Introduction
An abrupt reduction in renal function is the
hallmark of acute kidney injury (AKI), which occurs in 10-15% of
hospitalizations and affects >50% patients in intensive care in
the United States (1,2). Acute tubular necrosis, which is the
most common cause of AKI, may result from ischemia, exogenous
nephrotoxic agents (such as iodinated contrast media,
aminoglycosides, amphotericin B and vancomycin) or endogenous
nephrotoxic damage due to rhabdomyolysis and hemolysis (2). In particular, ischemia can arise in
the kidney after various urological treatments, including kidney
transplantation, partial nephrectomy and renal artery surgery
(3,4). Additionally, trauma, shock and sepsis
are also amongst the most commonly reported causes of kidney
ischemia (3-5).
Ischemia is considered to be one of the primary causes of AKI
(6). Renal damage first occurs
during ischemia, which is then exacerbated by the restoration of
blood flow (3). Diagnosis of renal
injury caused by ischemia/reperfusion (I/R) depends on clinical
assessment, urinary or blood biochemical markers, radiological
findings and eventually histologic examination (7,8).
Blood urea nitrogen (BUN) and serum creatinine are useful key
markers of renal I/R injury (8).
CT is not applicable for renal I/R injury diagnosis due to the
contrast toxicity seen in acute tubular necrosis (ATN) (7). Although MRI with contrasts can exert
toxic effects on kidney functions, T1 and
T2-weighted MRI can provide useful information regarding
the extent of damage induced by hypoxia (7,8).
Histopathologically, I/R injury frequently manifests as damage to
the tubular epithelium, primarily due to the high energy demands of
renal tubules. This can result in ATN and/or AKI (9).
Alterations in the mitochondrial oxidative
phosphorylation system during ischemia results in decreased
adenosine triphosphate (ATP) and antioxidant production (10). Furthermore, disruption of ion pumps
(Na+/K+/ATPase, Na+/H+,
Ca2+/ATPase pumps) leads to the accumulation of
hydrogen, sodium and calcium ions, resulting in cell swelling and
the activation of proteases (such as apoptosis protease-activating
factor) and phosphatases (such as serine/threonine-protein
phosphates) in the cytoplasm (4,10-12).
Activated enzymes then degrade the cytoskeleton and membrane
phospholipids, resulting in the production of reactive oxygen
species (ROS). With the resupply of oxygen following reperfusion,
ROS are produced by the xanthine oxidase system (due to the shift
from xanthine dehydrogenase to xanthine oxidoreductase under
ATP-deficient conditions during the hypoxic periods), by the
mitochondrial electron transport chain, the NADPH oxidase system
and unbound nitrite oxide synthase system (4,10).
The pathophysiology of ischemia-reperfusion
(I/R)-induced AKI is a highly complex process that has been
reported to involve the activation of neutrophils, release of
reactive oxygen species (ROS) and secretion of various inflammatory
mediators, including adhesion molecules (such as P-selectin and
ICAM-1) and cytokines (such as TNF-α and IL-6) (13). However, there is currently no
effective therapeutic option available for the treatment of renal
I/R injury (RIRI), other than supportive therapies such as renal
replacement therapy or hydration (14).
Lupeol is a biologically active triterpene that can
be found in various edible vegetables and fruits, such as mangoes,
cabbage, green peppers and strawberries (15). A previous study reported that
lupeol possesses anti-inflammatory, anticancer, cardioprotective,
hepatoprotective and wound-healing properties (16). Lupeol has been found to function
through the toll like receptor 4/myeloid differentiation primary
response 88/NF-κB p65, IL-1 receptor-associated kinase and p38 MAPK
pathways (16). It has been
previously tested in clinical studies for the treatment of cancer
(such as bone, liver, lung, colon, rectum and bladder), actinic
keratosis and nocturnal enuresis (17-19).
In a study conducted with actinic keratosis patients, the
birch-bark-containing Lupeol managed to clear 75% of the lesions
(18). It was determined that
there was a significant decrease in the number of day frequency,
nocturia and total incontinence with lupeol treatment (19). Lupeol has been demonstrated to
exert anti-cancer effects by promoting apoptosis, limiting cancer
cell migration and invasion, decreasing cell proliferation and
increasing cancer cell susceptibility to chemotherapy and
radiotherapy both in vitro and in vivo (17-19).
Lupeol has been investigated in previous studies for
its protective effects in an animal model of
hypercholesterolemia-induced kidney damage and its effects against
renal cell carcinoma in human cell culture via modulation of
mitochondrial dynamics by decreased cell viability and
mitochondrial fission (17,20).
In rats in which hypercholesterolemia was induced by feeding a
high-cholesterol diet, the decreased antioxidant status, increased
renal lysosomal acid hydrolase activities and acute phase proteins,
which indicates increased inflammation, were reversed by lupeol
treatment (20). However, to date,
to the best of our knowledge, no studies have evaluated its effects
on RIRI. Therefore, the present study aimed to assess the effects
of lupeol on this condition.
Materials and methods
Animals and treatments
Lupeol (purity, 99.31%) was purchased from TargetMol
Chemicals, Inc. and dissolved in olive oil using heat (37˚C) and
sonication at frequency of 42 kHz (3 h) as previously described by
Nitta et al (21). This
process was performed until it dissolved homogeneously with no
visible particles remaining in the solution. Previous studies have
attempted to administer lupeol through a variety of routes,
including the subcutaneous, intraperitoneal, topical and oral
routes (22-25).
It has been previously shown that an oral dose of 100 mg/kg is more
effective in inhibiting IL-2, IFN-gamma, and TNF-α in pleural
exudate than oral doses of 25-50-200 mg/kg, whilst an
intraperitoneal dose reaching as high as 200 mg/kg did not cause
toxicity in rats. Therefore, in the present study, an
intraperitoneal dose of 100 mg/kg was used (25,26).
The present experimental animal study was performed under the
supervision of expert veterinarians at the Gazi University Animal
Laboratory and Experimental Research Center and the animals'
respiration and heart rate were examined every 15 min. The Gazi
University Animal Experiments Ethics Committee granted ethical
approval (approval no. G.U.ET-22.006; Ankara, Turkey).
For the present study, a total of 24 healthy Wistar
Albino female rats (age, 3-4 months; weight, 200-250 g) were
obtained from the aforementioned center. The animals were housed in
cages with proper ambient temperature (at 20-21˚C) and humidity
(average 55±5%), with a 12 h light/dark cycle. Before and after the
procedure, all animals had free access to a normal diet and water.
For general anesthesia, intramuscular ketamine hydrochloride (50
mg/kg) and xylazine hydrochloride (5 mg/kg) were used. To maintain
sterility 10% povidone-iodine was used.
The rats were divided into the following four groups
at random (n=6): i) Sham group (group S), where no further
surgeries were performed apart from the median laparotomy; ii) the
lupeol group (group L), where at 1 h after the intraperitoneal
injection of 100 mg/kg lupeol, a median laparotomy was performed;
iii) the renal ischemia group (group I), where a median laparotomy
was performed at 1 h after the administration of intraperitoneal
saline, before ischemia was applied in right and left main renal
arteries for 45 min, and the rats were under anesthesia throughout
the 45 min; and iv) the lupeol therapy group (group T), where at 1
h after the intraperitoneal injection of 100 mg/kg lupeol, a median
laparotomy was performed under anesthesia, before right and left
main renal arteries were subjected to ischemia for 45 min.
