Introduction
Early detection of coronary artery disease (CAD) is
crucial for the prevention of cardiovascular mortality and
morbidity (1). Despite rapid
advancements in imaging technology, the clinical diagnosis of CAD
may be challenging due to certain patients having conditions that
make them unwilling or unable to undergo procedures such as
coronary angiography or coronary computed tomography angiography
(CTA). These conditions include severe allergies or a history of
anaphylactic reaction to contrast medium, bronchial asthma
(2), chronic kidney failure
(3) or purely psychological
reasons with no underlying physical disease. The current American
Heart Association/American College of Cardiology guidelines
recommend the use of a revised calculator to estimate the 10-year
risk of developing a first atherosclerotic cardiovascular disease
(ASCVD) event in individuals without prior ASCVD (4). However, for certain patients, the
cardiovascular risk factors are not known (5). Thus, alternative biomarkers such as
osteoprotegerin (OPG) have been explored.
OPG is a soluble glycoprotein that is a member of
the tumor necrosis factor cytokine superfamily (6-8).
Receptor activator of nuclear factor κB (RANK) and its ligand
(RANKL), the latter of which is also a ligand of OPG, were
initially described in the context of bone mass regulation
(9). RANKL is produced by
osteoblasts and other cell types, such as lymphocytes. The binding
of RANKL to RANK on the surface of osteoclast precursors stimulates
their differentiation into mature osteoclasts (10). However, OPG acts as a decoy
receptor for RANKL, thereby interfering with the binding of RANKL
to the RANK cell-surface receptor. This inhibits the formation and
activity of osteoclasts, reducing bone resorption. The balance
between RANKL and OPG is critical for the maintenance of bone
homeostasis (11).
OPG is also expressed in the vascular system,
notably within endothelial and smooth muscle cells. It is a crucial
survival factor for endothelial cells, as it is essential for
endothelial integrity and function (12). The presence of OPG within
atherosclerotic lesions indicates its possible involvement in the
pathogenesis of atherosclerosis, which suggests a broader
biological significance beyond bone health (13). Numerous human studies have
indicated that OPG is an independent predictor of cardiovascular
mortality and morbidity, particularly in populations with a high
risk of cardiovascular events (14,15).
Studies have also demonstrated that elevated serum OPG levels are
associated with a higher prevalence of CAD (16,17),
suggesting that OPG is a potential predictor of CAD. However, the
applicability of these findings to the Chinese population is yet to
be determined.
In typical conditions, RANKL is often not present in
normal vessels, noncalcified arteries or valves, yet its presence
has been identified in atherosclerotic lesions (18). RANKL has been indicated to act as a
chemotactic factor by promoting the release of chemokines and
stimulating the activity of matrix metalloproteinases (19-21).
Atherosclerosis is widely regarded as a chronic inflammatory
disease (22). However,
population-based studies on the role of soluble RANKL (sRANKL) in
CAD have yielded varied results. In some studies, no significant
association was found between RANKL concentration and coronary
artery calcification or ASCVD (23,24).
By contrast, other studies detected a positive or negative
correlation between RANKL levels and various forms of
cardiovascular disease (25,26).
This divergence in findings may be attributed to the interaction
between RANKL and OPG, indicating their complex, combined
pathogenic influence in the development of atherosclerosis. Further
supporting this notion, previous research has suggested that an
elevated RANKL:OPG ratio in the circulation could be a valuable
biomarker for CAD, potentially aiding in the assessment and
prediction of cardiac events (27,28).
The aim of the present study was to evaluate the
hypothesis that elevated serum OPG concentrations are associated
with the incidence of stable CAD, and that this association is
evident for various concentrations of sRANKL. The use of recursive
methods for the construction of smooth curves provides an intuitive
means for illustrating the relationship between dependent and
independent variables (29). In
the present study, OPG was used as the independent variable, stable
CAD as the dependent variable, and sRANKL as a stratifying factor.
This method enabled the plotting of a curve to visually illustrate
the curvilinear or linear relationship between OPG levels and
stable CAD at various sRANKL concentrations. Successful validation
of this hypothesis could be instrumental in the development of
novel predictive models for patients with stable CAD.
Patients and methods
Patients
The present case-control study was conducted between
June 2021 and December 2021 at the Department of Geriatrics, Union
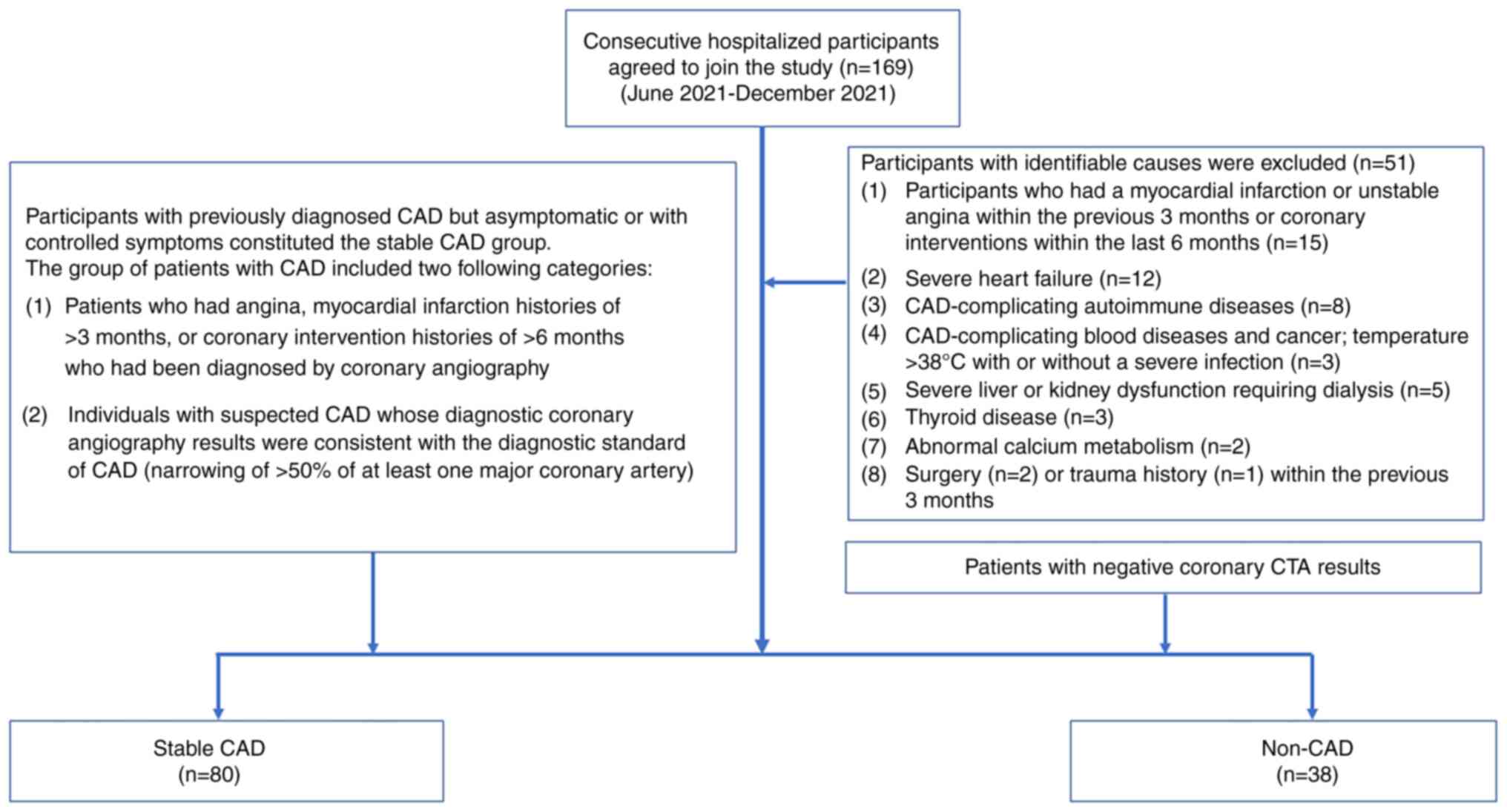
Hospital, Tongji Medical College (Wuhan, China). Fig. 1 shows the full process for the
inclusion and exclusion of research participants in the study.
