Introduction
Thyroid hormone concentrations in blood change
during acute illness. This typically involves a rapid decrease in
triiodothyronine (T3) with reciprocal increase of reverse-T3, a
slow decline of thyroxine (T4), while thyroid stimulating hormone
levels are preserved. The extent of these hormonal changes is
proportional to severity of disease and it remains unclear if this
contributes to disease, or represents an epiphenomenon (1-11).
Controlled clinical trials of T3 supplementation in
patients having cardiac surgery and in organ transplantation have
reported little effect (12,13).
A randomized, blinded, placebo-controlled trial of T3 (with and
without hydrocortisone) in a sheep model of septic shock
illustrated that increasing plasma T3 concentrations in blood did
not markedly alter hemodynamics, nor any other physiological
variable (14). Nevertheless,
further clinical studies of T3 have been proposed in patients with
COVID-19, sepsis, acute respiratory distress syndrome and
myocardial infarction (15).
While administration of T3 in critical illness has
appeared safe, there have been concerns about adverse effects
(11,16-18).
These have been based on clinical features seen in those with
prolonged exposure to excessive thyroid hormone and established
hyperthyroidism, such as tachycardia, increased metabolism and
hyperthermia. Studying the effects of short-term T3 supplementation
in a non-diseased state may further clarify safety, provide
insights why T3 has not been efficacious and facilitate studies of
thyroid hormone physiology. For these reasons we examined the
clinical effects of a 24-h infusion of T3 administered to a healthy
sheep receiving intensive care support.
Materials and methods
Study design
This was an observational prospective study of
administering T3 in a healthy sheep model of intensive care. Ethics
approval was obtained from the Institute of Medical and Veterinary
Science/Central Northern Adelaide Health Service and The University
of Adelaide Animal Ethics Committees (project numbers 157/08, 80/10
and M-2010-089). The study was conducted according to the
Australian Code of Practice for the Care and Use of Animals for
Scientific Purposes (2004) (19).
Sheep model
Five female sheep (Ovis aries) aged at least
3 years and not lactating, were randomly chosen from a herd of
similarly aged paddock-raised healthy agricultural stock, then
housed with other animals in pens with free access to food and
water. Individual sheep were managed according to an intensive care
model described previously (14).
Briefly, female sheep (weight, 56-65 kg) were anaesthetized with
isoflurane for insertion of carotid and pulmonary artery catheters,
tracheostomy, urinary catheter and venous cannula placed with
radiological guidance into the coronary sinus, renal, hepatic and
iliac veins. Following instrumentation (time 0 h), animals received
protocol-directed sedation [ketamine (7.1±2.3 mg/kg/h) and
midazolam (0.4±0.2 mg/kg/h)], ventilation, parenteral fluids and a
noradrenaline infusion titrated to maintain the normal mean
arterial pressure of sheep (75 mmHg).
After 2 h, animals were administered a 24-h
intravenous infusion of T3 (liothyronine; GlobalRx Inc.) 1 µg/kg/h
(time 2-26 h). This was the same dose administered previously in a
septic sheep model (14). A
pharmacist prepared the study drug solution each day of study.
Sacrifice with phenobarbitone (6.5 g, iv) was performed at the
completion of the study.
Endpoints
Clinical endpoints measured through the study period
included hemodynamic, respiratory, renal, hematological, metabolic
and endocrine parameters (Table
SI). These 45 clinical endpoints were compared with a
previously reported group of five non-septic sheep subjected to the
same protocol but not administered T3(14).
Assays
Biochemical analyses included serum electrolytes
(Na+, K+, Cl-,
HCO3-), creatinine, urea, bilirubin, alkaline
phosphatase, alanine aminotransferase (ALT) and lactate (Olympus
AU5400 chemistry analyzer; Olympus Corporation). Blood cell counts
were obtained with a SYSMEX-XE-2100 hematology analyzer (TOA
Medical Electronics, Co., Ltd.) and clotting times using STA-R
Evolution (Stago Diagnostica). Blood pH, gases and
haemoglobin-O2 saturation were analyzed on a RAPID-Point
405 Blood-gas analyzer (Siemens Healthineers).
Plasma free-T3 and free-T4 concentrations were
measured by Chemiluminescent Micro-particle Immuno-Assay using
commercially available reagents and calibration solutions (Unicel
DXi 800; Beckman Coulter, Inc.). Plasma cortisol concentrations
were determined by radioimmunoassay (14,20).
Blood cultures were taken aseptically from the
jugular vein at baseline, repeated at 12 and 26 h and analyzed on
the Bactec system (Becton, Dickinson and Company).
Statistical analysis
Data are presented as means (± SD in tables; ± SEM
in graphs). Group data over multiple time points were tested for
statistical difference by linear mixed-effects models. If a
statistically significant (P<0.05) ‘group x time’ interaction
effect was found, a post-hoc test with Bonferroni correction was
performed to determine the difference of adjusted means at each
time point. Data were analyzed with SPSS (version 21; IBM Corp.)
and GraphPad (version 8.2; Dotmatics).
Results
All sheep survived the study and maintained sterile
blood cultures. Study groups were of comparable size and received
similar amounts of parenteral fluids and sedation (Table SII).
