Introduction
Chronic obstructive pulmonary disease (COPD) is a
heterogeneous condition characterized by chronic respiratory
symptoms (dyspnea, cough and sputum production) caused by
abnormalities in the airways (bronchitis and bronchiolitis) and/or
alveoli (emphysema), which cause persistent, often progressive,
airflow obstruction (1).
Researchers have proposed adding the concept of ‘structural changes
due to failed regeneration by the distal airways progenitor cells’
to the updated definition of COPD, highlighting the core traits of
the disease and the demand for novel therapies (2). COPD is the most common chronic
disease of the respiratory system and has a heavy economic burden.
Factors contributing to the pathophysiology of COPD and leading to
the degradation and regeneration of lung tissue include an
imbalance in the protease and antiprotease systems, an imbalance in
oxidation-antioxidant activity, and continuous airway inflammation
(3).
Acute exacerbation of COPD (AECOPD) is mainly caused
by infection or the inhalation of toxic gases. During acute
exacerbation, the baseline symptoms of a patient, such as cough,
expectoration, wheezing and dyspnea, deteriorate significantly.
AECOPD is the leading cause of patient consultation and
hospitalization. This hospitalization can lead to further decline
in lung function, seriously affecting the quality of life of the
patient, and shortening their life expectancy. Frequent
hospitalization for COPD exacerbations has also been associated
with higher mortality risk (4).
Mortality in patients with severe AECOPD exceeds 20% within 1 year
and 50% within 5 years (5).
Compared with stable COPD (SCOPD), airway and systemic inflammation
in AECOPD is further increased, and inflammatory mediators,
cytokines and chemokines are released, causing inflammatory cells
to enter the lung parenchyma and resulting in systemic inflammatory
responses. Scholars worldwide are committed to identifying
additional specific biomarkers in the blood to evaluate the
disease, and have found that certain biomarkers have specific
reference values for evaluating COPD.
Interleukin (IL)-41, a novel cytokine with
immunoregulatory properties (6)
often linked to anti-inflammatory effects, is also known as
meteorin-like, glial cell differentiation regulator (Metrnl),
cometin, subfatin, meteorin and IL-39(7). IL-41 mediates both pro-inflammatory
and anti-inflammatory functions (8). The immune response is diminished when
the production of IL-41 by M2 macrophages is blocked. IL-41 mutant
mice experienced a more severe inflammatory injury in the liver,
kidney, and particularly the uterus when injected with adenoviral
vectors expressing LacZ or Metrnl (9). This implies that IL-41 is crucial for
maintaining anti-inflammatory balance. In a clinical trial,
increased inflammation and macrophage activity were primarily
responsible for high IL-41 levels observed in smokers with AECOPD
(10). However, compared with the
general population, individuals who were discharged after an acute
exacerbation had lower plasma IL-41 levels (11).
IL-6 is a cytokine with multiple functions that
regulates the immune response, inflammation and tumor growth
(5). Golestani et al
(11) found that the concentration
of IL-6 in exhaled breath condensate was markedly higher in
patients with AECOPD than in those with SCOPD. The endopeptidase
enzyme, matrix metalloproteinase-2 (MMP-2), separates extracellular
matrix proteins (12). In patients
with severe COPD, MMP-2 may facilitate processes that cause lung
tissue deterioration (13). Serum
amyloid A (SAA) is a precursor protein involved in AA amyloidosis
and a polymorphic acute-phase protein secreted by hepatocytes that
regulates innate immunity and cholesterol homeostasis (14). Prins et al (15) found that patients with AECOPD had
considerably high levels of SAA. C-reactive protein (CRP) is a
classic inflammatory marker for determining infection and
inflammatory responses and reflects the total burden of systemic
inflammation in an individual (16). Xu and Han (17) reported that CRP levels were related
to the severity of AECOPD complicated by pneumonia. Another study
revealed that the neutrophil percentage (NEU%),
neutrophil-to-lymphocyte ratio (NLR), and platelet-to-lymphocyte
ratio (PLR) in the AECOPD group were considerably higher than those
in the SCOPD and healthy control groups, suggesting NEU%, NLR and
PLR may have specific values for the clinical diagnosis and
treatment of AECOPD (18). The aim
of the present study was to explore the correlation between IL-41
and AECOPD.
Patients and methods
Study design
Patients with COPD and healthy controls were
enrolled at the First Affiliated Hospital of Ningbo University
(Ningbo, China) between March 2021 and November 2022. Among them,
51 patients (AECOPD group) and 56 (SCOPD group) were enrolled. A
total of 56 healthy individuals who visited the hospital for an
in-person checkup during the same period were selected as the
healthy control group at the same time. The average age of the
AECOPD group was (72.16±10.13) years, the average age of the SCOPD
group was (69.32±10.06) years and the average age of the healthy
control group was (70.07±6.12) years. The male/female ratio was
50/1 in the AECOPD group, 49/7 in the SCOPD group and 50/6 in the
healthy control group. There were no significant differences in
terms of gender and age among the three groups (P<0.05).
All individuals with COPD met the Global Initiative
for Chronic Obstructive Lung Disease (GOLD) diagnostic criteria
(19): i) The patient exhibited
symptoms such as long-term recurrent cough, expectoration and
dyspnea; ii) previous exposure to cigarette smoke and dust; and
iii) pulmonary function test results showing a forced expiratory
volume (FEV1)/forced vital capacity (FVC) ratio <70% after
bronchodilator inhalation. Patients with other diseases that could
cause similar symptoms and persistent airflow limitation were
excluded.
An event that meets the diagnostic criteria for
AECOPD was defined as one that worsens in <14 days; is
characterized by increased dyspnea, cough and sputum; and may be
accompanied by tachypnea and/or tachycardia. Such an event is
frequently associated with increased local and systemic
inflammation caused by infection, pollution, or other insults to
the airways (20). The diagnostic
criteria for SCOPD were as follows: Stable symptoms such as cough,
expectoration and shortness of breath, or stable symptoms 1 month
after an AECOPD event.
The exclusion criteria were as follows: i) Patients
with bronchial asthma, bronchiectasis, interstitial lung disease,
lung cancer or tuberculosis; ii) patients with severe immune
system, brain, kidney, heart or blood diseases; and iii) other
diseases that could cause elevated SAA, CRP, IL-6 and MMP-2 levels.