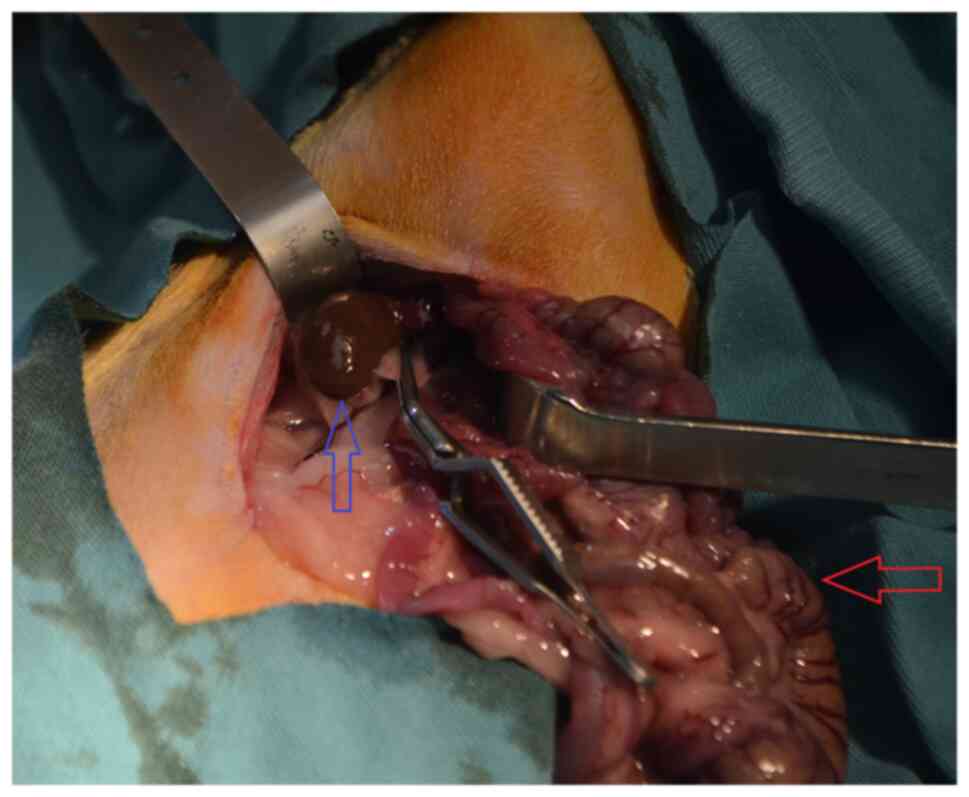
Experimental rat renal ischemia
model
Each animal was anesthetized and then placed in a
supine position on the surgical table. A 3-cm incision was made
through the midline of the abdomen. Renal ischemia was induced in
the rats in the I and T groups by bilaterally blocking their renal
pedicles, including the renal artery, using atraumatic
microvascular clamps for 45 min (Fig.
1). To provide reperfusion following ischemia, the atraumatic
microvascular clamps were removed. Subsequently, abdominal
incisions were repaired with 3/0 silk sutures in all groups. At the
end of the experiment, deep anesthesia was achieved in the rats by
confirming that they did not respond to tail clamping following the
administration of intramuscularly injected ketamine hydrochloride
(50 mg/kg) and xylazine hydrochloride (5 mg/kg; Alfazyne 2%).
Subsequently, the animals were euthanized by exsanguination through
intracardiac puncture. Death was confirmed by absence of heartbeats
determined by listening to cardiac sounds with the use of a
stethoscope (27,28). Bilateral nephrectomy was then
performed by re-laparotomy. Both kidneys were removed from each
animal, with one frozen in liquid nitrogen and kept at -80˚C for
further biochemical analysis, whilst the other was preserved in 10%
formalin at room temperature for 48 h for histological analysis.
The centrifugation of blood samples was performed at 2,110 x g for
10 min at 2-8˚C. Serum was stored in Eppendorf tubes at -80˚C for
TNF-α and IL-6 testing after BUN and creatinine levels were
measured for the assessment of renal function.
Histopathological evaluation
The kidney tissues were processed in standard
procedures with automatic tissue processers (Sakura Inc.). In
standard tissue processing, after formalin fixation, the samples
went through a series of graded ethanol solutions (70, 95 and 100%)
to dehydrate them. Alcohol was replaced by xylene to clear the
tissue samples. Molten paraffin wax was then infiltrated to
impregnate them with paraffin. Paraffinized tissue samples were
then blocked in paraffin. Slides were cut in microtomes from
paraffin blocks for 4 microns and were transferred to the staining
station.
The staining process started with deparaffinization
in a 60˚C laboratory oven for 5 min. The rest of the staining
process was performed using a Tissue-Tek Prisma (Sakura Inc.)
automatic tissue stainer at room temperature. The first step was
rehydration with a series of ethanol solutions (100, 95 and 70%)
and transferring to distilled water. The rehydrated slides were
immersed in hematoxylin solution at room temperature for 7 min and
then rinsed briefly in tap water. Slides were then dipped in acid
alcohol for a few sec, to remove excess hematoxylin and rinsed with
tap water. Counterstaining with eosin started with immersing in
eosin Y solution at room temperature for 3 min and rinsing with tap
water. Another step of dehydration was performed by transferring
through a series of ethanol solutions (70, 95 and 100%). Finally,
the slides were cleared in xylene and covered by Tissue-Tek (Sakura
Inc.) film slips in the automatic cover slipper.
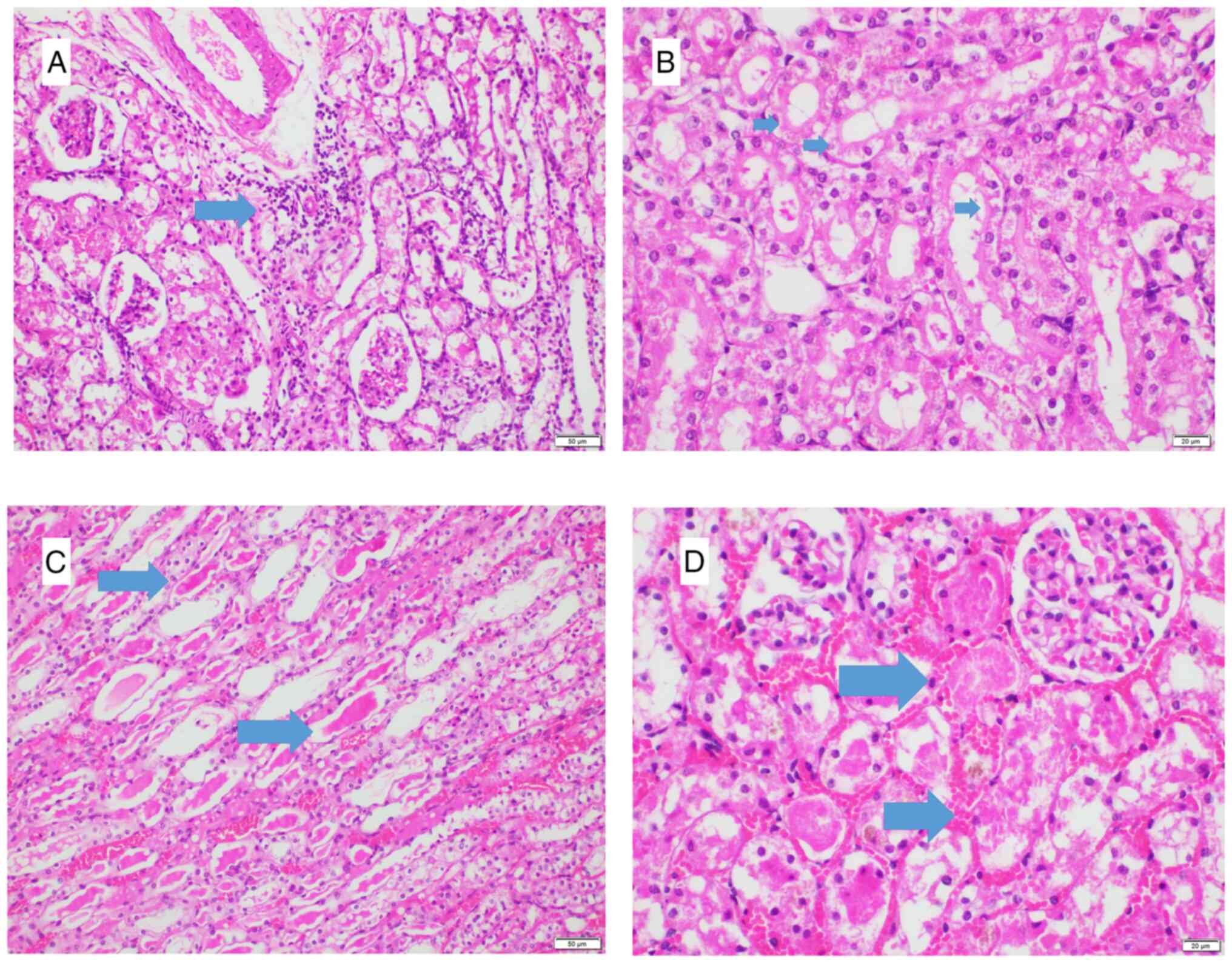
Histopathological analysis was performed by two
pathologists blinded to the subjects and each other. After overall
assessment of the injury, in the most harmed foci, 10 high power
fields were histopathologically analyzed under a light microscope
(Olympus BX53; Evident Inc.) to assess the degree of degeneration,
tubular dilatation, interstitial lymphocyte infiltration, protein
cylinders, necrosis and loss of brush borders (Fig. 2). Each criterion was evaluated
individually using a binary scale, depending only on the presence
or absence of the criteria (present, 1; absent, 0), without taking
into consideration the degree of severity of the histopathological
changes or increments. They were then summed to generate a
numerical score. A highly injured specimen would receive the
maximum score of 6. The injury was also scored according to the
percentage of the entire kidney which demonstrated damage.
