Introduction
Cisplatin, also known as cis-diaminedichloroplatinum
(CDDP), is an effective drug used to treat numerous types of cancer
(1). However, cisplatin easily
accumulates and is biotransformed in the kidneys (2), which can lead to serious side effects
including acute kidney injury (AKI). Of patients treated with
cisplatin, ~30% develop AKI (3).
This serious side effect limits the clinical application of
cisplatin (4).
Renal tubular epithelial cells (RTECs) are the
primary cellular target in cisplatin-induced AKI (1,5).
Cisplatin leads to the injury and death of RTECs by activating
complex signaling pathways, including apoptosis, necrosis,
inflammation and increased oxidative stress (6). Further, cisplatin promotes the
activation and cytosol-to-nucleus translocation of nuclear factor
(NF)-κB, in addition to promoting the production of inflammatory
cytokines and chemokines. This pathway contributes to
cisplatin-induced nephrotoxicity by increasing the production of
TNF-α in renal tubular cells (7,8).
Furthermore, cisplatin alters mitochondrial metabolism and produces
reactive oxygen species (ROS). Cisplatin-induced AKI is associated
with increased ROS production in the renal proximal convoluted
tubular cells (9) and AKI
development has been shown to depend on cell death. Cisplatin
induces necrosis and the intrinsic, extrinsic and endoplasmic
reticulum (ER) stress-associated apoptosis pathways (10). Despite several decades of research,
no stable drug treatment option is available to reduce the
cisplatin-induced AKI, which poses a serious concern for patients
receiving treatment with it (5).
Yishen Jiangzhuo decoction (YSJZD) is an empirical
prescription developed by Professor Shiwei Ruan, a traditional
Chinese medicine (TCM) doctor in Fujian Province, China, after
extensive clinical practice. This therapeutic is primarily used to
treat chronic renal failure (11).
YSJZD is comprised of a variety of herbs; False Starwort root,
Milkvetch root, Large-headed Atractylodes rhizome, Poria,
Mistletoe, Mulberry fruit, Achyranthes, Danshen, Chinese Angelica,
Rhubarb, Serissa foetida, Plantain seeds and Tangerine peel. each
of which contributes to its therapeutic effect, thus ‘invigorating
the kidney and spleen, reducing turbidity and removing blood
stasis’ in TCM terms (11).
The present study investigated the effect of YSJZD
on a cisplatin-induced AKI mouse model and examined the effects of
the optimal YSJZD concentration on renal function, pathology and
tubular epithelial cell ultrastructure in cisplatin-induced AKI
mice. The possible targets of YSJZD in cisplatin-induced AKI in
mice were identified by transcriptome sequencing and differential
expression analysis. The protective effects of YSJZD against
cisplatin-induced AKI and its primary mechanism of action were
revealed in vivo, thus providing theoretical and
experimental support for the therapeutic treatment of
cisplatin-induced AKI with YSJZD.
Materials and methods
Drug preparation
In the first round of preparation, the thirteen
herbs present in YSJZD (Table I)
were soaked in purified water for 1 h. The nascent decoction was
initially boiled at high heat, then simmered and decocted for 30
min. The decoction was then removed from heat. In the second step,
the herbs were soaked for 10 min, after which they were processed
following the same steps as in the first round. The second
decoction was mixed with the first. The combined solution was
filtered thrice through four layers of sterile surgical gauze.
After filtering, the solution was decocted over low heat and
alternately concentrated to volumes of 300, 150 and 75 ml,
corresponding to herb solutions of 0.7, 1.4 and 2.8 g/ml raw
medicine. The equivalent dose ratio of mice to 70 kg adults was
9.1. The dose calculation (209 g ÷ 70 kg x 9.1 = 27.17 g/kg)
indicated a working dose of 28 g/kg, following the pharmacological
experimental methodology described by Xu et al (12). Cisplatin injection was obtained
from Jiangsu Hausen Pharmaceutical Group Co Ltd. (cat. no.
H20040813).
 | Table IComponents of Yishen Jiangzhuo
decoction. |
Table I
Components of Yishen Jiangzhuo
decoction.
| Latin
binominal | English name | Part used | Origin of product
(province) | Type of
product | Weight (g) |
|---|
| Viscum
sp. | Mistletoe | Leaf with stem
branch | Heilongjiang | Raw (dry) | 15 |
| Mori
albae | Mulberry fruit | Fruit | Anhui | Raw (dry) | 15 |
| Achyranthes
bidentata | Achyranthes
root | Root | Henan | Raw (dry) | 15 |
| Pseudostellaria
heterophylla | False Starwort
root | Root | Fujian | Raw (dry) | 15 |
| Atractylodes
macrocephala | Large-headed
Atractylodes rhizome | Stem | Zhejiang | Raw (dry) | 15 |
| Poria
cocos | Poria | Sclerotium | Hubei | Raw (dry) | 15 |
|
Astragalus | Milkvetch Root | Root | Gansu | Raw (dry) | 30 |
| Angelicae
sinensis | Chinese
angelica | Root | Gansu | Raw (dry) | 10 |
| Citri
reticulatae | Tangerine peel | Peel | Guangdong | Raw (dry) | 10 |
|
Plantaginis | Plantain Seed | Seed | Sichuan | Raw (dry) | 15 |
| Salviae
miltiorrhizae | Danshen Root | Root | Shandong | Raw (dry) | 30 |
| Rheum | Rhubarb | Root and
rhizome | Sichuan | Raw (dry) | 9 |
| Serissa
japonica (Thunb.) | Serissa
Foetida | Whole herb | Hubei | Raw (dry) | 15 |
Construction of the cisplatin-induced
AKI murine model
A total of 181 specific pathogen-free male ICR mice
aged 7-8 weeks were purchased from the Laboratory Animal Center of
Fujian Medical University [Certificate No. SCXX (Min) 2016-0002].