As IL-41 is related to diabetes and coronary heart disease,
patients with these conditions were excluded. The screening
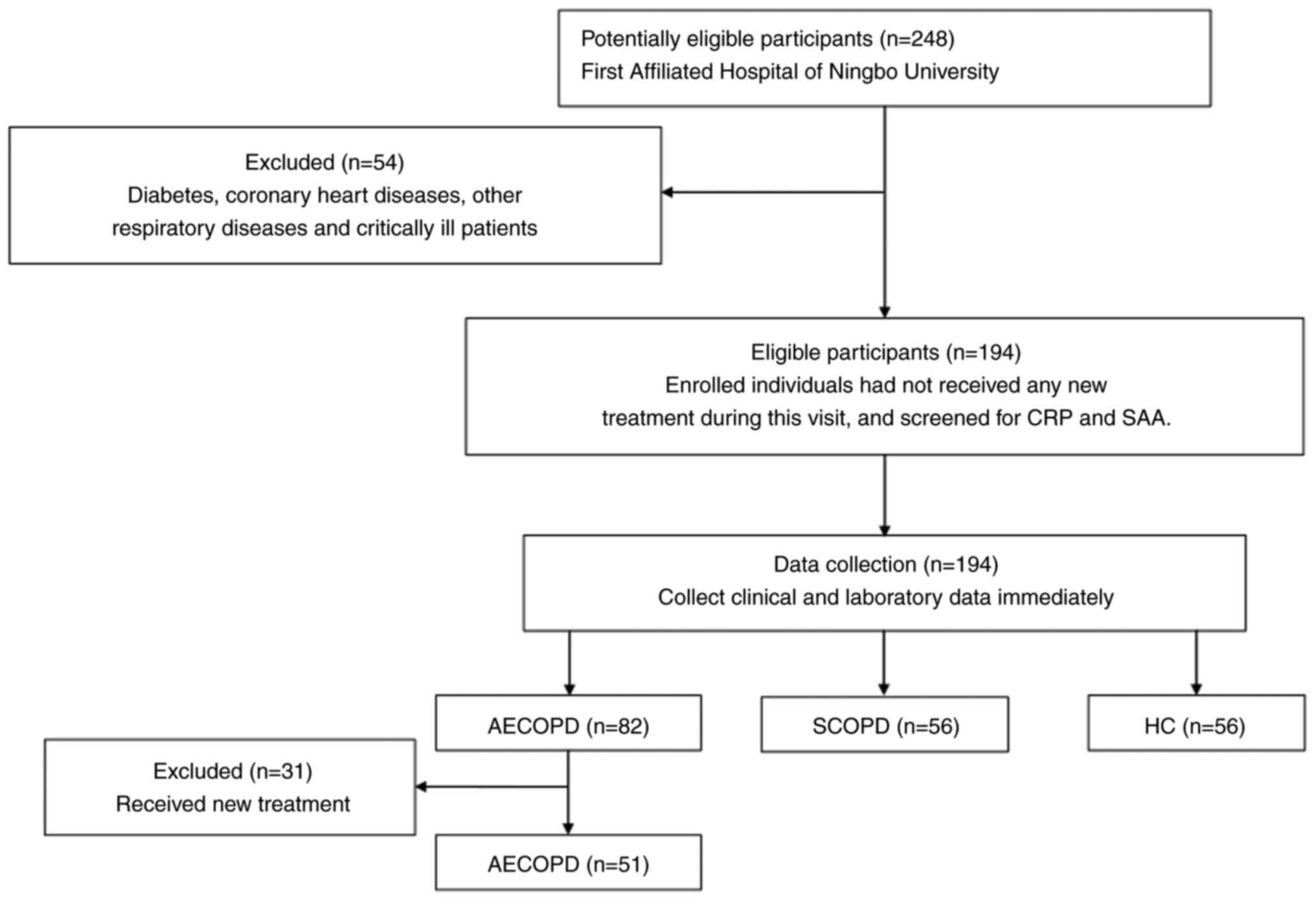
procedure is depicted in Fig.
1.
The Ethics Committee of the First Affiliated
Hospital of Ningbo University (Ningbo, China) granted consent for
the present study (approval no. 088RS-YJ01), and written informed
consent was obtained from all participants. The study was conducted
in accordance with the tenets put forth in the Declaration of
Helsinki of 1964 and any subsequent modifications.
For pulmonary function testing: i) The participants
sat during the examination, and pulmonary function tests were
performed using a pulmonary function instrument (Portable pulmonary
function testing instrument, X1; Xiamen XEEK Medical Equipment Co.,
Ltd.); ii) FVC, FEV1, FEV1/FVC (%) and FEV1% were recorded before
and after administration of the tracheal dilator (albuterol, 400
µg) three times with an interval of 3 min, and the three results
were averaged; iii) To reduce the influence of human factors,
examinations were performed by the same professional, and the same
pulmonary function instrument was used (calibration was required
before each examination). Lung function in patients with acute
exacerbations was measured 1 month after their condition had
stabilized.
AECOPD blood samples were obtained at the time of
admission, before antibiotics, hormones and other drugs were used.
The drugs used in the SCOPD group were mainly inhaled drugs and
oral expectorant drugs, and there was no study identified showing
that these inhaled drugs and oral expectorant drugs cause the
increase of CRP, SAA, IL-41 and MMP-2. IL-6 and MMP-2 levels were
measured using enzyme-linked immunosorbent assay (ELISA) kits (cat.
nos. KP00139 and KP00077, respectively; Wuhan Sanying
Biotechnology) according to the manufacturer's instructions. IL-41
levels in serum were also measured using ELISA kits (cat. no.
LV11160; Animalunion Biotechnology Co., Ltd) following the
manufacturer's instructions. Serum CRP levels were assessed in a
hospital laboratory using automated latex-enhanced
immunoturbidimetric assays (cat. no. 105-004860-00; Shenzhen
Mindray Biomedical Electronics Co., Ltd.), and mouse monoclonal
anti-CRP antibody at a concentration of 1.4 mg/ml bound latex was
included in the kit. Serum SAA levels were assessed in a hospital
laboratory using the automated latex immunoturbidimetric assay [at.
no. 8240-717(S); Ningbo Ruiyuan Biotechnology Co., Ltd.], and the
kit contained appropriate concentrations of latex particles coated
with mouse anti-human serum amyloid A antibody.
Statistical analysis
GraphPad Prism (version 9; Dotmatics), SPSS (version
25; IBM Corp.), and MedCalc (version 22.009; MedCalc Software) were
used for the data analysis, statistical analysis and plotting,
respectively. Data are presented as the mean ± standard deviation
(SD). Differences in continuous data between groups were compared
using Student's t-test or the Mann-Whitney U-test, and the
χ2-test was used for comparisons of categorical data.
One-way analysis of variance was used to compare differences
between multiple groups, and an independent samples t-test was used
to compare differences between two groups. The post-hoc test used
with ANOVA was Turkey's test. Pearson's correlation coefficient was
used to analyze data conforming to a normal distribution.
Spearman's rank correlation coefficient was used to analyze data
that did not conform to a normal distribution. ROC curve analysis
was used to examine the diagnostic value. P<0.05 was considered
to indicate a statistically significant difference.
