Introduction
According to World Health Organisation (WHO)
estimates, Cervical Cancer (CC) accounted for 604,000 new cases and
342,000 mortalities globally in 2020 alone. Of these new cases and
fatalities, ~90% are from low-and middle-income nations and are
identified at advanced stages (1).
Such patients have markedly worse prognosis, higher recurrence and
mortality rates compared with patients who are diagnosed at the
early stages (2). Based on the
guidelines of the International Federation of Obstetricians and
Gynecologists (FIGO) tumour staging system, the prognostic markers
for patients with CC include lymph node status, tumour size,
histological grade and depth of invasion (3,4).
Other characteristics, aside from the FIGO stage, can only be
assessed following surgery. However, clinical staging, particularly
in some patients with CC with advanced disease, is often inaccurate
in predicting the prognosis (5).
Therefore, identifying a vital pre-treatment parameter to assess
the likelihood of survival and prognosis of CC is necessary before
choosing a relevant clinical approach.
A number of malignancies are known to originate from
areas of chronic inflammation, irritation and infection.
Inflammation affects every stage of carcinogenesis from tumour
initiation and progression to metastatic dissemination (6). The metabolic demand increases as
malignancies spread and, if ignored, may lead to a gradual
deterioration in nutritional status, which is frequently noticeable
even before the patient is diagnosed. Studies have shown that ~20%
of deaths from gynaecological cancer may be associated with
malnutrition (7,8). Therefore, nutritional status is
recognised as a vital determinant of the quality of life of
survivors of cancer. Malnutrition, sarcopenia and cancer cachexia
are linked to higher rates (20%) of post-treatment complications,
poor clinical response, longer hospitalizations and shorter
survival in 2022(9). Several
studies have shown that numerous factors, including nutritional and
inflammatory indicators, predict the prognosis of various types of
cancer. Studies from Western countries have shown that 20-50% of
patients with gynaecological cancer present with malnutrition at
diagnosis (10). This proportion
is even higher in developing countries (62-88%) (11).
In recent years, the Prognostic Nutritional Index
(PNI), a simple and readily available marker, has gained interest
as a potential tool for assessing nutritional status and predicting
clinical outcomes in various malignancies (6). The PNI is calculated by combining
serum albumin levels (g/l) with the total lymphocyte count
(x109/l) using the formula: PNI=albumin+0.005 x
lymphocytes (12,13). The PNI is often associated with the
prognosis of several gastrointestinal and a few gynaecological
types of cancer and its score reflects both the protein reserve
(albumin) and cellular immunity (lymphocytes), providing a more
comprehensive assessment of the nutritional-immune state (8). While several studies have explored
the association between PNI and the prognosis of cervical cancer,
the findings remain inconclusive (6,8,10).
Thus, the aim of the current study was to evaluate the role of PNI
in predicting the overall survival (OS) and progression-free
survival (PFS) of women with cervical cancer.
Materials and methods
Research question
What is the predictive effect of PNI in predicting
various clinical outcomes in adult patients (>18 years)
diagnosed with cervical cancer?
Methods
The present study complied with the preferred
reporting items for systematic reviews and meta-analyses (PRISMA)
framework 2020(14). Ethical
approval was not necessary as the present study undertook a
secondary data analysis of the available literature. The present
study was registered at PROSPERO, with the number:
CRD42023423281.
Inclusion criteria
Prospective and retrospective studies conducted
among women with cervical cancer reporting PNI prior to therapy
were both included. Medline (https://www.ncbi.nlm.nih.gov), Google Scholar
(https://scholar.google.com), Science
Direct (https://www.sciencedirect.com) and
Cochrane Central databases (https://www.cochranelibrary.com/central/about-central)
were searched for articles available as free full text and
published in English from inception to April 2023. Conference
abstracts, narrative reviews, case reports and series and
randomised control trials were excluded. Studies that reported on
possible study treatments (concurrent chemoradiotherapy,
radiotherapy, surgery) were included.
Type of intervention
The PNI was calculated using the equation: PNI=10 x
albumin concentration (g/dl)+0.005 x total lymphocyte count (µl)
(15). All studies that had used
this accepted calculation of PNI were included in the present
study.
Outcome definitions
The main outcomes included evaluated overall
survival and progression-free survival.
Search strategy
Medline (https://www.ncbi.nlm.nih.gov), Google Scholar
(https://scholar.google.com), Science
Direct (https://www.sciencedirect.com) and
Cochrane Central databases (https://www.cochranelibrary.com/central/about-central)
were searched using the medical subject heading (MeSH) terms such
as: ‘Prognostic nutritional index’ OR ‘PNI’ AND ‘Cervical cancer’
OR ‘Cervical carcinoma’ OR ‘Cervical Ca’ OR ‘Cervical neoplasms’
AND ‘survival’ OR ‘Outcome’ OR ‘Progression-free survival’ AND
‘Observational studies’ OR ‘Retrospective studies OR ‘Prospective
studies’ along with free text terms as a filter. Cross-references
of primary studies were also searched for additional relevant
articles.
Selection of studies
The two authors independently performed the
preliminary title, abstract and keywords search screening. Full
texts of the relevant articles and their abstracts were then
screened for eligibility by both authors. All disagreements were
resolved by discussion. The two authors individually extracted all
data, monitored data entry and analysis and ensured quality.
Data extraction and management
The following information was retrieved from the
eligible studies by the primary investigator: i) General
information (authors and year of publication), ii) in the methods:
Study design and setting, iii) in the participants section: The
sample size and age distribution of participants, iv) in the
intervention section: details of PNI such as cut-offs used, the
formula used to calculate PNI, duration of follow up, and v) in the
outcomes section: Overall survival and progression-free
survival.
Risk of bias assessment in included
studies
The two authors measured the risk of bias in
relevant studies using the seven items Newcastle-Ottawa Scale (NOS)
for observational studies. The NOS scale that has three domains
(selection, comparability and outcome), was used to rate the
studies. A study was graded as good quality if it had 3 or 4 stars
in selection domain AND 1 or 2 stars in comparability domain AND 2
or 3 stars in outcome/exposure domain (16).