Consecutive hospitalized patients during this period who agreed to
participate in the study were considered for inclusion, and were
divided into two groups: Stable CAD and non-CAD. The non-CAD group
comprised patients who received coronary CTA and had no clear
coronary atherosclerosis. Participants who were previously
diagnosed with CAD and were asymptomatic or had their symptoms
under control due to undergoing long-term treatments with various
oral medications such as aspirin and statins, were classified into
the stable CAD group. The group of patients with CAD included the
following two categories: i) Patients with a history of angina,
myocardial infarction for >3 months, or coronary intervention
histories of >6 months, who had been diagnosed by coronary
angiography; and ii) individuals with suspected CAD who underwent
diagnostic coronary angiography, and had results consistent with
the diagnostic standard of CAD, with the narrowing of at least one
major coronary artery by >50% as indicated by coronary
angiography (30). Participants
with the following conditions were excluded from the study:
Myocardial infarction or unstable angina within the previous 3
months, or coronary interventions within the last 6 months; severe
heart failure; CAD-complicating autoimmune diseases, blood diseases
or cancer; temperature >38˚C with or without a severe infection;
severe liver or kidney dysfunction requiring dialysis; thyroid
disease; abnormal calcium metabolism; surgery or trauma history
within the previous 3 months. The study was approved by the Ethics
Committee of Tongji Medical School, Huazhong University of Science
and Technology (Wuhan, China; reference no. 2021/0569), and written
informed consent was obtained from all participants.
Baseline data
Demographic data and lifestyle characteristics,
including age, sex, smoking status and alcohol consumption, were
assessed using structured questionnaires. The height and weight of
the participants were measured and used to calculate the body mass
index (BMI). Serum levels of fasting plasma glucose (FPG),
low-density lipoprotein-cholesterol (LDL-C), alkaline phosphatase
(ALP), inorganic phosphorus (P) and calcium were examined using an
automated biochemical analysis system (AU5800; Beckman Coulter
Inc.). The presence of complicating diseases, including
hypertension, diabetes, osteoporosis, lacunar infarcts, peripheral
arteriosclerosis and chronic kidney disease, was also recorded in
the baseline data.
Enzyme-linked immunosorbent assays
(ELISAs)
Blood specimens were collected from the participants
in the morning after an overnight fast, and the serum samples were
stored at -70˚C. The double-antibody sandwich ELISA method was used
to determine the serum concentrations of OPG and sRANKL.
Commercially available kits for OPG (cat. no. H286; Nanjing
Jiancheng Bioengineering Institute) and sRANKL (cat. no. H284;
Nanjing Jiancheng Bioengineering Institute) were used. Briefly,
anti-human OPG or RANKL monoclonal antibodies were coated on the
microplate. The patient samples and standards were applied, the OPG
or RANKL present in the samples bound to the antibodies fixed on
the plate, and the unbound components were then washed away.
Biotin-labeled anti-human OPG or RANKL polyclonal antibodies were
subsequently added, which bound to the OPG or RANKL already
attached to the microplate, and the unbound components were washed
away. Next, streptavidin-labeled horseradish peroxidase, which
specifically recognizes biotin, was added to form a complex. After
washing away the unbound components, a chromogenic substrate
solution was added, which gradually turned the solution blue. The
addition of stop solution changed the color to yellow and halted
the reaction. The absorbance was measured using an ELISA reader at
a wavelength of 450 nm (Thermo Fisher Scientific, Inc.). To
determine the concentration of each sample, a standard curve was
drawn based on results obtained using the standard solution. The
results were expressed in pg/ml. All samples were measured in
duplicate, and the results were averaged.
Statistical analysis
Normally distributed variables are presented as the
mean ± standard deviation, while abnormally distributed variables
are presented as the median and quartiles. Qualitative data are
expressed as the number with the percentage in parentheses.
Comparisons between groups were made using unpaired Student's
t-test for normally distributed parameters, or Kruskal-Wallis test
for variables with a skewed distribution. For categorical
variables, the χ2 test was used to analyze differences
between the non-CAD and CAD groups, or Fisher's exact test was
employed for categorical variables with expected frequencies <5.
Spearman's correlation analysis was used to analyze the correlation
between two continuous variables. Pearson's χ2 test was
used to test the correlation between dichotomous variables.
Subsequently, a univariate analysis model was utilized to examine
the association of OPG and other risk factors with the presence of
CAD. The consistency of these associations was then explored in
various subgroups (stratified analyses). Globally, individuals aged
65 and above are categorized as elderly (31), with age groups typically classified
as under 65 and 65 and older. Smooth curve fitting analysis
revealed a biphasic relationship between FPG and CAD, characterized
by an initial decrease followed by an increase. This analysis
identified a nadir at 4.7 mmol/l, leading to the stratification of
FPG levels into two groups: <4.7 and ≥4.7 mmol/l. Similarly, BMI
exhibited a similar biphasic curve in relation to CAD, with a
minimum value of 28.96 kg/m2. Consequently, BMI values
were categorized into <28.96 kg/m2 and ≥28.96
kg/m2 groups, reflecting distinct risk profiles for CAD.
ALP, LDL-C, calcium and P were categorized into tertiles. The
association between serum OPG concentration and CAD in individuals
in different sRANKL-level groups was analyzed, with adjustment for
potential confounding factors through smooth curve fitting by the
recursive method. The potential confounders were determined based
on covariate screening criteria, which included effect factors
producing a >10% change when introducing or eliminating
covariates in the basic or regression models. Finally, a
multivariate piecewise linear regression model was conducted to
evaluate the independent relationship between OPG and the presence
of CAD in the total sample and in various sRANKL-level groups based
on smooth curve fitting.
