Introduction
Chronic kidney disease (CKD) remains a public health
challenge worldwide, the etiology of which is associated with
various factors, such as lifestyle, genetic predisposition and
metabolic dysregulation (1). CKD
is associated with substantial morbidity, mortality and healthcare
costs, making early identification of high-risk populations
particularly important for the timely initiation of preventive
strategies that may inhibit progressive renal decline (2).
It has been reported that lipid accumulation product
(LAP) index, an emerging biomarker that serves as a novel indicator
for the quantification of lipid overaccumulation, could potentially
predict metabolic aberrations (3).
LAP index is calculated using readily available clinical measures
(waist circumference and fasting triglycerides), making it an
easily accessible tool for clinical and epidemiological
applications (3,4). Its use extends beyond a mere lipid
profile index, offering an insight into the interplay between
adiposity and lipid metabolism, which is particularly pertinent in
the context of CKD (5).
The pathophysiological association between lipid
metabolism and renal function is further strengthened by the
evidence that lipid accumulation in renal cells can precipitate and
exacerbate renal injury (6).
Dyslipidemia, a common comorbidity in CKD, is implicated in the
pathogenesis of glomerulosclerosis and tubulointerstitial fibrosis
(7). Consequently, LAP index may
serve as a surrogate marker for detecting early renal impairment
(8).
The potential of LAP index as a predictive marker
for CKD has recently become a focus of research. Several
observational studies have highlighted an association between
elevated LAP index levels and the prevalence of CKD, suggesting a
dose-response relationship wherein higher quartiles of LAP index
are correlated with a higher risk of renal dysfunction (8-10).
This proposed gradient of risk highlights the need to assess the
magnitude and consistency of the association across diverse
populations.
The complexity of CKD etiology is further compounded
by the presence of diabetes mellitus (DM), a condition that
singularly accelerates the progression of nephropathy, and is a
primary cause of CKD in numerous regions (11). The close relationship between
hyperglycemia, insulin resistance and lipid disorders further
strengthens the need to evaluate the relationship between LAP index
and CKD in diabetic subpopulations (12), and to conduct subgroup analyses
based on the presence of DM. These analyses may improve risk
stratification and allow for the tailoring of preventive measures
in these high-risk cohorts.
Currently, the body of evidence regarding the
relationship between LAP index and CKD is based on studies of
varying designs, populations and outcomes (8-10).
To the best of our knowledge, no systematic review and
meta-analysis on the subject has been published to date. The
present study aimed to summarize the existing evidence on the
predictive capacity of LAP index for CKD, explore the risk gradient
conferred by LAP levels, and analyze the association between LAP
index and CKD in the diabetic subpopulation.
Materials and methods
Protocol registration and eligibility
criteria
The present study has been registered in PROSPERO
(registration no. CRD42023486707; https://www.crd.york.ac.uk/PROSPERO/display_record.php?RecordID=486707).
Studies satisfying the following criteria were considered eligible:
i) Studies reporting the diagnostic utility of the LAP index for
predicting CKD, or reporting the association of LAP index across
various quintiles or tertiles and CKD; ii) no restriction for study
design (trials/case-control/cohort/cross-sectional studies) or
study participants (irrespective of age, sex, comorbidities etc.);
iii) published full-text studies or abstracts; iv) no language
restriction; v) published until November 2023.
Search strategy
PubMed/MEDLINE (https://pubmed.ncbi.nlm.nih.gov), Scopus (https://www.scopus.com/search/form.uri?display=basic#basic),
Cochrane Library (https://www.cochranelibrary.com/search), ScienceDirect
(https://www.sciencedirect.com) and
Google Scholar (https://scholar.google.com) databases were searched.
Both medical subject heading and free-text terms were utilized for
the search. Examples of terms were ‘Lipid Accumulation Product’,
‘Chronic Kidney Disease’, ‘Diagnostic Accuracy’, ‘Utility’,
‘Declined Renal Function’, ‘Validation Studies’, ‘Chronic Renal
Failure’ and ‘LAP Index’. As mentioned in the eligibility criteria,
there were no language restrictions during the search, and the time
limit was from the starting year of the database until November 30,
2023. References of retrieved full texts were also identified and
screened.
Study selection strategy
In the initial phase of the review process, two
separate researchers meticulously examined the titles, key words
and abstracts of the relevant papers. In the second phase, the full
text of the selected articles was screened for eligibility. Any
differences of opinion regarding the selection of studies were
resolved through the adjudication of a third researcher.
Data extraction
The lead author was responsible for gathering
relevant details from the studies and entering the data into STATA
software 14.2 (StataCorp LLC). Data extraction encompassed a range
of variables, including the first author, year of publication,
study country, setting and region, design of the study, number of
participants, criteria for inclusion and exclusion, reference
standards, average age of participants, values of the LAP index,
incidence of CKD, as well as counts of true positives, true
negatives, false positives and false negatives. To ensure accuracy,
a thorough validation of the data was conducted by
cross-referencing the entered data with the corresponding reports
from each study.