Results
Study population
The present study included 107 patients with a
confirmed COPD diagnosis. There were 51 patients diagnosed with
AECOPD and 56 diagnosed with SCOPD. A total of 56 healthy controls
who visited the hospital for in-person checkups during the
recruitment period were also included in the analysis. There were
no significant differences in sex, age or body mass index among the
three groups (P>0.05). The baseline data of the patients are
presented in Table I.
 | Table IComparison of age, sex, BMI, smoking
Index, CRP, SAA, IL-6 and MMP-2 in the study groups. |
Table I
Comparison of age, sex, BMI, smoking
Index, CRP, SAA, IL-6 and MMP-2 in the study groups.
| Variable | Control | SCOPD | AECOPD |
|---|
| Age, years | 70.07±6.12 | 69.32±10.06 | 72.16±10.13 |
| Sex, male/female | 50/6 | 49/7 | 50/1 |
| BMI,
kg/m2 | 22.79±3.22 | 22.75±3.18 | 21.48±3.00 |
| Smoking index, pack
years | 6.07±14.48 |
28.84±28.51a |
35.62±28.95b |
| CRP, mg/l | 1.79±1.52 |
1.85±1.71a |
41.15±56.48b |
| SAA, mg/l | 3.33±1.98 |
3.83±2.85a |
113.0±107.3b |
| IL-6, ng/l | 17.28±6.52 |
18.37±6.12a |
36.37±34.33b |
| MMP-2, ng/l | 255±107.1 | 334.1±106.7 |
309.9±113.7b |
| WBC,
109/l | 5.92±1.53 |
6.36±1.76a |
7.66±3.00b |
| NEU% |
58.20±9.37c |
64.49±9.13a |
73.52±9.86b |
| NLR | 1.98±1.01 |
2.93±1.60a |
6.05±4.12b |
| PLR | 128.6±51.93 |
144.6±60.55a |
209.2±95.89b |
| FEV1/FVC, % | 85.83±4.37 |
55.83±10.22a |
51.22±11.86b |
| FEV1%pred | 108.1±16.79 |
52.33±21.10a |
40.87±15.85b |
Concentrations of IL-41 and other
inflammatory factors in serum
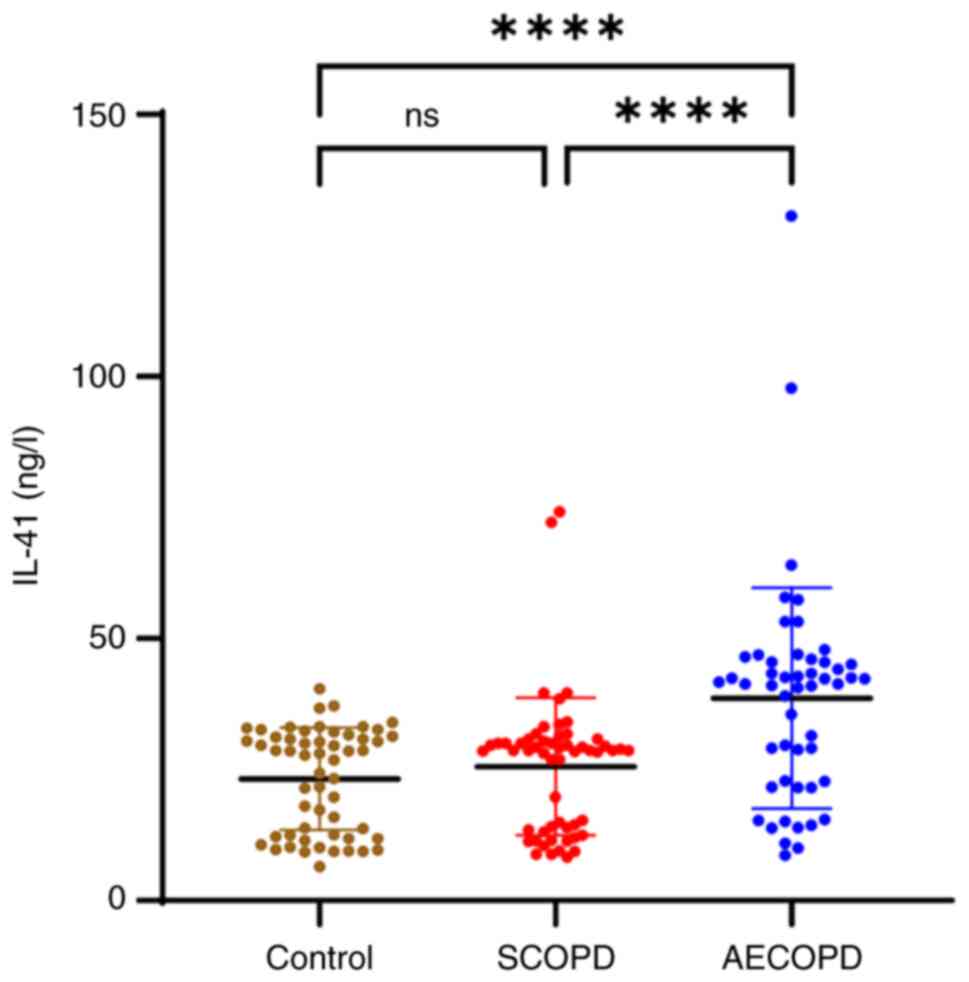
IL-41, IL-6, SAA, and CRP levels were higher in the
AECOPD group than those in the SCOPD and control groups
(P<0.0001). The NEU%, NLR and PLR were also higher in the AECOPD
group than in the SCOPD and control groups (P<0.05; Table I). Although the levels of IL-41,
IL-6, SAA and CRP were higher in the SCOPD group than those in the
control group, the differences were not statistically significant
(Figs. 2 and 3). The levels of MMP-2 were lower in the
control group than those in the SCOPD (P<0.001) and AECOPD
groups (P<0.05) (Fig. 3). There
was no significant difference in the level of MMP-2 between the
AECOPD and SCOPD groups (Fig.
3).
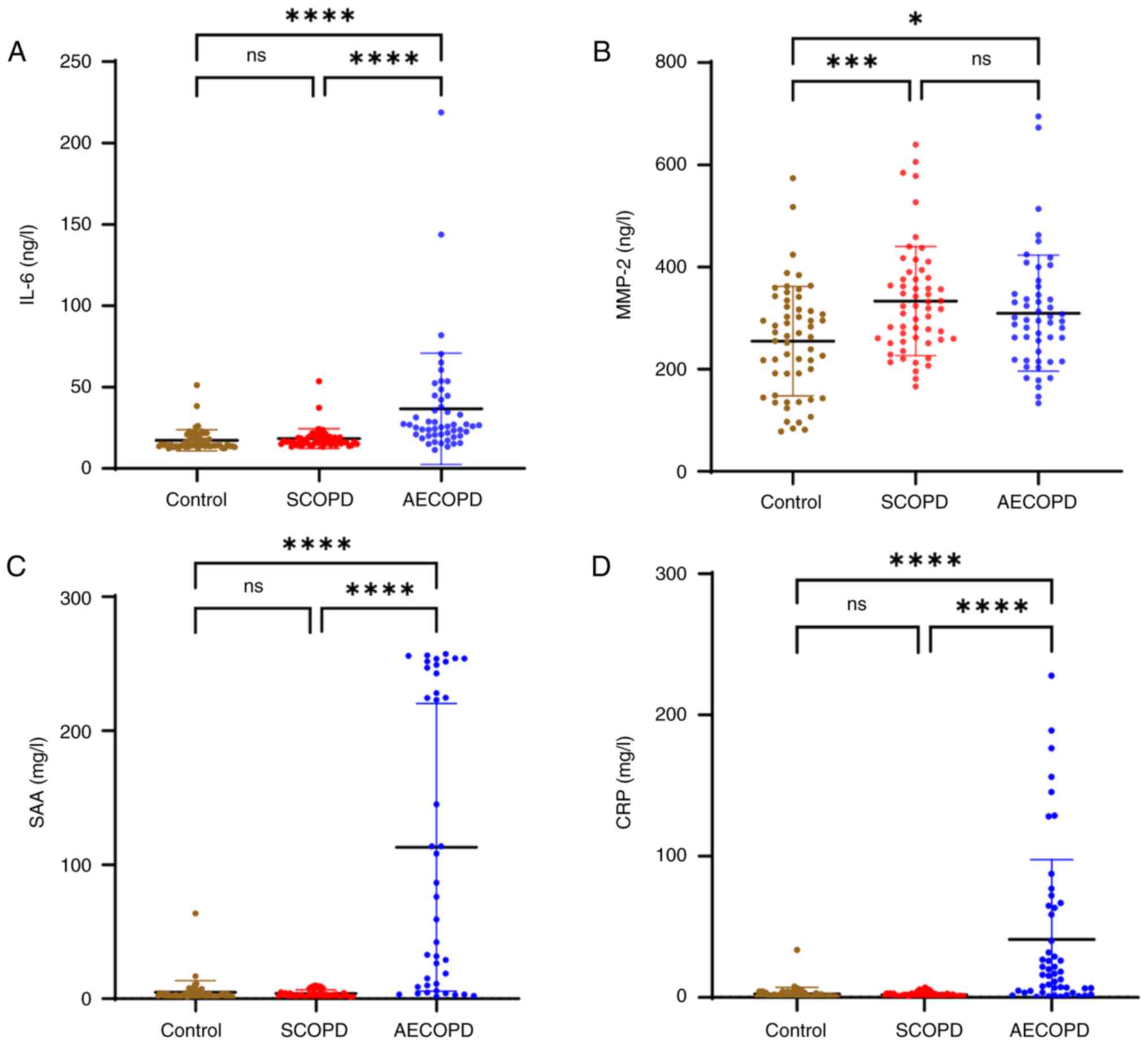 | Figure 3Concentrations of serum cytokines.