Statistical analysis
Data were extracted, entered into Microsoft Excel
and analysed using STATA 18 (StataCorp LLC). The primary and
secondary outcomes data was summarized as hazards ratio (HR) with
95% confidence interval (CI) for outcomes on overall survival and
progression-free survival. The estimates were pooled using a random
effects model with Mantel-Haenszel method as the present study
encountered considerate clinical heterogeneity and high statistical
heterogeneity. In cases of missing data, the author(s) of the trial
were contacted if possible. The prognostic effect of PNI on
selected cancer outcomes were separately using univariate and
multivariate estimates of HRs.
Assessment of heterogeneity
Between-study variance due to heterogeneity was
assessed by the Chi square test and I2 statistic.
I2 <25% was considered mild, 25-75%, moderate and
>75% as substantial heterogeneity. Study details and pooled
estimates were graphically represented by a forest plot.
Publication bias was assessed using funnel plot.
Sensitivity analysis
Sensitivity analysis was performed for the risk of
bias among the included studies. Hence, separate pooled estimates
were obtained by analysing studies with various risk of bias scores
according to NOS.
Results
Study selection
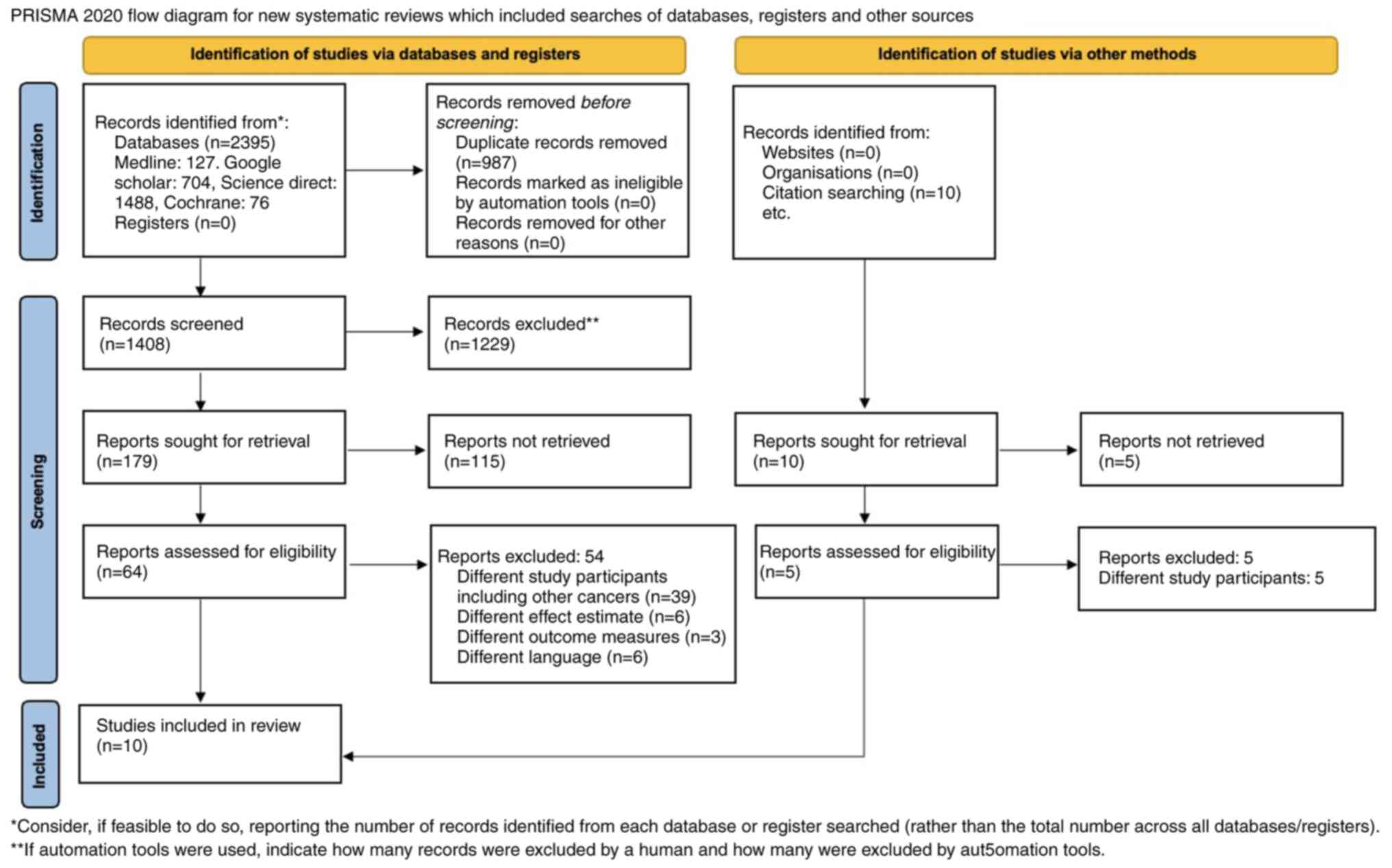
A total of 1,408 articles were identified by the
search. Of them, 1,229 duplicates were removed and 54 were excluded
at the stage of the preliminary title and abstract screening, as
they did not match the inclusion criteria. A study performed by
Haraga et al (17) in 2016,
reported study outcomes on OS and PFS separately for individuals
who were treated on concurrent chemoradiotherapy and radiotherapy
respectively. Thus the data were included as two differences
entries for the meta-analysis. Finally, nine articles were included
in the systematic review and 10 articles were included in the
meta-analysis (17-25).
A total number of subjects in all studies was 2,352. The PRISMA
2020 flow diagram is explained in Fig.
1 and the search strategy is summarized in Table SI.
Characteristics of the included
studies
Table I describes
the characteristics of the included studies. Of the 10 included
studies, seven studies were from China and the remaining three were
from Japan [as Haraga et al (17) reported on both chemotherapy and
radiotherapy treatment]. All studies included adult women diagnosed
with cervical cancer with an age distribution between 25-88 years.
All studies reported results in the English language. The number of
patients in the included studies ranged between 79-698. All studies
were of retrospective design and all reported on overall survival.
A total of five studies reported on the progression-free survival.
