Introduction
Inflammation is a complex biological defense system
activated by a pathogen, damaged cells and irritants (1,2). The
inflammatory response eliminates the initial cause of cell damage,
necrosis of cells and tissues damaged by the inflammatory process
and initiates tissue repair (3).
The inflammatory process is regulated by various cell types,
including macrophages, neutrophils, eosinophils and mononuclear
phagocytes (4,5). Macrophages are associated with the
initiation and maintenance of inflammation, the presentation of
antigens and the production of cytokines and growth factors
(6). Therefore, the regulation of
macrophages is essential for controlling the overall immune
response.
Heme oxygenase 1 (HO-1) is an enzyme responsible for
degrading heme into carbon monoxide, biliverdin and iron, playing
crucial roles as a tissue homeostatic regulator, immune function
regulator and inflammatory attenuator (7-9).
Multiple isozymes of HO-1 and HO-2 have been identified, each
encoded by different gene (10).
While HO-2 is predominantly expressed in the brain and testicles
and remains unstimulated by receptors or metabolism (10-12),
HO-1, characterized by low basal expression levels in most cells
and tissues, undergoes marked elevation in response to heme
substrate and various stress such as UV light, lipopolysaccharides
(LPS) or hydrogen peroxide (13,14).
Carbon monoxide produced by HO-1 plays an anti-inflammatory role by
inhibiting the secretion of LPS-induced inflammatory cytokines
including TNF-α, interleukin (IL)-1β and IL-6. Additionally, it
induces IL-10, which is known for its anti-inflammatory properties,
in macrophages (15-18).
When exposed to damage and injury, tissues activate macrophages and
produce prostaglandin E2 (PGE2) via cyclooxygenase-2 (COX-2), and
nitric oxide (NO) via inducible nitric oxide synthase (iNOS)
(19). Increased HO-1 expression
inhibits LPS-mediated expression of COX-2 and iNOS, thereby
inhibiting PGE2 and NO production (20,21).
Although it has been reported that HO-1 induces NO during cellular
senescence, the induction of HO-1 inhibits iNOS to reduce NO by
oxidative injury (22). CO and
biliverdin, the products of HO-1, are also known to inhibit iNOS
(23-25).
Therefore, controlling HO-1 expression is pivotal in modulating
anti-inflammatory responses.
Peperomia dindygulensis Miq. (P.
dindygulensis), a commonly found herb in southern China, has
been used in folk medicine to address various ailments, including
cough, asthma, phthisis and a range of cancers, such as stomach,
lung, breast and liver cancer (26). The ethanol extract of P.
dindygulensis repressed the growth of the lung cancer cell
lines A549 and Lovo (5). Some
compounds found in the extract demonstrate activity that hampers
the growth of liver cancer cells (27). Moreover, P. dindygulensis
ethanol extracts have demonstrated the capacity to inhibit cell
growth and impede angiogenesis at specific concentrations in human
umbilical vein endothelial cells (HUVECs) (28,29).
No studies, to the best of the authors' knowledge, have explored
the regulatory effects of P. dindygulensis methanol extract
(PDME) on inflammatory responses in raw 264.7 cells.
The present study demonstrated that PDME acts as an
anti-inflammatory effector in macrophages. This extract diminished
NOS activity and inflammatory factor expression, such as iNOS,
Cox-2 and TNF-α, in LPS-induced macrophages. The anti-inflammatory
effects of PDME are contingent upon the HO-1 translation level.
Based on these results, a new role was identified for PDME in
inducing anti-inflammatory reactions through the regulation of the
expression of HO-1.
Materials and methods
Cell culture
RAW 264.7 and U937 cells were purchased from the
American Type Culture Collection (cat. nos. TIB-71 and CRL-1593.2).
RAW 264.7 cells were cultured in Dulbecco's modified Eagle's medium
(Welgene, Inc.; cat. no. LM 001-05) containing 10% fetal bovine
serum (GenDEPOT, LLC; cat. no. F0900-050) and 1%
penicillin/streptomycin (Lonza Group, Ltd.; cat. no. 17-602E). U937
cells (30,31) were maintained in Dulbecco's
modified Eagle's medium (Welgene, Inc.; cat. no. LM 001-05)
containing 10% fetal bovine serum (GenDEPOT, LLC; cat. no.
F0900-050), 1% penicillin/streptomycin (Lonza Group, Ltd.; cat. no.
17-602E) and 1% beta-mercaptoethanol (cat. no. MER002; BioShop
Canada, Inc.). After exposure to 10 ng/ml PMA (MilliporeSigma; cat.
no. p8139) for 24 h and the U937 cells underwent differentiation
and were washed to eliminate remaining PMA. HUVECs were purchased
from PromoCell GmbH. HUVECs were maintained in medium M199
(MilliporeSigma; cat. no. M4530) containing 20% fetal bovine serum,
30 µg/ml ECGS (Corning, Inc.; cat. no. 306006) and 100 µg/ml
heparin (MilliporeSigma; cat. no. H3149). Cells were incubated in a
humidified 5% CO2 atmosphere at 37˚C incubator.
Plant material
P. dindygulensis was obtained from the Thang
Loi community, located in the Ha Lang district of Cao Bang
Province, Vietnam, in July 2015. It was identified by Dr Tran The
Bach, from the Institute of Ecology and Biological Resources in
Hanoi, Vietnam. Voucher specimens labelled as VK 6535 were archived
in the herbarium of the Korea Research Institute of Bioscience and
Biotechnology. The dried whole plant (50 g) was pulverized and
extracted with methanol (500 ml; HPLC grade) using an ultrasonic
extractor (SDN-900H; Sungdong Ultrasonic Co., Ltd.). The ultrasonic
extraction procedure was systematically conducted over 30 cycles,
with each cycle comprising a 15 min extraction phase followed by a
120 min standby period to optimize extraction efficiency and
prevent temperature elevation effects. After the P.
dindygulensis methanol extract was filtered, it was then
concentrated under reduced pressure, yielding 2.8 g of extract,
which corresponds to a 5.6% yield. The methanol extract of P.
dindygulensis was supplied by the International Biological
Material Research Center (cat. no. FBM259-078).
Reagents
SB203580 (cat. no. 559389), SP600125 (cat. no.
420119), U0126 (cat. no. U120), Cycloheximide (cat. no. 01810) and
actinomycin D (cat. no. A1410) were purchased from MilliporeSigma.
IX (ZnPP; cat. no. sc-200329) was purchased from Santa Cruz
Biotechnology, Inc.
NOS assay
RAW 264.7 cells were seeded at a density of
1x105/well in a 96-well plate and incubated for 24 h at
37˚C. Cells were treated with PDME (10 µg/ml) or ZnPP (10 µM) at
the indicated concentration and time, followed by treatment with
LPS (1 µg/ml). After a 24 h-incubation at 37˚C, 70 µl of the medium
from each well was transferred to a new 96-well plate, mixed with
50 µl of Griess reagent (40 µg/µl; MilliporeSigma; cat. no. G4410)
and incubated in a foil-wrapped plate for 15 min at room
temperature. Absorbance was measured at 540 nm using Spark (Tecan
Group, Ltd.). The amount of NO produced was calculated by using a
standard curve.