Results
Clinical and baseline characteristics
of the subjects
Table I presents
the clinical and baseline data of the study participants. A total
of 118 patients were enrolled. The patients with CAD were older
than those without CAD and had a higher likelihood of having a
history of diabetes mellitus and peripheral arteriosclerosis
(P<0.001). The patients with CAD also exhibited significantly
higher serum levels of OPG and sRANKL compared with the non-CAD
controls [OPG, median (interquartile range), CAD, 8.44 (5.10-14.87)
vs. non-CAD, 7.00 (4.49-9.63) pg/ml, P=0.005; sRANKL, median
(interquartile range), CAD, 31.65 (12.60-101.62) vs. non-CAD, 17.09
(8.66-39.01) pg/ml, P<0.001].
 | Table IDemographic characteristics and
biochemical data of the subjects. |
Table I
Demographic characteristics and
biochemical data of the subjects.
| Variables | Non-CAD (n=38) | Stable CAD
(n=80) |
P-valuea |
|---|
| Demographic
characteristics | | | |
|
Age, mean ±
SD, years | 55.9±13.5 | 69.4±12.4 | <0.001 |
|
Male, n
(%) | 29 (76.32) | 65 (81.25) | 0.534 |
|
Body mass
index, mean ± SD, kg/m2 | 26.22±2.87 | 26.65±3.37 | 0.495 |
| Current smoker, n
(%) | 18 (47.37) | 30 (37.50) | 0.308 |
| Current drinker, n
(%) | 9 (23.68) | 17 (21.25) | 0.766 |
| Complicating
disease history, n (%) | | | |
|
Hypertension | 30 (78.95) | 70 (87.50) | 0.227 |
|
Diabetes
mellitus | 0 (0.00) | 35 (43.75) | <0.001 |
|
Lacunar
infarcts | 16 (42.11) | 49 (61.25) | 0.051 |
|
Peripheral
arteriosclerosis | 12 (31.58) | 68 (85.00) | <0.001 |
|
Osteoporosis | 4 (10.53) | 21 (26.25) | 0.051 |
|
Chronic
kidney disease | 2 (5.26) | 10 (12.50) | 0.224 |
| Laboratory test
results | | | |
|
Fasting
plasma glucose, mean ± SD, mmol/l | 4.93±0.70 | 5.33±1.46 | 0.114 |
|
Alkaline
phosphatase, mean ± SD, U/l | 72.03±21.24 | 74.29±20.32 | 0.579 |
|
LDL-C, mean
± SD, mmol/l | 2.52±0.71 | 2.25±0.77 | 0.072 |
|
Total
calcium, mean ± SD, mmol/l | 2.24±0.11 | 2.26±0.12 | 0.304 |
|
Phosphorus,
mean ± SD, mmol/l | 1.15±0.17 | 1.16±0.17 | 0.901 |
|
OPG, median
(IQR), pg/ml | 7.00
(4.49-9.63) | 8.44
(5.10-14.87) | 0.005 |
|
sRANKL,
median (IQR), pg/ml | 17.09
(8.66-39.01) | 31.65
(12.60-101.62) | <0.001 |
Crude associations between serum OPG
levels and the presence of CAD in patient subgroups
Associations between different variables and CAD
were examined in the total study population by univariate analysis
using logistic regression analysis with CAD as the dependent
variable. Initially, a positive association was found between serum
OPG levels and the presence of CAD [odds ratio (OR), 1.11; 95%
confidence interval (CI), 1.03-1.19; P=0.009], as depicted in
Table SI. In addition,
significant positive associations were found for CAD with age,
peripheral arteriosclerosis, sRANKL and LDL-C (P<0.05). These
associations were further investigated through stratified analyses,
which consistently revealed a positive association between OPG and
CAD across various subgroups, as illustrated in Fig. 2. Unfortunately, the model was not
able to predict the association of CAD with diabetes mellitus, as
all the diabetic patients included in the study had been diagnosed
with CAD.
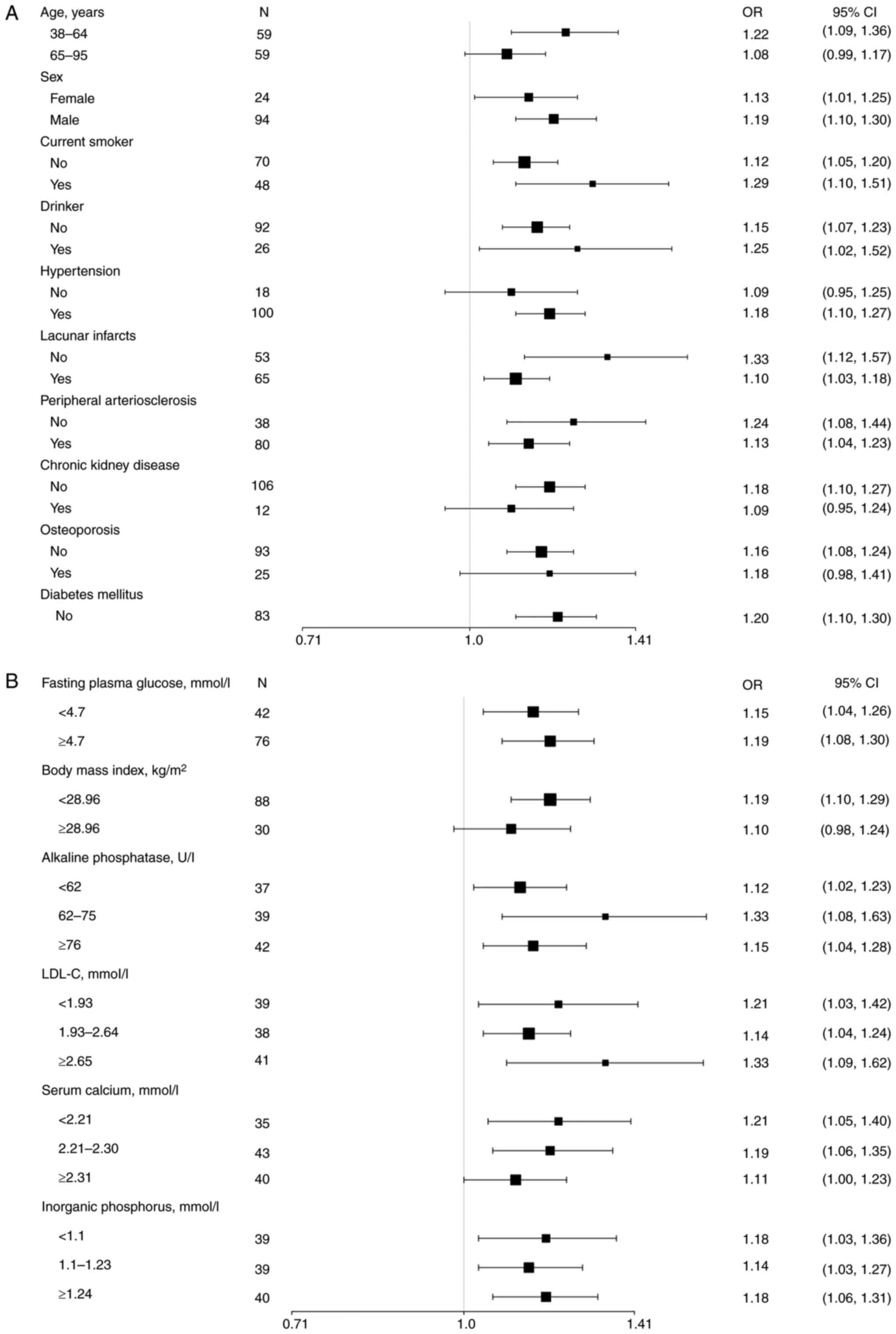 | Figure 2Univariate analysis models were used
to examine the relationship between OPG and the presence of CAD in
different subgroups using stratified analyses. (A) Stratification
was conducted based on the basic characteristics of the patients,
including age, sex, current smoking and drinking habits, as well as
comorbidities including hypertension, lacunar infarcts, peripheral
arteriosclerosis, chronic kidney disease and osteoporosis. The
model of association between OPG and CAD in individuals with
diabetes mellitus was unsuccessful, as all patients in this
subgroup had already been diagnosed with CAD. (B) Stratification