Risk of bias assessment
The evaluation of potential bias was conducted
independently by two evaluators using the Newcastle Ottawa Scale
for observational studies (13).
This assessment covered three main areas: Selection, for which up
to four stars could be awarded; comparability, with a maximum of
two stars; and outcome, for which up to three stars available. The
overall quality score ranged from zero to nine stars, with a score
between seven and nine indicating high quality, five to six
reflecting moderate quality, and a score from zero to four
suggesting low quality.
Statistical analysis
All analyses were executed using the ‘Midas’ command
package in STATA software (14).
In the analytical stage for binary outcomes, the number of events
and sample size for each group to deduce the pooled effect
estimate, expressed as an odds ratio (OR). These were visually
summarized in a forest plot. A random-effects model using
inverse-variance model with the DerSimonian-Laird estimator was
used (15). The presence of
heterogeneity was examined using the χ2 test and the
I2 statistic, which measures the degree of inconsistency
among the study results. Subgroup analysis was performed based on
DM status and study design. The potential for publication bias was
evaluated using a funnel plot.
For the diagnostic accuracy of the LAP index in
predicting CKD, a bivariate meta-analysis approach was employed.
This allowed us to compute pooled diagnostic indices, such as
sensitivity, specificity, the diagnostic odds ratio (DOR), and
likelihood ratios for positive (LR+) and negative