Mice were housed with free access to food and water under
controlled environmental conditions (temperature 22±2˚C; humidity
50-60%; 12-h light/dark cycle). The health and behavior of mice
were observed twice a day. The mice were adaptively fed for 7 days
and weighed 29-33 g at the time of the experiment. The experimental
protocols (approval no. FJ-TCM IACUC2020021) were approved by the
Fujian University of Traditional Chinese Medicine Laboratory Animal
Welfare and Ethics Committee. Fig.
S1 gives a flow chart of the experiments detailed below.
Mortality rates of mice induced by
different doses of cisplatin
A total of 40 mice were divided into cisplatin 20,
18.75, 18, 15, 0 mg/kg groups (n=8) and the cisplatin induced AKI
model was established by intraperitoneal injection of corresponding
cisplatin solution, the 10-day mortality of mice in 5 groups was
observed. 20 and 18.75 mg/kg cisplatin, which had the higher
mortality rates, were selected as the modeling doses of CDDP for
follow-up experiments. See ‘Effects of YSJZD on the survival rate
of cisplatin-induced AKI mice’.
Effects of YSJZD on the survival rate
of cisplatin (20 mg/kg)-induced AKI mice
A total of 40 mice were divided into 4 groups
(n=10), as follows: i) cisplatin, ii) cisplatin + YSJZD (7 g/kg),
iii) cisplatin + YSJZD (14 g/kg), iv) cisplatin + YSJZD (28 g/kg).
A single intraperitoneal injection of 20 mg/kg of cisplatin was
used to establish the model of cisplatin-induced AKI in mice.
Intragastric administration corresponding concentrations of YSJZD
began 30 min before modeling and continued once daily at the same
time after modeling. The cisplatin group was administered the
corresponding dose of purified water. The 10-day mortality of mice
in 4 groups was observed. The survival rate of mice in the
cisplatin 20 mg/kg + YSJZD 14 g/kg group was the highest; however,
no statistically significant difference was noted. The
intraperitoneal injection of 20 mg/kg cisplatin solution in mice
resulted in a high mortality rate; therefore, the dose of cisplatin
was subsequently reduced to 18.75 mg/kg. See ‘Effects of YSJZD on
the survival rate of cisplatin-induced AKI mice’.
Effects of YSJZD on the survival rate
of cisplatin (18.75 mg/kg)-induced AKI mice
A total of 45 mice were divided into 3 groups
(n=15), as follows: i) cisplatin, ii) cisplatin + YSJZD (7 g/kg),
iii) cisplatin + YSJZD (14 g/kg). A single intraperitoneal
injection of 18.75 mg/kg of cisplatin was used to establish the
model of cisplatin-induced AKI in mice. Intragastric administration
corresponding concentrations of YSJZD began 30 min before modeling
and continued once daily at the same time after modeling. The
cisplatin group was administered the corresponding dose of purified
water. The 15-day mortality of mice in three groups was
observed.
Short-term effects of YSJZD on
hepatotoxicity in mice
A total of 16 mice were divided into Normal control
and YSJZD groups (n=8) in a toxicological experimental. YSJZD group
was Intragastric administration of YSJZD 14 g/kg for 4 days, The
Normal control group was administered the corresponding dose of
purified water, then blood taken for alanine aminotransferase (ALT)
detection.
Effects of YSJZD therapy in cisplatin
(18.75 mg/kg)-induced AKI mice
A total of 40 mice were divided into five groups, as
follows: i) Normal control (NC; n=8), ii) cisplatin 2-days (n=8),
iii) cisplatin 4-days (n=8), iv) cisplatin + YSJZD 2-days (n=8) and
v) cisplatin + YSJZD 4-days (n=8). A single intraperitoneal
injection of 18.75 mg/kg of cisplatin was used to establish the
model of cisplatin-induced AKI in male ICR mice. Mice in the NC
group (n=8) were injected with corresponding doses of saline. In
the treated mice, YSJZD (14 g/kg) was administered intragastrically
30 min before modeling and continued daily after modeling. The
model and NC groups were administered corresponding doses of
purified water. After 2 and 4 days, the mice were anesthetized by
intraperitoneal injection of pentobarbital sodium (50 mg/kg) and
blood was then taken from the heart (~200 µl per mouse). Then the
mice were sacrificed by intraperitoneal injection of pentobarbital
sodium (150 mg/kg). After the mice had cardiac and respiratory
arrest and showed no nerve reflex, the kidneys were taken for
further study. The mice demonstrated the following humane
endpoints: The mice continued to lie down and there was a loss of
righting reflex. In addition, a toxicological experimental group
was set up to give YSJZD 14 g/kg for 4 days and then blood taken
from the heart for alanine aminotransferase (ALT) detection
following anesthesia and then sacrifice as aforementioned. Blood
samples were collected to assess renal function and ALT. A section
of kidney tissue was harvested for paraffin embedding and electron
microscopy. The other section of the kidney tissues were stored at
-80˚C for transcriptome sequencing, differential expression and
molecular analyses.
Renal function detection
Serum creatinine (SCr), blood urea nitrogen (BUN)
and ALT levels were measured using an automated biochemical
analyzer (Abbott Cil6200; Abbott Laboratories).