Percentages were scored as follows: i) 0, none; ii) #x003C;10%, 1;
iii) 11-25%, 2; iv) 26-45%, 3; v) 46-75%, 4; and vi) 76-100%, 5.
Numerical score of injury was multiplied with the percentage score
before the final histopathological injury score was recorded by
each pathologist (9). The maximum
possible histopathological injury score was 30. Any scores that did
not concur within pathologists were revised together on a
two-headed microscope and re-scored with consensus.
Biochemical parameters
The BUN and creatinine results in blood samples were
measured using Beckman Coulter kits (creatinine, cat. no. OSR6178;
BUN, cat. no. OSR6134) on an AU 480 Chemistry analyzer (Beckman
Coulter, Inc.) according to the manufacturer's protocols. The IL-6
(cat. no. E0135Ra) and TNF-α (cat. no. E0764Ra) levels in blood
samples were determined using commercial ELISA kits (Shanghai
Korain Biotech Co., Ltd.).
The kidney tissues were homogenized using PBS (pH
7.2) to create a 10% (w/v) homogenate. Homogenization was performed
in an ice bath using a tissue grinder with a Teflon pestle, and
then the supernatant was collected after centrifugation at 960 x g
for 15 min at 21-23˚C. Through the use of commercial ELISA kits
(Shanghai Korain Biotech Co., Ltd.), the levels of malondialdehyde
(MDA; cat. no. E0156Ra), glutathione (GSH; cat. no. E1101Ra) and
caspase-3 (cat. no. E1648Ra) were measured.
Statistical analysis
SPSS version 22.0 for Windows (IBM Corp.) was used
for the statistical analysis of the data. The results of each
experiment are reported as the mean ± standard deviation.
Histopathological injury scores are reported as median (Q1-Q3).
Shapiro-Wilk test was used to assess the normal distribution of
each data. Tukey's post hoc test was utilized after one-way
analysis of variance for the statistical analysis of normally
distributed data. In the event that the data were not normally
distributed, Kruskal-Wallis with Bonferroni post hoc test was
performed. P#x003C;0.05 was considered to indicate a statistically
significant difference.
Results
Biochemical analysis
The results revealed that group I had significantly
higher levels of BUN compared with those in group S
(P#x003C;0.001). Group T had lower BUN levels compared with those
in group I, but there was no significant difference (Table I). BUN levels were similar between
group S and Group L. Creatinine levels were found to be
significantly higher in group I compared with those in group S
(P#x003C;0.001), but there was no significant difference between
group T and group I, or between group L and group S. Regarding the
IL-6 levels, although there was no significant difference between
group S and group I, there was a statistically significant
difference between group T and group I (P#x003C;0.05).
Additionally, there were significant decreases in group L compared
with those in group S (P#x003C;0.05). Group I had significantly
higher levels of TNF-α compared with those in groups S and T
(P#x003C;0.05 and P#x003C;0.01, respectively). Although TNF-α was
lowest in group L, there was no significant difference between this
group and group S.
 | Table IEffects of lupeol on the levels of
kidney injury markers in serum or tissue samples from animals in
the experimental groups. |
Table I
Effects of lupeol on the levels of
kidney injury markers in serum or tissue samples from animals in
the experimental groups.
| Parameters
tested | Sham | Lupeol | Ischemia | Therapy |
|---|
| Serum Blood urea
nitrogen, mg/dl | 23.83±3.76 | 22.50±3.98 |
111.00±12.04a | 83.16±12.65 |
| Serum Creatinine,
mg/dl | 0.41±0.07 | 0.36±0.04 |
1.32±0.55a | 0.67±0.13 |
| Serum IL-6,
ng/l | 26.20±5.87 |
17.35±3.05b | 33.43±10.35 |
20.98±1.30c |
| Serum TNF-α,
ng/l | 184.68±17.76 | 168.93±20.26 |
217.18±13.69d |
177.15±19.75e |
| Tissue
Malondialdehyde, nmol/ml | 1.65±0.12 | 1.56±0.19 |
2.10±0.27f |
1.66±0.18e |
| Tissue Glutathione,
mg/l | 527.35±68.83 | 533.03±67.14 |
415.23±58.56d |
517.45±38.24c |
| Tissue Caspase 3,
ng/ml | 7.50±1.45 | 6.86±1.02 | 8.88±1.13 | 7.55±1.15 |
Group I had the highest MDA value. There was a
significant difference between this group and group S
(P#x003C;0.01), in addition to between group I and group T
(P#x003C;0.01). The lowest MDA value was obtained in group L, but
there was no significant difference between this group and group S.
GSH levels were found to be significantly lower in group I compared
with those in group S (P#x003C;0.05). Similarly, there was a
significant difference between the ischemia and treatment groups
(P#x003C;0.05), but there was no difference between groups S and L.
Although group I had the highest caspase-3 value, there was no
significant difference between group I and group S, or between
group I and T (Table I). The
results in group T were markedly lower even though there was no
statistically significant difference in BUN, creatinine or
caspase-3 levels when compared with group I.