Plasma hormone concentrations
Free-T3 plasma levels were markedly increased by T3
infusion, being nearly eight times higher than the group not given
T3 (Table I). Free-T4 decreased
over time and was not markedly different between groups. Cortisol
declined from elevated baseline levels before increasing by the end
of the study. This pattern of change and cortisol plasma
concentration did not markedly differ between groups.
 | Table IPlasma free-T3, free-T4 and cortisol
in healthy sheep with (n=5) and without (n=5) a 24-h intravenous
infusion of T3 (1 µg/kg/h commenced at 2 h). Means ± SD. |
Table I
Plasma free-T3, free-T4 and cortisol
in healthy sheep with (n=5) and without (n=5) a 24-h intravenous
infusion of T3 (1 µg/kg/h commenced at 2 h). Means ± SD.
| | | 0 h | 12 h | 26 h | Group x time | Time | Group |
|---|
| Free-T3,
pmol/) | No T3 | 5.6±0.5 | 4.8±0.4 | 4.4±0.3 | P<0.01 | | |
| | T3 | 4.1±0.8 |
26.9±14.4a |
34.9±9.9a | | | |
| Free-T4,
pmol/l | No T3 | 10.8±1.6 | 9.2±2.4 | 7.0±1.2 | P=0.26 | P<0.01 | P=0.15 |
| | T3 | 10.2±2.3 | 6.6±2.7 | 4.7±1.7 | | | |
| Cortisol,
nmol/l | No T3 | 241±45 | 37±27 | 144±169 | P=0.24 | P<0.01 | P=0.40 |
| | T3 | 150±41 | 66±44 | 126±70 | | | |
Clinical endpoints
There were no significant differences between study
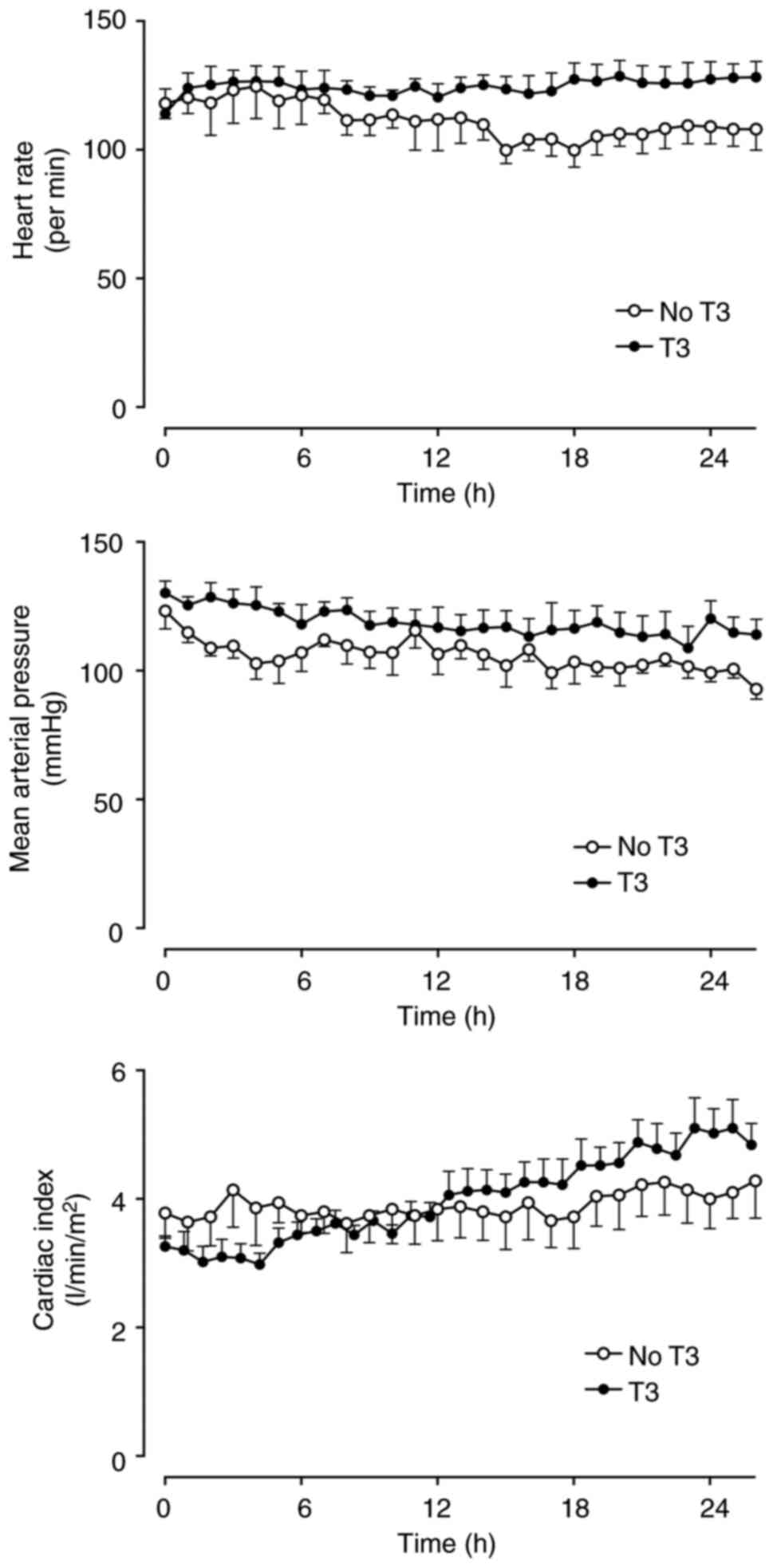
groups. Cardiac index, heart rate, circulatory pressures and the
derived hemodynamic indices did not differ between the two groups
over time (Figs. 1, S1 and S2). No animals required
noradrenaline.
Partial pressure of carbon dioxide
(PaCO2) was similar in both groups with no difference in
the amount of ventilation required or pulmonary compliance
(Fig. 2). Arterial pH was not
markedly different between groups, but lactate was slightly lower
in the T3 group in the first 8 h (Fig.
3).