MTT assay
RAW 264.7 cells were seeded at a density of
1x105/well in a 96-well plate. HUVECs were seeded at a
density of 0.5x104/well in a 96-well plate. The cells
were incubated for 24 h at 37˚C. PDME treatment was administered at
the indicated concentrations and times. Then, 20 µl of MTT reagents
(Merck KGaA; cat. no. 475989) was added per 96-well plate, with
reagents added to designated wells as a blank. After incubation for
3.5 h at 37˚C, 100 µl DMSO solvent was added to each well. The
foil-wrapped plate was incubated for 15 min at room temperature on
the shaker, and then the absorbance was measured at 590 nm using a
SPARK microplate reader (Tecan Group, Ltd.).
Western blot analysis and
antibodies
For western blotting, proteins were extracted from
cells lysed in lysis buffer [50 mM Tris (pH 7.4), 150 nM NaCl, 1%
NP-40, 1 mM EDTA, protease/phosphatase inhibitor cocktail (Cell
Signaling Technology, Inc.; cat. no. 5872S)] for 30 min on ice and
centrifuged at 21,000 x g for 15 min at 4˚C. Protein quantification
was performed using the Bradford reagent (Bio-Rad Laboratories,
Inc.; cat. no. 5000006) using a microplate reader (Bio-Rad
Laboratories, Inc.; model 680) and calculated using standard
curves. The cell lysate (25 µg/lane) was loaded onto an 8-12%
sodium dodecyl sulfate-polyacrylamide gel and transferred to a
polyvinylidene fluoride membrane (Bio-Rad Laboratories, Inc.; cat.
no. 1620177). The membrane was blocked with a 5% blocking reagent
(GenomicBase; cat. no. SKI400) for 1 h at room temperature and then
incubated with the specific primary antibody overnight at 4˚C. The
next day, the membrane was washed with PBS-T (0.2% Tween 20) and
incubated with the secondary antibody for 3 h at room temperature.
After incubation, the blot was washed with PBS-T and developed with
the Clarity ECL Substrate Kit (Bio-Rad Laboratories, Inc.; cat. no.
1705061) using KwikQuant Pro Imager (Kindle Biosciences, LLC; cat.
no. D1010). To western blot using other antibody on the same
membrane, the membrane was incubated with stripping buffer [2% SDS;
50 mM Tris (pH 6.8), 0.7% β-mercaptoethanol] for 10 min at 55˚C.
After incubation, the membrane was washed with PBS-T and every step
repeated from blocking to detection. Phosphorylated (p-)JNK, p-p38
and p-ERK1/2 levels were semi-quantified using ImageJ software
(version 1.53n; National Institutes of Health) and normalized to
the intensity of JNK, p38 and ERK1/2.
The antibodies used were: HO-1 (1:500; cat. no.
sc-390991), β-actin (1:2,000; cat. no. sc-47778), p-JNK (1:1,000;
cat. no. sc-6254), JNK (1:1,000; cat. no. sc-7345), p-p38 (1:1,000;
cat. no. sc-166182), p38 (1:1,000; cat. no. sc-7972), p-ERK1/2
(1:1,000; cat. no. sc-7383), ERK1/2 (1:1,000; cat. no. sc-514302),
α-tubulin (1:1,000; cat. no. sc-58666), iNOS (1:1,000; cat. no.
sc-7271) and COX-2 (1:1,000; cat. no. sc-514489). All antibodies
were anti-mouse IgG and purchased from Santa Cruz Biotechnology,
Inc. The mouse secondary antibody (HRP-linked antibody) was
purchased from Cell Signaling Technology, Inc. (1:2,000; cat. no.
7076S).
Reverse transcription-quantitative
(RT-q) PCR
The cells were seeded at a density of
1x104 in a 6-cm plate and incubated for 24 h at 37˚C.
Cells were treated with PDME (10 µg/ml) or ZnPP (10 µM) for 1 h at
37˚C, followed by treatment with LPS (1 µg/ml) for 24 h at 37˚C.
Total RNA for analysis was obtained using NucleoSpin RNA
(Macherey-Nagel; cat. no. 740955.250) according to the
manufacturer's instructions. RNA (1 µg) was used for complementary
DNA synthesis using an iScript cDNA Synthesis Kit (Bio-Rad
Laboratories, Inc.; cat. no. BR-170-8891). The reverse
transcription reaction was performed at 37˚C for 1 h and then
terminated at 95˚C for 5 min. RT-PCR analysis was performed with
cDNA and specific gene primers using IQ SYBR Green Supermix
(Bio-Rad Laboratories, Inc.; cat. no. BR1708882). Primers sequence
for RT-qPCR were; HO-1, 5'-TGAACACTCTGGAGATGACA-3' (sense) and
5'-AACAGGAAGCTGAGAGTGAG-3' (antisense); TNF-a,
5'-CAGGAGGGAGAACAGAAACTCCA-3' (sense) and 5'-CCTGGTTGGCTGCTTGCTT-3'
(antisense); iNOS, 5'-TGCATGGACCAGTATAAGGCAAGC-3' (sense) and
5'-CTCCTGCCCACTGAGTTCGTC-3' (antisense); COX-2,
5'-CCACTTCAAGGGAGTCTGGA-3' (sense) and 5'-AGTCATCTGCTACGGGAGGA-3'
(antisense); GAPDH, 5'-CATCACTGCCACCCAGAAGACTG-3' (sense) and
5'-ATGCCAGTGAGCTTCCCGTTCAG-3' (antisense). Pre-denaturation step
was conducted at 95˚C for 3 min, followed by denaturation and
extension steps at 95˚C for 15 sec and 60˚C for 1 min,
respectively, repeated for 40 cycles in a Rotor-Gene Q (Qiagen
GmbH). RT-qPCR experiments were repeated three times independently.
The RNA expression was calculated using the 2-ΔΔCq
method (32).
TNF-α ELISA
To measure the secretion level of TNF-α, Mouse TNF-α
Quantikine ELISA Kit was used (R&D Systems, Inc.; cat. no.
MTA00B) according to the manufacturer's protocol. First, the
standard reagent was prepared and the cultured medium obtained from
control, LPS-treatment and LPS with PDME treatment cells. Diluent
RD1-63 (50 µl) was added to each well and then 50 µl of standard or
sample medium added per well. The plate was gently tapped to mix
and incubated at room temperature for 2 h with adhesive strips
covering. Each well was washed four times with 400 µl wash buffer.
The wash buffer was completely removed and 100 µl Mouse TNF-α
conjugate added to each well. The wells were incubated at room
temperature for 2 h while covered with adhesive strips. After
incubation, each well was washed four times with 400 µl wash
buffer. Substrate solution (100 µl) was added to each well and the
foil-wrapped plates were incubated for 30 min at room temperature.
Stop solution (100 µl) was added to each well and gently tapped to
mix thoroughly. Absorbance was measured at 450 nm using Spark
(Tecan Group, Ltd.).
Statistical analysis
Statistical analyses were conducted using GraphPad
Prism version 7.04 (Dotmatics). For multiple comparisons, data were
subjected to one-way ANOVA followed by the Bonferroni post hoc test
to generate adjusted P-values. For comparisons between two groups,
data were analyzed using an unpaired two-tailed Student's t-test.
Error bars in all graphs represent the means of SEM. P-value was
denoted with symbols (*P<0.05,
**P<0.005, ***P<0.0005 and ‘n.s’
indicating no significance). All statistical analyses were
performed in triplicate. P<0.05 was considered to indicate a
statistically significant difference.