Concentrations of (A) IL-6, (B) MMP-2, (C) SAA and (D) CRP in
serum. *P<0.05, ***P<0.001 and
****P<0.0001. IL-6, interleukin 6; MMP-2, matrix
metalloproteinase-2; SAA, serum amyloid A; CRP, C-reactive protein;
SCOPD, stable chronic obstructive pulmonary disease; AECOPD, acute
exacerbation of chronic obstructive pulmonary disease; ns, not
significant. |
Correlation of the expression level of
IL-41 with the number of acute exacerbations, severity of the
exacerbation, PLR, smoking index, modified (British) Medical
Research Council (mMRC) scores and COPD assessment test (CAT)
scores
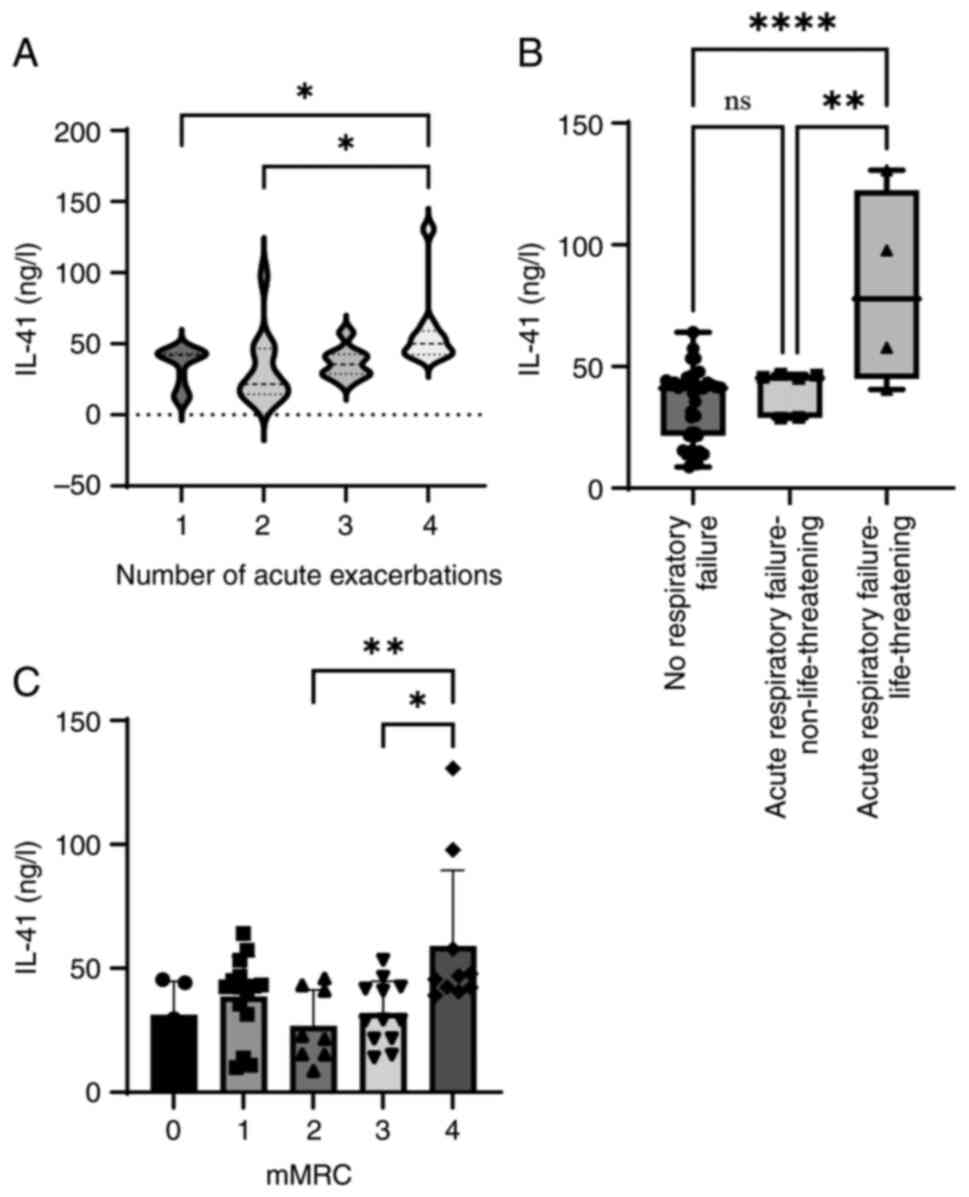
Only patients in the AECOPD group were included in
the correlation analysis. ‘Number of acute exacerbations’ refers to
the number of exacerbations in the past year. The degree of
exacerbation in hospitalized patients was determined according to
the clinical indicators of the patient as described in the 2023
GOLD report, which suggests three groups: No respiratory failure;
acute respiratory failure, non-life-threatening; and acute
respiratory failure, life-threatening (21).