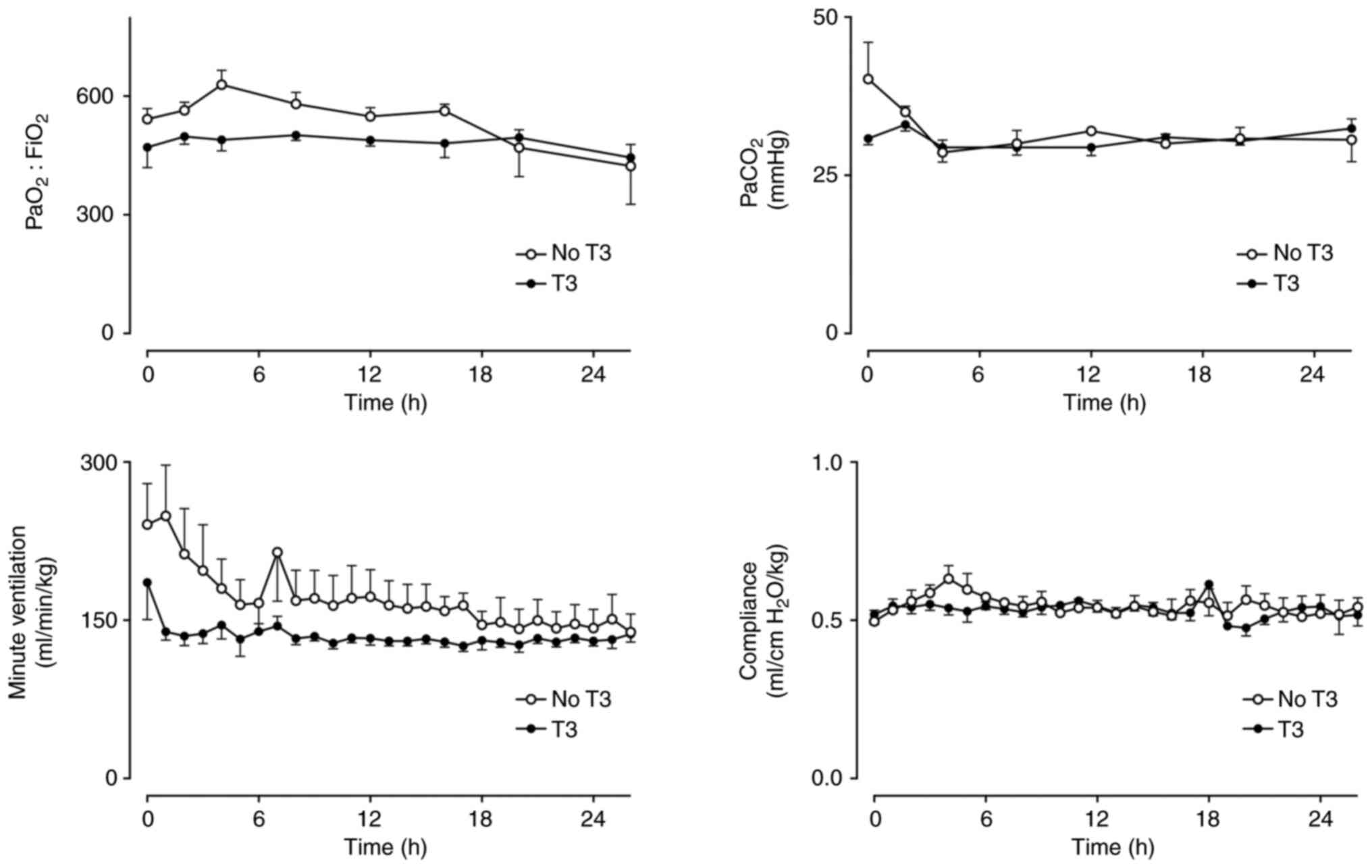 | Figure 2PaO2:FiO2,
PaCO2, VA and pulmonary compliance in a
healthy sheep model of intensive care with (n=5) and without (n=5)
a 24-h infusion of T3 (commenced at h 2). Mean ± SEM.
PaO2:FiO2: Group x Time P=0.46, Group P=0.24,
Time P=0.22. PaCO2: Group x Time P=0.24, Group P=0.31,
Time P=0.13. VA: Group x Time P=0.47, Group P=0.06, Time
P=0.04. Compliance: Group x Time P=0.77, Group P=0.55, Time P=0.22.
PaO2, partial pressure of oxygen; FiO2,
fraction of inspired oxygen; PaCO2, partial pressure of
carbon dioxide; VA, minute ventilation; T3,
triiodothyronine. |
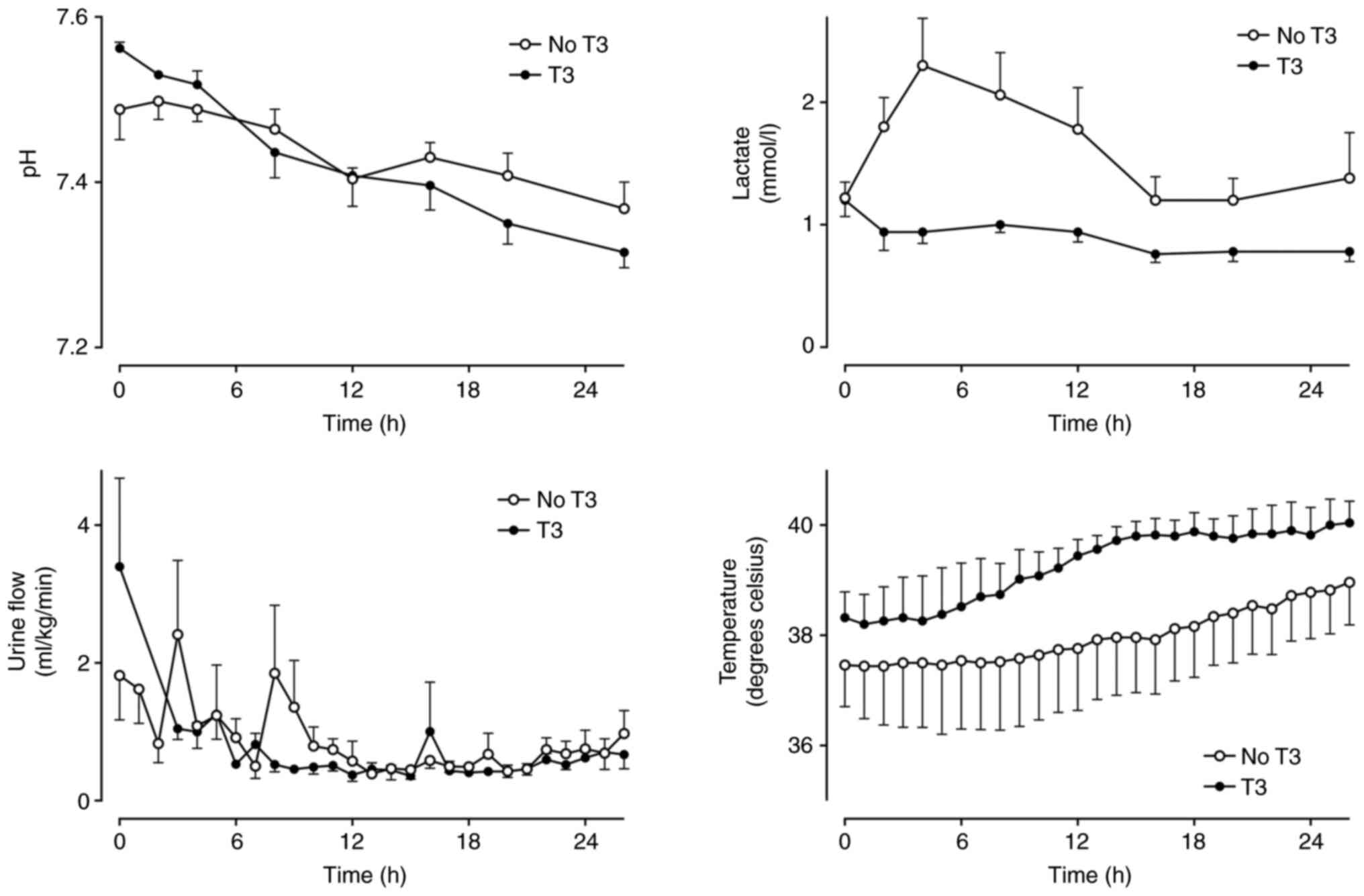 | Figure 3Arterial blood pH, serum lactate
concentration, urine flow and core temperature in a healthy sheep
model of intensive care with (n=5) and without (n=5) a 24-h
infusion of T3 (commenced at h 2). Mean ± SEM. Urine data are
missing for the T3 group at h 1 and 2. pH: Group x Time P=0.22,
Group P=0.92, Time P<0.01. Lactate: Group x Time P=0.04,
P<0.05 between groups at 2, 4 and 8 h. Urine: Group x Time
P=0.36, Group P=0.40, Time P<0.01. Temperature: Group x Time
P=0.95, Group P=0.28, Time P=0.01. T3, triiodothyronine. |
Urine flow, serum creatine, electrolytes, hematology
and temperature did not differ between groups over time (Table II, Fig. 3). There was a slight difference in
ALT between groups over time, but clinically insignificant.
Haemoglobin-O2 saturation in the pulmonary artery,
coronary sinus, renal, hepatic and iliac veins was no different
between the groups over time (Fig.
S3).
 | Table IIBiochemistry and haematological
parameters in healthy sheep with (n=5) and without (n=5) a 24-h
infusion of T3 (1 µg/kg/h commenced at 2 h). Means ± SD. |
Table II
Biochemistry and haematological
parameters in healthy sheep with (n=5) and without (n=5) a 24-h
infusion of T3 (1 µg/kg/h commenced at 2 h). Means ± SD.
| | | 0 h | 2 h | 12 h | 26 h | Group x Time | Time | Group |
|---|
| Na+,
mmol/l | No T3 | 146±1 | 147±1 | 147±2 | 148±1 | P=0.14 | P=0.83 | P<0.01 |
| | T3 | 143±1 | 143±1 | 142±3 | 142±1 | | | |
| K+,
mmol/l | No T3 | 3.8±0.3 | 3.3±0.4 | 3.7±0.5 | 3.8±0.2 | P=0.87 | P=0.11 | P=0.05 |
| | T3 | 3.5±0.3 | 3.0±0.2 | 3.5±0.3 | 3.6±0.3 | | | |
| Cl-,
mmol/l | No T3 | 107±3 | 110±2 | 118±2 | 121±3 | P=0.28 | P<0.01 | P=0.01 |
| | T3 | 105±2 | 106±2 | 112±2 | 118±3 | | | |
|
HCO3-, mmol/l | No T3 | 29±3 | 24±2 | 18±1 | 17±2 | P=0.27 | P<0.01 | P=0.06 |
| | T3 | 31±2 | 29±3 | 21±4 | 16±6 | | | |
| Anion Gap,
mmol/l | No T3 | 14±3 | 16±3 | 15±2 | 13±1 | P=0.89 | P=0.67 | P=0.10 |
| | T3 | 11±2 | 10±3 | 12±3 | 12±7 | | | |
| Urea, mmol/l | No T3 | 9±2 | 8±2 | 7±2 | 7±2 | P=0.72 | P<0.01 | P=0.02 |
| | T3 | 5±2 | 5±2 | 4±2 | 4±2 | | | |
| Creatinine,
µmol/l | No T3 | 97±19 | 84±10 | 75±14 | 76±16 | P=0.10 | P=0.22 | P=0.71 |
| | T3 | 83±20 | 77±15 | 84±40 | 110±39 | | | |
| ALP, U/l | No T3 | 85±29a | 64±16 | 51±16 | 42±10 | P=0.27 | P<0.01 | P=0.11 |
| | T3 | 53±23 | 45±14 | 38±8 | 32±17 | | | |
| Bilirubin,
µmol/l | No T3 | 4±2 | 7±1 | 11±5 | 10±3 | P=0.91 | P<0.01 | P=0.21 |