A total of four studies had surgery as a treatment option, while
four had radiotherapy and two studies used concurrent
chemoradiotherapy as treatment. The PNI cut off determined by the
included studies ranged between 45-55.
 | Table ICharacteristics of included
studies. |
Table I
Characteristics of included
studies.
| First author,
year | Country | Sample size and age
(median and range) | Type of study | PNI
calculation | PNI cut off | Low and high PNI
incidence | Treatment
course | Follow up period
(months) | Primary and
secondary outcomes | Quality of study
(NOS) | (Refs) |
|---|
| Gao et al,
2023 | China | 110 Age: Not
reported | Retrospective | Albumin+0.005 x
lymphocytes | 47.35 | Low PNI <47.35
High PNI >47.35 | Radiotherapy | 26 months | Overall survival
and progression- free survival | 6 | (18) |
| Guo et al,
2023 | China | 109 Age:
53.95±9.56 | Retrospective | Albumin+0.005 x
lymphocytes | 52.68 | Low PNI <52.68
High PNI >52.68 | Surgery | Not reported | Overall
survival | 7 | (19) |
| Haraga et
al, 2016 | Japan | 131 Age: 61.5 years
(25-88) | Retrospective | Albumin+0.005 x
lymphocytes | 48.55 | Not reported | Chemoradio-
therapy | Once in every 1-2
months | Overall survival
and progression- free survival | 6 | (17) |
| Haraga et
al, 2016 | Japan | 131 Age: 61.5 years
(25-88) | Retrospective | Albumin+0.005 x
lymphocytes | 48.55 | Not reported | Radiotherapy | Once in every 1-2
months | Overall survival
and progression- free survival | 6 | (17) |
| He X et al,
2018 | China | 229 Age: 44 years
(28-79) | Retrospective
lymphocytes | Albumin+0.005
x | 45 | Not reported | Surgery
reported | Not | Overall
survival | 5 | (20) |
| Ida N et al,
2018 | Japan | 79 Age: 52.4 years
(25-78) | Retrospective | Albumin+0.005 x
lymphocytes | 46.9 | Not reported | Concurrent
chemoradio- therapy | Median: 15 months,
range (2-93) | Overall
survival | 7 | (21) |
| Jiang et al,
2021 | China | 583 Age:
49.05±9.208 | Retrospective | Albumin+0.005 x
lymphocytes | 50.15 | Low PNI ≤50.15 High
PNI >50.15 | Surgery | Mean follow up:
68.34±26.93 | Overall survival
and progression- free survival | 7 | (22) |
| Wang et al,
2023 | China | 178 Age: 52.46
(9.06) | Retrospective | Albumin+0.005 x
lymphocytes | 55 | Low PNI ≤55 High
PNI >55 | Radiotherapy | Mean follow up: 50
months | Overall survival