With increased COPD severity, an overall increasing
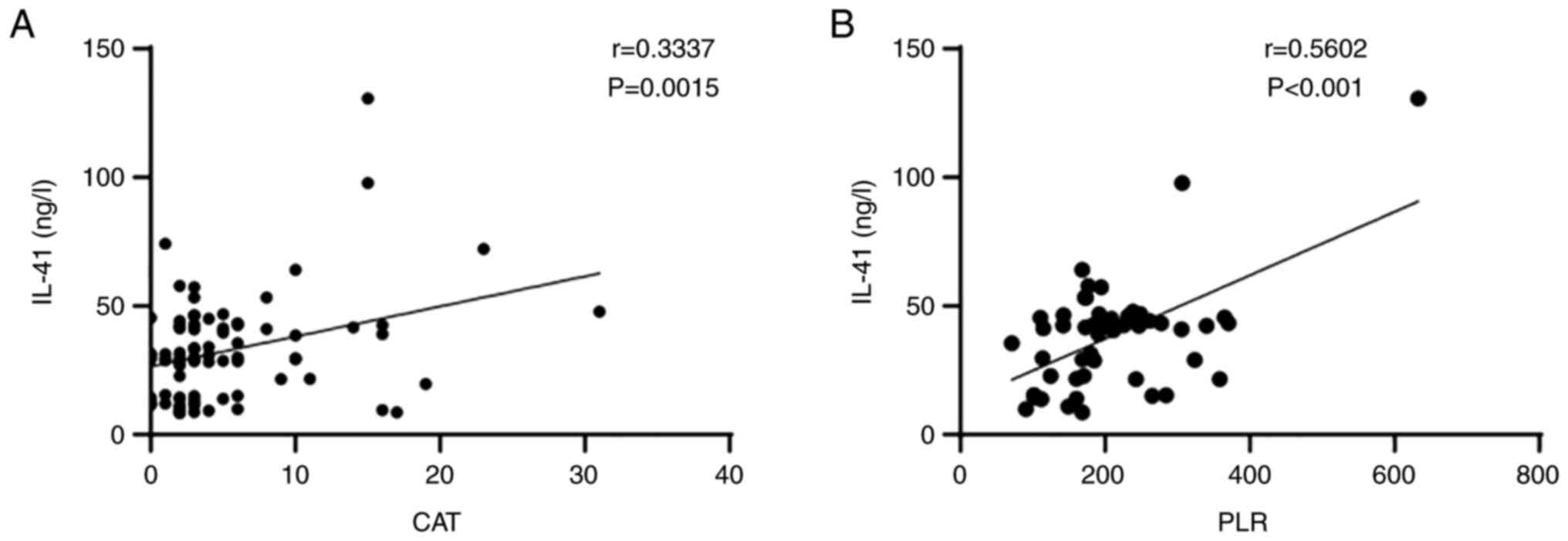
trend in the serum level of IL-41 was observed (Fig. 4). The correlations between the
expression level of IL-41 and other factors were also analyzed. It
was determined that the serum level of IL-41 was positively
correlated with PLR (r=0.5602, P<0.001) and CAT score (r=0.3337,
P<0.0015) in the AECOPD group (Fig.
5). It was also found that smoking index was positively
correlated with serum IL-41 (r=0.3178, P=0.0245), CRP (r=0.2568,
P=0.0011), and SAA (r=0.4249, P<0.0001) levels. However, smoking
index was not correlated with serum IL-6 (r=0.05883, P=0.4557) or
MMP-2 (r=0.1289, P=0.1011) levels (Fig. 6).
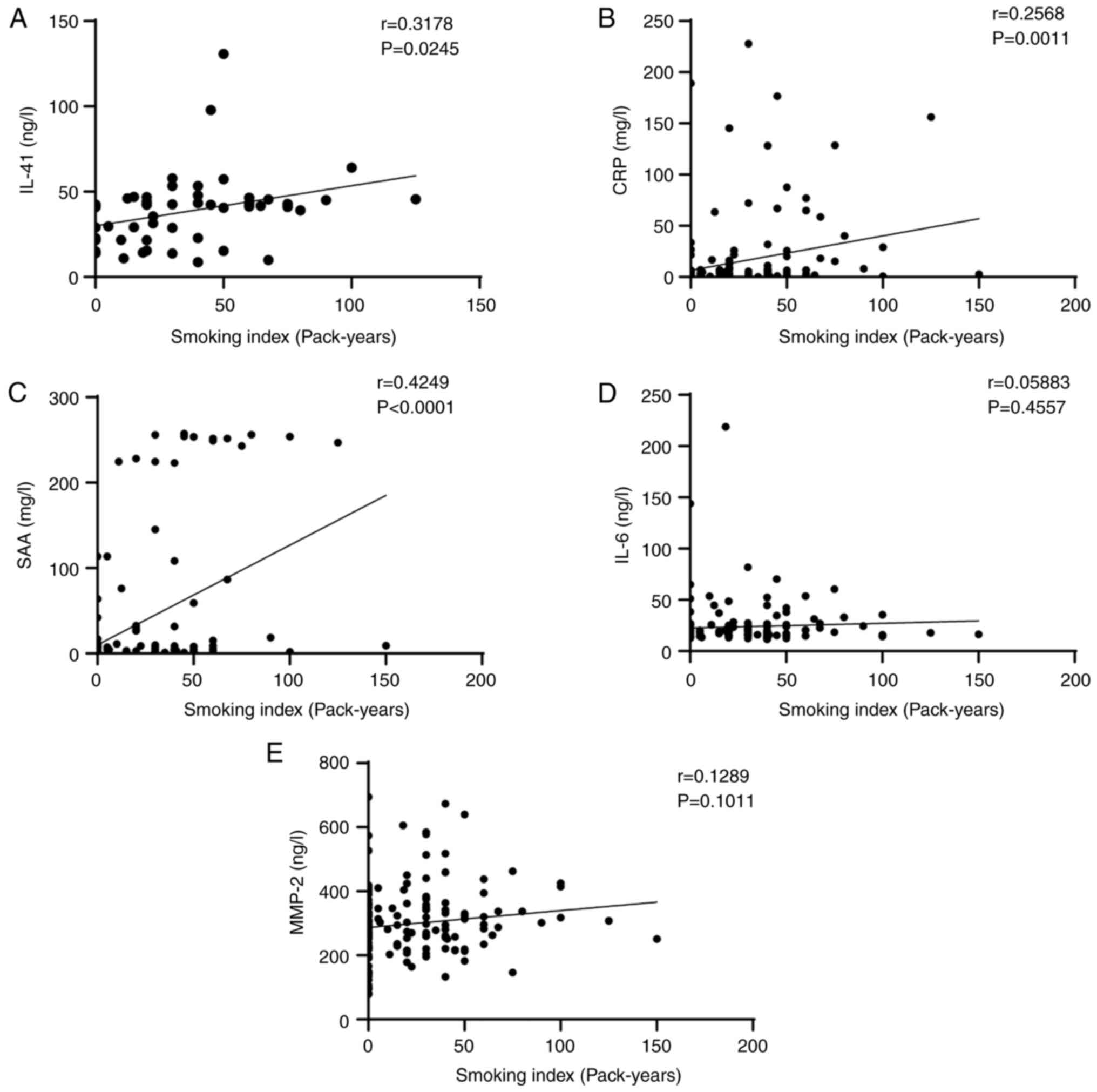 | Figure 6Correlation of smoking index and serum
cytokines. (A) Correlation of IL-41 with smoking index. r=0.3178,
P=0.0245. (B) Correlation of CRP with smoking index. r=0.2568,
P=0.0011. (C) Correlation of SAA with smoking index. r=0.4249,
P<0.0001. (D) Correlation of IL-6 with smoking index. r=0.05883,
P=0.4557. (E) Correlation of MMP-2 with smoking index. r=0.1289,
P=0.1011. IL-41, interleukin-41; CRP, C-reactive protein; SAA,
serum amyloid A; IL-6, interleukin 6; MMP-2, matrix
metalloproteinase-2. |
In conclusion, the levels of IL-41 were associated
with the severity of AECOPD, as indicated by the correlation
between IL-41 and the number of acute exacerbations, severity of
exacerbation, and CAT scores in the AECOPD group.