| | T3 | 2±1 | 4±2 | 8±5 | 8±8 | | | |
| ALT, U/l | No T3 | 11±3 | 12±4 | 19±6 | 25±7a | P=0.01 | | |
| | T3 | 10±7 | 12±6 | 12±7 | 13±7 | | | |
| aPTT, sec | No T3 | 33±9 | 33±4 | 34±6 | 39±5 | P=0.26 | P=0.02 | P=0.65 |
| | T3 | 30±4 | 32±6 | 41±9 | 42±8 | | | |
| PT, sec | No T3 | 22±1 | 22±1 | 23±1 | 24±2 | P=0.46 | P=0.08 | P=0.65 |
| | T3 | 22±4 | 22±3 | 26±9 | 26±8 | | | |
| Fibrinogen,
g/l | No T3 | 1.6±0.3 | 1.5±0.3 | 1.9±0.5 | 2.7±0.7 | P=0.42 | P<0.01 | P=0.12 |
| | T3 | 2.0±0.2 | 2.1±0.3 | 2.3±0.4 | 3.3±0.3 | | | |
| Hb, g/l | No T3 | 99±3 | 106±8 | 95±14 | 92±7 | P=0.06 | P=0.34 | P<0.01 |
| | T3 | 80±10 | 84±7 | 91±5 | 86±11 | | | |
| WCC,
x109/l | No T3 | 6.5±3.0 | 7.2±3.6 | 9.1±3.8 | 5.6±3.5 | P=0.57 | P<0.01 | P=0.31 |
| | T3 | 4.5±1.7 | 5.9±1.4 | 7.0±1.2 | 4.9±0.9 | | | |
| PLTs,
x109/l | No T3 | 163±79 | 160±61 | 115±36 | 106±27 | P=0.20 | P<0.01 | P=0.70 |
| | T3 | 192±46 | 174±35 | 85±38 | 108±26 | | | |
Discussion
A 24-h intravenous infusion of T3 in healthy sheep
managed with intensive care support produced supra-physiological
plasma T3 concentrations, yet was associated with little or no
significant physiological change when compared with a similarly
managed group of sheep not administered T3. This is an important
finding given the ongoing interest in clinical trials of T3 and
adds further evidence that short term administration of T3 appears
safe.
While there was no significant difference between
groups for the vast majority of the 45 endpoints measured, there
were some parameters that differed slightly. Mean arterial, central
venous and pulmonary artery pressures were slightly higher in the
T3 group. However, these parameters differed between groups before
the commencement of T3 infusion and did not change over time. Hence
it is unlikely there is an obvious treatment effect of T3 within 24
h on hemodynamic pressures. Lactate concentrations were markedly
higher in the group not given T3. This is unlikely to be important
given that differences appeared early in the present study,
concentrations were not markedly elevated and the large number of
other parameters that did not differ.
Failing to observe a significant increase of cardiac
index in animals given T3 was unexpected. Laboratory studies in
cardiac myocytes and isolated hearts have reported prompt increases
in contractility with T3 added to perfusate (21-24).
Subsequently, several clinical trials have been conducted in
patients having cardiac surgery. A meta-analysis of these studies
concluded that T3 increased cardiac index but had inconclusive
effects on other parameters (12).
Most of these clinical studies used intravenous T3 doses of
0.175-0.333 µg/kg/h for 6-9 h, which is less than 1 µg/kg/h used
for 24 h, and reported an effect on cardiac index at 4-6 h.
Possibly there was some aspect of the sheep model that limited the
ability to detect a change to cardiac output following T3.
Ketamine, used as a sedating agent, is known to increase cardiac
output in humans (25) and sheep
(26) and may have obscured any