Results
Inhibition of NOS activity by PDME in
murine macrophages
To evaluate the anti-inflammatory properties of
PDME, the present study conducted a NOS activity assay in Raw264.7
cells. LPS stimulation increased NOS activity. However, the
combination of PDME and LPS resulted in a dose-dependent reduction
in NOS activity (Fig. 1A). Cell
viability decreased only in cells treated with 20 µg/ml PDME, with
no changes observed at other concentrations (Fig. 1B). Cell cytotoxicity was measured
in HUVECs following treatment with the PDME, as there have been
reports indicating its impact on endothelial cell viability
(29). However, it was confirmed
that there was no cellular toxicity when treated with a
concentration of 10 µg/ml for 24 h, indicating no impairment in
endothelial cell function at the concentration demonstrating
anti-inflammatory activity (Fig.
S1). Collectively, these findings suggested that PDME exerted
anti-inflammatory effects on LPS-stimulated Raw264.7 cells.
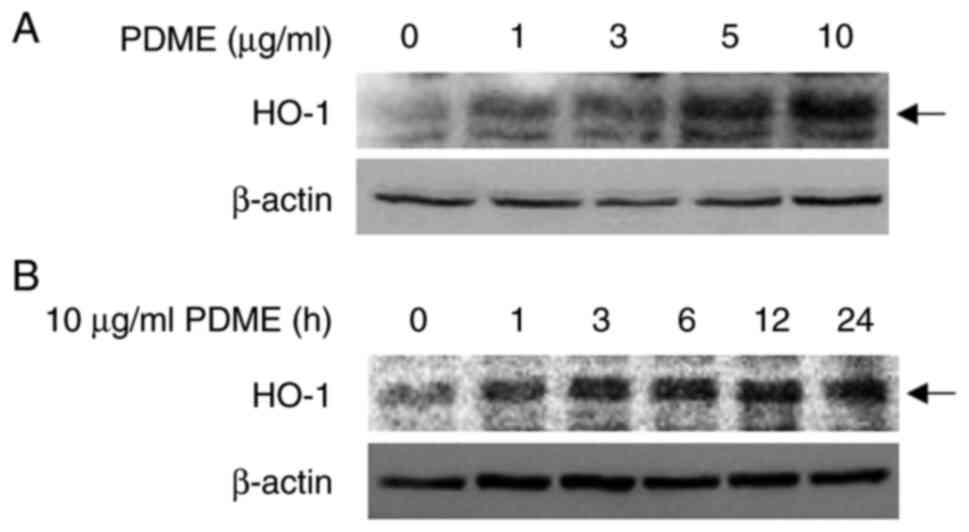
PDME induces HO-1 expression
HO-1 induces anti-inflammatory reactions in
macrophages (21). Therefore, the
present study tested whether PDME induced HO-1 expression in
Raw264.7 cells. The expression of HO-1 gradually increased with
increasing treatment doses of PDME from 1-10 µg/ml over 24 h
(Fig. 2A). Time course experiments
conducted with 10 µg/ml PDME showed an increasing HO-1 protein
level 1 h post-treatment, followed by a steady increase in
expression up to 24 h (Fig. 2B).
Furthermore, when human monocytic leukemia cells U937 were
differentiated and treated with 10 µg/ml of PDME at various time
points or with PDME ranging from 1-10 µg/ml for 24 h, the
expression of HO-1 increased similarly to the previous results
(Fig. S2A and B). These results indicated that PDME
modulated HO-1 expression in murine macrophages and human monocytic
leukemia cells.
Regulation of HO-1 expression by PDME
at the translation level
The expression of HO-1 is primarily controlled at
the transcriptional level through the MAPK signaling pathways,
including JNK, ERK and p38 kinase (33). Therefore, the present study
investigated the mRNA levels of HO-1 in a time- and dose-dependent
manner upon PDME treatment in Raw264.7 cells. Notably, PDME
treatment did not alter HO-1 mRNA expression (Fig. 3A and B). To determine whether the inhibition of
MAPKs regulates the PDME-induced increase in HO-1 expression,
Raw264.7 cells were treated with specific inhibitors: JNK inhibitor
SP600125, p38 inhibitor SB203580, or MEK inhibitor U0126 (Fig. 3C). Treatment with these inhibitors
and PDME partly affected HO-1 expression. In addition, PDME
treatment did not affect the expression or activity of JNK, p38 and
ERK1/2 (Fig. 3D and E). Furthermore, the present study
examined whether PDME-induced changes in HO-1 expression occurred