Renal pathological observation
The fixed kidney tissue was dehydrated by gradient
alcohol, cleared with xylene and embedded with paraffin. Paraffin
sections (4 µm) were stained with hematoxylin and eosin (H&E)
and periodic acid-Schiff (PAS) stains. H&E was staining with
hematoxylin for 5 min and eosin for 2 min at room temperature. PAS
was staining with periodic acid for 10 min and Schiff's solution
for 10 min at room temperature. Renal damage was graded using
kidney slices stained with H&E (n=8). Paller scores (13) were used to determine the degree of
renal tubule damage in H&E-stained kidney sections (n=8) and
the morphology was observed under a light microscope. A total of 10
non-overlapping renal tissue fields (magnification, x200) and 10
renal tubules were randomly selected from each field. In total, 100
renal tubules from each mouse were evaluated. The severity of renal
tubular damage was assessed by assigning points based on specific
criteria: Renal tubular dilatation and flattened tubular epithelial
cells and renal tubular epithelial brush border damage were
assigned one point each and shedding was assigned two points. Cast
formation in renal tubules was assigned two points and exfoliative
and necrotic cells in the lumen of renal tubules (without cast
formation or cell fragments) were scored at one point each. The
maximum possible score was five points. Higher scores indicated
more severe damage to the renal tubules.
Transmission electron microscopy
A total of three cortical kidney tissue samples were
randomly selected from each group. Kidney cortex tissues were fixed
in 2.5% glutaraldehyde (cat. no. G1102; Wuhan Servicebio Technology
Co., Ltd.) for 4 h at 4˚C and 1% osmic acid (cat. no. 18466, Ted
Pella Inc.) for 2 h at room temperature to examine the
ultrastructural alterations in the proximal tubular epithelial
cells. Subsequently, samples were dehydrated using a gradient of
alcohol and acetone and embedded in 812 epoxy resin. Following
ultrathin sectioning, the tissues were stained with lead citrate
(cat. no. 19312; Ted Pella Inc.) and uranyl acetate (cat. no.
19481; Ted Pella Inc.). Transmission electron microscopy was
applied to identify stained slices (HT-7700; Hitachi, Ltd.). Image
information acquisition using Transmission electron Microscopy
imaging system (Hitachi TEM system; Hitachi, Ltd.).
RNA extraction and RNA quantitative
and quality detection
A total of three cortical kidney tissue samples were
randomly selected from each group. Total ribonucleic acid (RNA) was
isolated from 50 mg of kidney tissue using 1 ml of Trizol reagent
(cat. no. B610409-0100; Sangon Biotech Co., Ltd., Shanghai, China),
following the manufacturer's instructions. An Agilent 5300 Fragment
Analyzer (Agilent Technologies Inc.) was used to perform
quantitative and quality RNA detection, comprising elucidation of
concentration, RNA integrity number (RIN) and 28 S to 18 S ratio
(28S/18S). A RIN value close to 10 indicates high sample integrity.
The 28 S/18 S is another indicator to evaluate sample integrity,
for which a eukaryotic ratio ≥1.5 indicates good RNA integrity.
Only high-quality whole RNA samples were used to generate
complementary deoxyribonucleic acid (cDNA) libraries.
RNA-seq and cDNA library creation
BGI Shenzhen Co., Ltd. prepared a cDNA library and
performed RNA-Seq on a DNBSEQ platform (BGI Shenzhen Co., Ltd. ) in
accordance with the manufacturer's instructions.
Bioinformatics analysis
Bioinformatics analysis tools, including SOAPnuke
(v1.5.6, https://github.com/BGI-flexlab/SOAPnuke), FastQC
(v0.11.7, http://www.bioinformatics.babraham.ac.uk/projects/fastqc/),
HISAT2 (v2.1.0, http://www.ccb.jhu.edu/software/hisat), Bowtie2
(v2.3.4.3, http://bowtie-bio.sourceforge.net/index.shtml), RSEM
(v1.3.1, http://deweylab.biostat.wisc.edu/rsem), DESeq2
(v1.4.5, http://www.bioconductor.org/packages/release/bioc/html/DESeq2.html)
and Phyper function in R package v 2.26.0 (http://github.com/jdstorey/qvalue), were employed in
the present study. Low-quality raw reads were removed using the BGI
SOAPnuke filtering program. The quality of the clean reads was
assessed using the FastQC software and the sequencing quality
values Q20 and Q30 were calculated to determine whether the
sequencing data were sufficient for subsequent analysis. The HISAT2
software was used to match clean reads to the mouse reference
genome and to check whether the mapping outcomes satisfied the
calibrated quality control. Clean reads were aligned to reference
gene sequences using Bowtie2 software and the gene expression
levels of each sample were calculated using RSEM software. The
fragments per kilobase of transcripts per million mapped fragments
(FPKM) was calculated to evaluate the transcript expression levels
for each sample. Typically, a transcript was considered to be
expressed if its FPKM value was >0.1. DESeq2 software was used
to identify differentially expressed genes (DEGs) between groups,
with the threshold set at a fold-change threshold ≥2 and a Q-value
<0.05. Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway
enrichment and Gene Ontology (GO) enrichment analyses of DEGs were
performed using the phyper function in R code.
Immunofluorescence
For immunofluorescence analysis, paraffin sections
were incubated with the following antibodies at 4˚C overnight:
Anti-Phospho-p65 (1:200; Cell Signaling Technology (CST); cat. no.
3033), then goat anti-rabbit IgG Alexa Fluor 594 (1:200;
Proteintech Group, Inc.; cat. no. SA00006-4) for 1 h. Following
DAPI counterstaining for 10 min at room temperature, the sections
were examined under a fluorescence microscope (EVOS M5000 Cell
Imaging System; Invitrogen; Thermo Fisher Scientific, Inc.).