Diagnostic values of IL-41
In the included population, with a cutoff of 40.10
ng/l, the AUC, sensitivity and specificity of IL-41 to discriminate
between AECOPD and SCOPD cases were 0.741 (95% confidence interval,
0.642-0.841; P<0.001), 58.82, and 96.43%, respectively (Fig. 7 and Table II).
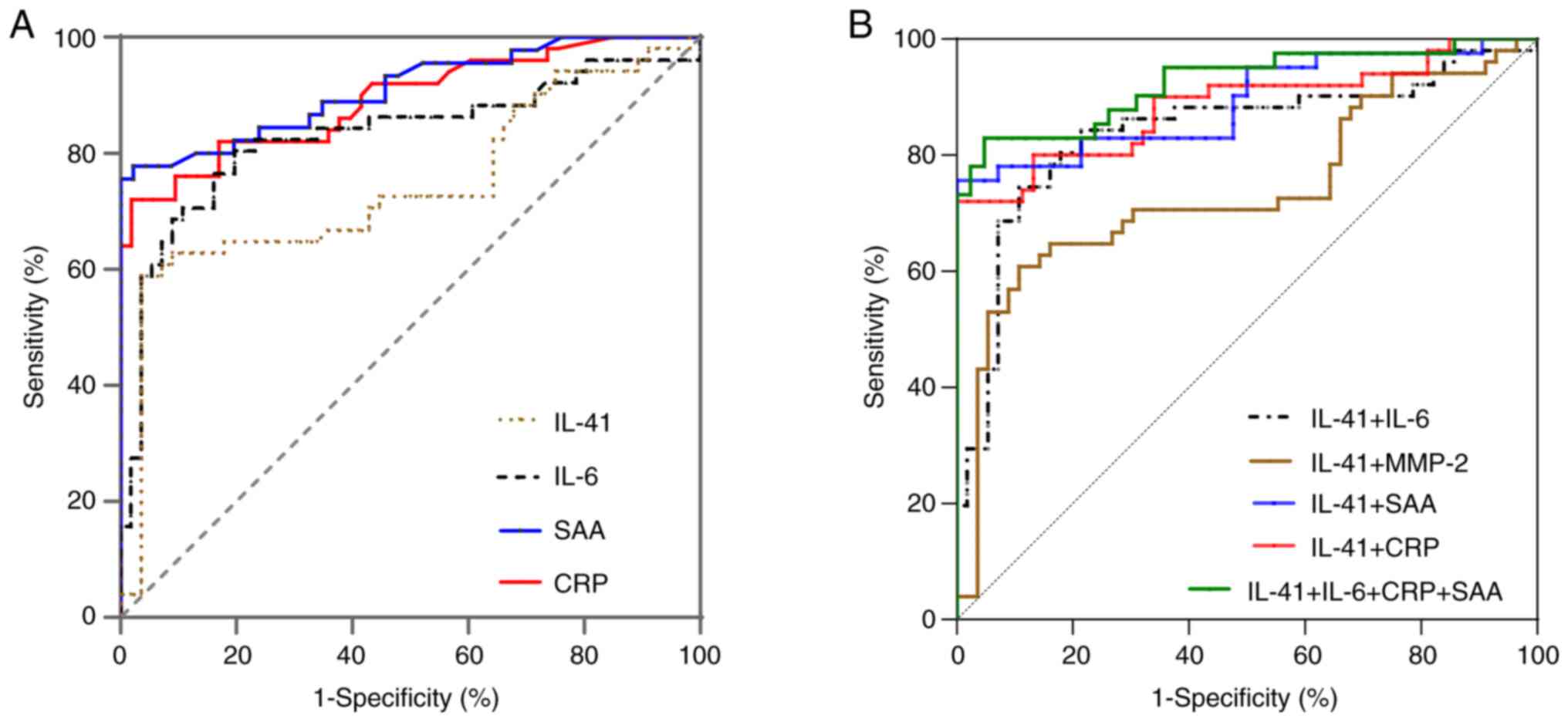 | Figure 7ROC curves. (A) ROC curves indicating
the diagnostic value of IL-41, IL-6, SAA and CRP in AECOPD and
SCOPD. (B) ROC curves indicating the diagnostic value of IL-41
combined with IL-6, MMP-2, SAA and CRP in AECOPD and SCOPD. ROC,
receiver operating characteristic; IL-41, interleukin-41; IL-6,
interleukin 6; MMP-2, matrix metalloproteinase-2; SAA, serum
amyloid A; CRP, C-reactive protein; AECOPD, acute exacerbation of
chronic obstructive pulmonary disease; SCOPD, stable chronic
obstructive pulmonary disease. |
 | Table IIDiagnostic performance of IL-41,
IL-6, SAA and CRP in differentiating AECOPD from SCOPD. |
Table II
Diagnostic performance of IL-41,
IL-6, SAA and CRP in differentiating AECOPD from SCOPD.
| Variables | AUC | Cutoff | Sensitivity, % | Specificity, % | Accuracy, % | Youden index | Std. error |
|---|
| IL-41 | 0.741 | 40.10 | 58.82 | 96.43 | 78.50 | 0.310 | 0.051 |
| IL-6 | 0.809 | 20.01 | 80.50 | 81.00 | 80.38 | 0.615 | 0.051 |
| SAA | 0.900 | 10.55 | 73.20 | 100.00 | 87.23 | 0.732 | 0.034 |
| CRP | 0.889 | 3.15 | 82.90 | 85.70 | 84.10 | 0.686 | 0.037 |
| IL-41 + IL-6 | 0.839 | N.A. | 74.50 | 89.30 | 82.24 | 0.630 | 0.042 |
| IL-41 + MMP-2 | 0.738 | N.A. | 62.70 | 91.10 | 77.57 | 0.538 | 0.051 |
| IL-41 + CRP | 0.885 | N.A. | 72.00 | 100.00 | 86.92 | 0.720 | 0.035 |
| IL-41 + SAA | 0.891 | N.A. | 75.60 | 100.00 | 88.79 | 0.756 | 0.037 |
| IL-41 + IL-6 +
MMP-2 + SAA + CRP | 0.925 | N.A. | 82.90 | 95.20 | 88.79 | 0.781 | 0.030 |
| IL-41 + IL-6 + SAA
+ CRP | 0.925 | N.A. | 82.90 | 95.20 | 88.79 | 0.782 | 0.030 |
There were significant differences in the AUC
between IL-41 and IL-41 combined with IL-6 [0.839 (0.757-0.922),
z=1.863, P=0.0625], IL-41 combined with SAA [0.891 (0.818-0.964),
z=2.994, P=0.0028], IL-41 combined with CRP [0.881 (0.805-0.957),
z=2.891, P=0.0038], and IL-41 combined with these three
inflammatory factors [0.925 (0.865-0.984), z=3.493, P=0.0005].