Pathological analysis
A difference was found between group I and group S
in terms of the numerical injury score (P#x003C;0.05). In group S,
the median histopathological injury score was 9.00 (3.75-12.75). In
group L, it was 8.00 (2.00-10.50). In group I, it was 17.50
(13.75-21.25) and in group T, it was 8.00 (7.50-11.25) (Table II). Group I had significantly
higher histopathological injury score compared with that in group S
(P#x003C;0.05). The levels of tubular dilatation, protein
cylinders, numerical score of injury and the percentage score in
group T were lower, despite the observation that there was no
significant difference between group T and the group I (Table II).
 | Table IIHistopathological evaluation scores
of renal tissues from each of the experimental groups. |
Table II
Histopathological evaluation scores
of renal tissues from each of the experimental groups.
| Parameters
tested | Sham | Lupeol | Ischemia | Therapy |
|---|
| Sub-categories of
numerical injury score | | | | |
|
Loss of
brush borders | 1.00
(1.00-1.00) | 1.00
(1.00-1.00) | 1.00
(1.00-1.00) | 1.00
(1.00-1.00) |
|
Lymphocyte
infiltration | 0.00
(0.00-0.25) | 0.00
(0.00-1.00) | 0.00
(0.00-0.00) | 0.00
(0.00-0.00) |
|
Degeneration | 1.00
(1.00-1.00) | 1.00
(1.00-1.00) | 1.00
(1.00-1.00) | 1.00
(1.00-1.00) |
|
Tubular
dilatation | 0.00
(0.00-0.25) | 0.00
(0.00-0.00) | 1.00
(1.00-1.00) | 1.00
(0.00-1.00) |
|
Protein
cylinders | 0.50
(0.00-1.00) | 0.50
(0.00-1.00) | 1.00
(1.00-1.00) | 0.50
(0.00-1.00) |
|
Necrosis | 1.00
(0.75-1.00) | 1.00
(0.00-1.00) | 1.00
(1.00-1.00) | 1.00
(1.00-1.00) |
| Total numerical
injury score | 3.50
(3.00-4.25) | 3.50
(2.00-5.00) | 5.00
(5.00-5.00)a | 4.00
(3.75-5.00) |
| Percentage
score | 2.50
(1.00-3.25) | 2.00
(1.00-2.25) | 3.50
(2.75-4.25) | 2.00
(2.00-2.25) |
| Histopathological
injury score | 9.00
(3.75-12.75) | 8.00
(2.00-10.50) | 17.50
(13.75-21.25)a | 8.00
(7.50-11.25)b |
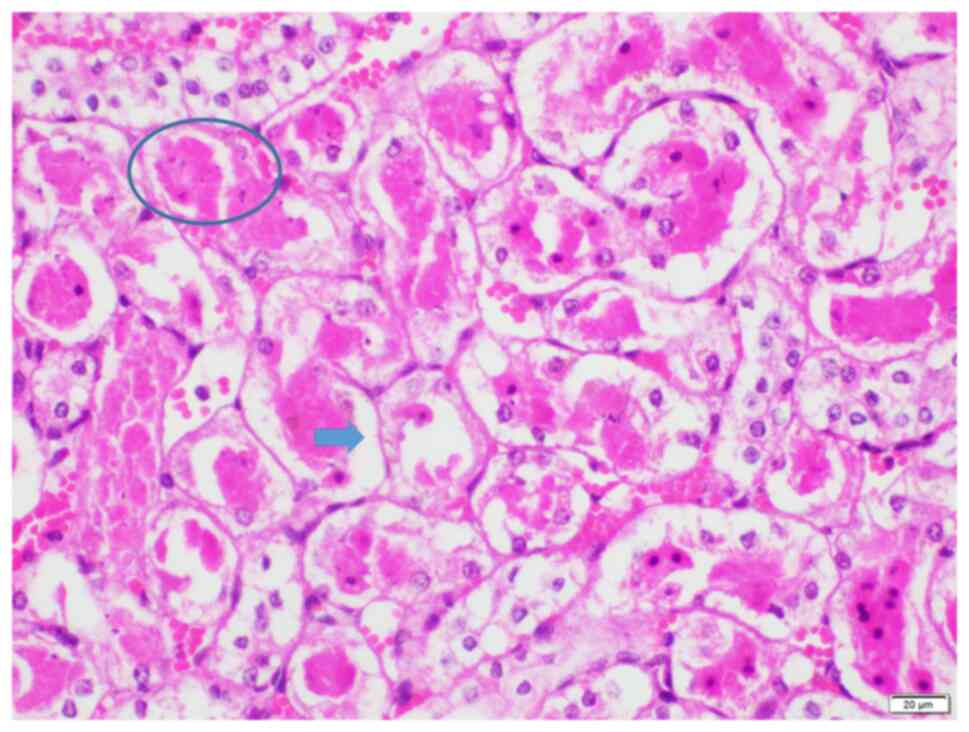
The highest observed histopathological injury score
of 25 was observed in two animals in group I (Figs. 3 and 4A), indicating an extensively damaged
kidney histology, which was characterized by loss of brush borders,
necrosis, degeneration, tubular dilatation and protein casts.
Conversely, the lowest histopathological injury score of 2 was
observed in two animals in group L, where brush border loss and
mild degeneration were noted (Fig.
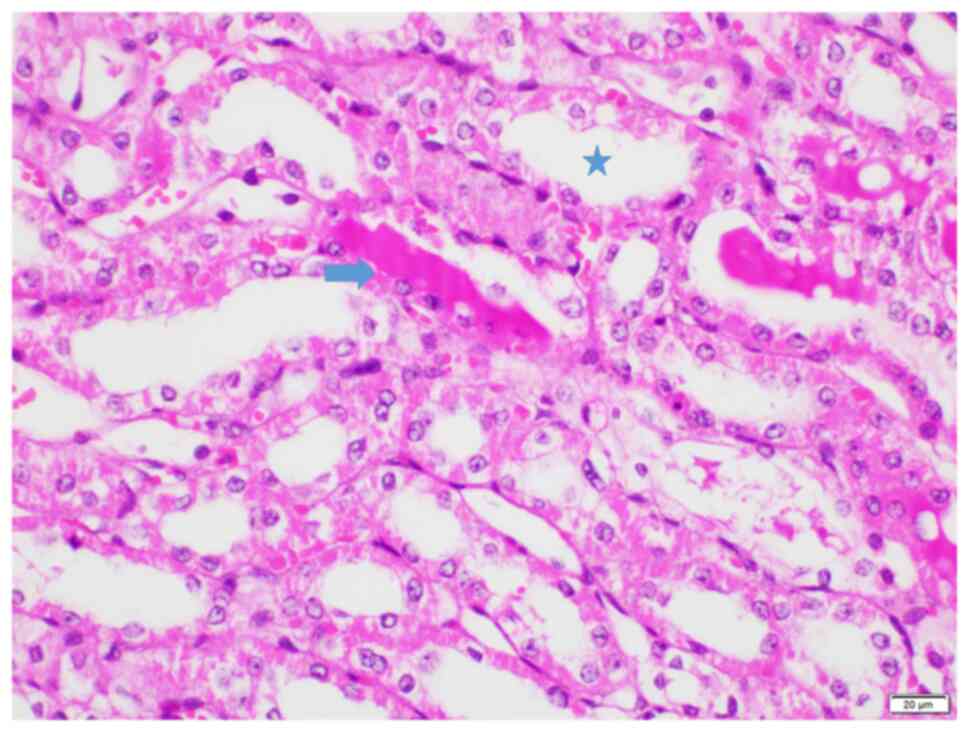
4B). Groups S (score range, 3-20; Fig. 4C) and T (score range, 8-25;
Figs. 4D and 5) exhibited average histopathological
injury scores, with variations in percentages observed among the
kidneys. No glomerular necrosis was observed in any group.