TdT-mediated dUTP nick end labeling
(TUNEL) assay
Apoptosis in kidney sections was determined using an
in situ cell death detection kit (Roche Diagnostics GmbH),
in accordance with the manufacturer's recommendations. Briefly, the
sections were deparaffinized in xylene, rehydrated with decreasing
grades of ethanol and permeabilized with proteinase K at a
concentration of 20 µg/ml in 10 mM Tris-HCl (pH 7.4-8) for 30 min
at room temperature. The slices were then washed and incubated in
the TUNEL reaction mixture at 37˚C for 1 h. DAPI was used as a
nuclear counterstain for 10 min at room temperature. Images were
captured using an EVOS M5000 Cell Imaging System (Invitrogen;
Thermo Fisher Scientific, Inc.) A total of six randomly selected
fields (magnification, x200) from each kidney were counted to
determine the number of TUNEL-stained apoptotic renal tubular
epithelial cells. ImageJ software (version 1.49; National
Institutes of Health) was used to analyze the images.
Determination of glutathione (GSH),
superoxide dismutase (SOD) and malondialdehyde (MDA) levels
Kidney tissue was homogenized and lysed, after which
samples were centrifuged at 1,120 x g for 10 min at 4˚C. The levels
of GSH, SOD and MDA in the supernatant were measured using
commercial GSH, SOD (Nanjing Jiancheng Bioengineering Institute)
and MDA (Beyotime Institute of Biotechnology) assay kits,
respectively, according to the manufacturer's instructions.
Western blotting (WB) assay. Renal tissue was added
to ice-cold RIPA lysis buffer (Beyotime Institute of Biotechnology)
and homogenized using a tissue homogenizer (IKA Werke GmbH &
Co. KG) at speed in second gear under cold conditions. An
ultrasonic cell processor (Sonics & Materials, Inc.) was used
to apply ultrasound for 5 sec x 3 times at 30% power on ice (20
KHz, with intervals of 10 sec). Protein concentrations were
determined using a bicinchoninic acid (BCA) protein assay kit
(Pierce; Thermo Fisher Scientific, Inc.). Subsequently, total
protein samples were adjusted to the same concentration and a
loading buffer was added to the samples before boiling for 10 min
at 100˚C to denature them. Protein samples (30 µg per lane) were
then separated on a 10 or 15% sodium dodecyl sulfate-polyacrylamide
gel electrophoresis (SDS-PAGE) separator and subsequently
transferred to a 0.22 µm polyvinylidene fluoride (PVDF) membrane
(MilliporeSigma). The PVDF membranes were blocked for 1 h at room
temperature with 5% nonfat milk in PBST (PBS + 0.05% Tween-20).
Membranes were then treated with the following antibodies overnight
at 4˚C: Anti-TNFα antibody (1:1,000; Abcam; cat. no. ab215188),
anti-Phospho-p65 antibody p65 (1:1,000; Ser536; CST; cat. no.
3033), anti-p65 (1:1,000; CST; cat. no. 8242), anti-p38 antibody
(phospho T180+Y182) (1:1,000; Abcam; cat. no. ab195049), anti-p38
mitogen-activated protein kinase (MAPK; 1:1,000; CST; cat. no.
8690), anti-caspase 3/P17/P19 (1:500; Proteintech Group, Inc.; cat.
no. 19677-1-AP), anti-Sirt3 (1:1,000; Abcam; cat. no. ab246522) and
anti-β-actin (1:1,000; Santa Cruz Biotechnology, Inc.; cat. no.
sc-47778). Membranes were then exposed to appropriate secondary
horseradish peroxidase-conjugated antibodies for 1 h at room
temperature and observed using an ultrasensitive
electrochemiluminescence kit (Beyotime Institute of Biotechnology).
An iBright1500 imaging system (Invitrogen; Thermo Fisher
Scientific, Inc.) was used to detect signals and ImageJ software
(version 1.51j8; National Institutes of Health) was used to
quantify the band intensities.
Statistical analyses
Data analyses were performed using GraphPad Prism
8.0 (Dotmatics) or SPSS software (version 22.0; IBM Corp.).
Normally distributed data are expressed as the mean ± standard
deviation and were analyzed using a one-way analysis of variance
(ANOVA) and Tukey's post hoc test. For non-normally distributed
data, the Kruskal-Wallis test was used. P<0.05 was considered to
indicate a statistically significant difference.
