >0.999 |
| Chromosomal
anomalies | 0 (0%) | 0 (0%) | |
Correlation between maternal use of
letrozole and neonatal birth outcomes
With Spearman's rank correlation analysis, the
possible correlation between maternal use of letrozole and neonatal
birth outcomes was explored. As presented in Table IV, after controlling for etiology
of infertility, duration of infertility, age, history of previous
IUI and body mass index, no significant correlation was observed
between maternal use of letrozole and neonatal birth outcomes (all
P>0.05). However, the P-value for the correlation between
maternal use of letrozole and neonatal complications was marginal
(P=0.051).
 | Table IVCorrelation analysis between maternal
use of letrozole and neonatal birth outcomes. |
Table IV
Correlation analysis between maternal
use of letrozole and neonatal birth outcomes.
| | n=348 |
|---|
| Investigated
factors | | Delivery mode | Full-term or
preterm birth | Neonatal sex | Birth length | Birth weight | Apgar score | Neonatal
complicationsa |
|---|
| Letrozole use
to | Correlation
coefficient | 0.059 | 0.066 | 0.033 | 0.071 | 0.012 | 0.068 | 0.126 |
| induce
ovulationb | P-value | 0.361 | 0.306 | 0.604 | 0.270 | 0.852 | 0.290 | 0.051 |
Effect of maternal use of letrozole on
neonatal complications of singletons
Considering the marginal P-value (P=0.051) for the
correlation between maternal use of letrozole and neonatal
complications, this correlation was further investigated using the
logistic regression analysis. As presented in Table V, results from logistic regression
analysis confirmed that maternal use of letrozole was not a
significant contributor for neonatal complications, independent of
statistical adjustment (crude OR, 1.436; 95% CI, 0.803-2.569;
P=0.223 vs. adjusted OR, 1.406; 95% CI, 0.748-2.643; P=0.290).
 | Table VEffect of maternal use of letrozole
on neonatal complications of singletons. |
Table V
Effect of maternal use of letrozole
on neonatal complications of singletons.
| | Neonatal
complications of singletonsa (n=348) |
|---|
| Factors
investigated | Crude OR (95%
CI) | P-value | Adjusted OR (95%
CI) | P-value |
|---|
| Letrozole use to
induce ovulationb | 1.436
(0.803-2.569) | 0.223 | 1.406
(0.748-2.643) | 0.290 |
Discussion
Currently, the investigation of the safety of
letrozole administration for ovulation induction is limited in the
Chinese cohort. The present study is the first report of such kind.
Overall, it was found that birth outcomes of neonates born to
mothers using letrozole were not inferior when compared with
neonates born to mothers using non-letrozole ovulation-inducing
strategies. This finding indicated that maternal use of letrozole
is not associated with poorer birth outcomes, which is consistent
with numerous previous studies (1,10-13).
Noteworthily, the percentage of caesarean section deliveries in the
letrozole group was significantly lower than that of the
non-letrozole group (Table II).
This phenomenon may be partly attributed to the younger age of
women in the letrozole group (Table
I), since previous studies have demonstrated that advanced age
of puerpera is associated with elevated possibilities of a
caesarean section delivery (18).
As one of the important aims of the present study,
the incidence of congenital anomalies between the letrozole and the
non-letrozole group was evaluated. It was discovered that incidence
of major and minor congenital anomalies between the two groups were
comparable (Table III). This
finding supported the fact that, as an ovulation-inducing drug,
letrozole is equally safe when compared with other counterparts.
Maternal use of letrozole does not appear to be associated with
increased risk of congenital anomaly. This finding is in agreement
with numerous previous studies (8).
In the present study, a case of major congenital
anomaly (congenital intestinal atresia) and a case of minor
congenital anomaly (finger anomaly) were observed in the letrozole
group. By contrast, a total of 3 cases of minor congenital
anomalies (a case of ankylotia, a case of undersized right ear and
a case of strephenopodia) were observed in the non-letrozole group.
The overall incidence of congenital anomaly in the letrozole and
the non-letrozole group were 1.03 and 2.59% respectively, which are
lower than those observed in the general population (19). This discrepancy may be due to the
fact that only live singletons were included and analyzed in the
present study. Congenital anomalies of twins or triplets were
excluded in the present study to avoid any biases that originated
from multiple births. This exclusion inevitably reduced the
incidence of congenital anomaly observed herein.