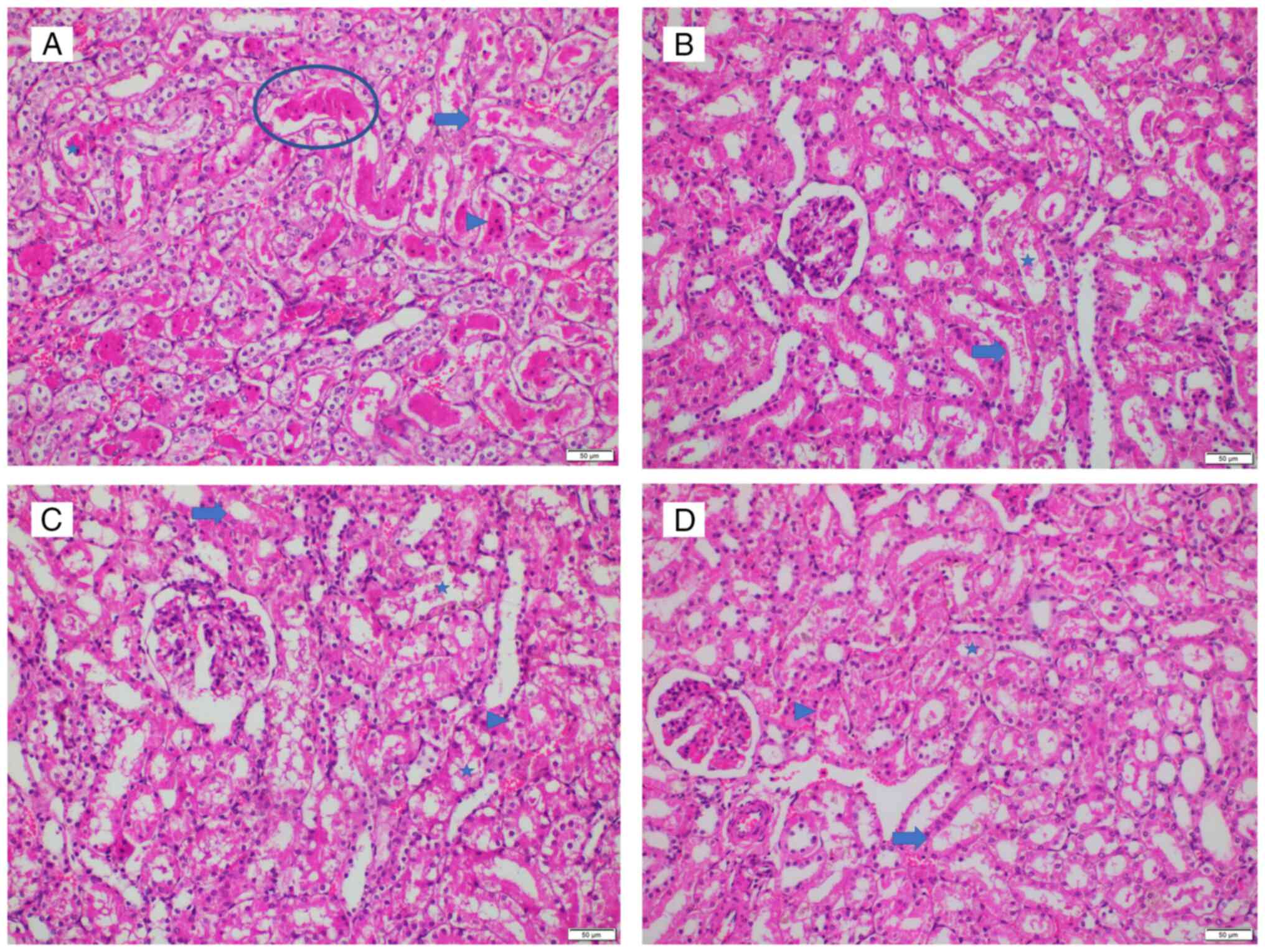 | Figure 4Representative histopathological
image from each group. (A) Group I, score 25. Prominent hyalin
casts and dilatation of tubules with degeneration, necrosis and
loss of brush borders are indicated. (B) Group L, score 2.
Locations of degeneration and loss of brush borders are shown. (C)
Group S, score 12. Locations of degeneration, loss of brush
borders, necrosis, and dilatation are indicated. (D) Group T, score
12. Locations of degeneration, loss of brush borders, necrosis, and
dilatation are indicated. Magnification, x200. Ovals, prominent
hyalin casts; asterisks, dilatation of tubules with degeneration;
arrowhead, necrosis; arrows, loss of brush borders. |
Discussion
In the present study, it was found that renal damage
occurred due to the high levels of various markers observed in the
kidney tissues and blood samples from rats in group I, namely BUN,
creatinine, MDA, TNF-α and caspase-3. Elevated BUN level during
ischemia may be associated with tubular blockage or reverse tubular
leakage. This suggests oxidative, inflammatory, biochemical and
cellular damage. In addition, it was found that the levels of GSH,
which is an antioxidant marker, were decreased in renal tissue
following ischemia (29). Lupeol
treatment was then found to exert antioxidant, anti-inflammatory
and general protective effects against ischemia.
In previous studies, reperfusion was performed 30-60
min after ischemia in models of experimental RIRI (30-32).
In addition, following 45 min of ischemia in rats, kidney damage
commenced at 4 h and reached its maximal extent at 24 h (33). Therefore, the present study opted
to terminate the ischemia procedure at 45 min followed by 24 h of
reperfusion prior to euthanasia.
Renal I/R is a multifactorial process that results
in a cascade of renal damage, both histopathological and functional
(34). During ischemia, ROS from
the xanthine oxidase system, mitochondrial electron transport
chain, NADPH oxidase system and uncoupled nitrite oxide synthase
(NOS) system may accumulate in ischemic cells due to low
antioxidant agent concentration (10). Following tissue reperfusion, local
inflammation occurs and ROS production becomes amplified, which
then enters the systemic circulation to induce cell damage (renal
structural damage) via apoptosis and necrosis (35). Numerous agents, such as allicin,
urolithin A, empagliflozin, asiaticoside and dapsone, have all been
used before and after renal ischemia to prevent this type of
damage. These studies are experimental and further studies are
required for clinical use (36-40).
Lupeol is a bioactive triterpene that can be found
in various edible vegetables and fruits, such as mangoes, cabbage,
green peppers, strawberries, olives and grapes. It can also be
found in various medicinal plants, such as Bowdichia
virgilioides and Crataeva nurvala (15,41,42).
Previous in vivo and in vitro experimental studies
have reported anti-inflammatory, antimicrobial, antioxidant,
anticancer, cardioprotective, hepatoprotective, antiarthritic and
wound healing effects of lupeol and its therapeutic potential
(16). In the present study,
lupeol demonstrated its antioxidant effects by lowering MDA levels
whilst increasing those of GSH in group T compared with those in
group I. To the best of our knowledge, the present study was the
first in which lupeol was found to exert anti-inflammatory and
antioxidant effects in a renal I/R model. Lupeol (100 mg/kg) has
been previously reported to confer combined antioxidant potential
and hepatoprotective effects, such as silymarin in aflatoxin
B1-induced liver injury, following the detection of
oxidant (MDA and catalase), antioxidant [GSH and superoxide
dismutase (SOD)] parameters and histopathological evaluation
(42). In another study that
previously tested the effects of lupeol for wound healing in
diabetic rats, antioxidant effects were observed and stimulatory
effects were exerted on wound healing (41). It has also been experimentally
shown that lupeol treatment can confer antioxidant effects against
acetaminophen (AAP)-induced liver injury, middle cerebral artery
occlusion-induced cerebral ischemia and selenite-induced cataract
and myocardial ischemia (29,43-45).
In addition, antioxidant effects of lupeol have been previously
demonstrated in models of liver damage, where it was observed to
restore the levels of antioxidant enzymes, reduce lipid
peroxidation and ROS formation, which in turn maintained the redox
balance (29). Lupeol has been
shown to inhibit oxidative stress-induced NF-κB activation in
culture of isolated lymphocytes obtained from peripheral blood of
healthy non-smoking donors (46).