at the transcriptional or translational level by co-treating cells
with actinomycin D (ActD) and cycloheximide (CHX) (Fig. 3F). While the expression of HO-1
remained unaffected when the transcription inhibitor ActD was
administered, PDME-induced HO-1 expression was diminished when the
translation inhibitor CHX was used. Collectively, these findings
indicated that PDME specifically regulates HO-1 expression at the
translational level.
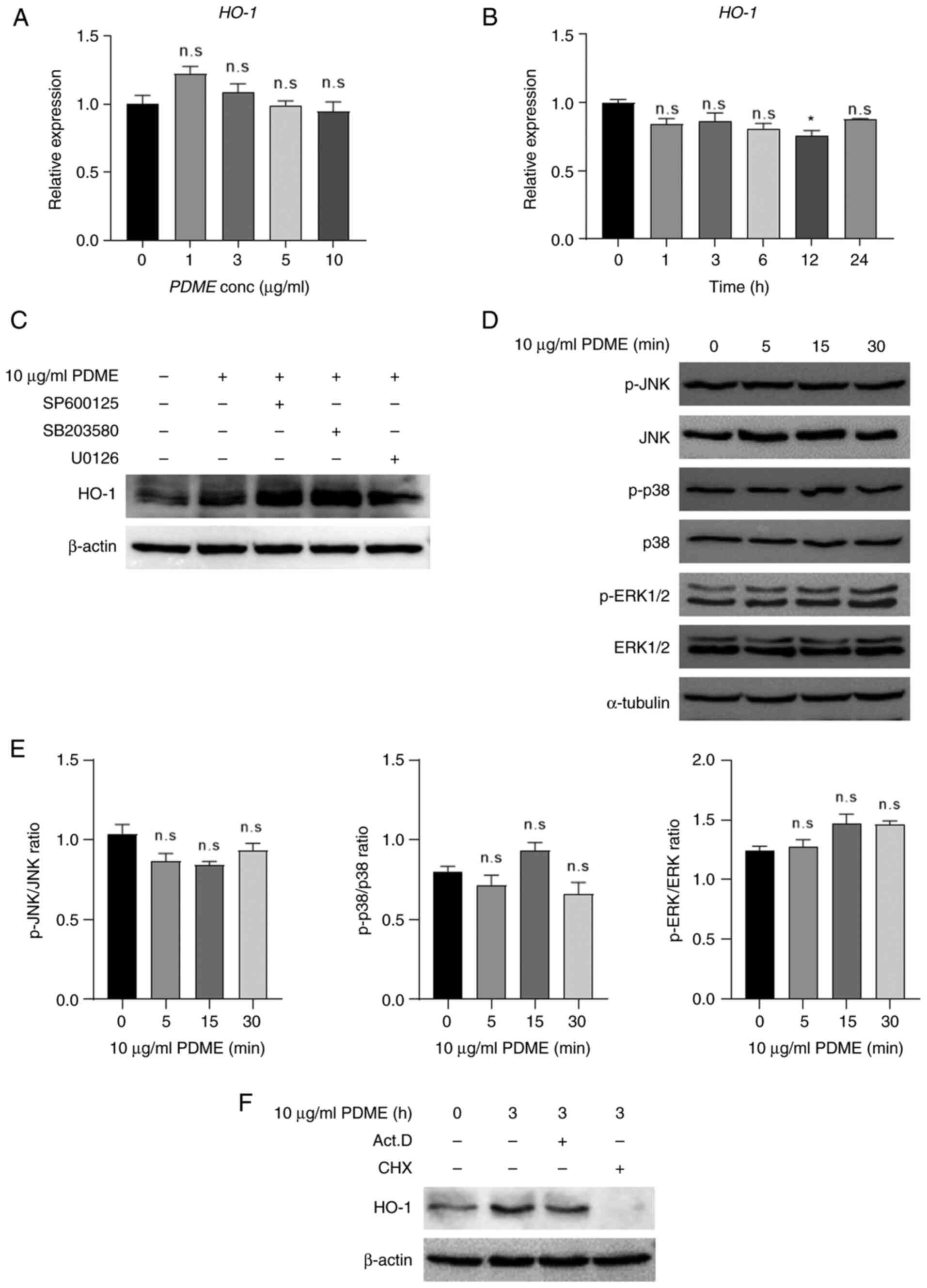 | Figure 3PDME regulate HO-1 expression at the
translation level. (A) The RNA expression of HO-1 was evaluated in
PDME-treated cells with concentrations from 0-10 µg/ml for 24 h and
(B) PDME (10 µg/ml)-treated cells with time from 0-24 h. For all
controls, the solution used for extraction and dilution was treated
in equal amounts. Data are presented as the mean ± SEM and
statistically analyzed using one-way ANOVA; n.s, not significant
and *P<0.05 vs. control. (C) RAW246.7 cells were
pre-treated with SP600125 (JNK inhibitor, 10 µM), SB203580 (p38
inhibitor, 10 µM), or U0126 (MEK inhibitor, 10 µM) for 30 min and
treated with PBVN14063 (10 µg/ml) for 24 h. Subsequently, HO-1
protein expression was determined by western blotting using total
lysate. (D) The cells were treated with PDME (10 µg/ml) for the
indicated time (0-30 min), and the expression and phosphorylation
levels of JNK, p38 and ERK1/2 were detected using western blotting.
(E) Ratio of phospho-kinase/kinase semi-quantified using ImageJ.
All data are presented as the mean ± SEM and were statistically
analyzed using one-way ANOVA with the Bonferroni post hoc test. (F)
The cells were pre-treated with Act. D (50 ng/ml) or CHX (10 µg/ml)
for 30 min and treated with PDME (10 µg/ml) for 3 h. Subsequently,
HO-1 protein expression was determined by western blotting using
total lysate. PDME, P. dindygulensis methanol extracts;
HO-1, heme oxygenase 1; Act. D, actinomycin D; CHX, cycloheximide;
n.s, not significant; p-, phosphorylated. |
PDME suppresses the expression of
iNOS, COX-2 and TNF-α induced by LPS
The present study investigated the effect of PDME
treatment on the expression levels of iNOS, COX-2 and TNF-α
stimulated by LPS. The combination of PDME with LPS for 24 h
resulted in a notable decrease in the mRNA expression of iNOS,
COX-2 and TNF-α in Raw 264.7 cells (Fig. 4A). Furthermore, LPS-induced protein
expression of iNOS and COX-2 was attenuated following PDME
treatment for either 24 h (Fig.
4B) or 6 h (Fig. S3). In
addition, even in U937 cells, the expression of iNOS and COX-2
stimulated by LPS was reduced with PDME treatment (Fig. S2C). The secretion of TNF-α, which
was increased by LPS, is also decreased by treatment with 10 µg/ml
PDME for 24 h (Fig. 4C). These
findings demonstrated that PDME effectively reduced both mRNA and
protein expression levels of iNOS, COX-2 and TNF-α induced by LPS