Despite numerous previous studies having
investigated the impact of maternal use of letrozole on congenital
anomaly, the relationship between maternal use of letrozole and
neonatal complications still remains largely unknown (1,10-13).
Therefore, as another important purpose of the present study, the
relation between maternal use of letrozole and neonatal
complications was analyzed. With Spearman's rank correlation
analysis, a marginal P-value (P=0.051) for the correlation between
maternal use of letrozole and neonatal complications was found
(Table IV). To further clarify
this ambiguity, the authors went on validating this correlation
using the logistic regression model. Results from both unadjusted
and adjusted regression analyses revealed that maternal use of
letrozole is not a significant predictor for neonatal complications
(Table V). These findings appear
to suggest that maternal use of letrozole for ovulation does not
correlate with increased risk of neonatal complications. These
findings provided us with additional evidences to support the
safety of letrozole as an ovulation-inducing drug.
At present, the major concern about application of
letrozole as an ovulation-inducing drug centers on its debatable
teratogenic effects (6). However,
for a teratogenic effect to occur, teratogen must be present at the
time of embryogenesis or organogenesis (4,5). The
median half-life of letrozole is ~45 h (30-60 h) (4). Generally, letrozole is administrated
in the early follicular phase to induce ovulation, and it should be
completely eliminated from the body by the time of embryogenesis or
organogenesis. It is unlikely that letrozole may directly affect
fetal embryogenesis or organogenesis (20). The findings of the present study
are in agreement with this theory and indicate that letrozole could
be a safe drug for ovulation induction.
The present study has its own strengths and
limitations. As congenital anomalies were more prevalent among
multiple births (10). Only
neonatal data of live singletons were included for analysis in the
study, while those from multiple gestations were excluded so as to
avoid any possible biases originated from multiple gestations. In
addition, unlike multicenter studies, in the present study all the
pregnancy-related attempts were conducted and completed in the same
hospital by the same medical team to ensure the consistency of the
data and minimize potential biases as much as possible.
However, there are limitations to the present study.
One of the limitations is the retrospective design of the study. It
is likely that recall bias or misinterpretation bias may occur
during interview, as information collected through interview may
not always be accurate. To address this problem, data were obtained
again from medical archives and subsequently used for verification
against those collected from the interview. This validation
procedure was carried out to reduce the influence of recall bias or
misinterpretation bias. However, owning to the retrospective design
of the present study, additional prospective studies are needed to
further confirm the current finding. The second limitation is the
relatively small sample size; further researches with sufficient
sample size are necessary to confirm the findings of the present
study.
In conclusion, the results appeared to suggest that
maternal use of letrozole for ovulation induction does not
associate with poorer birth outcomes or increased risk of
congenital anomalies and neonatal complications. The present study
provided additional evidence to support the safety of letrozole as
an ovulation-inducing drug, and helped to restore letrozole as a
low-risk medicine for ovulation induction by reducing the concern
about its teratogenic effects.
Supplementary Material
Questionnaire of follow-up.
Acknowledgements
Not applicable.
Funding
Funding: The present study was supported by Guangdong Science
and Technology Department (grant no. 2016A020218015).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
BW and ZL designed the study. BW, RX, HL and SL
collected and analyzed the data. BW, HL and SL drafted the initial
manuscript. ZL reviewed and edited the article. ZL and SL served as
guarantors, accepted full responsibility for the work and
controlled the decision to publish. All authors read and approved
the final manuscript. BW and ZL confirmed the authenticity of all
the raw data.
Ethics approval and consent to
participate
The present study was approved (approval no. 2015)
by the Institutional Ethics Committee of The First Affiliated
Hospital of Shantou University Medical College (Shantou, China).