was performed according to body mass index or various laboratory
results associated with CAD or its comorbidities. Data are
expressed as OR (95% CI). OR, odds ratio; CI, confidence interval;
OPG, osteoprotegerin; CAD, coronary artery disease; LDL-C,
low-density lipoprotein-cholesterol. |
Correlation between serum OPG level
and the presence of CAD
The adjusted smoothed plots in Fig. 3 suggest a nonlinear relationship
between serum OPG and the presence of CAD after adjustment for
confounding variables. Specifically, the presence of CAD increased
as serum OPG levels increased up to the turning point at 18 pg/ml,
as shown by the curve exhibiting one breakpoint and a two-stage
change. For serum OPG levels >18 pg/ml, the estimated
dose-response curve was consistent with a horizontal line. We
further divided OPG <18 pg/ml into two equal groups (<9 and
9-18 pg/ml) in order to obtain the OR value by generalized linear
regression. Furthermore, in analyses adjusted for age and sex,
individuals with middle levels of OPG (9-18 pg/ml) had an OR for
CAD of 2.46 (95% CI, 0.82-7.39; P=0.110) compared with those with
low levels of OPG (<9 pg/ml), as shown in Table II. However, following progressive
adjustment for various cardiovascular risk factors, namely smoking,
drinking, peripheral arteriosclerosis, osteoporosis, FPG, LDL-C,
and hypertension, the patients with middle levels of OPG had an OR
for CAD of 6.66 compared with those with low levels of OPG (95% CI,
1.35-32.79; P=0.020, P-value for trend=0.013; Table II). No significant association was
found between serum OPG levels and increasing age or other risk
factors.
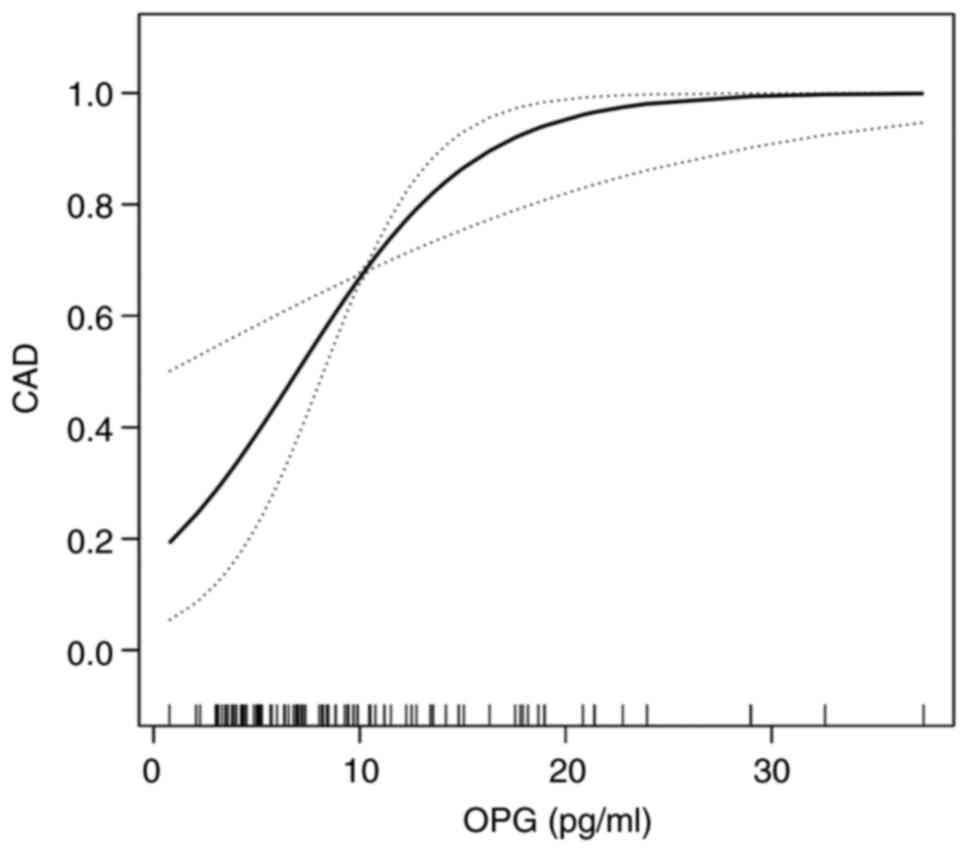 | Figure 3Smoothed plots for the nonlinear
relationship between serum OPG and the presence of CAD after
adjustment for certain variables. The presence of CAD increased
with serum OPG level up to the turning point (OPG=18 pg/ml), as
shown by the curve exhibiting one breakpoint and a two-stage
change. With serum OPG >18 pg/ml, the estimated dose-response
curve was consistent with a horizontal line. Adjustments were made
for age (smooth), sex, smoking, drinking, peripheral
arteriosclerosis, osteoporosis, fasting plasma glucose, low-density
lipoprotein cholesterol and hypertension. OPG, osteoprotegerin;
CAD, coronary artery disease. |
 | Table IIThreshold effect analysis of the
association between OPG and CAD using piecewise linear
regression. |
Table II
Threshold effect analysis of the
association between OPG and CAD using piecewise linear
regression.
| | Crude model | Model 1 | Model 2 |
|---|
| Variable | OR (95% CI) | P-value | OR (95% CI), | P-value | OR (95% CI), | P-value |
|---|
| OPG ≤18 pg/ml | 1.05 (0.94,
1.17) | 0.367 | 1.11 (0.98,
1.26) | 0.102 | 1.32 (1.07,
1.63) | 0.011 |
| OPG categories,
pg/ml | | | | | | |
|
<9
(n=69) | Reference | | Reference | | Reference | |
|
9-18
(n=31) | 1.35 (0.55,
3.30) | 0.511 | 2.46 (0.82,
7.39) | 0.11 | 6.66 (1.35,
32.79) | 0.02 |
| P-value for
trend | | 0.511 | | 0.11 | | 0.013 |
Association between serum OPG level
and the presence of CAD in individuals with low and high serum
sRANKL levels
To investigate the association between serum OPG and
the presence of CAD, sRANKL levels were divided into low and high
concentrations. Specifically, the participants were split into two
groups, each comprising 59 individuals. Those with sRANKL levels
between 8.66 and 24.69 pg/ml were assigned to the low concentration
group, while the remaining with sRANKL concentrations ranging from
25.2 to 101.62 pg/ml were classified as the high concentration
group. Notably, the adjusted smoothed plots in Fig. 4 indicate that the percentage of
individuals with higher sRANKL levels who had CAD was higher
compared with that of individuals with lower sRANKL levels,
following adjustment for various cardiovascular risk factors.