treatment.
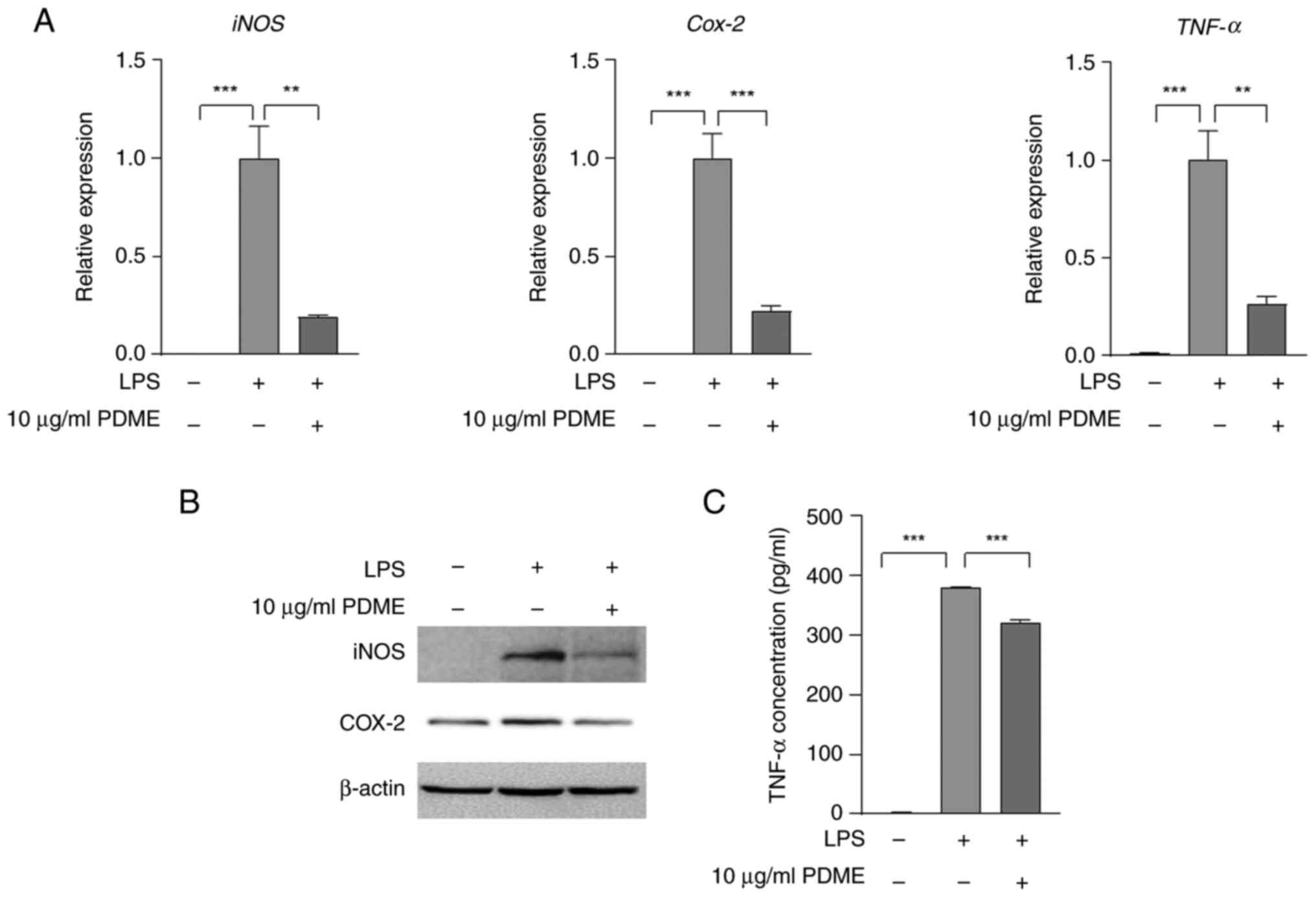 | Figure 4PDME reduces LPS-mediated iNOS, COX-2
and TNF-α expression. (A) RAW246.7 cells were pre-treated with PDME
(10 µg/ml) for 30 min and treated with LPS (1 µg/ml) for 24 h. For
all controls, the solution used for extraction and dilution was
treated in equal amounts. Next, the mRNA expression of iNOS, COX-2
and TNF-α was determined using RT-qPCR. Columns are presented with
the mean ± SEM and statistical analysis using one-way ANOVA;
**P<0.005, ***P<0.0005, Statistical
analyses was compared among the columns shown using triplicate
results. (B) RAW246.7 cells were pre-treated with PDME (10 µg/ml)
for 30 min treated with LPS (1 µg/ml) for 24 h. Next, the protein
expression of iNOS and COX-2 was determined using a western
blotting. (C) The cells were pre-treated with PDME (10 µg/ml) for
30 min and treated with LPS for 24 h. Next, the incubation medium
was obtained from cells and TNF-α secretion was detected using
ELISA. Columns are presented with the mean ± SEM and statistical
analysis using one-way ANOVA. ***P<0.0005. PDME,
P. dindygulensis methanol extracts; LPS,
lipopolysaccharides; iNOS. inducible nitric oxide synthase; COX-2,
cyclooxygenins-2; RT-qPCR, reverse transcription-quantitative
PCR. |
Inhibition of HO-1 activity suppresses
the anti-inflammatory effect stimulated by PDME
In RAW 264.7, PDME demonstrated its
anti-inflammatory effect by upregulating HO-1 expression. To
establish whether the anti-inflammatory function induced by PDME
was mediated by HO-1, the inflammatory response following treatment
with ZnPP, a specific inhibitor of HO-1 was assessed. The increase
in NO levels induced by LPS decreased when PDME was administered
concurrently, whereas NO levels increased when ZnPP was
administered as a pretreatment (Fig.
5A). Additionally, the mRNA expression of iNOS, COX-2 and TNF-α
was analyzed under identical conditions and it was verified that
the inflammatory factors induced by PDME were reduced by the
addition of ZnPP (Fig. 5B). These
findings strongly indicated that the anti-inflammatory response
triggered by PDME relies on HO-1 in macrophages.
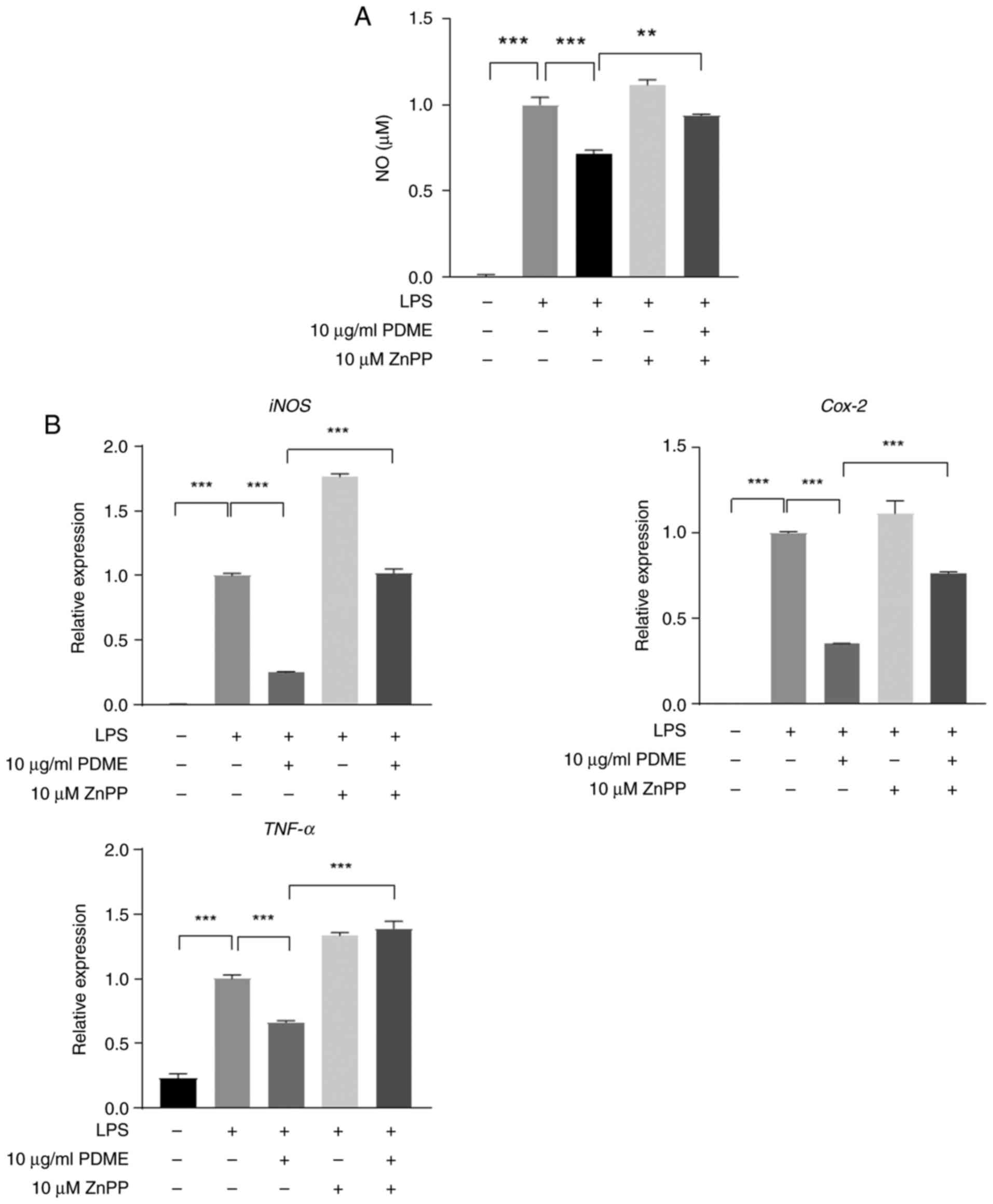 | Figure 5Inhibition of HO-1 activity decreases
PDME-mediated anti-inflammatory effects in RAW264.7 cells. (A) The
RAW264.7 cells were pre-treated with PDME (10 µg/ml) and ZnPP (10
µM) for 1 h. For all controls, the solution used for extraction and
dilution was treated in equal amounts. NOS activity was measured in
LPS (1 µg/ml) -stimulated cells for 24 h. (B) The cells were
pre-treated with PDME (10 µg/ml) and ZnPP (10 µM) for 1 h and then
stimulated with LPS (1 µg/ml) for 24 h. Next, the mRNA expression
of iNOS, COX-2 and TNF-α were measured using RT-PCR. Data are
presented as the mean ± SEM and statistically analyzed using
one-way ANOVA; **P<0.005, ***P<0.0005.