hemodynamic effect of T3.
T3 rapidly stimulates
Na+/K+-ATPase on alveolar membranes (27,28)
and renal tubule cells (29) in
vitro. This was not clinically apparent in the present study,
as supra-physiological plasma T3 levels were not associated with
any significant change to respiratory or renal function, or serum
biochemistry. Lack of change to these variables has also been
reported in clinical trials of T3 in neonatal respiratory failure
(30) and renal transplant
dysfunction (31).
Concerns that supplementing T3 would stimulate
metabolism and O2 demand (16), were not supported in the present
study. While temperatures were higher in the group of sheep given
T3, this did not differ between groups over time and likely
reflects baseline differences and data variability. Sheep given T3
did not require more ventilation to control PaCO2
compared with those not given T3. Haemoglobin-O2
saturation of mixed venous blood and from the different organs
remained stable over the duration of the study and did not differ
between groups.
There were no obvious effects of supplementary T3 on
plasma concentrations of T4 or cortisol. T3 can increase
deiodinase-1 synthesis (32,33)
and upregulate deiodinase-3 activity (34) favoring increased clearance of T4.
Exogenous T3 can also suppress deiodinase-2 which would limit
deiodination of T4(35). Thus, T3
therapy could theoretically increase and/or decrease deiodination
of T4. In the present study, T3 did not alter plasma levels of T4
and understanding how supra-physiological T3 alters the deiodinase
enzymes requires further investigation. T3 can also upregulate
steroid dehydrogenase and reductase enzymes leading to increased
clearance of cortisol (36).
However, this may take more than 24 h to become evident, or be
balanced by increased cortisol secretion, as there were no changes
to plasma cortisol concentrations in sheep given T3.
Why would the supra-physiological plasma levels of
T3 achieved in this healthy sheep model not produce obvious
clinical changes? First, a 24-h infusion of T3 may have been too
short to allow T3 to exert any noticeable effect. Although T3 has
rapid cell membrane effects in vitro (37), these may not manifest in
vivo within 24 h in sheep. The genomic and mitochondrial
effects of T3 may be responsible for clinical change, but require
>24 h to manifest. Of note, studies that have reported
T3-induced changes in heart rate (38), venous compliance (39), diastolic function (40), myocardial contractility (41) and metabolic rate (42) have administered hormone for at
least seven days. Second, cellular concentration of T3 may not
reflect those in plasma. Cells may exhibit homeostatic mechanisms,
such as deiodination, modulation of cell receptors and hormone
transport mechanisms, that may limit exposure to excess plasma
hormone concentrations. This will require further study. Third, an
acute clinical change following T3 administration may be more
likely when restoring hormone levels in established hypothyroid
states (38,42), rather than in euthyroid animals.