and progression- free survival | 7 | (23) |
| Zhang et al,
2018 | China | 235 Age: 46 years
(29-78) | Retrospective | Albumin+0.005 x
lymphocytes | 50.38 | Low-PNI: 76.2%
High-PNI: 23.8% | Surgery | Median: 77 months,
range (32-96) | Overall survival
and progression- free survival | 6 | (24) |
| Zhang et al,
2021 | China | 698 Age: 51
years | Retrospective | Albumin+0.005 x
lymphocytes | 48.55 | Low-PNI: >48.55
High-PNI: ≤48.55 | Radiotherapy | Median: 56.2 months
(range: 4.9-186.9 months) | Overall
survival | 6 | (25) |
Excluded studies
Of 64 full-text potentially eligible articles, 54
studies were excluded. Of them, 39 studies were excluded because of
the variability in the diagnosis of study participants (a mix of
different gynaecological cancers), six studies had effect estimates
other than HR, three studies reported on outcomes other than OS and
PFS and six were in languages other than English.
Risk of bias in included studies
Table I summarizes
the risk of bias in the included studies. The studies were
categorised as high quality if NOS score ≥6, while studies with the
lesser scores were categorised as low quality. Of the nine studies,
eight were of high quality.
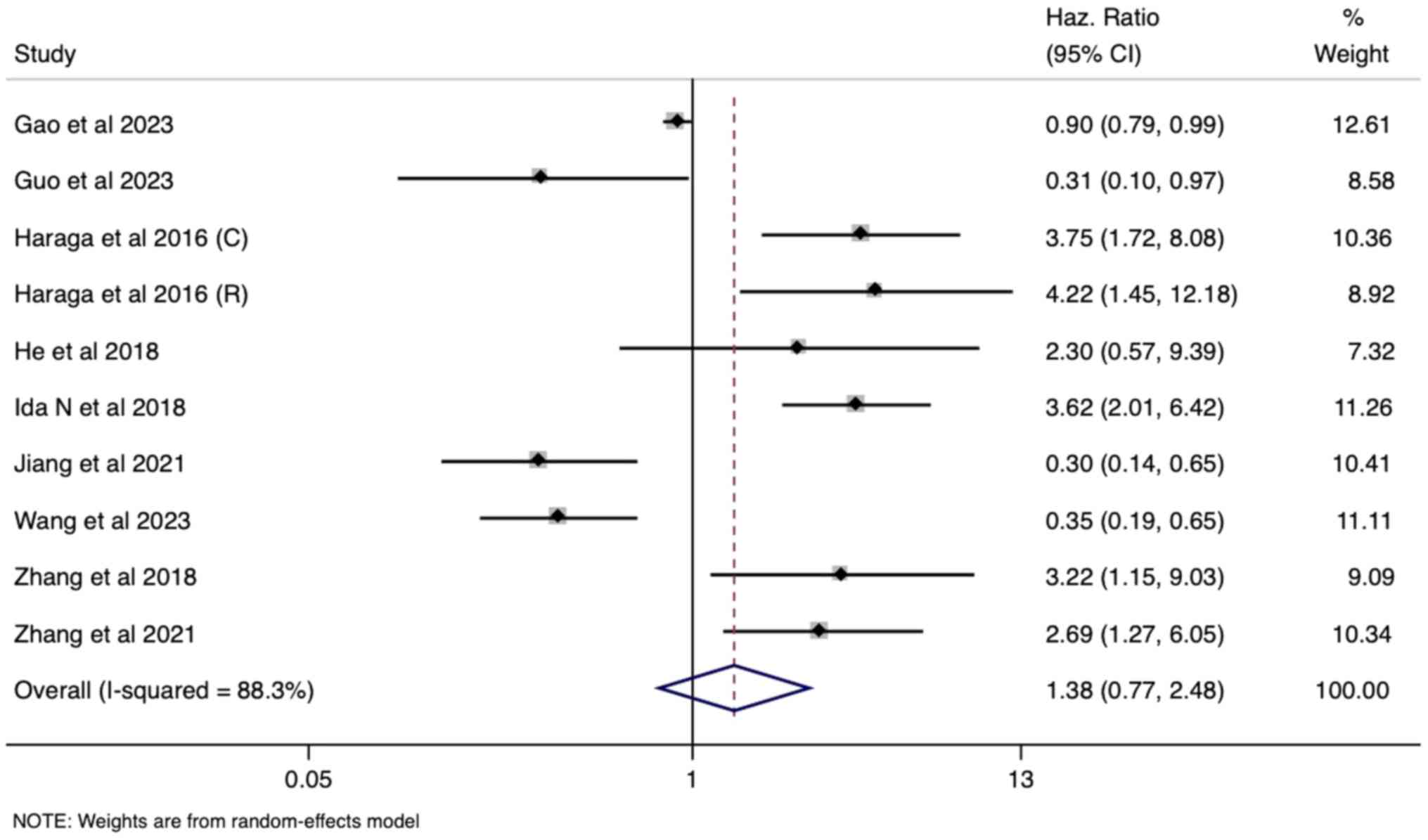
Prognostic utility of PNI and OS
The meta-analysis examined the association between
pre-operative PNI and OS in patients with cervical cancer using
data from 10 studies. Univariate analysis revealed a pooled hazard
ratio (HR) of 1.38 [95% confidence interval (CI): 0.77-2.48;
Fig. 2]. However, this result did
not reach statistical significance, indicating no independent
prognostic effect of PNI on OS at baseline. This was further
supported by the non-significant pooled HR of 1.06 (95% CI:
0.64-1.76) in the multivariate analysis (eight studies), suggesting
that PNI's predictive ability for OS might be mitigated by the
influence of other prognostic factors included in the adjusted
models (Fig. 3). Notably, high
heterogeneity was observed in both univariate and multivariate
analyses (I²=88% and 83%, respectively; P<0.001), which required
the use of a random-effects model for pooled estimate
generation.
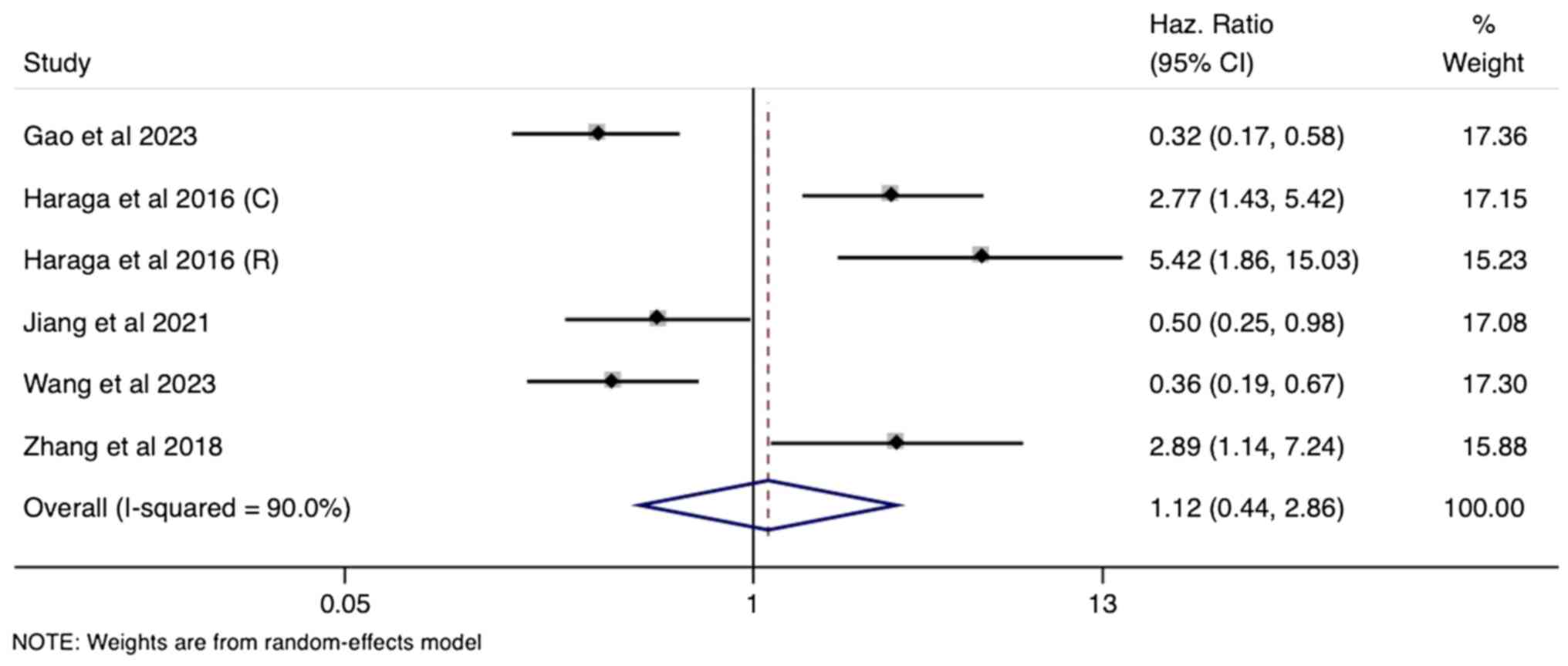
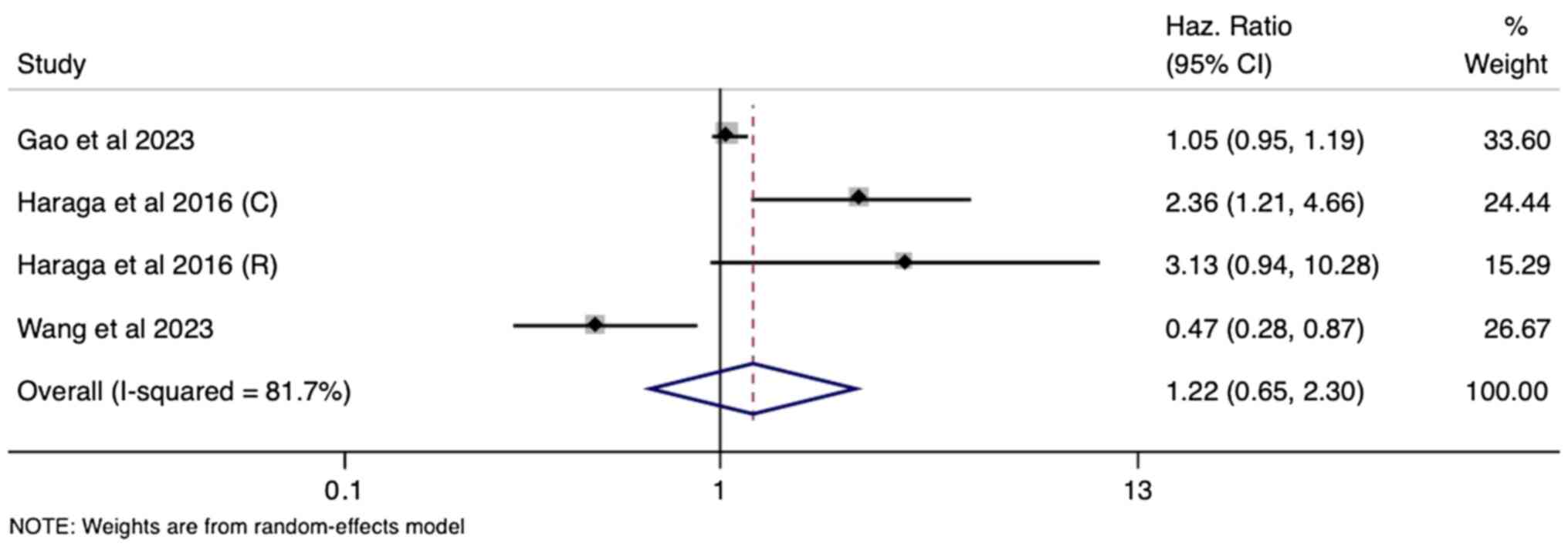
Prognostic utility of PNI and PFS
A total of six studies within the meta-analysis