Written informed consent was provided by all participants. The
present study was carried out according to the principles of The
Declaration of Helsinki.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Takeshima K, Ezoe K, Kawasaki N, Hayashi
H, Kuroda T and Kato K: Perinatal outcomes and congenital anomalies
associated with letrozole and natural cycles in single fresh
cleaved embryo transfers: A single-center, 10-year cohort study. F
S Rep. 3:138–144. 2022.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Gonen Y and Casper RF: Sonographic
determination of a possible adverse effect of clomiphene citrate on
endometrial growth. Hum Reprod. 5:670–674. 1990.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Weiss NS, van Vliet MN, Limpens J, Hompes
PGA, Lambalk CB, Mochtar MH, van der Veen F, Mol BWJ and van Wely
M: Endometrial thickness in women undergoing IUI with ovarian
stimulation. How thick is too thin? A systematic review and
meta-analysis. Hum Reprod. 32:1009–1018. 2017.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Tulandi T and DeCherney AH: Limiting
access to letrozole-is it justified? Fertil Steril. 88:779–780.
2007.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Kar S: Current evidence supporting
‘letrozole’ for ovulation induction. J Hum Reprod Sci. 6:93–98.
2013.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Biljan MM, Hemmings R and Brassard N: The
outcome of 150 babies following the treatment with letrozole or
letrozole and gonadotropins. Fertil Steril. 84 (Suppl
1.1)(S95)2005.
|
|
7
|
Casper RF and Mitwally MF: A historical
perspective of aromatase inhibitors for ovulation induction. Fertil
Steril. 98:1352–1355. 2012.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Pundir J, Achilli C, Bhide P, Sabatini L,
Legro RS, Rombauts L, Teede H, Coomarasamy A, Zamora J and
Thangaratinam S: Risk of foetal harm with letrozole use in
fertility treatment: A systematic review and meta-analysis. Hum
Reprod Update. 27:474–485. 2021.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Yang AM, Cui N, Sun YF and Hao GM:
Letrozole for female infertility. Front Endocrinol (Lausanne).
12(676133)2021.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Sharma S, Ghosh S, Singh S, Chakravarty A,
Ganesh A, Rajani S and Chakravarty BN: Congenital malformations
among babies born following letrozole or clomiphene for infertility
treatment. PLoS One. 9(e108219)2014.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Akbari Sene A, Ghorbani S and Ashrafi M:
Comparison of the pregnancy outcomes and the incidence of fetal
congenital abnormalities in infertile women treated with letrozole
and clomiphene citrate. J Obstet Gynaecol Res. 44:1036–1041.
2018.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Tulandi T, Martin J, Al-Fadhli R, Kabli N,
Forman R, Hitkari J, Librach C, Greenblatt E and Casper RF:
Congenital malformations among 911 newborns conceived after
infertility treatment with letrozole or clomiphene citrate. Fertil
Steril. 85:1761–1765. 2006.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Yun J, Choi YS, Lee I, Won YB, Lee JH, Seo
SK, Cho S, Lee BS and Yun BH: Comparison of congenital
malformations among babies born after administration of letrozole
or clomiphene citrate for infertility treatment in a Korean cohort.
Reprod Toxicol. 82:88–93. 2018.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Wang B and Li Z: Comparison of
dual-trigger and human chorionic gonadotropin-only trigger among
polycystic ovary syndrome couples who underwent controlled ovarian
stimulation and intrauterine insemination: A retrospective cohort
study. Medicine (Baltimore). 102(e32867)2023.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Wang B and Li Z: Hypersecretion of basal
luteinizing hormone and an increased risk of pregnancy loss among
women with polycystic ovary syndrome undergoing controlled ovarian
stimulation and intrauterine insemination. Heliyon.
9(e16233)2023.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Papile LA: The Apgar score in the 21st
century. New Engl J Med. 344:519–520. 2001.PubMed/NCBI View Article : Google Scholar
|
|
17
|
World Health Organization (WHO):
International statistical classification of diseases and related
health problems. 10th Revision, fifth edition. WHO, Geneva, 2016.
https://apps.who.int/iris/handle/10665/246208.
Accessed August 27, 2023.
|
|
18
|
Marchi J, Berg M, Dencker A, Olander EK
and Begley C: Risks associated with obesity in pregnancy, for the
mother and baby: A systematic review of reviews. Obes Rev.
16:621–638. 2015.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Jacqz-Aigrain E and Koren G: Effects of
drugs on the fetus. Semin Fetal Neonatal Med. 10:139–147.
2005.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Dean JCS: Catalog of teratogenic agents. J
Med Genet. 39(454)2002.
|