Fourth, the effect of exogenous T3 may be species-specific, with
sheep having limited response to acute therapy. When tested on
hypothyroid lambs, T3 increased high energy phosphate compounds
within 20 min but did not alter hemodynamics, coronary blood flow
or myocardial O2 consumption (43). Another sheep study reported
increased cardiac output 2 h after a T3 bolus (1.2 µg/kg) that
yielded much higher plasma concentrations (80 pmol/l) than the
present study (44). Finally, most
studies of T3 administered to euthyroid animals and humans have
been unblinded and not randomized. This may have led to a greater
likelihood to report an effect of T3 therapy. Notably, the only
randomized, blinded, placebo-controlled study of T3 (100 µg i.v.
bolus) administered to euthyroid human volunteers, reported no
effect on cardiac output, heart rate or mean arterial pressure over
45 min (45). It is thus
reasonable to conclude that T3 does not exert an acute effect on
hemodynamics in euthyroid subjects.
There were several limitations to the present study.
It was not randomized or blinded and the group of sheep given T3
were compared with a group of historical controls studied up to
three years earlier. Despite the lag time between studies, sheep
were obtained from the same herd, were of a similar size, received
an identical management protocol and staffing expertise of the
model was consistent over all studies. The number of sheep in each
group was relatively small, which limits power of the study to
detect a subtle effect of T3. A total of five animals were chosen,
as this was the number of non-septic sheep in the earlier model
validation studies.
The effects of supplementary T3 were studied in a
group of healthy sheep receiving intensive care supports and
compared with an earlier group not administered T3. The two groups
of animals had similar baseline characteristics and were managed
with identical protocols. The group receiving a 24-h infusion of T3
developed supra-physiological plasma levels while hemodynamic,
metabolic and all other physiological parameters did not differ
markedly between the groups over time. Administration of T3 for 24
h does not have substantial physiological effect in this healthy
animal model and further study is required to understand cellular
function and thyroid hormone metabolism with short-term T3
supplementation.
Supplementary Material
CVP and mean PAP in sheep with (n=5)
and without (n=5) a 24-h infusion of T3 (commenced at h 2). Mean ±
SEM. CVP: Group x Time P=0.05. PAP: Group x Time P=0.25, Group
P<0.01 Time P=0.01. CVP, central venous pressure; PAP, pulmonary
artery pressure; T3, triiodothyronine.
SVRI, PVRI, LVSWI and RVSWI in sheep,
with (n=5) and without (n=5) a 24-h infusion of T3 (commenced at h
2). Mean ± SEM. SVRI: Group x Time P=0.07, Group P=0.74, Time
P=0.16. PVRI: Group x Time P=0.69, Group P=0.53, Time P=0.46.
LVSWI: Group x Time P=0.39, Group P=0.94, Time P=0.49. RVSWI: Group
x Time P=0.59, Group P=0.39, Time P<0.01. SVRI, systemic
vascular resistance index; PVRI, pulmonary vascular resistance
index; LVSWI, left ventricular stroke work index; RVSWI, right
ventricular stroke work index; T3, triiodothyronine.
Hb-O2 saturation in blood from MV, CS,
RV, HV and IV in sheep, with (n=5) and without (n=5) a 24-h
infusion of T3 (commenced at h 2). Mean ± SEM. MV: Group x Time
P=0.22, Group P=0.07, Time P=0.97. CS: Group x Time P=0.81, Group
P=0.88, Time P=0.76. RV: Group x Time P=0.88, Group P=0.15, Time
P=0.31. HV: Group x Time P=0.33, Group P=0.06, Time P=0.94. IV:
Group x Time P=0.22, Group P=0.48, Time P=0.80. Hb, hemoglobin; O2,
oxygen; MV, mixed venous; CS, coronary sinus; RV, renal vein; HV,
hepatic vein; IV, iliac vein; T3, triiodothyronine.
Physiological variables measured in an
ovine model of intensive care.
Characteristics of sheep managed with
ICU supports for 26 h, with (n=5) and without (n=5) an intravenous
infusion of T3 (1 μg/kg/h for 24 h). Mean ± SD.
Acknowledgements
Ms Alison Ankor, Mr Luke Chester, Mr Jason Edwards
and Mr Alex Poole (Royal Adelaide Hospital ICU; Adelaide,
Australia) provided staffing assistance in the sheep laboratory.
Study drug preparation was overseen by Mr Peter Slobodian
(Department of Pharmacy, Investigational Drugs, Royal Adelaide
Hospital; Adelaide, Australia). Ms Alix Rao (Department of
Physiology, Monash University; Clayton, Australia) performed
hormonal assay of sheep plasma. Mr Tom Sullivan (Discipline of
Public Health, University of Adelaide; Adelaide, Australia)
provided statistical advice.
Funding
Funding: The present study received funding from a Royal
Adelaide Hospital Research Committee Project Grant (grant no.
6047), a donation from Ms. Deidre Tidswell (dec.) and institutional
departmental funds.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
MJM was responsible for study conception, model
development, study design and conduct, data collection and analysis
and manuscript preparation. MJM, DJT and GLL were responsible for
study design, project supervision and manuscript review. IJC was
responsible for study design, assays and manuscript review. CHN was
responsible for study conduct, data collection and manuscript
review. LM was responsible for study conduct, animal care and
manuscript review. SP was responsible for study conduct, animal
care, data collection and manuscript review. BC was responsible for
study conduct, animal care, data collection and manuscript review.