With neuronal cells in rat models of cerebral ischemia, lupeol
treatment was found to reduce the expression levels of oxidation
(by increasing SDO, GSH and decreasing ROS production) and
inflammation markers (by decreasing TNF-α and IL-1β) through the
activation of nuclear factor erythroid 2-related factor 2, a key
antioxidant in vascular cells, through the inhibition of p38 MAPK
signaling (43).
The anti-inflammatory activity of lupeol has also
been investigated in the nervous system, digestive system and
cardiovascular diseases. Lupeol has been documented to inhibit the
expression of inflammatory genes and proteins through the
TLR4/MyD88/NF-κB P65 signaling pathway, IRAK (interleukin-1
receptor-associated kinase)-mediated TLR (toll like receptor)
inflammatory signaling, P38 MAPK, and JNK (c-Jun N-terminal kinase)
pathways, whilst reducing that of cytokines, such as TNF-α, IFN-γ
and IL-2(16). Kim et al
(47) previously demonstrated in a
model of cerulein-induced acute pancreatitis that lupeol treatment
could reduce the degree of pancreatic edema and neutrophil
infiltration, whilst inhibiting the release of pro-inflammatory
cytokines such as TNF-α, IL-1β, and IL-6. In another study, Ahmad
et al (26) reported that
the optimal dose to reduce leukocyte count, IL-2, IFN-γ and TNF-α
production in their cytometric investigation was 100 mg/kg of
lupeol compared to 25-50-200 mg/kg. In the present study, lupeol
was used to assess the possibility of these aforementioned effects
in the kidney. Consistently in the present study, a significant
decrease in the levels of in IL-6 and TNF-α was observed in the
therapy group compared with the ischemia group.
Sudhahar et al (20) previously established a model of
renal damage induced by hypercholesterolemia, where the initiation
of oxidative damage in hypercholesterolemic rats was indicated by
an increase in MDA levels (a product of lipid peroxidation) and a
decrease in antioxidant levels (such as SOD and GSH). Additionally,
Sudhahar et al (20) found
that the expression of acute-phase proteins, including fibrinogen
and C-reactive protein, in addition to the activity of renal
lysosomal acid hydrolases (such as acid phosphatase,
β-glucuronidase, β-galactosidase, N-acetyl glucosaminidase and
cathepsin D), were all increased in proportion to the degree of
inflammation. Subsequent histopathological findings revealed that
kidney damage occurred in a hypercholesterolemic state (20), which lead to the conclusion that
hypercholesterolemia increased oxidative stress and inflammation,
triggering glomerulosclerosis and renal damage. By contrast, lupeol
and lupeol linoleate treatment lead to the effective reversals of
these aforementioned abnormalities (20).
Renal tubules can become damaged during episodes of
ischemia (48). Since the
inhibition of cell pyroptosis has been shown to exert a protective
effect against renal I/R damage, Ni et al (48) previously measured the number of
pyroptotic cells after hydrogen sulfide treatment. Decreased
numbers of pyroptotic cells and reduced expression of pyroptosis
protein markers, such as caspase-1, gasdermin D, IL-1β and IL-18,
were observed (48). In the
present study, caspase-3 expression was examined as a marker of
pyroptosis, which is an indicator of inflammatory cell death
(49). Caspase 3 levels were found
to be lower in group T, indicating that lupeol reduced the extent
of cell death. To the best of our knowledge, the only previous
study on the effects of lupeol on kidney damage was the
aforementioned hypercholesterolemia-induced kidney damage model,
which did not examine caspase-3 expression (20). However, lupeol has been shown to
inhibit caspase-3 in a rat cerebral I/R model (50).
In the present study, it was found that group I had
higher BUN and creatinine levels. Increased blood levels of BUN and
creatinine during ischemia may be caused by tubular blockage or
reverse tubular leakage, but the exact underlying mechanism of this
phenomenon remains unknown. However, acute renal failure, as
indicated by increased BUN and creatinine levels, has been shown to
occur before tubular necrosis develops (51). In previous studies with the effects
of hydrogen sulfide and lycopene on renal I/R injury, BUN and
creatinine values were found to be lower in the treatment groups
(4,48). Similarly, lupeol has been shown to
decrease serum BUN and creatinine values following
hypercholesterolemia-induced renal damage (20). Although results from the present
study did not reveal a statistically significant difference in the
BUN and creatinine levels, the values were markedly decreased in
group T. The reason for this improvement may be the sum of the
antioxidant, anti-inflammatory and cytoprotective effects exerted
by lupeol.
Previous studies have examined histological
characteristics of renal I/R, such as tubular dilatation, tubular
vacuolization, brush border loss, glomerular necrosis and tubular
necrosis. After lycopene and hydrogen sulfide were administered to
the treatment group following ischemia, a reduction in the extent
of brush boundary loss, tubular vacuolization and tubular
dilatation was observed (4,48).
In addition, tubular epithelial denudation with casts attributable
to lipemic-oxidative damage, and damage to the tubules predisposing
to hypoxic injury were found in a model of
hypercholesterolemia-induced renal injury, whilst intact renal
architecture comparable to that of healthy kidneys was noted in the
lupeol treatment groups (20). In
the present study, the histopathological injury score was
determined, where the highest values were found in group I. By
contrast, scores close to Sham group level were obtained following
lupeol treatment. The antioxidant and anti-inflammatory properties
of lupeol may be partly responsible for such protective effects,
similar to those previously observed in other organs, such as the
eyes, brain, heart, liver and skin (16,20).
Lupeol has been shown to be effective even when
applied through different routes, such as subcutaneously,
topically, orally and intraperitoneally. In vivo, no
systemic toxicity was observed upon treatment with 200 mg/kg
intraperitoneally in rats or 2,000 mg/kg orally in mice (25). Yokoe et al (52) previously showed that subcutaneously
administering lupeol can prevent local tumor progression and
distant metastasis by canine oral malignant melanoma in dogs,
whereas Jesus et al (53)
reported that intraperitoneally administering lupeol could protect
the liver and spleen against leishmaniasis. Beserra et al
(41) showed that topically
administering lupeol could accelerate wound healing, whilst Asha
et al (44) found that
orally administering lupeol alleviated selenite-induced cataracts.
Another previous study, in which 200 mg/kg lupeol was administered
orally to mice, found the level of lupeol in both plasma and organs
such as kidney, liver, small and large intestine to be remains in
high concentration and constant over time
(T1/2 for lupeol was 13.564±2.912
h) (54). Although lupeol can be
found in various fruits, consumption of ≥555 g/kg mango fruit is
needed to reach the dose of 100 mg/kg lupeol in humans, which was
used in the present experimental study. By contrast, the oral
bioavailability of lupeol was previously reported to be #x003C;1%
(18). Therefore, lupeol may be
more appropriately applied as a drug instead of a dietary
supplement. The demonstration of antioxidant, anti-inflammatory,
anticancer and anti-apoptotic effects of lupeol in both
experimental in vivo and in vitro human cell cultures
has supported its use for a number of human diseases. Lupeol has
been tested in various clinical studies on hepatocellular cancer
and actinic keratoses treatment. In addition, this drug has been
previously tested a randomized placebo-controlled clinical study
for the treatment of nocturnal enuresis in children (16-19,55).
In the present study, lupeol was shown to exert
protective effects in the kidney against RIRI by reducing the
histopathological damage score and oxidative stress, whilst
suppressing the production of inflammatory proteins. In conclusion,
results from the present study support the clinical use of lupeol
for AKI.
Acknowledgements
Not applicable.