There was a significant difference in the AUC between IL-41
combined with IL-6, and IL-41 combined with the three inflammatory
factors [0.925 (0.865-0.984), z=2.812, P=0.0049] (Tables II and III).
 | Table IIIDifferences between the area under
the curves. |
Table III
Differences between the area under
the curves.
| Variables | P-value | Z-value |
|---|
| IL-41 and IL-41+
IL-6 | 0.0625 | 1.863 |
| IL-41 and IL-41 +
MMP-2 | 0.4694 | 0.723 |
| IL-41 and IL-41 +
SAA | 0.0028 | 2.994 |
| IL-41 and IL-41 +
CRP | 0.0038 | 2.891 |
| IL-41 and IL-6 +
SAA + CRP | 0.0005 | 3.493 |
| IL-41 + IL-6 and
IL-41 + IL-6 + SAA + CRP | 0.0049 | 2.812 |
| IL-41 + SAA and
IL-41 + IL-6 + SAA + CRP | 0.1082 | 1.606 |
| IL-41+ CRP and
IL-41 + IL-6 + SAA + CRP | 0.1456 | 1.455 |
Discussion
The World Health Organization has estimated that 65
million individuals worldwide have mild-to-severe COPD, and its
prevalence is increasing (22). In
severe cases, admission to an intensive care center with passive or
mechanical ventilation is necessary, resulting in considerable
medical and economic burdens. Moreover, the diagnosis of COPD is
subjective. Therefore, it is important to identify the biomarkers
associated with AECOPD. There are two types of biomarkers for
AECOPD: Pulmonary and systemic. Pulmonary biomarkers include IL-6,
IL-8 and myeloperoxidase (23).
Systemic biomarkers include CRP, procalcitonin, NLR and platelet
distribution width values (24).
However, no single biomarker has been widely used.
A previous study by Xu et al (25) also found that the FEV1, FEV1/FVC,
and serum CRP, IL-6 and tumor necrosis factor (TNF)-α levels of
patients with COPD in the smoking group were significantly lower
than those of patients with COPD in the non-smoking group. The
levels of CD+4 and CD+4/CD+8 were
also significantly lower in smokers than those in non-smokers. It
has been suggested that smoking may lead to impaired lung function
in patients with COPD, which may subsequently aggravate airway
inflammation and weaken T-lymphocyte immune function. Xian and Chen
(26) found that FEV1 and
diffusing capacity for carbon monoxide (DLCO%) of patients with
AECOPD in the smoking group were significantly lower than those of
patients with AECOPD in the non-smoking group and healthy controls.
Furthermore, it was also revealed that the expression levels of
serum cytokines such as procalcitonin, CRP, IL-6 and TNF-α were
significantly higher in the smoking group than in the non-smoking
and control groups. These findings indicate that the smoking status
of patients with AECOPD is an important indicator of the expression
of inflammatory factors. The findings in the present study revealed
that the smoking index was positively correlated with serum IL-41,
CRP and SAA levels.
IL-41 production is blocked in M2 macrophages, which
reduces the immunological response. Numerous studies on the
mechanism of action of macrophages in COPD have been published,
drawing attention to the significance of macrophage polarization in
the disease. ‘Macrophage polarization’ describes the geographical
and temporal patterns of macrophage activation (27). In response to the synthesis of
several signaling molecules and inflammatory factors, both
conventionally activated (M1) and alternatively activated (M2)
macrophage polarization is possible, demonstrating their high
plasticity. In the lungs, M2 macrophages suppress inflammatory
responses, whereas M1 macrophages promote them (28). By controlling macrophage
polarization, IL-41 may contribute to the pulmonary inflammation
associated with COPD; however, its specific role and mechanism
require further study.
The findings of the present study indicate that,
although IL-41 levels did not differ between the SCOPD and control
groups, the IL-41 level was higher in the AECOPD group than those
in the SCOPD and control groups. IL-6, SAA, CRP, NEU%, NLR and PLR
values were higher in the AECOPD group than those in the SCOPD and
control groups. IL-41 levels were also correlated with AECOPD
severity. Compared with common inflammatory factors such as IL-6,
CRP and SAA, IL-41 did not exhibit obvious superiority in
diagnostic efficacy, but it did have high specificity. Use of a
combination of factors significantly improved the efficiency of
AECOPD diagnosis. A previous study by the authors also revealed
that IL-41, which suppresses cigarette smoke-induced pulmonary
inflammation in vivo in mice, can be used therapeutically to
treat inflammatory lung conditions (29). According to earlier investigations,
IL-41 level has been linked to diabetes and coronary heart disease,
and the plasma IL-41 level in patients with AECOPD with diabetes
mellitus and coronary heart disease was found to be lower than that
in patients with AECOPD without diabetes and coronary heart disease
(9). This implies that, during the
acute phase, patients with AECOPD with diabetes and coronary heart
disease do not generate a strong anti-inflammatory response.
Therefore, we hypothesized that IL-41 level is elevated in patients
with AECOPD and associated with AECOPD. IL-41 may inhibit
inflammation by promoting macrophage polarization in the M2
direction and upregulate gene expression of M2 macrophages and
their markers via a signal transduction pathway.
The present study did have some limitations. First,
patients with diabetes or coronary heart disease were excluded.
Second, the number of participants in this study was relatively
limited, and the included population was predominantly male,
indicating the study may have been affected by selection bias. The
role of IL-41 in the pathophysiology of COPD requires further
studies with larger sample sizes. The correlation between IL-41
level and sex also requires further exploration. Third, due to
ethical reasons, it was very difficult to obtain tracheoscopic or
ventilator secretions from COPD patients and analyze the
correlation with the serum level of IL-41. The relationship between
tracheoscopic or ventilator secretions from patients with COPD and
the serum level of IL-41 may be addressed in a future study.
In conclusion, the expression level of IL-41 was
increased in the serum of patients with AECOPD, indicating it may
play a significant role in the inflammatory process of COPD and,
especially, AECOPD. In the future, IL-41 may be a useful biomarker
for the monitoring, evaluation and treatment of patients with
AECOPD.
Acknowledgements
Not applicable.
Funding
Funding: This work was supported by the Natural Science
Foundation of Ningbo (grant nos. 2021J242 and 2021J080) and the Key
Research and Development Plan of Ningbo city (grant no.
2023Z179).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
TC, MH and HM designed the study, wrote the
manuscript. MH, ML and JJ collected clinical information and serum
of the patients. ML, JJ, QD and DL designed and performed the
statistical analysis. LF and SW performed the experiments. HM
revised the manuscript (for intellectual content) and gave final
approval for publication. TC, HM, JJ, ML, QD, DL, LF, SW and HM
confirmed the authenticity of all the raw data. All authors have
read and approved the final manuscript.
Ethics approval and consent to
participate
The Ethics Committee of the First Affiliated
Hospital of Ningbo University (Ningbo, China) granted consent for
the present study (approval no. 088RS-YJ01), and written informed
consent was obtained from all participants. It is attested that the
study was carried out considering the Declaration of Helsinki of
1964 and any subsequent changes.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Celli B, Fabbri L, Criner G, Martinez FJ,
Mannino D, Vogelmeier C, Montes de Oca M, Papi A, Sin DD, Han MK
and Agusti A: Definition and nomenclature of chronic obstructive
pulmonary disease: Time for its revision. Am J Respir Crit Care
Med. 206:1317–1325. 2022.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Confalonieri M, Braga L, Salton F, Ruaro B
and Confalonieri P: Chronic obstructive pulmonary disease
definition: Is it time to incorporate the concept of failure of
lung regeneration? Am J Respir Crit Care Med. 207:366–367.