TRK was responsible for model development, animal surgery, animal
care and study conduct. All authors (TRK deceased) read and
approved the final manuscript. MJM and CHN confirm the authenticity
of all the raw data.
Ethics approval and consent to
participate
Ethics approval was obtained from the Institute of
Medical and Veterinary Science/Central Northern Adelaide Health
Service and The University of Adelaide Animal Ethics Committees
(approval nos. 157/08, 80/10 and M-2010-089). The study was
conducted according to the Australian Code of Practice for the Care
and Use of Animals for Scientific Purposes (2004).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Maiden MJ and Torpy DJ: Thyroid hormones
in critical illness. Crit Care Clin. 35:375–388. 2019.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Ray DC, Macduff A, Drummond GB, Wilkinson
E, Adams B and Beckett GJ: Endocrine measurements in survivors and
non-survivors from critical illness. Intensive Care Med.
28:1301–1308. 2002.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Meyer S, Schuetz P, Wieland M, Nusbaumer
C, Mueller B and Christ-Crain M: Low triiodothyronine syndrome: A
prognostic marker for outcome in sepsis? Endocrine. 39:167–174.
2011.PubMed/NCBI View Article : Google Scholar
|
|
4
|
den Brinker M, Dumas B, Visser TJ, Hop WC,
Hazelzet JA, Festen DA, Hokken-Koelega AC and Joosten KF: Thyroid
function and outcome in children who survived meningococcal septic
shock. Intensive Care Med. 31:970–976. 2005.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Joosten KF, de Kleijn ED, Westerterp M, de
Hoog M, Eijck FC, Hop WCJ, Voort EV, Hazelzet JA and Hokken-Koelega
AC: Endocrine and metabolic responses in children with
meningoccocal sepsis: Striking differences between survivors and
nonsurvivors. J Clin. Endocrinol. Metab. 85:3746–3753.
2000.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Yildizdas D, Onenli-Mungan N, Yapicioglu
H, Topaloglu AK, Sertdemir Y and Yuksel B: Thyroid hormone levels
and their relationship to survival in children with bacterial
sepsis and septic shock. J Pediatr Endocrinol Metab. 17:1435–1442.
2004.PubMed/NCBI
|
|
7
|
Angelousi AG, Karageorgopoulos DE,
Kapaskelis AM and Falagas ME: Association between thyroid function
tests at baseline and the outcome of patients with sepsis or septic
shock: A systematic review. Eur J Endocrinol. 164:147–155.
2011.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Fliers E and Boelen A: An update on
non-thyroidal illness syndrome. J Endocrinol Invest. 44:1597–1607.
2021.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Beltrao FEL, Beltrao DCA, Carvalhal G,
Beltrão FEL, Brito ADS, Capistrano KHRD, Bastos IHA, Hecht F,
Daltro CHDC, Bianco AC, et al: Thyroid hormone levels during
hospital admission inform disease severity and mortality in
COVID-19 patients. Thyroid. 31:1639–1649. 2021.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Van den Berghe G: Non-thyroidal illness in
the ICU: A syndrome with different faces. Thyroid. 24:1456–1465.
2014.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Adler SM and Wartofsky L: The nonthyroidal
illness syndrome. Endocrinol Metab Clin North Am. 36:657–672, vi.
2007.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Kaptein EM, Sanchez A, Beale E and Chan
LS: Clinical review: Thyroid hormone therapy for postoperative
nonthyroidal illnesses: A systematic review and synthesis. J Clin
Endocrinol Metab. 95:4526–4534. 2010.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Macdonald PS, Aneman A, Bhonagiri D, Jones
D, O'Callaghan G, Silvester W, Watson A and Dobb G: A systematic
review and meta-analysis of clinical trials of thyroid hormone
administration to brain dead potential organ donors. Crit Care Med.
40:1635–1644. 2012.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Maiden MJ, Chapman MJ, Torpy DJ, Kuchel
TR, Clarke IJ, Nash CH, Fraser JD and Ludbrook GL: Triiodothyronine
administration in a model of septic shock: A randomized blinded
placebo-controlled trial. Crit Care Med. 44:1153–1160.
2016.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Maiden MJ and Forehan S: The use of
triiodothyronine during critical illness. Curr Opin Clin Nutr Metab
Care. 27:163–167. 2024.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Burman KD and Wartofsky L: Thyroid
function in the intensive care unit setting. Crit Care Clin.
17:43–57. 2001.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Gardner DF, Kaplan MM, Stanley CA and
Utiger RD: Effect of tri-iodothyronine replacement on the metabolic
and pituitary responses to starvation. N Engl J Med. 300:579–584.
1979.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Stathatos N, Levetan C, Burman KD and
Wartofsky L: The controversy of the treatment of critically ill
patients with thyroid hormone. Best Pract Res Clin Endocrinol
Metab. 15:465–478. 2001.PubMed/NCBI View Article : Google Scholar
|
|
19
|
National Health and Medical Research
Council (Australian Government): Australian Code of Practice for
the Care and Use of Animals for Scientific Purposes. 7th edition.
National Health and Medical Research Council, Canberra, p85,
2004.
|
|
20
|
Bocking AD, McMillen IC, Harding R and
Thorburn GD: Effect of reduced uterine blood flow on fetal and
maternal cortisol. J Dev Physiol. 8:237–245. 1986.PubMed/NCBI
|
|
21
|
Snow TR, Deal MT, Connelly TS, Yokoyama Y
and Novitzky D: Acute inotropic response of rabbit papillary muscle
to triiodothyronine. Cardiology. 80:112–117. 1992.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Tielens ET, Forder JR, Chatham JC,
Marrelli SP and Ladenson PW: Acute L-triiodothyronine
administration potentiates inotropic responses to beta-adrenergic
stimulation in the isolated perfused rat heart. Cardiovasc Res.