explored the association of PNI with PFS in cervical cancer. As
with OS, the univariate analysis yielded a non-significant pooled
HR of 1.12 (95% CI: 0.44-2.86), suggesting no independent
prognostic value of PNI for predicting PFS (Fig. 4). This lack of association
persisted in the multivariate analysis (four studies), with a
pooled HR of 1.22 (95% CI: 0.65-2.30) failing to reach statistical
significance (Fig. 5). Since both
univariate and multivariate analyses showed substantial
heterogeneity (I²=90% and 82%, respectively; P<0.001), a
random-effects model was used for pooling the estimates.
Subgroup analysis
Due to variability in the treatment and estimated
cut offs, a subgroup analysis was performed for the prognostic
utility of PNI in determining OS (univariate and multivariate) and
PFS (univariate) based on the region, PNI cut off used (≤48 and
>48), sample size (≤150 and >150), grade of evidence and
treatment protocol followed. PNI was efficient as a prognostic tool
in predicting OS in studies conducted in Japan (univariate and
multivariate) and in studies that report concurrent
chemoradiotherapy as the treatment of patients with CC (univariate
and multivariate). Other subgroups did not show any significant
prognostic value for PNI (Fig.
S1, Fig. S2, Fig. S3, Fig. S4, Fig. S5, Fig. S6, Fig. S7 and Fig. S8). Similar results were obtained
in terms of the prognostic value of PNI in predicting PFS. Japanese
studies and studies that used concurrent chemoradiotherapy
indicated that PNI had a significant prognostic value (Fig. S9, Fig. S10, Fig. S11 and Fig. S12). The present study did not
perform subgroup analysis for pooled multivariate HRs due to the
limited number of studies (four).
Sensitivity analysis
A sensitivity analysis showed that there was not
much difference in the pooled effect estimate between the overall
risk estimate and the pooled estimate among high-risk studies. This
suggests the robustness of the pooled estimate irrespective of the
quality of individual studies. (Fig.
S13, Fig. S14 and Fig. S15)
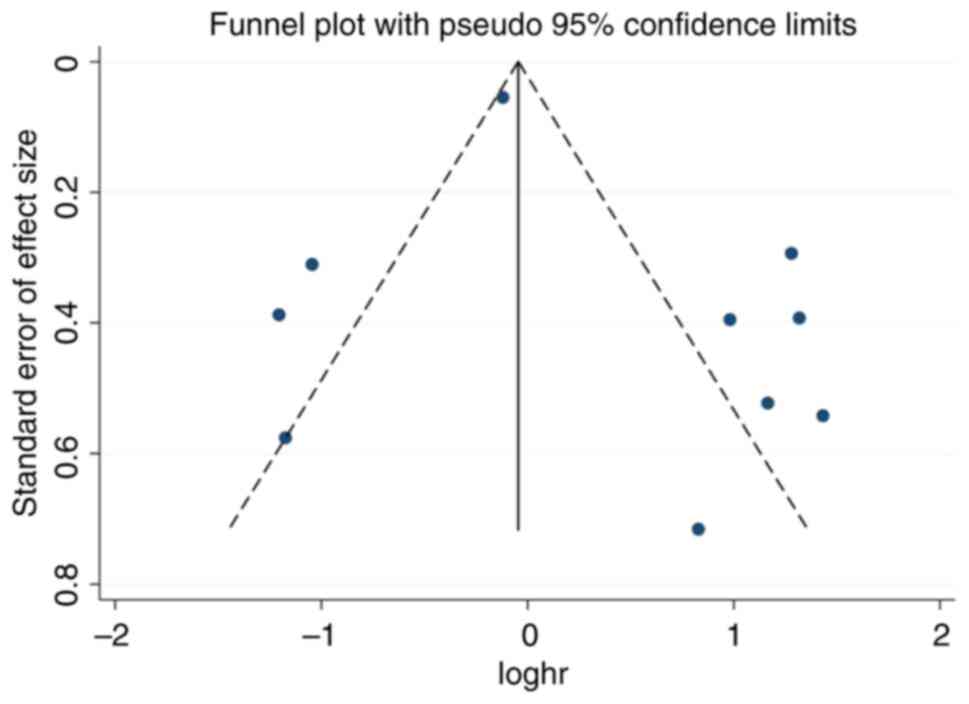
Publication bias
Publication bias for the univariate analysis
component of OS was evaluated using the funnel plot. The funnel
plot showed symmetry among the included studies indicating absence
of publication bias (Fig. 6).