Furthermore, after limiting OPG to ≤18 pg/ml, the results showed
that for every one-unit increase in serum OPG, there was a 52%
increase in the presence of CAD in the low sRANKL group (OR, 1.52;
95% CI, 1.06-2.17; P=0.022) and a 61% increase in the presence of
CAD in the high sRANKL group (OR, 1.61; 95% CI, 1.04-2.50; P=0.032)
(Table III).
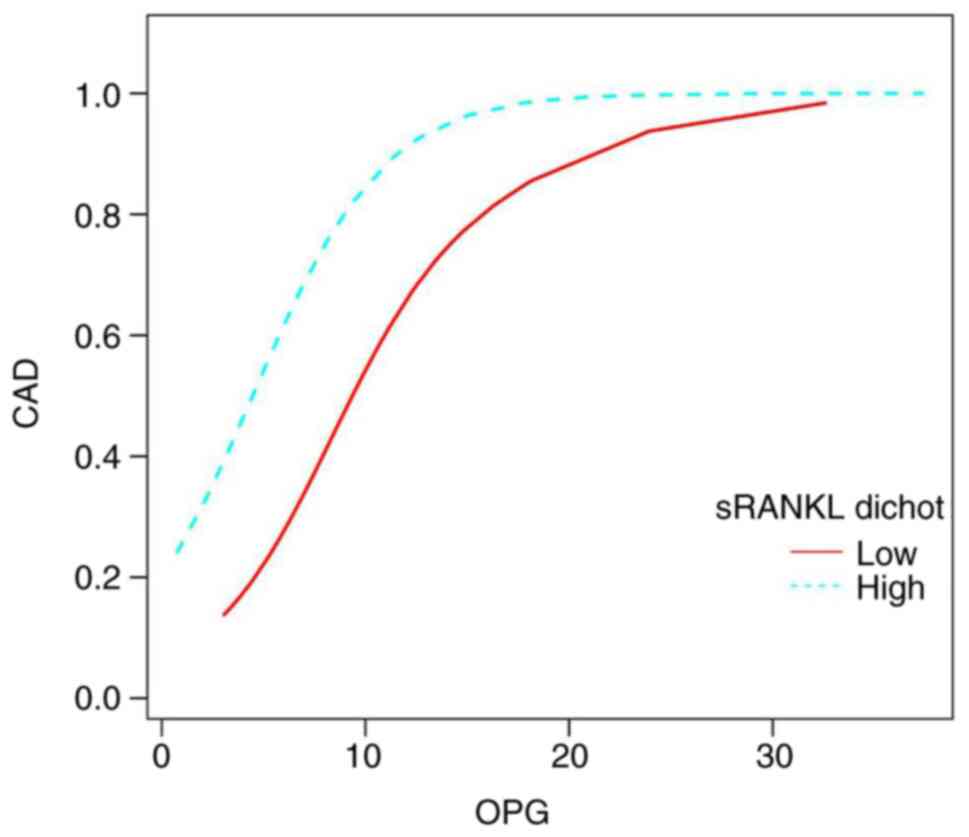 | Figure 4Smoothed plots for the correlation
between serum OPG and the presence of CAD in patients with low and
high sRANKL levels. The low sRANKL group had levels of 8.66-24.69
pg/ml (n=59), while the high sRANKL group had levels of 25.2-101.62
pg/ml (n=59). Serum OPG levels <18 pg/ml were positively
associated with stable CAD, regardless of sRANKL levels.
Additionally, higher sRANKL levels were positively associated with
a greater presence of stable CAD compared with lower sRANKL levels,
at the same serum OPG levels, when adjustment for age (smooth),
sex, smoking, drinking, peripheral arteriosclerosis, osteoporosis,
fasting plasma glucose, low-density lipoprotein cholesterol and
hypertension. CAD, coronary artery disease; OPG, osteoprotegerin.
sRANKL, soluble receptor activator of nuclear factor-kB ligand;
dichot, dichotomous. |
 | Table IIIAssociation between OPG and the
presence of coronary artery disease at low and high sRANKL
concentrations analyzed using multivariate regression. |
Table III
Association between OPG and the
presence of coronary artery disease at low and high sRANKL
concentrations analyzed using multivariate regression.
| | Model 1 | Model 2 |
|---|
| sRANKL
concentration, pg/ml | OR (95% CI) | P-value | OR (95% CI) | P-value |
|---|
| 8.66-24.69 | 1.46 (1.03,
2.08) | 0.036 | 1.52 (1.06,
2.17) | 0.022 |
| 25.2-101.62 | 1.15 (0.92,
1.43) | 0.213 | 1.61 (1.04,
2.50) | 0.032 |
No correlation was found between OPG and sRANKL
using Spearman's correlation analysis (Fig. S1). Spearman's correlation analysis
was then applied to further clarify whether serum OPG or sRANKL
levels correlated with the CAD risk factors collected in the
present study (Figs. S2 and
S3). The analysis showed that age
was positively correlated with sRANKL (P<0.0001; Fig. S3A), but not with OPG (Fig. S2A). No significant correlation of
OPG or sRANKL with other continuous variables including FBG, LDL-C,
BMI, ALP, serum calcium and P was detected (Figs. S2 and S3). Pearson's χ2 test showed
that serum OPG was associated with peripheral arteriosclerosis
(χ2=0.082, P=0.018) and diabetes mellitus
(χ2=6.447, P=0.040), while sRANKL was associated with
lacunar infarction (χ2=5.789, P=0.016), peripheral
arteriosclerosis (χ2=7.608, P=0.006) and osteoporosis
(χ2=4.111, P=0.043).
Discussion
Using the recursive method, the present study
revealed a nonlinear relationship between serum OPG levels and the
presence of CAD. A positive association between the presence of CAD
and serum OPG was observed when the serum OPG level was ≤18 pg/ml.
However, when the serum OPG concentration was >18 pg/ml, the
incidence of CAD no longer increased with increasing serum OPG
concentration. Additionally, patients with higher sRANKL levels
tended to have a higher risk of CAD compared with those with lower
sRANKL levels, at the same serum OPG levels.
The present study used the recursive method for
curve fitting and demonstrated that OPG and CAD had a curvilinear
relationship, in which the turning point of the curve occurred at a
serum OPG concentration of 18 pg/ml. The turning point was
identified using the threshold selection method derived from curve
fitting (32-36).