(LR-) results. The diagnostic value of the LAP index was
presented graphically via forest plots, which included both
study-specific and aggregated estimates. In addition, the summary
receiver operating characteristic (sROC) curve was illustrated to
measure the area under the ROC curve (AUROC). P<0.05 was
considered to indicate a statistically significant difference.
Diagnostic accuracy related analysis was performed using the
‘Midas’ package in STATA.
Results
Search results
A flowchart of the study selection process is
summarized in Fig. 1. Initially,
1,514 records were identified from various databases. Of them, 342
were removed as duplicates. Abstracts of the remaining 1,172
records were searched, and full texts of 140 studies were assessed
for eligibility. Finally, 17 studies were included in the review
(8-10,12,16-28).
Characteristics of the included
studies
As summarized in Table
SI, the included 17 studies had cross-sectional, prospective,
retrospective and cohort designs, and originated from diverse
geographical locations, including Iran, the United States, China,
Cameroon, South Korea and Taiwan. The age ranges of the
participants varied between studies, with some studies reporting
data of patients aged ≥60 years, while others included a broader
age range (≥18 years). Sample sizes across these studies ranged
from 200 to 14,068 patients. Diagnostic criteria for CKD primarily
relied on estimated glomerular filtration rates and
albumin-to-creatinine ratios, with varying cut-off points for
different studies (Table SI).