Statistical analyses were compared among the bars using triplicate
results. HO-1, heme oxygenase 1; PDME, P. dindygulensis
methanol extracts; NOS, nitric oxide synthase; LPS,
lipopolysaccharides; iNOS. inducible nitric oxide synthase; COX-2,
cyclooxygenins-2; RT-qPCR, reverse transcription-quantitative
PCR. |
Discussion
P. dindygulensis, a traditional medicinal
herb from southern China, has historically been employed to
alleviate conditions such as cough, asthma and pharyngitis
(26). Previous reports have
highlighted the anticancer properties of compounds extracted from
P. dindygulensis, particularly against lung and liver
cancers (5,27-29).
Despite the close relationship between its anticancer properties
and its anti-inflammatory function, there has been limited
investigation into the anti-inflammatory effects of natural
products derived from P. dindygulensis. Traditionally, P.
dindygulensis has been recognized for its pharmacological
effectiveness in diseases associated with inflammation. Studies
have indicated that treatment with an ethanol extract of this plant
alleviate atherosclerosis by inhibiting the formation of the
NOD-like receptor pyrin 3 inflammasome (26,34,35).
Furthermore, Lin et al (28) demonstrated the potential for the
structural constituents of ethanol extracts to act as regulators of
the IFN-γ/STAT1 and IL-6/STAT3 pathway. Treatment of endothelial
cells with the ethanol extract reduced angiogenic ability, such as
tube formation (29). However, the
efficacy of the methanol extract is unknown and changes in
representative factors of the inflammatory response, such as HO-1
and NO, have not yet been studied. Hence, the objective of the
present study was to investigate the anti-inflammatory effects and
underlying mechanism of action of a methanol extract derived from
P. dindygulensis against LPS-induced inflammation. While the
anticancer effects are closely linked to its anti-inflammatory
properties, the extent of the anti-inflammatory effects of PDME
remain relatively unexplored.
LPS binds to Toll-like receptor 4, triggering
inflammatory signals and inducing the expression and secretion of
pro-inflammatory cytokines such as TNF-α, IL-1β and IL-6 (36,37).
Upon stimulation by LPS, macrophages activate inflammatory pathways
including NF-κB, MAPKs and AKT through Toll-like receptor 4,
leading to the secretion of various pro-inflammatory mediators such
as NO, PGE2, iNOS and COX-2 (38,39).
HO-1, an enzyme that catalyzes heme degradation, is a potent
protective enzyme upregulated in response to various cellular
stress conditions (4). HO-1 and
its by-product, carbon monoxide, both possess anti-inflammatory
activity by inhibiting LPS-induced expression of NO, PGE2, iNOS and
COX-2 (20,21). Natural extracts exert their
anti-inflammatory effects by upregulating HO-1(40).
The present study observed that the treatment of
macrophages with PDME reduced LPS-induced NOS activity and
increased HO-1 production in RAW 264.7 and U937 cells. HO-1
expression is subjected to regulation at the mRNA level by various
transcription factors, including activator protein-1, NF-κB,
hypoxia-inducible factor and notably, nuclear factor erythroid
2-related factor 2. Additionally, its regulation extends to the
protein level (41,42). No significant changes were observed
in HO-1 regulation by PDME at the mRNA level. The upregulation of
HO-1 expression is typically associated with the activities of JNK,
ERK and p38, which are the three representative kinases of the MAPK
pathway. For instance, in mouse hepatocytes, sodium arsenite
activates the JNK pathway to modulate HO-1 expression (43). Similarly, HO-1 is upregulated in
rat hepatocytes through both the JNK and p38 pathways (43,44).
In chicken hepatoma cells, arsenite triggers the ERK and p38
pathways, leading to increased HO-1 expression (45). However, in the present study, no
significant changes in HO-1 expression were observed when MAPK
inhibitors (JNK, p38 and MEK) were administered. Li et al
(46) demonstrated that
Fucoxanthin, a marine seaweed extract, exhibits anti-inflammatory
effects by modulating pro-inflammatory factors and regulating
TLR4/MyD88 signaling in RAW264.7 cells. Exposure to NO inhibits the
activity of iron regulatory protein 1 and increases the expression
of HO-1 in mouse lymphoma cells (47). The increase in the mRNA levels of
HO-1 upon complete inhibition of inducible HO-1 expression with NO
scavenger treatment demonstrate the presence of a direct regulatory
system for HO-1 against NO exposure (48). The present study showed that
resistance to LPS-induced inflammatory responses was not influenced
by MAPK. These results demonstrated that PDME may trigger
anti-inflammatory responses through TLR4 signaling or direct
modulation of HO-1 and NO. The translation of HO-1 can be regulated
through alternative mechanisms in the 5'-untranslated region and
HO-1 protein levels may be modulated via proteasomal degradation
(49,50). The expression of HO-1 decreased
with CHX treatment, a translation inhibitor, while it remained
unaffected by ActD, a transcription inhibitor. These results
suggested that PDME regulates HO-1 protein expression in
macrophages.
Furthermore, PDME treatment exhibited its capacity
to inhibit the LPS-induced increase in NO, COX-2 and TNF-α.
Notably, the effect of PDME was negated by the administration of
ZnPP, an HO-1 inhibitor. Collectively, these results indicated that
PDME induces anti-inflammatory effects in macrophages by
upregulating HO-1 expression. Additional investigation
concentrating on the specific compounds present in PDME is
necessary. Nevertheless, the present study highlighted the
anti-inflammatory capacity of PDME and could aid in uncovering
novel natural therapeutics.
The present study confirmed the anti-inflammatory
effects of whole methanol extracts obtained from P.
dindygulensis. Although this marks the first validation of the
anti-inflammatory effect of PDME, to the best of the authors'
knowledge, this extract comprises various compounds. Therefore, it
is necessary to analyze the distribution of individual compounds
using experimental methods such as LC/MS and isolate them to
ascertain their singular anti-inflammatory effect. The present
study verified the anti-inflammatory effect of PDME against
LPS-stimulated inflammation in human monocyte leukemia cells U937.
PDME increased HO-1 expression and suppressed the upregulation of
COX-2 and iNOS induced by LPS-mediated inflammation in U937 cells.
However, further studies such as changes in the expression and
release of TNF-α by PDME in U937 cells and effects by ZnPP are
needed to identify clear efficacy in human cells. In addition, the
effectiveness of PDME has not been confirmed in animal models.
Verifying the anti-inflammatory effects of natural products in
animal models will be an important step in confirming their
effectiveness as well as their stability in organisms. These
further investigations serve as a foundation for safer and more
efficacious utilization of the anti-inflammatory properties of
these extracts.
Supplementary Material
PDME does not affect the viability of
endothelial cells. HUVECs viability was evaluated in PDME-treated
cells according to the indicated concentration for 24 h. Data are
presented as the mean ± SEM and statistically analyzed using
t-test; n.s, not significant PDME 10 μg/ml vs. PDME 0
μg/ml. PDME, P. dindygulensis methanol extracts;
HUVECs, human umbilical vein endothelial cells.