Funding
Funding: The present study was sponsored by the Scientific
Research Foundation of Gazi University (grant no. 6/2022-7842).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
AK and RK designed the study. CK and SE performed
the experiments. ZT and KS analyzed the data. AK, RK and CK wrote
the manuscript. AK, CK and SE searched the literature. MAI and OG
analyzed the tissues. All authors have read and approved the final
manuscript. AK and CK confirm the authenticity of all the raw
data.
Ethics approval and consent to
participate
The Gazi University Animal Experiments Ethics
Committee granted ethical approval (approval no. G.U.ET-22.006;
Ankara, Turkey). All methods were performed in accordance with the
relevant guidelines and regulations.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Ronco C, Bellomo R and Kellum JA: Acute
kidney injury. Lancet. 394:1949–1964. 2019.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Shaikhouni S and Yessayan L: Management of
acute kidney injury/renal replacement therapy in the intensive care
unit. Surg Clin North Am. 102:181–198. 2022.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Thapa K, Singh TG and Kaur A: Targeting
ferroptosis in ischemia/reperfusion renal injury. Naunyn
Schmiedebergs Arch Pharmacol. 395:1331–1341. 2022.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Kaya C, Karabulut R, Turkyilmaz Z, Sonmez
K, Kulduk G, Gülbahar Ö, Köse F and Basaklar AC: Lycopene has
reduced renal damage histopathologically and biochemically in
experimental renal ischemia-reperfusion injury. Ren Fail.
37:1390–1395. 2015.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Kinra M, Mudgal J, Arora D and Nampoothiri
M: An insight into the role of cyclooxygenase and lipooxygenase
pathway in renal ischemia. Eur Rev Med Pharmacol Sci. 21:5017–5020.
2017.PubMed/NCBI
|
|
6
|
Miao AF, Liang JX, Yao L, Han JL and Zhou
LJ: Hypoxia-inducible factor prolyl hydroxylase inhibitor
roxadustat (FG-4592) protects against renal ischemia/reperfusion
injury by inhibiting inflammation. Ren Fail. 43:803–810.
2021.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Katagiri D, Wang F, Gore JC, Harris RC and
Takahashi T: Clinical and experimental approaches for imaging of
acute kidney injury. Clin Exp Nephrol. 25:685–699. 2021.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Tao Q, Zhang Q, An Z, Chen Z and Feng Y:
Multi-Parametric MRI for evaluating variations in renal structure,
function, and endogenous metabolites in an animal model with acute
kidney injury induced by ischemia reperfusion. J Magn Reson
Imaging: Oct 26, 2023 (Epub ahead of print).
|
|
9
|
Bonventre JV and Yang L: Cellular
pathophysiology of ischemic acute kidney injury. J Clin Invest.
121:4210–4221. 2011.PubMed/NCBI View
Article : Google Scholar
|
|
10
|
Wu MY, Yiang GT, Liao WT, Tsai AP, Cheng
YL, Cheng PW, Li CY and Li CJ: Current mechanistic concepts in
ischemia and reperfusion injury. Cell Physiol Biochem.
46:1650–1667. 2018.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Nath KA and Norby SM: Reactive oxygen
species and acute renal failure. Am J Med. 109:665–678.
2000.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Zhu H, Tan Y, Du W, Li Y, Toan S, Mui D,
Tian F and Zhou H: Phosphoglycerate mutase 5 exacerbates cardiac
ischemia-reperfusion injury through disrupting mitochondrial
quality control. Redox Biol. 38(101777)2021.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Malek M and Nematbakhsh M: Renal
ischemia/reperfusion injury; from pathophysiology to treatment. J
Renal Inj Prev. 4:20–27. 2015.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Lin Y, Xu L, Lin H, Cui W, Jiao Y, Wang B,
Li H, Wang X and Wu J: Network pharmacology and experimental
validation to investigate the mechanism of Nao-Ling-Su capsule in
the treatment of ischemia/reperfusion-induced acute kidney injury.
J Ethnopharmacol. 326(117958)2024.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Ruiz-Rodríguez MA, Vedani A,
Flores-Mireles AL, Cháirez-Ramírez MH, Gallegos-Infante JA and
González-Laredo RF: In Silico prediction of the toxic potential of
lupeol. Chem Res Toxicol. 30:1562–1571. 2017.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Liu K, Zhang X, Xie L, Deng M, Chen H,
Song J, Long J, Li X and Luo J: Lupeol and its derivatives as
anticancer and anti-inflammatory agents: Molecular mechanisms and
therapeutic efficacy. Pharmacol Res. 164(105373)2021.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Sohag AAM, Hossain MT, Rahaman MA, Rahman
P, Hasan MS, Das RC, Khan MK, Sikder MH, Alam M, Uddin MJ, et al:
Molecular pharmacology and therapeutic advances of the pentacyclic
triterpene lupeol. Phytomedicine. 99(154012)2022.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Park JS, Rehman IU, Choe K, Ahmad R, Lee
HJ and Kim MO: A triterpenoid lupeol as an antioxidant and
anti-neuroinflammatory agent: Impacts on oxidative stress in
Alzheimer's disease. Nutrients. 15(3059)2023.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Schloss J, Ryan K, Reid R and Steel A: A
randomised, double-blind, placebo-controlled clinical trial
assessing the efficacy of bedtime buddy® for the treatment of
nocturnal enuresis in children. BMC Pediatr. 19(421)2019.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Sudhahar V, Ashok Kumar S, Varalakshmi P
and Sujatha V: Protective effect of lupeol and lupeol linoleate in
hypercholesterolemia associated renal damage. Mol Cell Biochem.
317:11–20. 2008.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Nitta M, Azuma K, Hata K, Takahashi S,
Ogiwara K, Tsuka T, Imagawa T, Yokoe I, Osaki T, Minami S and
Okamoto Y: Systemic and local injections of lupeol inhibit tumor
growth in a melanoma-bearing mouse model. Biomed Rep. 1:641–645.
2013.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Sunitha S, Nagaraj M and Varalakshmi P:
Hepatoprotective effect of lupeol and lupeol linoleate on tissue
antioxidant defence system in cadmium-induced hepatotoxicity in
rats. Fitoterapia. 72:516–523. 2001.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Murtaza I, Saleem M, Adhami VM, Hafeez BB
and Mukhtar H: Suppression of cFLIP by lupeol, a dietary
triterpene, is sufficient to overcome resistance to TRAIL-mediated
apoptosis in chemoresistant human pancreatic cancer cells. Cancer
Res. 69:1156–1165. 2009.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Saleem M, Afaq F, Adhami VM and Mukhtar H:
Lupeol modulates NF-kappaB and PI3K/Akt pathways and inhibits skin
cancer in CD-1 mice. Oncogene. 23:5203–5214. 2004.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Siddique HR and Saleem M: Beneficial
health effects of lupeol triterpene: A review of preclinical
studies. Life Sci. 88:285–293. 2011.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Ahmad SF, Pandey A, Kour K and Bani S:
Downregulation of pro-inflammatory cytokines by lupeol measured
using cytometric bead array immunoassay. Phytother Res. 24:9–13.
2010.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Al-Mousawi AM, Kulp GA, Branski LK, Kraft
R, Mecott GA, Williams FN, Herndon DN and Jeschke MG: Impact of
anesthesia, analgesia, and euthanasia technique on the inflammatory
cytokine profile in a rodent model of severe burn injury. Shock.
34:261–268. 2010.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Rampil IJ and Laster MJ: No correlation
between quantitative electroencephalographic measurements and
movement response to noxious stimuli during isoflurane anesthesia
in rats. Anesthesiology. 77:920–925. 1992.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Kumari A and Kakkar P: Lupeol protects
against acetaminophen-induced oxidative stress and cell death in
rat primary hepatocytes. Food Chem Toxicol. 50:1781–1789.