Most studies received no stars in the
representativeness and sample size domains, indicating a common
issue with these domains across the studies. A significant number
of studies managed to secure at least 1 star in ascertainment of
exposure and control of confounding, reflecting a moderate level of
detail in these domains. Eight out of the 17 studies had a low risk
of bias (Table SII).
Association between LAP index and
CKD
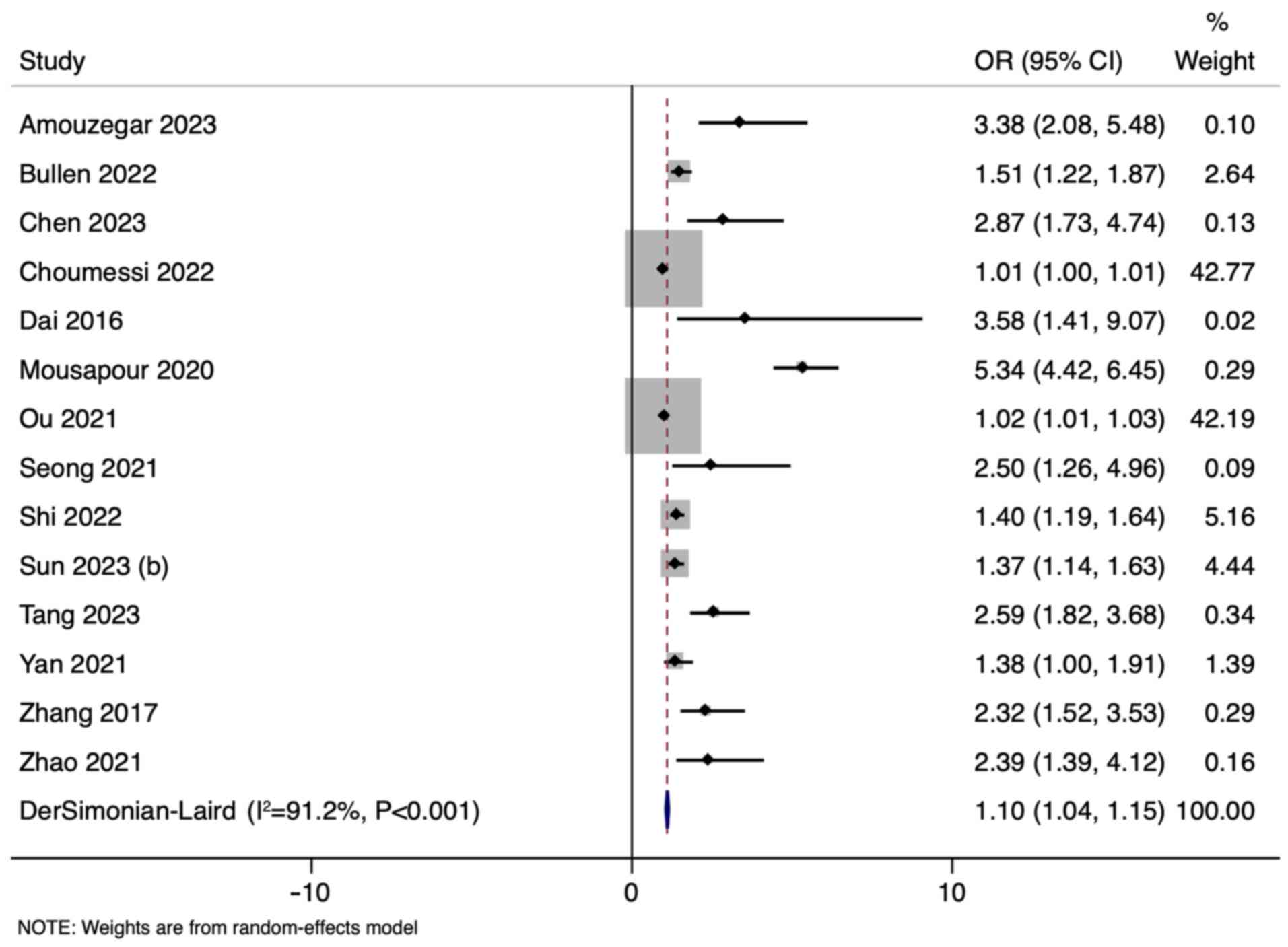
The results of the random-effects inverse-variance
model with the DerSimonian-Laird estimator for τ² showed an OR of
1.10 (95% CI: 1.04-1.15) across all studies that reported the
association (14/17), indicating a modest association between the
higher quintiles or tertiles of LAP index and CKD (Fig. 2). However, the heterogeneity among
the included studies was substantial, as indicated by an I² of
91.2%. The funnel plot was asymmetrical indicating publication bias
(Fig. S1).
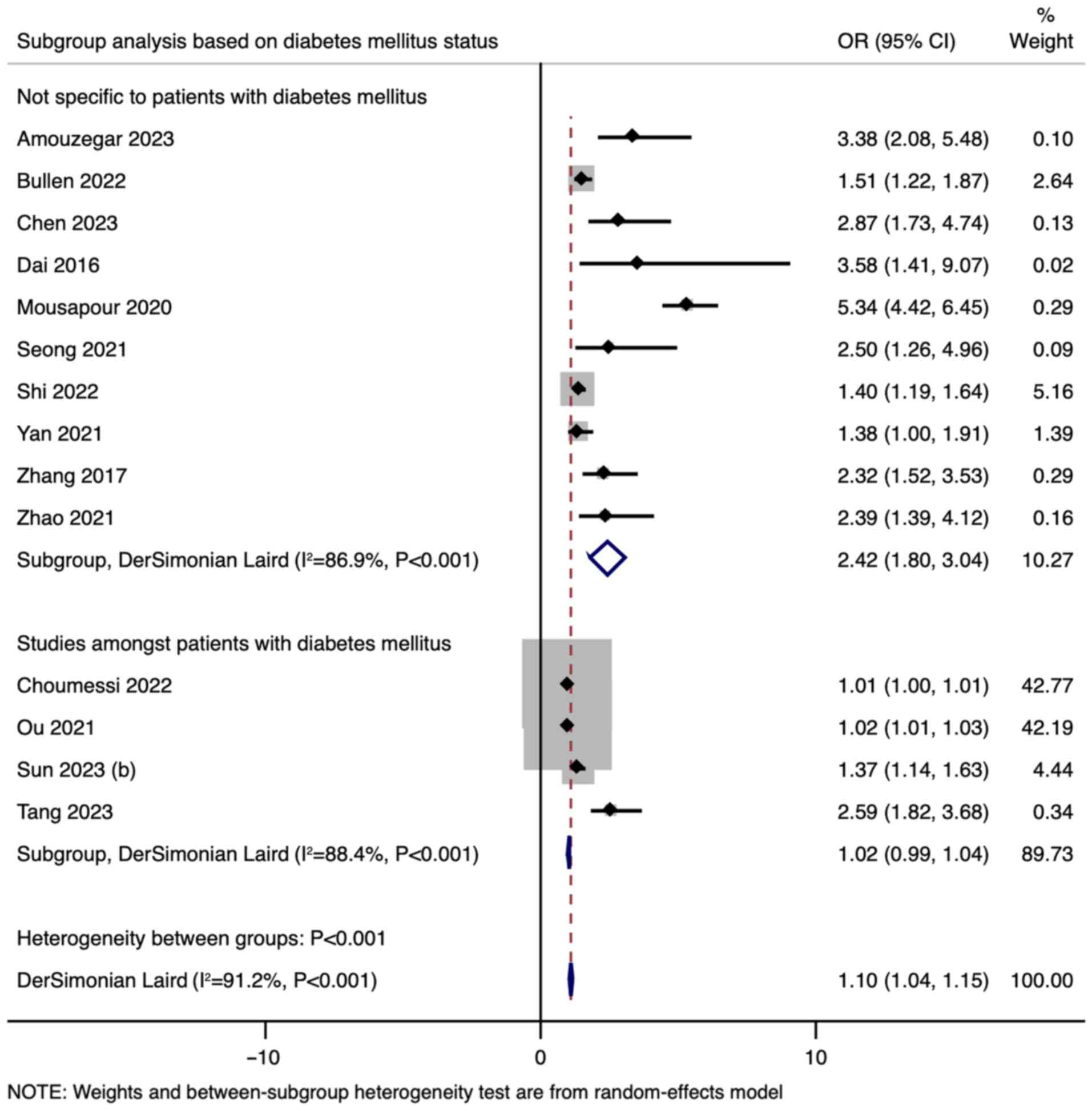
In the subgroup analysis based on DM status, studies
not specific to patients with DM demonstrated a higher pooled OR
(OR=2.42, 95% CI: 1.80-3.04) compared with studies among patients
with DM (OR=1.02, 95% CI: 0.99-1.04) (Fig. 3). This indicates a significant
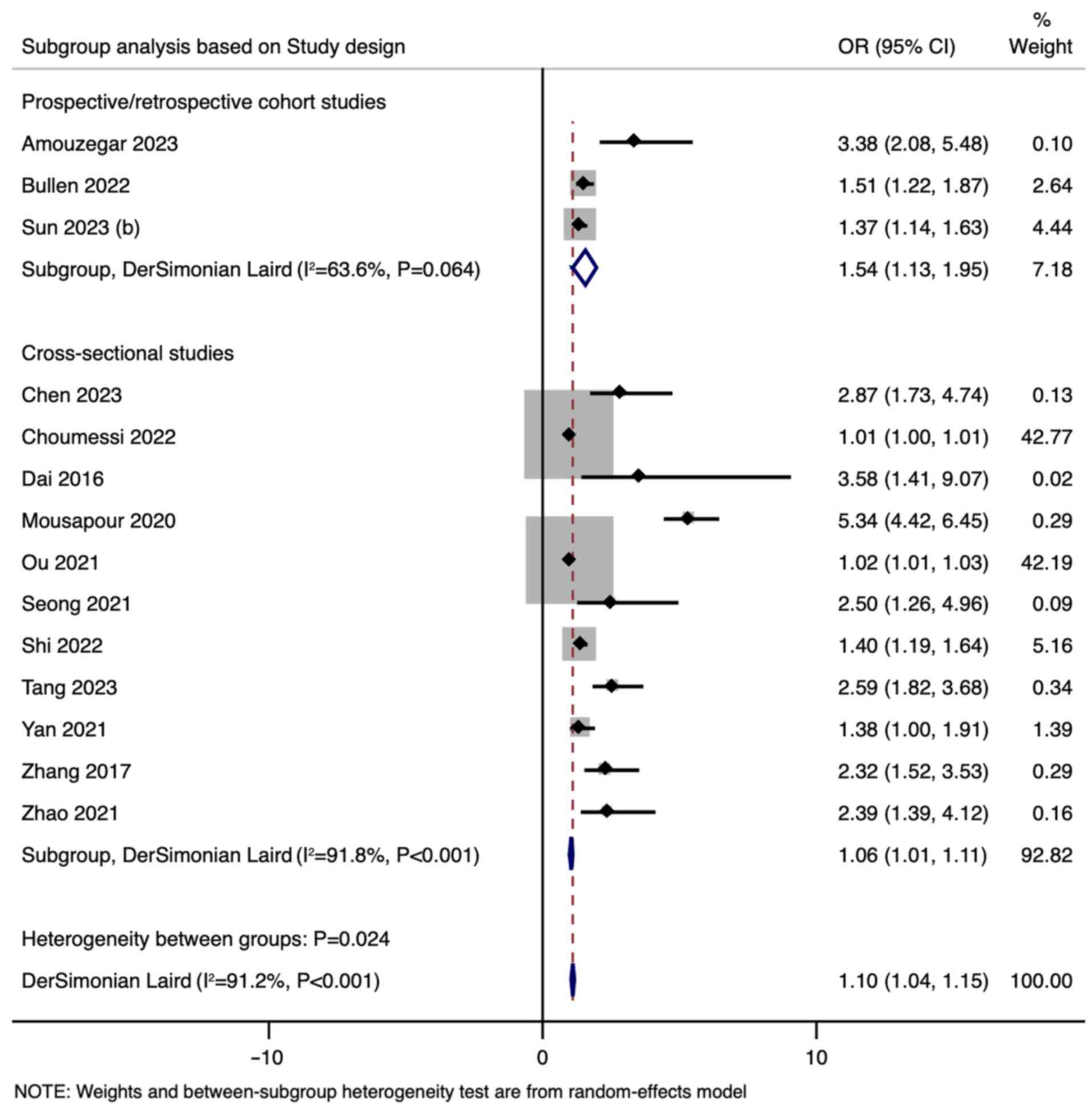
difference between the subgroups (P<0.001). Subgroup analysis
was also performed based on study design; the pooled OR for
prospective/retrospective cohort studies was 1.54 (95% CI:
1.13-1.95), whereas cross-sectional studies had a lower pooled OR
of 1.06 (95% CI: 1.01-1.11) (Fig.