PDME induces HO-1 expression and
reduces LPS-mediated iNOS and COX-2 expression in U937 cells. (A)
Differentiated U937 cells treated with PDME indicated concentration
for 24 h and HO-1 protein expression determined using western
blotting. (B) The cells were treated with PDME (10 μg/ml)
for the indicated time and HO-1 protein expression was determined
using western blotting. (C) Differentiated U937 cells were
pre-treated with PDME (10 μg/ml) for 30 min and treated with
LPS (1 μg/ml) for 24 h. Next, the protein expression of iNOS
and COX-2 was determined using western blotting. For control, the
solution used for extraction and dilution was treated in equal
amounts. PDME, P. dindygulensis methanol extracts; HO-1,
heme oxygenase 1; LPS, lipopolysaccharides; COX-2,
cyclooxygenins-2; iNOS. inducible nitric oxide synthase.
PDME reduces LPS-mediated iNOS and
COX-2 expression. RAW246.7 cells were pre-treated with PDME (10
μg/ml) for 30 min and treated with LPS (1 μg/ml) for
6 h. For control, the solution used for extraction and dilution was
treated in equal amounts. Next, the protein expression of iNOS and
COX-2 was determined using western blotting. PDME, P.
dindygulensis methanol extracts; LPS, lipopolysaccharides;
iNOS. inducible nitric oxide synthase; COX-2,
cyclooxygenins-2.
Acknowledgements
Not applicable.
Funding
Funding: The present study was funded by grants from the
National Research Foundation (NRF) of Korea (grant no.
NRF-2021R1C1C1006516). It was supported by the Technology
Innovation Program (grant no. 20022828; Research and Development of
micronized human acellular dermal matrix preserving collagen and
growth factor for soft tissue filling) funded by the Ministry of
Trade, Industry & Energy (MOTIE, Korea).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
WHM and CYK conducted the experiments. WHM, CYK and
HJK validated and curated the data. HJJ, HJK and CH conceived and
designed the study. HK, JHL, HJK and CH conceptualized and
supervised the study. HJJ, JHL, HJK and CH wrote the original
manuscript. TTB, LNH, HKK and HJK participated in the manuscript
modification of the important points and analyzed the data. HJJ,
TTB, LNH, HKK, HJK and CH confirm the authenticity of all the raw
data. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Meram C and Wu J: Anti-inflammatory
effects of egg yolk livetins (α, β, and γ-livetin) fraction and its
enzymatic hydrolysates in lipopolysaccharide-induced RAW 264.7
macrophages. Food Res Int. 100:449–459. 2017.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Dewanjee S, Dua TK and Sahu R: Potential
anti-inflammatory effect of Leea macrophylla Roxb. leaves: A wild
edible plant. Food Chem Toxicol. 59:514–520. 2013.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Cooke JP: Inflammation and its role in
regeneration and repair. Circ Res. 124:1166–1168. 2019.PubMed/NCBI View Article : Google Scholar
|
|
4
|
McGlade EA, Miyamoto A and Winuthayanon W:
Progesterone and inflammatory response in the oviduct during
physiological and pathological conditions. Cells.
11(1075)2022.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Chen L, Zhou Y and Dong JX: Chemical
constituents of Peperomia dindygulensis. Zhong Cao Yao. 38:491–493.
2007.(In Chinese).
|
|
6
|
Fujiwara N and Kobayashi K: Macrophages in
inflammation. Curr Drug Targets Inflamm Allergy. 4:281–286.
2005.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Tenhunen R, Marver HS and Schmid R: The
enzymatic conversion of heme to bilirubin by microsomal heme
oxygenase. Proc Natl Acad Sci USA. 61:748–755. 1968.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Campbell NK, Fitzgerald HK and Dunne A:
Regulation of inflammation by the antioxidant haem oxygenase 1. Nat
Rev Immunol. 21:411–425. 2021.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Funes SC, Rios M, Fernández-Fierro A,
Covián C, Bueno SM, Riedel CA, Mackern-Oberti JP and Kalergis AM:
Naturally derived heme-oxygenase 1 inducers and their therapeutic
application to immune-mediated diseases. Front Immunol.
11(1467)2020.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Cruse I and Maines M: Evidence suggesting
that the two forms of heme oxygenase are products of different
genes. J Biol Chem. 263:3348–3353. 1988.PubMed/NCBI
|
|
11
|
Trakshel GM, Kutty RK and Maines MD:
Purification and characterization of the major constitutive form of
testicular heme oxygenase. The noninducible isoform. J Biol Chem.
261:11131–11137. 1986.PubMed/NCBI
|
|
12
|
Ryter SW, Alam J and Choi AM: Heme
oxygenase-1/carbon monoxide: From basic science to therapeutic
applications. Physiol Rev. 86:583–650. 2006.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Choi AM and Alam J: Heme oxygenase-1:
Function, regulation, and implication of a novel stress-inducible
protein in oxidant-induced lung injury. Am J Respir Cell Mol Biol.
15:9–19. 1996.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Alam J, Igarashi K, Immenschuh S,
Shibahara S and Tyrrell RM: Regulation of heme oxygenase-1 gene
transcription: Recent advances and highlights from the
international conference (Uppsala, 2003) on Heme Oxygenase.
Antioxid Redox Signal. 6:924–933. 2004.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Lee TS, Tsai HL and Chau LY: Induction of
heme oxygenase-1 expression in murine macrophages is essential for
the anti-inflammatory effect of low dose 15-deoxy-Delta
12,14-prostaglandin J2. J Biol Chem. 278:19325–19330.
2003.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Wiesel P, Foster LC, Pellacani A, Layne
MD, Hsieh CM, Huggins GS, Strauss P, Yet SF and Perrella MA:
Thioredoxin facilitates the induction of heme oxygenase-1 in
response to inflammatory mediators. J Biol Chem. 275:24840–24846.
2000.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Morse D, Pischke SE, Zhou Z, Davis RJ,
Flavell RA, Loop T, Otterbein SL, Otterbein LE and Choi AM:
Suppression of inflammatory cytokine production by carbon monoxide
involves the JNK pathway and AP-1. J Biol Chem. 278:36993–36998.
2003.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Lee TS and Chau LY: Heme oxygenase-1
mediates the anti-inflammatory effect of interleukin-10 in mice.
Nat Med. 8:240–246. 2002.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Lee JA, Lee MY, Shin IS, Seo CS, Ha H and
Shin HK: Anti-inflammatory effects of Amomum compactum on RAW 264.7
cells via induction of heme oxygenase-1. Arch Pharm Res.
35:739–746. 2012.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Suh GY, Jin Y, Yi AK, Wang XM and Choi AM:
CCAAT/enhancer-binding protein mediates carbon monoxide-induced
suppression of cyclooxygenase-2. Am J Respir Cell Mol Biol.
35:220–226. 2006.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Oh GS, Pae HO, Lee BS, Kim BN, Kim JM, Kim
HR, Jeon SB, Jeon WK, Chae HJ and Chung HT: Hydrogen sulfide
inhibits nitric oxide production and nuclear factor-kappaB via heme
oxygenase-1 expression in RAW264.7 macrophages stimulated with
lipopolysaccharide. Free Radic Biol Med. 41:106–119.
2006.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Li Volti G, Sorrenti V, Murabito P,
Galvano F, Veroux M, Gullo A, Acquaviva R, Stacchiotti A, Bonomini
F, Vanella L and Di Giacomo C: Pharmacological induction of heme
oxygenase-1 inhibits iNOS and oxidative stress in renal
ischemia-reperfusion injury. Transplant Proc. 39:2986–2991.