Results
Effects of YSJZD on the survival rate
of cisplatin-induced AKI mice
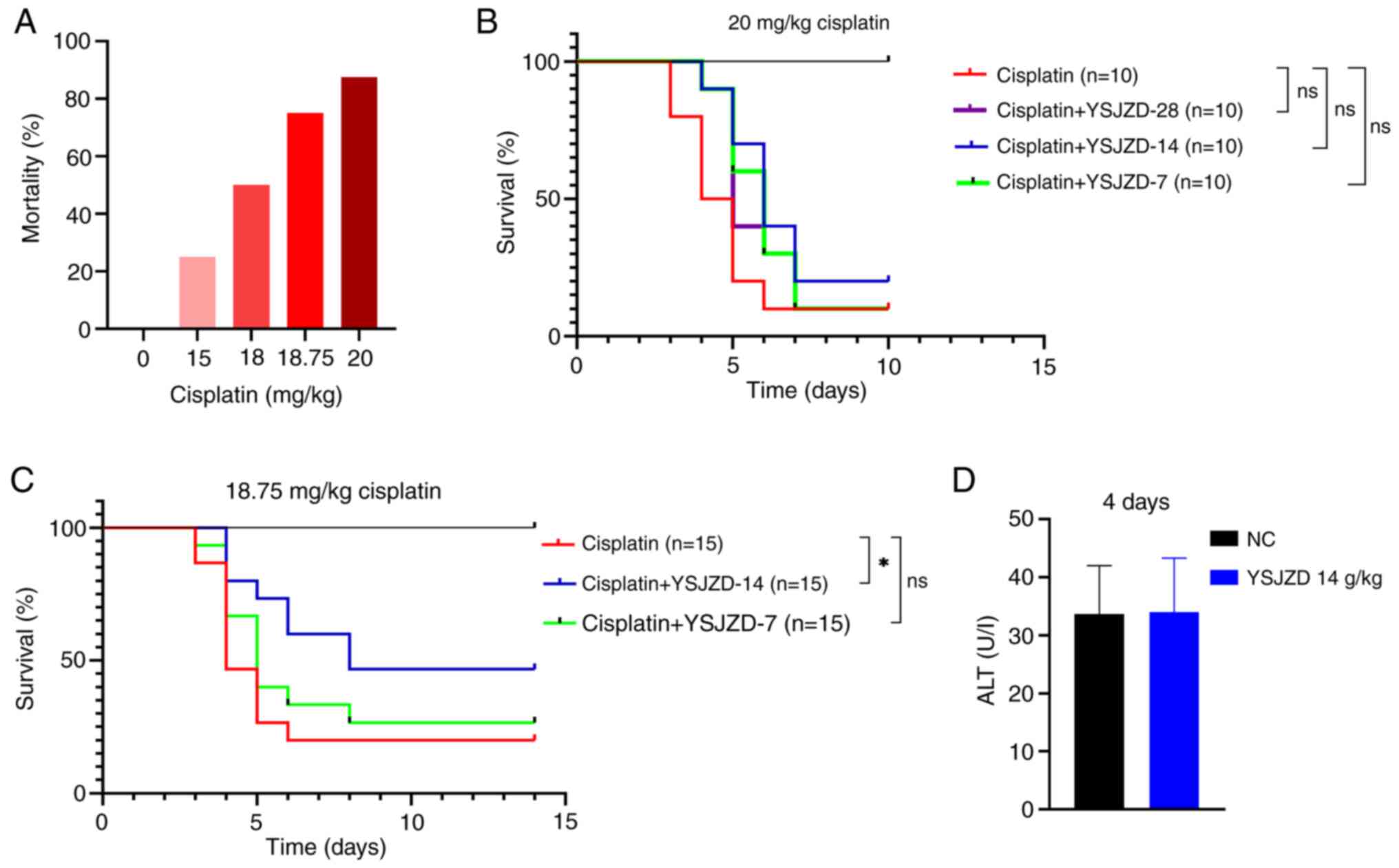
In the present study, The mortality rates in the 20,
18.75, 18 and 15 mg/kg cisplatin groups (n=8 each) were 87.5, 75,
50 and 25%, respectively (Fig.
1A). Therefore, 20 and 18.75 mg/kg, which had the higher
mortality rates, were selected as the modeling doses of CDDP for
follow-up experiments.
As shown in Fig.
1B, the survival rate of mice in the cisplatin + YSJZD 14 g/kg
group was the highest; however, no statistically significant
difference was noted between the two groups. The intraperitoneal
injection of 20 mg/kg cisplatin solution in mice resulted in a high
mortality rate; therefore, the dose of cisplatin was subsequently
reduced to 18.75 mg/kg. YSJZD at 28 g/kg concentration was no more
effective in improving survival rates than concentrations of 14 and
7 g/kg, and was therefore excluded from further experiments. The
cisplatin (18.75 mg/kg)-induced AKI mouse model was selected for
future investigations of the effect of YSJZD on the survival rate
of these mice and YSJZD 14 g/Kg and YSJZD 7 g/Kg were selected for
medication intervention.
As shown in Fig.
1C, the survival rate of the cisplatin + YSJZD 14 g/kg group
was significantly higher than that of the cisplatin group
(P<0.05). The cisplatin + YSJZD 7 g/kg group had a higher
survival rate than the model group (P<0.05). Based on these
results, the YSJZD concentration of 14 g/kg was selected for
subsequent experiments to observe its effects on the indices of
cisplatin (18.75 mg/kg)-induced AKI.
Fig. 1D
demonstrates that short-term YSJZD administration has no
hepatotoxicity in normal unrestricted diet mice (n=8). No mice died
except in the cisplatin group and cisplatin + YSJZD group in the
mortality observation experiment. In the cisplatin 4-day group and
cisplatin + YSJZD 4-day group, one mouse in each group was
harvested blood and kidney and sacrificed at 84-96 h due to humane
endpoint being reached.
Effect of YSJZD on renal function,
renal pathology and renal tubular epithelial cell ultrastructure in
mice with cisplatin-induced AKI
Serum BUN and SCr levels in the model group were
significantly higher than those in the NC group on the second day
following AKI induction (P<0.01). Compared with the cisplatin
group, the serum BUN and SCr levels in the cisplatin + YSJZD group
were reduced; however, the difference did not reach significance.
On the fourth day following AKI, serum BUN and SCr levels were
significantly higher in the cisplatin group than in the NC group
(P<0.01), while the serum BUN and SCr level was significantly
lower in the cisplatin + YSJZD group than in the model group
(P<0.05). The results revealed that YSJZD reduced serum BUN and
SCr levels and improved renal function in cisplatin-induced AKI
mice (Fig. 2A).