Discussion
Cervical cancer is associated with a significant
health burden worldwide. Identifying potential prognostic factors
that can predict outcomes is crucial for guiding treatment
decisions and improving patient care. In recent years, the
prognostic nutritional index (PNI) has gained attention as a
potential predictor of prognosis in various malignancies. The
present analysis aimed to investigate the predictive value of the
PNI for CC outcomes. It included nine articles and showed that PNI
does not markedly determine OS of PFS in patients with CC. Subgroup
analysis showed that PNI served as a useful prognostic tool in
predicting OS in studies conducted in Japan and when the patients
were treated with concurrent chemoradiotherapy.
Study results. The univariate analysis of the
pooled hazard ratios (HRs) for OS revealed an insignificant
prognostic utility of the preoperative PNI. The pooled HR was 1.38
(95% CI: 0.77 to 2.48), indicating that the PNI was not a
significant predictor of overall survival. This finding was
consistent with the multivariate analysis estimates provided by
eight studies, which also showed an insignificant utility of the
PNI in predicting OS after adjusting for other confounders. The
pooled HR in the multivariate analysis was 1.06 (95% CI: 0.64 to
1.76). Similarly, the results of the present study demonstrated
that the prognostic utility of the PNI in predicting PFS was
insignificant. In the univariate analysis, the pooled HR for PFS
was 1.12 (95% CI: 0.44 to 2.68), indicating that the PNI was not a
significant predictor of PFS. A total of four studies provided
multivariate estimates, which resulted in a pooled HR of 1.22 (95%
CI: 0.65 to 2.30) for PNI in predicting PFS.
Malnutrition is seen at alarming rates among
patients with gynaecological cancer. Studies from the USA and
Australia have shown that almost 20 and 53% of patients with
gynaecological cancers, respectively, suffer from at least mild
malnutrition and ~20% of all mortality is linked to malnutrition
(8,26). Inability to absorb sufficient
nutrition frequently results in malnutrition among patients with
cancer. Cancer treatment causes reduction in appetite, thereby
reducing food intake. Surgery also necessitates starvation.
Therefore, as a results of reduced food intake, protein catabolism
develops postoperatively depending on the duration of starvation
before surgery. This can be further worsened by bowel obstruction,
leading to malabsorption. However, as the cancer progresses, there
is an increased metabolic demand, triggering catabolism of stored
proteins. All these factors build up the negative nutritional
balance resulting in the deterioration of nutritional status
(27).
Studies from various settings have shown that
malnutrition serves as a prognostic determinant of several
gynaecological cancers (20,21,28,29).
Albumin, total protein, haemoglobin and transferrin are among the
widely studied nutritional factors in patients with gynaecological
cancers in general and CC in particular (30). Malnutrition and inflammation lead
to the decrease in the levels of serum albumin, that reflect the
nutritional state of the patient and the severity, course and
prognosis. IL-6 has the ability to control albumin synthesis and
lower serum albumin levels (31).
Similarly, serum albumin levels that strongly correlate with body
immunity and nutritional state, may rise and cause inflammation
(32,33). In studies of lung, breast,
colorectal, ovarian and cervical cancers as well as other
malignancies, serum albumin has been found to be a reliable
predictor of clinical outcomes (34). The present study used the PNI, a
composite indicator calculated from serum albumin and lymphocyte
levels.
Comparison with other studies. The present
study demonstrated that PNI is not markedly linked with the OS or
PFS of patients with cervical cancer. Its results differ from the
conclusions of the previous meta-analysis on the same topic
(35). However, our study
population included only cervical cancer cases, whereas the
previous study included all gynaecological cancers. Furthermore,
while PNI is a well-established prognostic marker for ovarian and
endometrial cancers, its predictive value for cervical cancers
patients is still unclear. In addition, it was noted that some
studies (18,22,23)
have shown that PNI has poor prognostic utility in determining the
survival of patients. Therefore, there is a need to identify
additional comprehensive nutritional indicators to prognosticate
cervical cancer outcomes. The present study results were in
agreement with findings reported by individual studies performed in
patients with cervical cancer (17,20,23).
In our study, subgroup analysis did not show any significant
prognostic values for PNI in predicting PFS.
There could be several factors that may explain the
lack of significant prognostic value of PNI in predicting OS and
PFS in patients with cervical cancer. Firstly, the PNI is
calculated based on two components: Serum albumin levels and total
lymphocyte count. While these markers reflect nutritional status
and immune function, they may not fully capture the complex
interplay between nutrition, inflammation and tumor biology in
cervical cancer. Other nutritional indicators, such as body mass
index, weight loss, or specific micronutrient levels, could
potentially provide a more comprehensive assessment of the
nutritional status and its impact on cancer outcomes. Therefore,
considering alternative nutritional indicators in future studies
may shed light on their potential prognostic value in cervical
cancer. Additionally, the lack of standardized cut-off values for
the PNI across the included studies may have contributed to the
non-significant findings of the present study. Different studies
may have utilized different cut-off values, leading to
heterogeneity in the results. Establishing consensus on
standardized cut-off values for the PNI in cervical cancer could
potentially improve its prognostic utility and facilitate
comparisons across studies.
The present study had several strengths. It is one
of the few reviews that has attempted to generate evidence on the
prognostic utility of PNI in OS and PFS of patients with CC. While
a previous review evaluated the use of PNI in gynaecological
cancers, the present review is more comprehensive, has updated the
results and is focused on cervical cancer alone (35). Additional subgroup and sensitivity
analysis (using NOS) was performed that adds to the limited
literature available. Funnel plot showed no publication bias which
adds to the strength of the present study. The large sample size of
the included studies added robustness to the results and increased
generalizability. All studies were reviewed separately by the two
authors. Furthermore, although the present study reported negative
outcomes, it is important to note that the prognostic utility of
the PNI in patients with cervical cancer is an area of continuing
debate. Therefore, our robust meta-analysis methodology ensured the
reliability and validity of the results and provided crucial
information for comprehensive understanding and informed
decision-making in clinical practice. The present study does come
with a few limitations. First, there was evidence of high
heterogeneity among the included studies, which might have had an
impact on the combined results. Although subgroup analysis and
sensitivity analyses were used to adjust for heterogeneity, the
underlying variability in patient characteristics, treatment
provided and cut off used should be taken into account when
interpreting the results. All included studies were retrospective
in nature, which may add biases and restrict the capacity to
demonstrate causation. All included studies were from China and
Japan, which may have affected the generalizability of the results.