2007.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Datta PK, Koukouritaki SB, Hopp KA and
Lianos EA: Heme oxygenase-1 induction attenuates inducible nitric
oxide synthase expression and proteinuria in glomerulonephritis. J
Am Soc Nephrol. 10:2540–2550. 1999.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Lee DS, Kim BN, Lim S, Lee J, Kim J, Jeong
JG and Kim S: Effective suppression of nitric oxide production by
HX106N through transcriptional control of heme oxygenase-1. Exp
Biol Med. 240:1136–1146. 2015.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Luo W, Wang Y, Yang H, Dai C, Hong H, Li
J, Liu Z, Guo Z, Chen X, He P, et al: Heme oxygenase-1 ameliorates
oxidative stress-induced endothelial senescence via regulating
endothelial nitric oxide synthase activation and coupling. Aging
(Albany NY). 10:1722–1744. 2018.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Duan Z, Wang Y and Huang X: The Peperomia
dindygulensis: a review of phytochemistry and pharmacology
perspectives. Asian J Tradit Med. 14:193–201. 2019.
|
|
27
|
Wu JL, Li N, Hasegawa T, Sakai J, Mitsui
T, Ogura H, Kataoka T, Oka S, Kiuchi M, Tomida A, et al: Bioactive
secolignans from Peperomia dindygulensis. J Nat Prod. 69:790–794.
2006.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Lin MG, Yu DH, Wang QW, Lu Q, Zhu WJ, Bai
F, Li GX, Wang XW, Yang YF, Qin XM, et al: Secolignans with
antiangiogenic activities from Peperomia dindygulensis. Chem
Biodivers. 8:862–871. 2011.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Wang QW, Yu DH, Lin MG, Zhao M, Zhu WJ, Lu
Q, Li GX, Wang C, Yang YF, Qin XM, et al: Antiangiogenic
polyketides from Peperomia dindygulensis Miq. Molecules.
17:4474–4483. 2012.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Park S, Han HT, Oh SS, Kim DH, Jeong JW,
Lee KW, Kim M, Lim JS, Cho YY, Hwangbo C, et al: NDRG2 sensitizes
myeloid leukemia to arsenic trioxide via GSK3β-NDRG2-PP2A complex
formation. Cells. 8(495)2019.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Choi SC, Kim KD, Kim JT, Oh SS, Yoon SY,
Song EY, Lee HG, Choe YK, Choi I, Lim JS and Kim JW: NDRG2 is one
of novel intrinsic factors for regulation of IL-10 production in
human myeloid cell. Biochem Biophys Res Commun. 396:684–690.
2010.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Dulak J, Loboda A and Jozkowicz A: Effect
of heme oxygenase-1 on vascular function and disease. Curr Opin
Lipidol. 19:505–512. 2008.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Sun RF, Zhu CC, Yang Y and Yu NJ: Novel
secolignans from peperomia dindygulensis and their inhibitory
activities on JAK-STAT signaling pathways. Fitoterapia. 122:80–84.
2017.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Yan J, Li M, Wang XD, Lu ZY and Ni XL:
Peperomin E (PepE) protects against high fat diet-induced
atherosclerosis in Apolipoprotein E deficient (ApoE(-/-)) mice
through reducing inflammation via the suppression of NLRP3
signaling pathway. Biomed Pharmacother. 105:862–869.
2018.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Beutler B and Rietschel ET: Innate immune
sensing and its roots: The story of endotoxin. Nat Rev Immunol.
3:169–176. 2003.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Medzhitov R and Janeway C Jr: Innate
immune recognition: Mechanisms and pathways. Immunol Rev.
173:89–97. 2000.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Liu S, Yang T, Ming TW, Gaun TKW, Zhou T,
Wang S and Ye B: Isosteroid alkaloids with different chemical
structures from Fritillariae cirrhosae bulbus alleviate LPS-induced
inflammatory response in RAW 264.7 cells by MAPK signaling pathway.
Int Immunopharmacol. 78(106047)2020.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Hu TY, Ju JM, Mo LH, Ma L, Hu WH, You RR,
Chen XQ, Chen YY, Liu ZQ, Qiu SQ, et al: Anti-inflammation action
of xanthones from Swertia chirayita by regulating
COX-2/NF-κB/MAPKs/Akt signaling pathways in RAW 264.7 macrophage
cells. Phytomedicine. 55:214–221. 2019.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Kim Y, Sung J, Sung M, Choi Y, Jeong HS
and Lee J: Involvement of heme oxygenase-1 in the anti-inflammatory
activity of Chrysanthemum boreale Makino extracts on the expression
of inducible nitric oxide synthase in RAW264.7 macrophages. J
Ethnopharmacol. 131:550–554. 2010.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Medina MV, Sapochnik D, Sola MG and Coso
O: Regulation of the expression of heme oxygenase-1: Signal
transduction, gene promoter activation, and beyond. Antioxid Redox
Signal. 32:1033–1044. 2020.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Yang Q and Wang W: The nuclear
translocation of heme oxygenase-1 in human diseases. Front Cell Dev
Biol. 10(890186)2022.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Kietzmann T, Samoylenko A and Immenschuh
S: Transcriptional regulation of heme oxygenase-1 gene expression
by MAP kinases of the JNK and p38 pathways in primary cultures of
rat hepatocytes. J Biol Chem. 278:17927–17936. 2003.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Ohlmann A, Giffhorn-Katz S, Becker I, Katz
N and Immenschuh S: Regulation of heme oxygenase-1 gene expression
by anoxia and reoxygenation in primary rat hepatocyte cultures. Exp
Biol Med (Maywood). 228:584–589. 2003.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Shan Y, Pepe J, Lu TH, Elbirt KK,
Lambrecht RW and Bonkovsky HL: Induction of the heme oxygenase-1
gene by metalloporphyrins. Arch Biochem Biophys. 380:219–227.
2000.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Li X, Huang R, Liu K, Li M, Luo H, Cui L,
Huang L and Luo L: Fucoxanthin attenuates LPS-induced acute lung
injury via inhibition of the TLR4/MyD88 signaling axis. Aging
(Albany NY). 13:2655–2667. 2020.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Lipinski P, Starzynski RR, Drapier JC,
Bouton C, Bartlomiejczyk T, Sochanowicz B, Smuda E, Gajkowska A and
Kruszewski M: Induction of iron regulatory protein 1 RNA-binding
activity by nitric oxide is associated with a concomitant increase
in the labile iron pool: Implications for DNA damage. Biochem
Biophys Res Commun. 327:349–355. 2005.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Bouton C and Demple B: Nitric
oxide-inducible expression of heme oxygenase-1 in human cells.
Translation-independent stabilization of the mRNA and evidence for
direct action of nitric oxide. J Biol Chem. 275:32688–32693.
2000.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Kramer M, Sponholz C, Slaba M, Wissuwa B,
Claus RA, Menzel U, Huse K, Platzer M and Bauer M: Alternative 5'
untranslated regions are involved in expression regulation of human
heme oxygenase-1. PLoS One. 8(e77224)2013.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Lin PH, Chiang MT and Chau LY:
Ubiquitin-proteasome system mediates heme oxygenase-1 degradation
through endoplasmic reticulum-associated degradation pathway.
Biochim Biophys Acta. 1783:1826–1834. 2008.PubMed/NCBI View Article : Google Scholar
|