2023.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Christenson SA, Smith BM, Bafadhel M and
Putcha N: Chronic obstructive pulmonary disease. Lancet.
399:2227–2242. 2022.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Perera WR, Hurst JR, Wilkinson TM,
Sapsford RJ, Müllerova H, Donaldson GC and Wedzicha JA:
Inflammatory changes, recovery and recurrence at COPD exacerbation.
Eur Respir J. 29:527–534. 2007.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Wei Y, Wang S, Wang D and Liu C:
Expression and clinical significance of serum amyloid A and
interleukin-6 in patients with acute exacerbation of chronic
obstructive pulmonary disease. Exp Ther Med. 19:2089–2094.
2020.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Bridgewood C, Russell T, Weedon H,
Baboolal T, Watad A, Sharif K, Cuthbert R, Wittmann M, Wechalekar M
and McGonagle D: The novel cytokine Metrnl/IL-41 is elevated in
Psoriatic Arthritis synovium and inducible from both entheseal and
synovial fibroblasts. Clin Immunol. 208(108253)2019.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Zheng SL, Li ZY, Song J, Liu JM and Miao
CY: Metrnl: A secreted protein with new emerging functions. Acta
Pharmacol Sin. 37:571–579. 2016.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Ushach I, Arrevillaga-Boni G, Heller GN,
Pone E, Hernandez-Ruiz M, Catalan-Dibene J, Hevezi P and Zlotnik A:
Meteorin-like/Meteorin-β is a novel immunoregulatory cytokine
associated with inflammation. J Immunol. 201:3669–3676.
2018.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Rao RR, Long JZ, White JP, Svensson KJ,
Lou J, Lokurkar I, Jedrychowski MP, Ruas JL, Wrann CD, Lo JC, et
al: Meteorin-like is a hormone that regulates immune-adipose
interactions to increase beige fat thermogenesis. Cell.
157:1279–1291. 2014.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Kerget B, Afşin DE, Kerget F, Aşkın S and
Akgün M: Is metrnl an adipokine involved in the anti-inflammatory
response to acute exacerbations of COPD? Lung. 198:307–314.
2020.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Golestani R, Razavian M, Ye Y, Zhang J,
Jung JJ, Toczek J, Gona K, Kim HY, Elias JA, Lee CG, et al: Matrix
metalloproteinase-targeted imaging of lung inflammation and
remodeling. J Nucl Med. 58:138–143. 2017.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Alrumaihi F: A cheminformatics-biophysics
correlate to identify promising lead molecules against matrix
metalloproteinase-2 (MMP-2) enzyme: A promising anti-cancer target.
Saudi Pharm J. 31:1244–1253. 2023.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Zhang Y, Li Y, Ye Z and Ma H: Expression
of matrix metalloproteinase-2, matrix metalloproteinase-9, tissue
inhibitor of metalloproteinase-1, and changes in alveolar septa in
patients with chronic obstructive pulmonary disease. Med Sci Monit.
26(e925278)2020.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Tanaka M, Takarada T, Nadanaka S, Kojima
R, Hosoi K, Machiba Y, Kitagawa H and Yamada T: Influences of
amino-terminal modifications on amyloid fibril formation of human
serum amyloid A. Arch Biochem Biophys. 742(109615)2023.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Prins HJ, Duijkers R, Kramer G, Boerhout
E, Rietema FJ, de Jong PA, Schoorl MI, van der Werf TS and Boersma
WG: Relationship between biomarkers and findings on low-dose
computed tomography in hospitalised patients with acute
exacerbation of COPD. ERJ Open Res. 8:00054–2022. 2022.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Tan Q, Wang B, Ye Z, Mu G, Liu W, Nie X,
Yu L, Zhou M and Chen W: Cross-sectional and longitudinal
relationships between ozone exposure and glucose homeostasis:
Exploring the role of systemic inflammation and oxidative stress in
a general Chinese urban population. Environ Pollut.
329(121711)2023.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Xu B and Han L: Predictive Value of CRP,
PCT and ESR on piperacillin-tazobactam in treating chronic
obstructive pulmonary disease with pneumonia. Clin Lab.
69:2023.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Han Fang and Bin Zhao: Donghong Liu(2020)
To explore the clinical value of NLR and PLR in the treatment of
acute exacerbation of chronic obstructive pulmonary disease.
General practice clinical and education. 18:694–697. 2020.
|
|
19
|
Venkatesan P: GOLD COPD report: 2024
update. Lancet Respir Med. 12:15–16. 2024.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Celli BR, Fabbri LM, Aaron SD, Agusti A,
Brook R, Criner GJ, Franssen FME, Humbert M, Hurst JR, O'Donnell D,
et al: An updated definition and severity classification of chronic
obstructive pulmonary disease exacerbations: The rome proposal. Am
J Respir Crit Care Med. 204:1251–1258. 2021.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Agustí A, Celli BR, Criner GJ, Halpin D,
Anzueto A, Barnes P, Bourbeau J, Han MK, Martinez FJ, Montes de Oca
M, et al: Global initiative for chronic obstructive lung disease
2023 report: GOLD executive summary. Eur Respir J.
61(2300239)2023.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Gautam SS and O'Toole RF: Convergence in
the epidemiology and pathogenesis of COPD and pneumonia. COPD.
13:790–798. 2016.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Koutsokera A, Kostikas K, Nicod LP and
Fitting JW: Pulmonary biomarkers in COPD exacerbations: A
systematic review. Respir Res. 14(111)2013.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Ramya PA, Mohapatra MM, Saka VK, Kar R,
Chakkalakkoombil SV and Vemuri MB: Haematological and inflammatory
biomarkers among stable COPD and acute exacerbations of COPD
patients. Sultan Qaboos Univ Med J. 23:239–244. 2023.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Xu Haifeng, Sun Yifeng and Lin Huan:
Effects of smoking on immune inflammatory response and pulmonary
function in patients with chronic obstructive pulmonary disease. J
Clin Pulmonol. 25:58–61. 2020.
|
|
26
|
Xian Shaojing and Chen Qingyun: Changes of
lung function and serum cytokine levels in patients with acute
exacerbation of chronic obstructive pulmonary disease and their
correlation with smoking and body mass index. J Clin &
Pathology. 42:144–150. 2021.
|
|
27
|
Murray PJ: Macrophage Polarization. Annu
Rev Physiol. 79:541–566. 2017.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Bazzan E, Turato G, Tinè M, Radu CM,
Balestro E, Rigobello C, Biondini D, Schiavon M, Lunardi F, Baraldo
S, et al: Dual polarization of human alveolar macrophages
progressively increases with smoking and COPD severity. Respir Res.
18(40)2017.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Cen T, Mai Y, Jin J, Huang M, Li M, Wang S
and Ma H: Interleukin-41 diminishes cigarette smoke-induced lung
inflammation in mice. Int Immunopharmacol. 124(Pt
A)(110794)2023.PubMed/NCBI View Article : Google Scholar
|