4).
Diagnostic accuracy of LAP index for
CKD
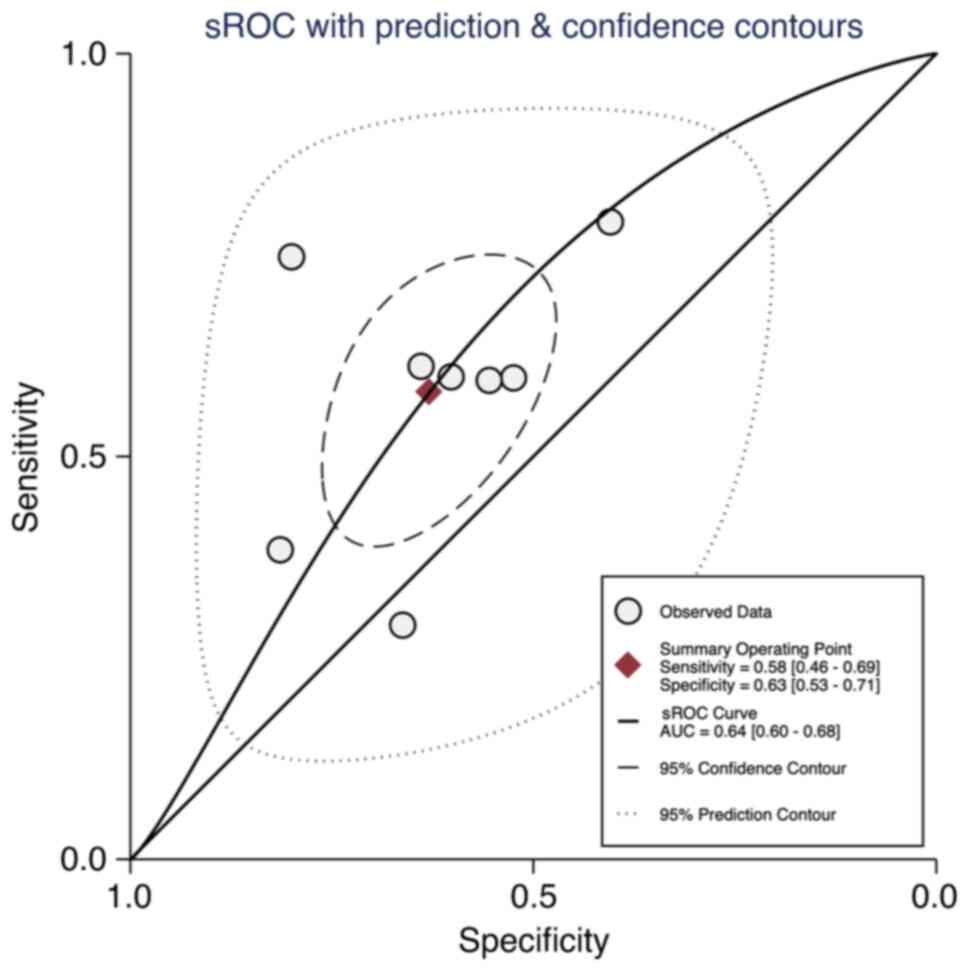
The diagnostic performance of LAP index was
evaluated using sROC curves. The calculated AUROC of 0.64 indicated
moderate accuracy (Fig. 5). The
95% CI for the AUROC ranged from 0.60 to 0.68, suggesting a degree
of uncertainty around the estimate. The heterogeneity of the
included studies, as assessed by the χ2 test, was
extremely high (P<0.001), with an I2 value of 100%
(data not shown).
As shown in Fig. 5,
the estimated sensitivity of the test was 0.58, (95% CI:
0.46-0.69), suggesting that the test correctly identifies the
condition 58% of the time when the condition is present. The
specificity was estimated at 0.63 (95% CI: 0.53-0.71), indicating
the test correctly identifies the absence of the condition 63% of
the time.