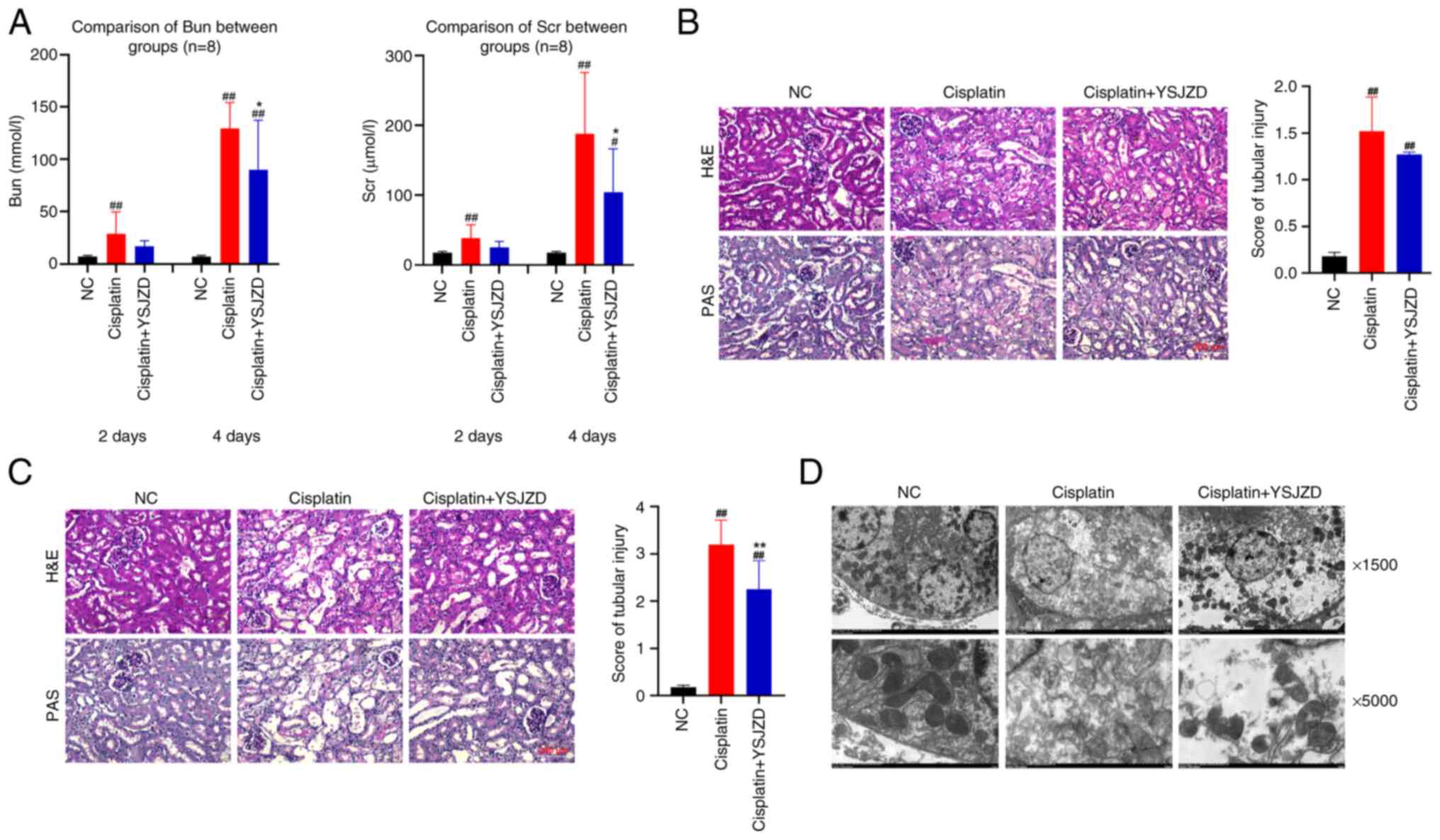 | Figure 2Effect of YSJZD on renal function,
pathology and tubular epithelial cell ultrastructure in mice with
cisplatin-induced AKI. (A) The effects of YSJZD therapy for 2 and 4
days on BUN (left panel) and SCr levels (right panel) in
cisplatin-induced AKI mice (n=8). The results are from 2-day and
4-day groups. (B) Effect of a 2-days course of YSJZD therapy on the
renal pathology (left panel) and the renal tubular damage score
(right panel) of mice with cisplatin-induced AKI (n=8;
magnification, x200; Scale bar, 200 µm). (C) Effect of a 4-days
course of YSJZD therapy on the renal pathology (left panel) and the
renal tubular damage score (right panel) of mice with
cisplatin-induced AKI (n=8). (In left panel of C, the top three
images show H&E staining. The bottom three images showed PAS
staining. Images were acquired using a microscope at 200x
magnification. Scale bar, 200 µm). (D) Effect of YSJZD treatment
for 4 days on the ultrastructure of renal tubular epithelial cells
in cisplatin-induced AKI mice (n=3). Images were acquired at x1,500
and x5,000 under transmission electron microscopy. The top three
pictures are x1,500; the bottom three pictures are x5,000.