Recursive methods for smooth curve fitting were first applied,
followed by segmental modeling to identify any turning points. It
was observed that when the serum OPG concentration was >18
pg/ml, the curve became horizontal. Multivariate linear regression
analysis yielded a result of 8,028 (0, Inf) with P=0.999,
suggesting that at OPG concentrations >18 pg/ml, OPG levels are
infinitely close to those associated with CAD. Conversely, when the
serum concentration of OPG was ≤18 pg/ml, curve fitting revealed a
linear correlation between OPG and CAD. Multivariate linear
regression with adjustment for age, sex and various cardiovascular
risk factors revealed an OR of 1.32 (95% CI 1.07-1.63; P=0.011),
further supporting the hypothesis that elevated OPG is an
independent predictive factor for CAD.
The curved relationship displayed between serum OPG
levels and the incidence of CAD in the present study suggests that
the association between these two factors exhibits a saturation
effect. This finding is supported by a study of mice conducted by
Morony et al (37), which
observed similar results. In that study, LDL receptor-knockout mice
were fed an atherogenic diet and subsequently treated with
recombinant OPG or vehicle for 5 months, and plasma OPG levels were
measured from the initiation of the atherogenic diet. The study
found a significant increase in plasma OPG levels in the first
month after the start of the diet, but no further increase in OPG
levels despite the progression of atherosclerosis in the
vehicle-treated mice. The results of the present study combined
with the findings in mice indicate that OPG may act as a biomarker
of CAD within a certain range.
It has been suggested that an increase in serum OPG
might occur in response to vascular calcification or
atherosclerosis rather than being a cause of these conditions, and
may be intended to regulate the disease process (14). We hypothesize that the elevation of
OPG could be a protective response to endothelial dysfunction in
patients with CAD, as endothelial dysfunction is a common
complication in these patients (38). Therefore, the elevation of OPG may
be indicative of the cumulative burden of risk factors in patients
with CAD. The present study found consistent ORs for the
associations between OPG levels and CAD in various subgroups,
including those for age, sex, smoking, drinking, hypertension,
lacunar infarcts, peripheral arteriosclerosis, chronic kidney
disease, osteoporosis, FPG, BMI, ALP, LDL-C levels, serum calcium
and P levels. The study indicated that elevated OPG levels increase
the burden of CAD independently of the aforementioned traditional
risk factors.
OPG acts in combination with one of its ligands,
RANKL. However, previous studies of the relationship between RANKL
and CAD have not yielded conclusive results (39,40).
The present study provides clinical evidence demonstrating that the
incidence of CAD among patients with higher levels of sRANKL was
elevated compared with that among patients with lower sRANKL
levels, even when serum OPG levels were the same. This suggests
that OPG acts in conjunction with RANKL in stable CAD. However, no
correlation was found between serum OPG and sRANKL concentrations.
It may be speculated that the concentrations of OPG and/or sRANKL
are associated with the expression of RANK and the quantity of
RANKL expressed by cells such as T cells, or that they are
influenced by other inflammatory factors within the human body
(39,24). The present found that serum sRANK
levels were positively associated with age, peripheral
arteriosclerosis, lacunar infarction and osteoporosis, further
suggesting that sRANKL is an inflammation-related factor.
Therefore, we hypothesize that the role of OPG in the
neutralization of RANKL activity via inhibition of its binding to
RANK can be attributed to three effects. First, the binding of
RANKL to RANK promotes the pathological differentiation of healthy
vascular smooth muscle cells (VSMCs) into calcified cells with an
osteoblastic phenotype (24,40),
and OPG inhibits this calcification. Second, RANKL significantly
increases matrix metalloproteinase activity in VSMCs from patients
with unstable angina, and OPG counteracts the effect of RANKL by
inhibiting its binding to RANK (41,42).
Last, the binding of OPG with RANKL may inhibit the rapid clearance
of RANKL from the serum, thereby stabilizing its levels and
enhancing its actions (41). These
studies indicate that OPG and sRANKL antagonize each other. Since
OPG contributes to an incomplete compensatory mechanism in
atherosclerosis, the present research suggests that RANKL is
indispensable in the role of OPG in CAD.
The present study primarily highlights the
relationships between OPG, sRANKL and asymptomatic or
well-controlled symptomatic CAD. Therefore, the measurement of OPG
and sRANKL serum levels in asymptomatic individuals in addition to
the analysis of traditional cardiovascular risk factors may serve
as an initial screening method for patients with this type of CAD
in clinical practice. However, it is important to note that further
confirmation of this conclusion is required through large-scale
prospective cohort studies.
There were some limitations to the present study.
First, as this was a case-control study, the number of patients in
the two groups was not exactly matched. The patients were recruited
from inpatient wards, and most of them were undergoing regular
check-ups due to pre-existing diseases. Although there were also
some patients who underwent regular check-ups despite not having
CAD, the number of patients with CAD was higher than those without
CAD during the study period. This imbalance might have led to an
underrepresentation of the non-CAD population, resulting in a
situation where a particular group or demographic within a
population was not adequately represented, which could potentially
impact the findings of the study. Second, the number of patients in
the study with diabetes was limited. As a result, the conclusions
of the study cannot be applied to the diabetic population. However,
it is widely accepted that diabetes mellitus is ‘CAD-risk
equivalent’ (43). Therefore,
additional biomarkers may not be necessary for assessing the
presence of CAD in diabetic patients, although recent guidelines
recommend further CAD risk stratification in patients with type 2
diabetes mellitus (44). Third,
although the study did not assess the degree of coronary artery
sclerosis in the group of patients with CAD due to them having been
diagnosed prior to the study, a relationship between OPG levels and
the degree of coronary artery atherosclerosis has previously been
reported (16). Therefore, the
curve derived in the study is not suitable for establishing if OPG
and sRANKL are associated with the degree of coronary artery
sclerosis. Additionally, due to the small size of the study
population, it was not possible to include another group of
patients with CAD to validate the predictive value of OPG for CAD,
as recommended by the TRIPOD statement for prediction model studies
(45). Finally, it is important to
note that the subjects who were physically examined had reasonable
control over cardiovascular risk factors such as FPG and lipid
levels, indicating a high level of concern for their health.
However, the study did not account for potential confounding
factors such as medication use.
In conclusion, independently of age, sex, smoking,
drinking, lacunar infarcts, peripheral arteriosclerosis,
osteoporosis, FBP, hypertension, LDL-C and BMI, a higher prevalence
of CAD is associated with serum OPG levels ≤18 pg/ml. Moreover, the
incidence of CAD among patients with higher sRANKL levels was
higher than that among patients with lower sRANKL levels at the
same concentration of OPG. However, further studies are necessary
to establish a predictive model for CAD based on OPG and sRANKL
levels.
Supplementary Material
Scatter plot for serum OPG and sRANKL
concentrations reveals no correlation between the two variables.
OPG, osteoprotegerin. sRANKL, soluble receptor activator of nuclear
factor-kB ligand.