The LR+ of 1.6, with a 95% CI of 1.2-2.0,
suggested a moderate increase in the odds of having the condition
when the test is positive. Conversely, the LR- was 0.67,
with a 95% CI of 0.52-0.85, indicating that a negative test result
is moderately useful for ruling out the condition. The diagnostic
odds ratio (DOR) was estimated at 2.00, with a 95% CI of 1.00-4.00,
showing a moderate effect size. A DOR of 2 indicates that the test
is twice as likely to give a positive result in those with the
condition compared to those without it (data not shown).
Discussion
The present meta-analysis revealed a modest but
significant association between LAP index and CKD, with a pooled OR
of 1.098. Notably, the subgroup analysis demonstrated that this
association was significantly higher in patients without DM. The
findings further emphasize the nuanced relationship between lipid
metabolism and renal function, particularly in different clinical
backgrounds.
The association between LAP index and CKD observed
in the present study adds a significant layer to the existing body
of knowledge. Previous research has primarily focused on
traditional lipid profiles, often overlooking the potential of LAP
index as a predictor for CKD. The present findings align with Fang
et al (29), who reported a
similar level of association and accuracy for visceral adiposity
index and CKD. Notably, the present subgroup analysis indicated the
differential impact of lipid accumulation in diabetic versus
non-diabetic individuals, suggesting a possible interplay between
metabolic status and renal health. This differential effect
confirms previous findings that highlighted the amplified risk of
CKD in non-diabetic populations with dyslipidemia (30).
The interplay between lipid metabolism and renal
dysfunction identified in the present study underscores the
multifaceted nature of CKD. LAP index, as a surrogate marker of
abnormal lipid accumulation, may reflect a broader spectrum of
metabolic disorders, including insulin resistance and inflammation,
which are all known contributors to renal pathology (31). The difference between diabetic and
non-diabetic subgroups in the present analysis could be attributed
to the underlying differences in metabolic and inflammatory
profiles between these populations. The results suggested that LAP
index might be more than a mere marker of lipid imbalance,
potentially providing an insight into the complex metabolic
interplay of CKD pathogenesis.
The moderate diagnostic accuracy of the LAP index,
as indicated by an AUROC of 0.64, makes it a potentially useful,
although not definitive, tool in CKD diagnosis. The sensitivity and
specificity values suggested a balanced ability to detect CKD and
to exclude its absence, respectively. However, the DOR of 2, while
indicative of moderate effectiveness, calls for the use of the LAP
index in conjunction with other diagnostic measures, rather than as
a standalone test.
While LAP index alone demonstrated moderate
diagnostic accuracy for CKD, its efficiency might be enhanced when
combined with other biomarkers or clinical parameters. For example,
combining LAP index with traditional markers, such as glomerular
filtration rate and albuminuria, could potentially yield a more
comprehensive risk assessment tool (32). This approach could be particularly
advantageous in primary care settings, where simple and
cost-effective tools are essential for early disease detection.
Age, blood pressure and body weight are other pertinent factors
that, when combined with LAP index, could provide a more targeted
screening tool for CKD (33). The
incorporation of these parameters may potentially refine the
specificity of CKD diagnosis, as they are known to be associated
with disease progression and patient outcomes.
It is important to acknowledge that the metabolic
interplay associated with CKD is complex and multifactorial.
Therefore, expanding the diagnostic toolkit beyond a single
biomarker to a composite index that includes demographic and
clinical variables, such as age, hypertension status and weight,
could allow for a more stratified risk assessment. This
comprehensive approach aligns with the precision medicine
initiative, which emphasizes tailoring medical treatment to the
individual characteristics of each patient. Furthermore, exploring
the utility of LAP index in different stages of CKD could provide
insights into its role across the disease spectrum, possibly
identifying stages where its predictive value is maximized.
From a clinical standpoint, the present results
suggested that monitoring LAP index could be integral in early CKD
detection, particularly in non-diabetic patients. This could lead
to timely interventions, potentially altering the disease
trajectory. In the public health domain, these findings advocate
for a more nuanced approach to CKD screening, possibly
incorporating LAP index assessment into routine health checks,
particularly for populations at higher risk due to lipid metabolism
disorders. Such targeted screening programs could be more effective
and cost-efficient.
Considering the rising prevalence of CKD globally
and its profound impact on healthcare systems, the findings of the
present study carry significant implications for individual patient
management and public health strategies. In clinical settings,
incorporating LAP index assessment into routine evaluations could
facilitate early identification of individuals at an elevated risk
for CKD, particularly among those not traditionally categorized as
high risk, such as non-diabetic patients. This early detection is
crucial, as it can result in more timely interventions, potentially
slowing disease progression and improving outcomes. From a public
health perspective, these findings advocate for the inclusion of
lipid management in CKD prevention programs. Such initiatives could
encompass not only pharmacological interventions, but also
lifestyle modifications, such as weight reduction and improved
dietary habits. Given the substantial economic and quality-of-life
burdens associated with CKD, integrating such preventive strategies
could yield significant benefits at the individual and societal
level.