#P<0.05, ##P<0.01 vs. NC group;
*P<0.05, **P<0.01 vs. the cisplatin
group. YSJZD, Yishen Jiangzhuo decoction; AKI, acute kidney injury;
BUN, blood urea nitrogen; SCr, serum creatinine; H&E
hematoxylin & eosin; PAS, periodic acid-Schiff; NC, normal
control. |
H&E and PAS staining of renal tissues treated
with 14 g/kg YSJZD for 2 and 4 days revealed that the kidney tissue
of the NC group exhibited a well-structured appearance with
morphologically normal renal tubules and a regular cell
arrangement. In the cisplatin group treated for 2 days, focal
shedding of renal tubular epithelial cells, brush border shedding,
slight dilation of the lumen, thinning of the renal tubular wall
and tubular cast formation were observed in the renal cortex. The
renal tubular damage score of the cisplatin group was higher than
that of the NC group (P<0.01). Renal tubular epithelial cell
shedding, lumen dilatation and cast formation were marginally
improved in the cisplatin + YSJZD group compared to the cisplatin
group. The renal tubular injury score in the cisplatin + YSJZD
group was lower than that in the cisplatin group; however, this
difference was not statistically significant (Fig. 2B).
After 4 days of modeling, renal tubular epithelial
cells in the cisplatin group showed diffuse shedding, a disordered
arrangement of renal tubular epithelial cells, significant lumen
dilatation, wall thinning, naked basement membrane and an abundance
of tubular casts in the renal cortex of the cisplatin group. The
renal tubular injury score in the cisplatin group was significantly
higher in the cisplatin group than that in the NC group
(P<0.01). Compared with the cisplatin group, the cisplatin +
YSJZD group showed significantly reduced shedding of renal tubular
epithelial cells, lumen dilation and tubular casting. Furthermore,
the cisplatin + YSJZD group showed a significantly lower score
(P<0.01). These findings demonstrated that YSJZD considerably
ameliorated renal pathological alterations and lowered the renal
tubular damage score in cisplatin-induced AKI mice (Fig. 2C).
Transmission electron microscopy was performed to
examine the effect of 4-days-YSJZD treatment on the ultrastructure
of renal tubular epithelial cells in cisplatin-induced AKI mice. As
shown in Fig. 2D, electron
microscopy revealed that the mitochondria of renal tubular
epithelial cells in the cisplatin group were significantly reduced
and the cytoplasm and organelles were disordered. By contrast, the
cisplatin + YSJZD group showed significantly decreased
ultrastructural damage to the cells. Thus, YSJZD alleviated damage
to the ultrastructure of renal tubular epithelial cells and
protected mitochondria in cisplatin-induced AKI mice.
GO and KEGG pathway enrichment analysis of DEGs in
cisplatin-induced AKI mice following YSJZD therapy. To identify
DEGs, a threshold of fold change ≥2 and Q-value < 0.05 was used.
The selected DEGs were plotted as bar graphs and volcanic plots. In
total, 4,702 DEGs were identified in the cisplatin group compared
to the NC group, of which 2,631 were upregulated and 2,071 were
downregulated (Fig. 3A and
B). Additionally, 2,754 DEGs were
identified when comparing the cisplatin + YSJZD group and the
cisplatin group; of these, 1,058 were upregulated and 1,696 were
downregulated (Fig. 3D and
E).
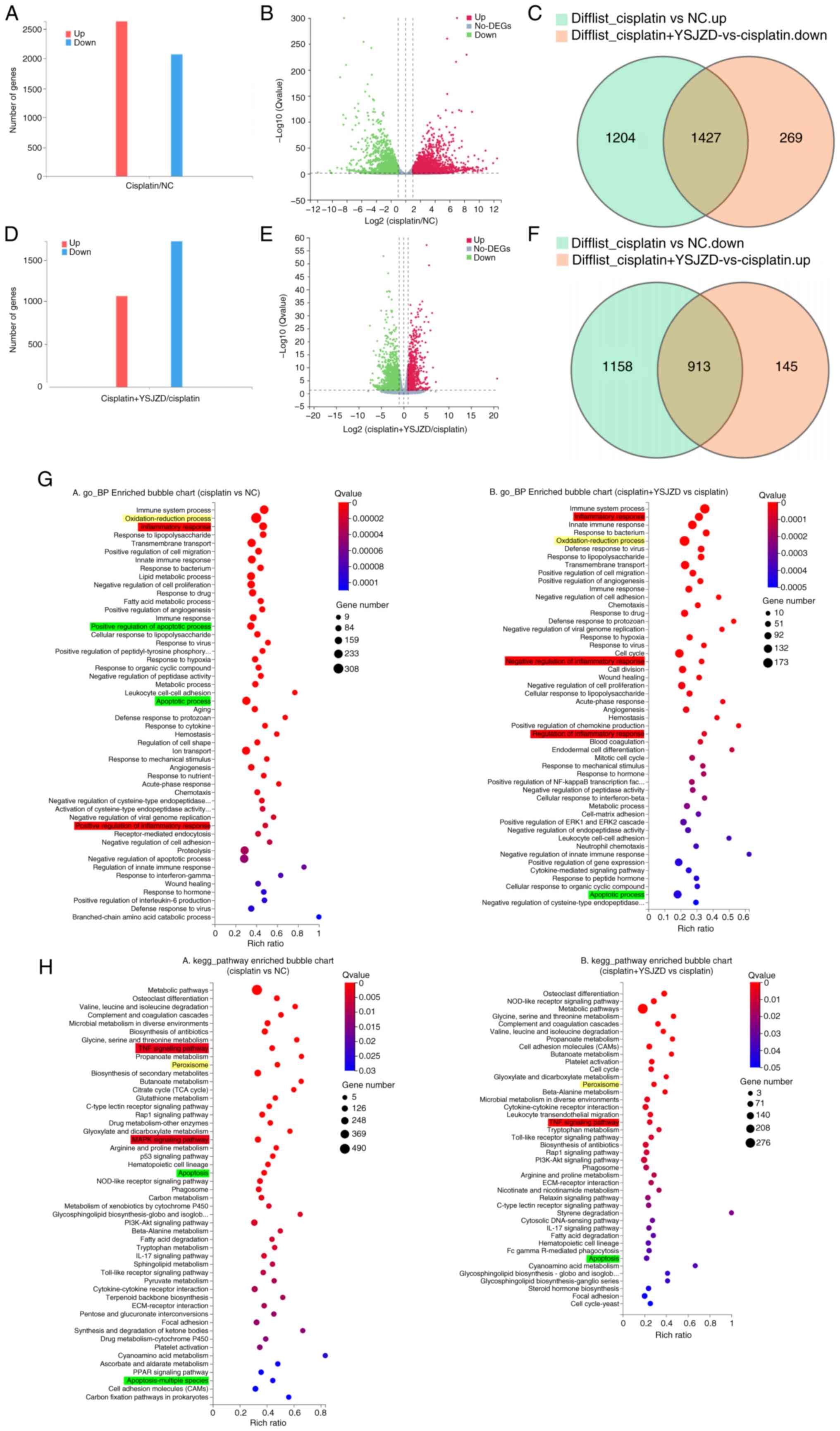 | Figure 3GO and KEGG pathway enrichment
analysis of DEGs in cisplatin-induced AKI mice following YSJZD
therapy. (A) DEGs in the cisplatin group compared with those in the
NC group are shown as bar graphs with the number of related DEGs on
the Y-axis. (B) Volcano plot of DEGs in the cisplatin group
compared with the NC group; the X-axis is log2(fold change) and the
Y-axis is -log10 (Q-value). (C) Downregulated DEGs in the cisplatin
+ YSJZD group compared to the cisplatin group, intersecting with