32:306–310. 1996.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Wang YG, Dedkova EN, Fiening JP, Ojamaa K,
Blatter LA and Lipsius SL: Acute exposure to thyroid hormone
increases Na+ current and intracellular Ca2+ in cat
atrial myocytes. J Physiol. 546:491–499. 2003.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Walker JD, Crawford FA Jr, Mukherjee R and
Spinale FG: The direct effects of 3,5,3'-triiodo-L-thyronine (T3)
on myocyte contractile processes. Insights into mechanisms of
action. J Thorac Cardiovasc Surg. 110:1369–1380. 1995.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Sigtermans M, Dahan A, Mooren R, Bauer M,
Kest B, Sarton E and Olofsen E: S(+)-ketamine effect on
experimental pain and cardiac output: A population
pharmacokinetic-pharmacodynamic modeling study in healthy
volunteers. Anesthesiology. 111:892–903. 2009.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Coulson NM, Januszkiewicz AJ and Ripple
GR: Physiological responses of sheep to two hours anaesthesia with
diazepam-ketamine. Vet Rec. 129:329–332. 1991.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Folkesson HG, Norlin A, Wang Y, Abedinpour
P and Matthay MA: Dexamethasone and thyroid hormone pretreatment
upregulate alveolar epithelial fluid clearance in adult rats. J
Appl Physiol (1995). 88:416–424. 2000.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Lei J, Nowbar S, Mariash CN and Ingbar DH:
Thyroid hormone stimulates Na-K-ATPase activity and its plasma
membrane insertion in rat alveolar epithelial cells. Am J Physiol
Lung Cell Mol Physiol. 285:L762–L772. 2003.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Lin HH and Tang MJ: Thyroid hormone
upregulates Na,K-ATPase alpha and beta mRNA in primary cultures of
proximal tubule cells. Life Sci. 60:375–382. 1997.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Amato M, Guggisberg C and Schneider H:
Postnatal triiodothyronine replacement and respiratory distress
syndrome of the preterm infant. Horm Res. 32:213–217.
1989.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Acker CG, Flick R, Shapiro R, Scantlebury
VP, Jordan ML, Vivas C, Greenberg A and Johnson JP: Thyroid hormone
in the treatment of post-transplant acute tubular necrosis (ATN).
Am J Transplant. 2:57–61. 2002.PubMed/NCBI View Article : Google Scholar
|
|
32
|
St Germain DL and Galton VA: The
deiodinase family of selenoproteins. Thyroid. 7:655–668.
1997.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Debaveye Y, Ellger B, Mebis L, Visser TJ,
Darras VM and Van den Berghe G: Effects of substitution and
high-dose thyroid hormone therapy on deiodination,
sulfoconjugation, and tissue thyroid hormone levels in prolonged
critically ill rabbits. Endocrinology. 149:4218–4228.
2008.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Tu HM, Legradi G, Bartha T, Salvatore D,
Lechan RM and Larsen PR: Regional expression of the type 3
iodothyronine deiodinase messenger ribonucleic acid in the rat
central nervous system and its regulation by thyroid hormone.
Endocrinology. 140:784–790. 1999.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Kim SW, Harney JW and Larsen PR: Studies
of the hormonal regulation of type 2 5'-iodothyronine deiodinase
messenger ribonucleic acid in pituitary tumor cells using
semiquantitative reverse transcription-polymerase chain reaction.
Endocrinology. 139:4895–4905. 1998.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Kronenberg H and Williams RH: Williams
Textbook of Endocrinology. Saunders Elsevier, Philadelphia, PA,
2008.
|
|
37
|
Bassett JH, Harvey CB and Williams GR:
Mechanisms of thyroid hormone receptor-specific nuclear and extra
nuclear actions. Mol Cell Endocrinol. 213:1–11. 2003.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Brooks I, Flynn SB, Owen DA and Underwood
AH: Changes in cardiac function following administration of thyroid
hormones in thyroidectomised rats: Assessment using the isolated
working rat heart preparation. J Cardiovasc Pharmacol. 7:290–296.
1985.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Gay RG, Raya TE, Lancaster LD, Lee RW,
Morkin E and Goldman S: Effects of thyroid state on venous
compliance and left ventricular performance in rats. Am J Physiol.
254:H81–H88. 1988.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Ojamaa K, Kenessey A, Shenoy R and Klein
I: Thyroid hormone metabolism and cardiac gene expression after
acute myocardial infarction in the rat. Am J Physiol Endocrinol
Metab. 279:E1319–E1324. 2000.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Marcus RH, Butkow N, Wheatley AM, Lippe I,
Norton G and Rosendorff C: Independent mechanisms for the
chronotropic and inotropic responses in hyperthyroidism. Basic Res
Cardiol. 82:261–270. 1987.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Ladenson PW, Goldenheim PD and Ridgway EC:
Rapid pituitary and peripheral tissue responses to intravenous
L-triiodothyronine in hypothyroidism. J Clin Endocrinol Metab.
56:1252–1259. 1983.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Portman MA, Qian K, Krueger J and Ning XH:
Direct action of T3 on phosphorylation potential in the sheep heart
in vivo. Am J Physiol Heart Circ Physiol. 288:H2484–H2490.
2005.PubMed/NCBI View Article : Google Scholar
|
|
44
|
DiPierro FV, Bavaria JE, Lankford EB,
Polidori DJ, Acker MA, Streicher JT and Gardner TJ:
Triiodothyronine optimizes sheep ventriculoarterial coupling for
work efficiency. Ann Thorac Surg. 62:662–669. 1996.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Schmidt BM, Martin N, Georgens AC,
Tillmann HC, Feuring M, Christ M and Wehling M: Nongenomic
cardiovascular effects of triiodothyronine in euthyroid male
volunteers. J Clin Endocrinol Metab. 87:1681–1686. 2002.PubMed/NCBI View Article : Google Scholar
|