The strength of the present study lies in its
comprehensive approach, integrating data from diverse studies to
provide a robust estimate of the association between LAP index and
CKD. The use of a random-effects model further enhances the
generalizability of the findings. However, the substantial
heterogeneity among the included studies and the indication of
publication bias necessitate a cautious interpretation. These
limitations underscore the need for more uniform research
methodologies in future studies.
Future research should focus on longitudinal studies
to clarify the causal relationship between LAP index and CKD
development. Furthermore, exploring ways to enhance the diagnostic
accuracy of the LAP index, possibly through a combination with
other biomarkers, could significantly impact clinical practice.
Studies in diverse populations, such as in patients with
hypertension and in overweight individuals, may also be valuable to
validate and extend the present findings.
In conclusion, the present study highlighted the
potential of the LAP index as a marker for CKD risk, particularly
in non-diabetic individuals. The findings advocate for its
incorporation into CKD screening protocols, possibly in combination
with other diagnostic tools. This research paves the way for more
targeted and effective public health strategies in CKD prevention
and management.
Supplementary Material
Funnel plot.
Included studies for
meta-analysis.
Risk of bias assessment.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
FW conceived and designed the study. CC, JW and YW
collected the data and performed the literature search. FW was
involved in the writing of the manuscript. All authors have read
and approved the final version of the manuscript. FW, CC, JW and YW
confirm the authenticity of all the raw data.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Webster AC, Nagler EV, Morton RL and
Masson P: Chronic kidney disease. Lancet. 389:1238–1252.
2017.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Luyckx VA, Tonelli M and Stanifer JW: The
global burden of kidney disease and the sustainable development
goals. Bull World Health Organ. 96:414–422D. 2018.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Khanmohammadi S, Tavolinejad H,
Aminorroaya A, Rezaie Y, Ashraf H and Vasheghani-Farahani A:
Association of lipid accumulation product with type 2 diabetes
mellitus, hypertension, and mortality: A systematic review and
meta-analysis. J Diabetes Metab Disord. 21:1943–1973.
2022.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Raposo MA, Guimarães NS and Tupinambás U:
Lipid accumulation product index to predict metabolic syndrome in
people living with HIV. Clin Med Res. 18:120–125. 2020.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Gai Z, Wang T, Visentin M, Kullak-Ublick
GA, Fu X and Wang Z: Lipid accumulation and chronic kidney disease.
Nutrients. 11(722)2019.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Bobulescu IA: Renal lipid metabolism and
lipotoxicity. Curr Opin Nephrol Hypertens. 19:393–402.
2010.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Rosenstein K and Tannock LR: Dyslipidemia
in chronic kidney disease. In: Endotext. Feingold KR, Anawalt B,
Blackman MR, et al. (eds.) MDText.com, Inc., South
Dartmouth (MA), 2000.
|
|
8
|
Bullen AL, Katz R, Kumar U, Gutierrez OM,
Sarnak MJ, Kramer HJ, Shlipak MG, Ix JH, Judd SE, Cushman M and
Garimella PS: Lipid accumulation product, visceral adiposity index
and risk of chronic kidney disease. BMC Nephrol.
23(401)2022.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Sun Y, Yan Y, Liao Y, Chu C, Guo T, Ma Q,
Wang Y, Wang D, Jia H and Mu J: The new visceral adiposity index
outperforms traditional obesity indices as a predictor of
subclinical renal damage in Chinese individuals: A cross-sectional
study. BMC Endocr Disord. 23(78)2023.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Tang M, Yao S, Cao H, Wei X, Zhen Q, Tan
Y, Liu F, Wang Y, Peng Y and Fan N: Interrelation between the lipid
accumulation product index and diabetic kidney disease in patients
with type 2 diabetes mellitus. Front Endocrinol (Lausanne).
14(1224889)2023.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Thomas MC, Brownlee M, Susztak K, Sharma
K, Jandeleit-Dahm KA, Zoungas S, Rossing P, Groop PH and Cooper ME:
Diabetic kidney disease. Nat Rev Dis Primers.
1(15018)2015.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Sun Z, Wang K, Yun C, Bai F, Yuan X, Lee Y
and Lou Q: Correlation between the variability of different obesity
indices and diabetic kidney disease: A retrospective cohort study
based on populations in Taiwan. Diabetes Metab Syndr Obes.
16:2791–2802. 2023.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Lo CK, Mertz D and Loeb M:
Newcastle-Ottawa scale: Comparing reviewers' to authors'
assessments. BMC Med Res Methodol. 14(45)2014.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Vogelgesang F, Schlattmann P and Dewey M:
The evaluation of bivariate mixed models in meta-analyses of
diagnostic accuracy studies with SAS, Stata and R. Methods Inf Med.
57:111–119. 2018.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Cumpston M, Li T, Page MJ, Chandler J,
Welch VA, Higgins JP and Thomas J: Updated guidance for trusted
systematic reviews: A new edition of the cochrane handbook for
systematic reviews of interventions. Cochrane Database Syst Rev.