Scatter plots depicting the
correlation between serum OPG concentration and factors associated
with coronary artery disease. OPG correlation with (A) age, (B)
FPG, (C) LDL-C, (D) BMI, (E) alkaline phosphatase, (F) total
calcium and (G) inorganic phosphorus. OPG, osteoprotegerin; FPG
fasting plasma glucose; LDL-C low-density lipoprotein-cholesterol;
BMI, body mass index.
Scatter plots depicting the
correlation between sRANKL concentration and factors associated
with coronary artery disease. sRANKL correlation with (A) age, (B)
FPG, (C) LDL-C, (D) BMI, (E) alkaline phosphatase, (F) total
calcium and (G) inorganic phosphorus. sRANKL, soluble receptor
activator of nuclear factor-kB ligand; FPG fasting blood glucose;
LDL-C low-density lipoprotein-cholesterol; BMI, body mass
index.
Crude correlations between variables
and coronary artery disease.
Acknowledgements
The authors would like to thank Dr Xinglin Chen
(Technical director, Empower States, Inc.) for providing
instruction on the statistical analysis.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
XF and SG conceived and designed the experiments.
All authors performed the experiments. HW and SZ collected data,
and XF and MZ analyzed the data. XF, HW and SZ drafted the paper,
and BW revised the paper. All authors read and approved the final
version of the manuscript. HW, SZ and XF confirm the authenticity
of all the raw data.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of Tongji Medical School, Huazhong University of Science
and Technology (Wuhan, China; approval no. 2021/0569). Written
informed consent was obtained from all subjects.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Bergstrom G, Persson M, Adiels M, Björnson
E, Bonander C, Ahlström H, Alfredsson J, Angerås O, Berglund G,
Blomberg A, et al: Prevalence of subclinical coronary artery
atherosclerosis in the general population. Circulation.
144:916–929. 2021.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Morcos SK, Thomsen HS and Webb JA:
Contrast Media Safety Committee of the European Society of
Urogenital Radiology. Prevention of generalized reactions to
contrast media: A consensus report and guidelines. Eur Radiol.
11:1720–1728. 2001.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Davenport MS, Perazella MA, Yee J, Dillman
JR, Fine D, McDonald RJ, Rodby RA, Wang CL and Weinreb JC: Use of
intravenous iodinated contrast media in patients with kidney
disease: consensus statements from the american college of
radiology and the national kidney foundation. Kidney Med. 2:85–93.
2020.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Arnett DK, Blumenthal RS, Albert MA,
Buroker AB, Goldberger ZD, Hahn EJ, Himmelfarb CD, Khera A,
Lloyd-Jones D, McEvoy JW, et al: 2019 ACC/AHA guideline on the
primary prevention of cardiovascular disease: A Report of the
American College of Cardiology/American Heart association task
force on clinical practice guidelines. Circulation. 140:e596–e646.
2019.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Petretta M, Fiumara G, Petretta MP and
Cuocolo A: Detection of silent myocardial ischemia: Is it
clinically relevant? J Nucl Cardiol. 20:707–710. 2013.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Proposed standard nomenclature for new
tumor necrosis factor members involved in the regulation of bone
resorption. The American Society for Bone and Mineral Research
President's Committee on Nomenclature. Bone. 27:761–764.
2000.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Boyce BF and Xing L: Biology of RANK,
RANKL, and osteoprotegerin. Arthritis Res Ther. 9 (Suppl
1)(S1)2007.PubMed/NCBI View
Article : Google Scholar
|
|
8
|
Hofbauer LC and Heufelder AE: Role of
receptor activator of nuclear factor-kappaB ligand and
osteoprotegerin in bone cell biology. J Mol Med (Berl). 79:243–253.
2001.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Theoleyre S, Wittrant Y, Tat SK, Fortun Y,
Redini F and Heymann D: The molecular triad OPG/RANK/RANKL:
Involvement in the orchestration of pathophysiological bone
remodeling. Cytokine Growth Factor Rev. 15:457–475. 2004.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Ikebuchi Y, Aoki S, Honma M, Hayashi M,
Sugamori Y, Khan M, Kariya Y, Kato G, Tabata Y, Penninger JM, et
al: Coupling of bone resorption and formation by RANKL reverse
signalling. Nature. 561:195–200. 2018.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Simonet WS, Lacey DL, Dunstan CR, Kelley
M, Chang MS, Lüthy R, Nguyen HQ, Wooden S, Bennett L, Boone T, et
al: Osteoprotegerin: A novel secreted protein involved in the
regulation of bone density. Cell. 89:309–19. 1997.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Bucay N, Sarosi I, Dunstan CR, Morony S,
Tarpley J, Capparelli C, Scully S, Tan HL, Xu W, Lacey DL, et al:
osteoprotegerin-deficient mice develop early onset osteoporosis and
arterial calcification. Genes Dev. 12:1260–1268. 1998.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Van Campenhout A and Golledge J:
Osteoprotegerin, vascular calcification and atherosclerosis.
Atherosclerosis. 204:321–329. 2009.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Ma T, Zhao J, Yan Y, Liu J, Zang J, Zhang
Y, Ruan K, Xu H and He W: Plasma osteoprotegerin predicts adverse
cardiovascular events in stable coronary artery disease: The PEACE
trial. Front Cardiovasc Med. 10(1178153)2023.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Nybo M and Rasmussen LM: The capability of
plasma osteoprotegerin as a predictor of cardiovascular disease: A
systematic literature review. Eur J Endocrinol. 159:603–608.
2008.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Jono S, Ikari Y, Shioi A, Mori K, Miki T,
Hara K and Nishizawa Y: Serum osteoprotegerin levels are associated
with the presence and severity of coronary artery disease.
Circulation. 106:1192–1194. 2002.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Hofbauer LC and Schoppet M: Clinical
implications of the osteoprotegerin/RANKL/RANK system for bone and
vascular diseases. JAMA. 292:490–495. 2004.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Collin-Osdoby P: Regulation of vascular
calcification by osteoclast regulatory factors RANKL and
osteoprotegerin. Circ Res. 95:1046–1057. 2004.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Kambayashi Y, Fujimura T, Furudate S, Lyu
C, Hidaka T, Kakizaki A, Sato Y, Tanita K and Aiba S: The
expression of matrix metalloproteinases in receptor activator of
nuclear factor Kappa-B Ligand (RANKL)-expressing Cancer of Apocrine
Origin. Anticancer Res. 38:113–120. 2018.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Qian Y and Huang HZ: The role of RANKL and
MMP-9 in the bone resorption caused by ameloblastoma. J Oral Pathol
Med. 39:592–598. 2010.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Ohshiba T, Miyaura C, Inada M and Ito A:
Role of RANKL-induced osteoclast formation and MMP-dependent matrix
degradation in bone destruction by breast cancer metastasis. Br J
Cancer. 88:1318–1326. 2003.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Heusch G, Libby P, Gersh B, Yellon D, Böhm
M, Lopaschuk G and Opie L: Cardiovascular remodelling in coronary
artery disease and heart failure. Lancet. 383:1933–1943.