Lastly, the present study included only free full-text
English-language articles and did not include grey literature.
The results of the present systematic review and
meta-analysis showed that there is inconclusive evidence on the
prognostic utility of PNI in determining OS and PFS of patients
with cervical cancer. Factors such as the complexity of the tumor
microenvironment, the choice of nutritional indicators and the lack
of standardized cut-off values may have contributed to these
findings. Thus, future prospective studies using PNI cut-off values
that are standardised are recommended to increase the clinical
efficacy of PNI as a predictive tool.
Supplementary Material
Forest plot showing prognostic utility
of PNI (univariate analysis) across region for overall survival.
PNI, prognostic nutritional index; Haz, Hazard; CI, confidence
interval.
Forest plot showing prognostic utility
of PNI (univariate analysis) across PNI cut offs for overall
survival. PNI, prognostic nutritional index; Haz, Hazard; CI,
confidence interval.
Forest plot showing prognostic utility
of PNI (univariate analysis) across sample size for overall
survival. PNI, prognostic nutritional index; Haz, Hazard; CI,
confidence interval.
Forest plot showing prognostic utility
of PNI (univariate analysis) across treatment for overall survival.
PNI, prognostic nutritional index; Haz, Hazard; CI, confidence
interval.
Forest plot showing prognostic utility
of PNI (multivariate analysis) across region for overall survival.
PNI, prognostic nutritional index; Haz, Hazard; CI, confidence
interval.
Forest plot showing prognostic utility
of PNI (multivariate analysis) across PNI cut offs for overall
survival. PNI, prognostic nutritional index; Haz, Hazard; CI,
confidence interval.
Forest plot showing prognostic utility
of PNI (multivariate analysis) across sample size for overall
survival. PNI, prognostic nutritional index; Haz, Hazard; CI,
confidence interval.
Forest plot showing prognostic utility
of PNI (multivariate analysis) across treatment for overall
survival. PNI, prognostic nutritional index; Haz, Hazard; CI,
confidence interval.
Forest plot showing prognostic utility
of PNI (univariate analysis) across region for progression-free
survival. PNI, prognostic nutritional index; Haz, Hazard; CI,
confidence interval.
Forest plot showing prognostic utility
of PNI (univariate analysis) across cut offs for progression-free
survival. PNI, prognostic nutritional index; Haz, Hazard; CI,
confidence interval.
Forest plot showing prognostic utility
of PNI (univariate analysis) across sample size for
progression-free survival. PNI, prognostic nutritional index; Haz,
Hazard; CI, confidence interval.
Forest plot showing prognostic utility
of PNI (univariate analysis) treatment for progression-free
survival. PNI, prognostic nutritional index; Haz, Hazard; CI,
confidence interval.
Forest plot showing prognostic utility
of PNI (univariate analysis) across NOS grade for overall survival.
PNI, prognostic nutritional index; NOS, Newcastle-Ottawa Scale;
Haz, Hazard; CI, confidence interval.
Forest plot showing prognostic utility
of PNI (multivariate analysis) across NOS for overall survival.
PNI, prognostic nutritional index; NOS, Newcastle-Ottawa Scale;
Haz, Hazard; CI, confidence interval.
Forest plot showing prognostic utility
of PNI (univariate analysis) across NOS for progression-free
survival. PNI, prognostic nutritional index; NOS, Newcastle-Ottawa
Scale; Haz, Hazard; CI, confidence interval.
Search strategy
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
DC conceived and designed the study. DC and QD
collected the data and performed the literature search. DC was
involved in the writing of the manuscript. The two authors have
read and approved the final manuscript. DC and QD confirm the
authenticity of all the raw data.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Xu T, Yang X, He X and Wu J: The study on
cervical cancer burden in 127 countries and its socioeconomic
influence factors. J Epidemiol Glob Health. 13:154–161.
2023.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Arbyn M, Castellsagué X, de Sanjosé S,
Bruni L, Saraiya M, Bray F and Ferlay J: Worldwide burden of
cervical cancer in 2008. Ann Oncol. 22:2675–2686. 2011.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Li C, Liu W and Cheng Y: Prognostic
significance of metastatic lymph node ratio in squamous cell
carcinoma of the cervix. Onco Targets Ther. 9:3791–3797.
2016.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Wang XJ, Xiong Y, Ma ZB, Xia JC and Li YF:
The expression and prognostic value of protein tyrosine kinase 6 in
early-stage cervical squamous cell cancer. Chin J Cancer.
35(54)2016.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Tangjitgamol S, Katanyoo K, Laopaiboon M,
Lumbiganon P, Manusirivithaya S and Supawattanabodee B: Adjuvant
chemotherapy after concurrent chemoradiation for locally advanced
cervical cancer. Cochrane Database Syst Rev.
2014(CD010401)2014.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Grivennikov SI, Greten FR and Karin M:
Immunity, inflammation, and cancer. Cell. 140:883–899.
2010.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Nho JH, Kim SR and Kwon YS: Depression and
appetite: Predictors of malnutrition in gynecologic cancer. Support
Care Cancer. 22:3081–3088. 2014.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Ottery FD: Cancer cachexia: Prevention,
early diagnosis, and management. Cancer Pract. 2:123–131.
1994.PubMed/NCBI
|
|
9
|
Ravasco P: Nutrition in cancer patients. J
Clin Med. 8(1211)2019.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Laky B, Janda M, Bauer J, Vavra C,
Cleghorn G and Obermair A: Malnutrition among gynaecological cancer
patients. Eur J Clin Nutr. 61:642–646. 2007.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Alphs HH, Zahurak ML, Bristow RE and
Díaz-Montes TP: Predictors of surgical outcome and survival among
elderly women diagnosed with ovarian and primary peritoneal cancer.