10(ED000142)2019.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Choumessi AT, Saha BUF, Navti LK, Tibi AS,
Njeck AT and Nantia EA: Assessment of visceral adiposity index and
lipid accumulation product index as markers of chronic kidney
disease among diabetic and hypertensive patients at the Bamenda
Regional Hospital, Cameroon: A cross-sectional study. Pan Afr Med
J. 42(228)2022.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Zhao S, Ren Z, Yu S, Chi C, Tang J,
Maimaitiaili R, Teliewubai J, Li J, Xu Y and Zhang Y: Association
between lipid accumulation product and target organ damage in
elderly population: The northern Shanghai study. Clin Interv Aging.
16:1769–1776. 2021.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Yu P, Meng X, Kan R, Wang Z and Yu X:
Association between metabolic scores for visceral fat and chronic
kidney disease: A cross-sectional study. Front Endocrinol
(Lausanne). 13(1052736)2022.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Shi Y, Hu L, Li M, Zhou W, Wang T, Zhu L,
Bao H, Cheng X and Li P: Association between the surrogate markers
of insulin resistance and chronic kidney disease in Chinese
hypertensive patients. Front Med (Lausanne).
9(831648)2022.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Yan P, Xu Y, Miao Y, Tang Q, Wu Y, Bai X,
Zhang Z, Li Q and Wan Q: Association of lipid accumulation product
with chronic kidney disease in Chinese community adults: A report
from the REACTION study. Lipids Health Dis. 20(131)2021.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Chen T, Wang X, Wang X, Chen H, Xiao H,
Tang H, Feng L, Xiang Z, Zou H and Shao X: Comparison of novel
metabolic indices in estimation of chronic kidney diseases in a
southern Chinese population. Diabetes Metab Syndr Obes.
13:4919–4927. 2020.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Seong JM, Lee JH, Gi MY, Son YH, Moon AE,
Park CE, Sung HH and Yoon H: Gender difference in the association
of chronic kidney disease with visceral adiposity index and lipid
accumulation product index in Korean adults: Korean national health
and nutrition examination survey. Int Urol Nephrol. 53:1417–1425.
2021.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Jin J, Woo H, Jang Y, Lee WK, Kim JG, Lee
IK, Park KG and Choi YK: Novel Asian-specific visceral adiposity
indices are associated with chronic kidney disease in Korean
adults. Diabetes Metab J. 47:426–436. 2023.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Mousapour P, Barzin M, Valizadeh M,
Mahdavi M, Azizi F and Hosseinpanah F: Predictive performance of
lipid accumulation product and visceral adiposity index for renal
function decline in non-diabetic adults, an 8.6-year follow-up.
Clin Exp Nephrol. 24:225–234. 2020.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Amouzegar A, Honarvar M, Masoumi S, Tohidi
M, Mehran L and Azizi F: Sex-specific trajectories of insulin
resistance markers and reduced renal function during 18 years of
follow-up: TLGS. J Clin Endocrinol Metab. 108:e230–e239.
2023.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Zhang K, Li Q, Chen Y, Wang N and Lu Y:
Visceral adiposity and renal function: An observational study from
SPECT-China. Lipids Health Dis. 16(205)2017.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Dai D, Chang Y, Chen Y, Chen S, Yu S, Guo
X and Sun Y: Visceral adiposity index and lipid accumulation
product index: Two alternate body indices to identify chronic
kidney disease among the rural population in northeast China. Int J
Environ Res Public Health. 13(1231)2016.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Ou YL, Lee MY, Lin IT, Wen WL, Hsu WH and
Chen SC: Obesity-related indices are associated with albuminuria
and advanced kidney disease in type 2 diabetes mellitus. Ren Fail.
43:1250–1258. 2021.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Fang T, Zhang Q, Wang Y and Zha H:
Diagnostic value of visceral adiposity index in chronic kidney
disease: A meta-analysis. Acta Diabetol. 60:739–748.
2023.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Suh SH and Kim SW: Dyslipidemia in
patients with chronic kidney disease: An updated overview. Diabetes
Metab J. 47:612–629. 2023.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Anoop SS, Dasgupta R, Rebekah G, Jose A,
Inbakumari MP, Finney G and Thomas N: Lipid accumulation product
(LAP) as a potential index to predict risk of insulin resistance in
young, non-obese Asian Indian males from southern India:
Observations from hyperinsulinemic-euglycemic clamp studies. BMJ
Open Diabetes Res Care. 9(e002414)2021.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Hallan SI, Ritz E, Lydersen S, Romundstad
S, Kvenild K and Orth SR: Combining GFR and albuminuria to classify
CKD improves prediction of ESRD. J Am Soc Nephrol. 20:1069–1077.
2009.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Jallow AW, Bah AN, Bah K, Hsu CY and Chu
KC: Machine learning approach for chronic kidney disease risk
prediction combining conventional risk factors and novel metabolic
indices. Appl Sci. 12(12001)2022.
|