2012.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Liu H, Wang L, Weng X, Chen H, Du Y, Diao
C, Chen Z and Liu X: Inhibition of Brd4 alleviates renal
ischemia/reperfusion injury-induced apoptosis and endoplasmic
reticulum stress by blocking FoxO4-mediated oxidative stress. Redox
Biol. 24(101195)2019.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Zheng Y, Zhang N and Bai F: Gastrodin
pretreatment alleviates renal ischemia-reperfusion injury. Urol
Int. 106:630–637. 2022.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Feng W, Remedies CE, Obi IE, Aldous SR,
Meera SI, Sanders PW, Inscho EW and Guan Z: Restoration of afferent
arteriolar autoregulatory behavior in ischemia-reperfusion injury
in rat kidneys. Am J Physiol Renal Physiol. 320:F429–F441.
2021.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Williams P, Lopez H, Britt D, Chan C,
Ezrin A and Hottendorf R: Characterization of renal
ischemia-reperfusion injury in rats. J Pharmacol Toxicol Methods.
37:1–7. 1997.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Chatterjee PK: Novel pharmacological
approaches to the treatment of renal ischemia-reperfusion injury: A
comprehensive review. Naunyn Schmiedebergs Arch Pharmacol.
376:1–43. 2007.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Eryilmaz S, Turkyilmaz Z, Karabulut R,
Gulburun MA, Poyraz A, Gulbahar O, Arslan B and Sonmez K: The
effects of hydrogen-rich saline solution on intestinal anastomosis
performed after intestinal ischemia reperfusion injury. J Pediatr
Surg. 55:1574–1578. 2020.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Shan Y, Chen D, Hu B, Xu G, Li W, Jin Y,
Jin X, Jin X and Jin L: Allicin ameliorates renal
ischemia/reperfusion injury via inhibition of oxidative stress and
inflammation in rats. Biomed Pharmacother.
142(112077)2021.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Zhang Y, Liu M, Zhang Y, Tian M, Chen P,
Lan Y and Zhou B: Urolithin A alleviates acute kidney injury
induced by renal ischemia reperfusion through the p62-Keap1-Nrf2
signaling pathway. Phytother Res. 36:984–995. 2022.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Wang Q, Ju F, Li J, Liu T, Zuo Y, Abbott
GW and Hu Z: Empagliflozin protects against renal
ischemia/reperfusion injury in mice. Sci Rep.
12(19323)2022.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Tang S, Xie X, Wang M, Yang L and Wei W:
Protective effects of asiaticoside on renal ischemia reperfusion
injury in vivo and in vitro. Bioengineered. 13:10235–10243.
2022.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Nezamoleslami S, Sheibani M, Jahanshahi F,
Mumtaz F, Abbasi A and Dehpour AR: Protective effect of dapsone
against renal ischemia-reperfusion injury in rat. Immunopharmacol
Immunotoxicol. 42:272–279. 2020.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Beserra FP, Vieira AJ, Gushiken LFS, de
Souza EO, Hussni MF, Hussni CA, Nóbrega RH, Martinez ERM, Jackson
CJ, de Azevedo Maia GL, et al: Lupeol, a dietary triterpene,
enhances wound healing in streptozotocin-induced hyperglycemic rats
with modulatory effects on inflammation, oxidative stress, and
angiogenesis. Oxid Med Cell Longev. 2019(3182627)2019.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Preetha SP, Kanniappan M, Selvakumar E,
Nagaraj M and Varalakshmi P: Lupeol ameliorates aflatoxin
B1-induced peroxidative hepatic damage in rats. Comp Biochem
Physiol C Toxicol Pharmacol. 143:333–339. 2006.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Zhang Z, Xu C, Hao J, Zhang M, Wang Z, Yin
T, Lin K, Liu W, Jiang Q, Li Z, et al: Beneficial consequences of
Lupeol on middle cerebral artery-induced cerebral ischemia in the
rat involves Nrf2 and P38 MAPK modulation. Metab Brain Dis.
35:841–848. 2020.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Asha R, Gayathri Devi V and Abraham A:
Lupeol, a pentacyclic triterpenoid isolated from Vernonia cinerea
attenuate selenite induced cataract formation in Sprague Dawley rat
pups. Chem Biol Interact. 245:20–29. 2016.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Li J, Ma X, Yang J, Wang L, Huang Y and
Zhu Y: Lupeol alleviates myocardial ischemia-reperfusion injury in
rats by regulating NF-[Formula: See text]B and Nrf2 pathways. Am J
Chin Med. 50:1269–1280. 2022.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Srivastava AK, Mishra S, Ali W and Shukla
Y: Protective effects of lupeol against mancozeb-induced
genotoxicity in cultured human lymphocytes. Phytomedicine.
23:714–724. 2016.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Kim MJ, Bae GS, Choi SB, Jo IJ, Kim DG,
Shin JY, Lee SK, Kim MJ, Song HJ and Park SJ: Lupeol protects
against cerulein-induced acute pancreatitis in mice. Phytother Res.
29:1634–1639. 2015.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Ni J, Jiang L, Shen G, Xia Z, Zhang L, Xu
J, Feng Q, Qu H, Xu F and Li X: Hydrogen sulfide reduces pyroptosis
and alleviates ischemia-reperfusion-induced acute kidney injury by
inhibiting NLRP3 inflammasome. Life Sci. 284(119466)2021.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Zhang B, Wan S, Liu H, Qiu Q, Chen H, Chen
Z, Wang L and Liu X: Naringenin alleviates renal ischemia
reperfusion injury by suppressing ER stress-induced pyroptosis and
apoptosis through activating Nrf2/HO-1 signaling pathway. Oxid Med
Cell Longev. 2022(5992436)2022.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Wang Z, Han Y, Tian S, Bao J, Wang Y and
Jiao J: Lupeol alleviates cerebral ischemia-reperfusion injury in
correlation with modulation of PI3K/Akt pathway. Neuropsychiatr Dis
Treat. 16:1381–1390. 2020.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Karimi G, Ramezani M and Tahoonian Z:
Cisplatin nephrotoxicity and protection by milk thistle extract in
rats. Evid Based Complement Alternat Med. 2:383–386.
2005.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Yokoe I, Azuma K, Hata K, Mukaiyama T,
Goto T, Tsuka T, Imagawa T, Itoh N, Murahata Y, Osaki T, et al:
Clinical systemic lupeol administration for canine oral malignant
melanoma. Mol Clin Oncol. 3:89–92. 2015.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Jesus JA, da Silva TNF, Sousa IMO,
Ferreira AF, Laurenti MD, da Costa PC, de Carvalho Ferreira D and
Passero LFD: Nanostructured lipid carriers as robust systems for
lupeol delivery in the treatment of experimental visceral
leishmaniasis. Pharmaceuticals (Basel). 16(1646)2023.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Cháirez-Ramírez MH, Gallegos-Infante JA,
Moreno-Jiménez MR, González-Laredo RF and Rocha-Guzmán NE:
Absorption and distribution of lupeol in CD-1 mice evaluated by
UPLC-APCI+ -MS/MS. Biomed Chromatogr.
33(e4432)2019.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Yang X, Feng Y, Liu Y, Ye X, Ji X, Sun L,
Gao F, Zhang Q, Li Y, Zhu B and Wang X: Fuzheng Jiedu Xiaoji
formulation inhibits hepatocellular carcinoma progression in
patients by targeting the AKT/CyclinD1/p21/p27 pathway.
Phytomedicine. 87(153575)2021.PubMed/NCBI View Article : Google Scholar
|