Gynecol Oncol. 103:1048–1053. 2006.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Feng Z, Wen H, Ju X, Bi R, Chen X, Yang W
and Wu X: The preoperative prognostic nutritional index is a
predictive and prognostic factor of high-grade serous ovarian
cancer. BMC Cancer. 18(883)2018.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Miao Y, Li S, Yan Q, Li B and Feng Y:
Prognostic significance of preoperative prognostic nutritional
index in epithelial ovarian cancer patients treated with
platinum-based chemotherapy. Oncol Res Treat. 39:712–719.
2016.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Page MJ, McKenzie JE, Bossuyt PM, Boutron
I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan
SE, et al: The PRISMA 2020 statement: An updated guideline for
reporting systematic reviews. BMJ. 372(n71)2021.PubMed/NCBI View
Article : Google Scholar
|
|
15
|
Nozoe T, Ninomiya M, Maeda T, Matsukuma A,
Nakashima H and Ezaki T: Prognostic nutritional index: A tool to
predict the biological aggressiveness of gastric carcinoma. Surg
Today. 40:440–443. 2010.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Stang A: Critical evaluation of the
Newcastle-Ottawa scale for the assessment of the quality of
nonrandomized studies in meta-analyses. Eur J Epidemiol.
25:603–605. 2010.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Haraga J, Nakamura K, Omichi C, Nishida T,
Haruma T, Kusumoto T, Seki N, Masuyama H, Katayama N, Kanazawa S
and Hiramatsu Y: Pretreatment prognostic nutritional index is a
significant predictor of prognosis in patients with cervical cancer
treated with concurrent chemoradiotherapy. Mol Clin Oncol.
5:567–574. 2016.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Gao Z, Zhao M, Yang X and Fu J: Assessment
of peripheral platelet to lymphocyte ratio and prognostic
nutritional index in the efficacy and prognosis of radiotherapy for
cervical cancer. Curr Oncol. 30:2834–2844. 2023.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Guo J, Lv W, Wang Z, Shang Y, Yang F,
Zhang X, Xiao K, Zhang S, Pan X, Han Y, et al: Prognostic value of
inflammatory and nutritional markers for patients with early-stage
poorly-to moderately-differentiated cervical squamous cell
carcinoma. Cancer Control. 30(10732748221148912)2023.PubMed/NCBI View Article : Google Scholar
|
|
20
|
He X, Li JP, Liu XH, Zhang JP, Zeng QY,
Chen H and Chen SL: Prognostic value of C-reactive protein/albumin
ratio in predicting overall survival of Chinese cervical cancer
patients overall survival: Comparison among various inflammation
based factors. J Cancer. 9:1877–1884. 2018.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Ida N, Nakamura K, Saijo M, Kusumoto T and
Masuyama H: Prognostic nutritional index as a predictor of survival
in patients with recurrent cervical cancer. Mol Clin Oncol.
8:257–263. 2018.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Jiang Y, Gu H, Zheng X, Pan B, Liu P and
Zheng M: Pretreatment C-reactive protein/albumin ratio is
associated with poor survival in patients with 2018 FIGO stage
IB-IIA HPV-positive cervical cancer. Pathol Oncol Res.
27(1609946)2021.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Wang HB, Xu XT, Tian MX, Ding CC, Tang J,
Qian Y and Jin X: Prognostic values of the prognostic nutritional
index, geriatric nutritional risk index, and systemic inflammatory
indexes in patients with stage IIB-III cervical cancer receiving
radiotherapy. Front Nutr. 10(1000326)2023.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Zhang W, Liu K, Ye B, Liang W and Ren Y:
Pretreatment C-reactive protein/albumin ratio is associated with
poor survival in patients with stage IB-IIA cervical cancer. Cancer
Med. 7:105–113. 2018.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Zhang G, Zhang Y, He F, Wu H, Wang C and
Fu C: Preoperative controlling nutritional status (CONUT) score is
a prognostic factor for early-stage cervical cancer patients with
high-risk factors. Gynecol Oncol. 162:763–769. 2021.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Gupta D, Lis CG, Vashi PG and Lammersfeld
CA: Impact of improved nutritional status on survival in ovarian
cancer. Support Care Cancer. 18:373–381. 2010.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Billson HA, Holland C, Curwell J, Davey
VL, Kinsey L, Lawton LJ, Whitworth AJ and Burden S: Perioperative
nutrition interventions for women with ovarian cancer. Cochrane
Database Syst Rev. 2013(CD009884)2013.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Liu Y, Chen S, Zheng C, Ding M, Zhang L,
Wang L, Xie M and Zhou J: The prognostic value of the preoperative
c-reactive protein/albumin ratio in ovarian cancer. BMC Cancer.
17(285)2017.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Zorlini R, Akemi Abe Cairo A and Salete
Costa Gurgel M: Nutritional status of patients with gynecologic and
breast cancer. Nutr Hosp. 23:577–583. 2008.PubMed/NCBI
|
|
30
|
Ayhan A, Günakan E, Alyazıcı İ, Haberal N,
Altundağ Ö and Dursun P: The preoperative albumin level is an
independent prognostic factor for optimally debulked epithelial
ovarian cancer. Arch Gynecol Obstet. 296:989–995. 2017.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Barber MD, Ross JA and Fearon KC: Changes
in nutritional, functional, and inflammatory markers in advanced
pancreatic cancer. Nutr Cancer. 35:106–110. 1999.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Silva Jde A, Trindade EB, Fabre ME,
Menegotto VM, Gevaerd S, Buss Zda S and Frode TS: Fish oil
supplement alters markers of inflammatory and nutritional status in
colorectal cancer patients. Nutr Cancer. 64:267–273.
2012.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Alberici Pastore C, Paiva Orlandi S and
González MC: Association between an inflammatory-nutritional index
and nutritional status in cancer patients. Nutr Hosp. 28:188–193.
2013.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Xiao Y, Ren YK, Cheng HJ, Wang L and Luo
SX: Modified glasgow prognostic score is an independent prognostic
factor in patients with cervical cancer undergoing
chemoradiotherapy. Int J Clin Exp Pathol. 8:5273–5281.
2015.PubMed/NCBI
|
|
35
|
Wang X and Wang Y: The prognostic
nutritional index is prognostic factor of gynecological cancer: A
systematic review and meta-analysis. Int J Surg. 67:79–86.
2019.PubMed/NCBI View Article : Google Scholar
|