2014.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Lieb W, Gona P, Larson MG, Massaro JM,
Lipinska I, Keaney JF Jr, Rong J, Corey D, Hoffmann U, Fox CS, et
al: Biomarkers of the osteoprotegerin pathway: Clinical correlates,
subclinical disease, incident cardiovascular disease, and
mortality. Arterioscler Thromb Vasc Biol. 30:1849–1854.
2010.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Raaz-Schrauder D, Schrauder MG, Stumpf C,
Lewczuk P, Kilian T, Dietel B, Garlichs CD, Schlundt C, Achenbach S
and Klinghammer L: Plasma levels of sRANKL and OPG are associated
with atherogenic cytokines in patients with intermediate
cardiovascular risk. Heart Vessels. 32:1304–1313. 2017.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Kiechl S, Schett G, Schwaiger J, Seppi K,
Eder P, Egger G, Santer P, Mayr A, Xu Q and Willeit J: Soluble
receptor activator of nuclear factor-kappa B ligand and risk for
cardiovascular disease. Circulation. 116:385–391. 2007.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Zhao F, Zhang R, Zhao H, Liu T, Ren M,
Song Y, Liu S and Cong H: Relationship between serum levels of
osteoproteins, inflammatory cytokines and coronary heart disease
and disease severity. Zhonghua Wei Zhong Bing Ji Jiu Yi Xue.
31:588–593. 2019.PubMed/NCBI View Article : Google Scholar : (In Chinese).
|
|
27
|
Quercioli A, Montecucco F, Bertolotto M,
Ottonello L, Pende A, Mach F and Dallegri F: Coronary artery
calcification and cardiovascular risk: The role of RANKL/OPG
signalling. Eur J Clin Invest. 40:645–654. 2010.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Mohammadpour AH, Shamsara J, Nazemi S,
Ghadirzadeh S, Shahsavand S and Ramezani M: Evaluation of RANKL/OPG
serum concentration ratio as a new biomarker for coronary artery
calcification: A pilot study. Thrombosis.
2012(306263)2012.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Yu X, Cao L and Yu X: Elevated cord serum
manganese level is associated with a neonatal high ponderal index.
Environ Res. 121:79–83. 2013.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Fihn SD, Gardin JM, Abrams J, Berra K,
Blankenship JC, Dallas AP, Douglas PS, Foody JM, Gerber TC,
Hinderliter AL, et al: 2012 ACCF/AHA/ACP/AATS/PCNA/SCAI/STS
guideline for the diagnosis and management of patients with stable
ischemic heart disease: Executive summary: A report of the American
College of Cardiology Foundation/American Heart Association task
force on practice guidelines, and the American College of
Physicians, American Association for Thoracic Surgery, Preventive
Cardiovascular Nurses Association, Society for Cardiovascular
Angiography and Interventions, and Society of Thoracic Surgeons.
Circulation. 126:3097–3137. 2012.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Xiaoying Li. Geriatric Medicine (a
standardized training textbook for specialists). Beijing: People's
Medical Publishing House, 2015.
|
|
32
|
Lin L, Chen CZ and Yu XD: Analysis of
threshold effects using empower stats software. Zhonghua Liu Xing
Bing Xue Za Zhi. 34:1139–1141. 2013.PubMed/NCBI(In Chinese).
|
|
33
|
Liu Y, Kong X, Wang W, Fan F, Zhang Y,
Zhao M, Wang Y, Wang Y, Wang Y, Qin X, et al: Association of
peripheral differential leukocyte counts with dyslipidemia risk in
Chinese patients with hypertension: Insight from the China stroke
primary prevention trial. J Lipid Res. 58:256–266. 2017.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Wu J, Geng J, Liu L, Teng W, Liu L and
Chen L: The relationship between estimated glomerular filtration
rate and diabetic retinopathy. J Ophthalmol.
2015(326209)2015.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Yu XD, Zhang J, Yan CH and Shen XM:
Prenatal exposure to manganese at environment relevant level and
neonatal neurobehavioral development. Environ Res. 133:232–238.
2014.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Hou X, Wang C, Wang S, Yang W, Ma Z, Wang
Y, Li C, Li M, Zhang X, Zhao X, et al: Fluctuation between fasting
and 2-H postload glucose state is associated with glomerular
hyperfiltration in newly diagnosed diabetes patients with HbA1c
<7%. PLoS One. 9(e111173)2014.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Morony S, Tintut Y, Zhang Z, Cattley RC,
Van G, Dwyer D, Stolina M, Kostenuik PJ and Demer LL:
Osteoprotegerin inhibits vascular calcification without affecting
atherosclerosis in ldlr(-/-) mice. Circulation. 117:411–420.
2008.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Oikonomou E, Siasos G, Tsigkou V, Bletsa
E, Panoilia ME, Oikonomou IN, Sinanidis I, Spinou M, Papastavrou A,
Kokosias G, et al: Coronary artery disease and endothelial
dysfunction: Novel diagnostic and therapeutic approaches. Curr Med
Chem. 27:1052–1080. 2020.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Sandberg WJ, Yndestad A, Oie E, Smith C,
Ueland T, Ovchinnikova O, Robertson AK, Müller F, Semb AG, Scholz
H, et al: Enhanced T-cell expression of RANK ligand in acute
coronary syndrome: Possible role in plaque destabilization.
Arterioscler Thromb Vasc Biol. 26:857–863. 2006.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Panizo S, Cardus A, Encinas M, Parisi E,
Valcheva P, López-Ongil S, Coll B, Fernandez E and Valdivielso JM:
RANKL increases vascular smooth muscle cell calcification through a
RANK-BMP4-dependent pathway. Circ Res. 104:1041–1048.
2009.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Venuraju SM, Yerramasu A, Corder R and
Lahiri A: Osteoprotegerin as a predictor of coronary artery disease
and cardiovascular mortality and morbidity. J Am Coll Cardiol.
55:2049–2061. 2010.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Kiechl S, Schett G, Wenning G, Redlich K,
Oberhollenzer M, Mayr A, Santer P, Smolen J, Poewe W and Willeit J:
Osteoprotegerin is a risk factor for progressive atherosclerosis
and cardiovascular disease. Circulation. 109:2175–2180.
2004.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Saely CH, Aczel S, Koch L, Schmid F, Marte
T, Huber K and Drexel H: Diabetes as a coronary artery disease risk
equivalent: Before a change of paradigm? Eur J Cardiovasc Prev
Rehabil. 17:94–99. 2010.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Jung CH and Mok JO: Recent updates on
vascular complications in patients with type 2 diabetes mellitus.
Endocrinol Metab (Seoul). 35:260–271. 2020.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Collins GS, Reitsma JB, Altman DG and
Moons KG: Transparent reporting of a multivariable prediction model
for individual prognosis or diagnosis (TRIPOD): The TRIPOD
statement. BMJ. 350(g7594)2015.PubMed/NCBI View Article : Google Scholar
|