upregulated DEGs in the cisplatin group compared to the NC group.
(D) Bar chart of DEGs in the cisplatin + YSJZD group compared to
the cisplatin group; number of corresponding DEGs in Y-axis. (E)
Volcano plot of DEGs in the cisplatin + YSJZD group compared to
those in the cisplatin group. (F) Upregulated DEGs in the cisplatin
+ YSJZD group compared to the cisplatin group, intersecting with
downregulated DEGs in the cisplatin group compared to the NC group.
(G) GO_ BP-enriched bubble charts of DEGs in comparison groups. (H)
KEGG pathway enrichment bubble charts of DEGs in the comparison
groups. The X-axis represents the rich ratio and the Y-axis
represents the GO or KEGG terms. The bubble size represents the
number of DEGs in a GO term or KEGG pathway, the color represents
the Q-value; red represents a smaller Q-value and blue represents a
larger Q-value. Red, yellow and green represent inflammatory
response, oxidative stress and apoptotic processes or pathways,
respectively. GO, Gene Ontology; KEGG, Kyoto Encyclopedia of Genes
and Genomes; DEGs, differentially expressed genes; AKI, acute
kidney injury; YSJZD, Yishen Jiangzhuo decoction; NC, normal
control; BP, biological processes. |
Further analysis of the identified DEGs was
conducted to determine the primary genes targeted by YSJZD in
cisplatin-induced AKI. The intersection of upregulated DEGs after
modeling and downregulated DEGs after YSJZD treatment identified
1,427 genes were downregulated following YSJZD treatment (Fig. 3C). The intersection of
downregulated DEGs after modeling and upregulated DEGs after YSJZD
treatment identified 913 genes upregulated after YSJZD treatment
(Fig. 3F).
The primary biological functions of the candidate
genes were determined by GO enrichment analysis. A comparison of
DEGs between the cisplatin and NC groups using GO analysis revealed
that 325 terms (Q-value <0.05) were enriched in biological
processes (BP). The top 50 terms were selected to construct the
bubble chart. The results showed enrichment in biological processes
including the oxidation-reduction process, inflammatory response,
positive regulation of the inflammatory response, apoptosis and
positive regulation of apoptosis. Similarly, a comparison of the
DEGs between the cisplatin and cisplatin + YSJZD groups using GO
analysis revealed that 259 terms (Q-value <0.05) were enriched
in biological processes. The top 50 terms were selected to create a
bubble chart. The results showed enrichment in multiple pathways,
including the oxidation-reduction process, inflammatory response,
regulation of inflammatory response, negative regulation of
inflammatory response and apoptosis (Fig. 3G).
The KEGG database was used as the primary accessible
database for pathway analysis. The signaling pathways used by the
potential genes were identified using KEGG pathway enrichment
analysis. Fig. 3H shows the KEGG
pathway enrichment analysis of the DEGs identified when the
cisplatin and NC groups were compared. In total, 56 pathways showed
significant changes (Q value < 0.05). The top 50 pathways were
then selected to construct a bubble chart. Relevant signaling
pathways were screened based on the results of the GO-BP analysis.
Peroxisomes, TNF signaling, MAPK signaling, apoptosis and multiple
forms of apoptosis were identified as the principal mechanisms
involved. The DEGs identified in the comparison between the
cisplatin + YSJZD and cisplatin groups were analyzed using KEGG
pathway enrichment, with significant changes observed in 42
pathways (Q-value <0.05), which were subsequently visualized
using a bubble chart. These pathways primarily involved the
peroxisome, the TNF signaling pathway and apoptosis.
In summary, GO-BP enrichment of DEGs in the
cisplatin vs. NC group and the cisplatin + YSJZD vs. cisplatin
group was related to inflammation, oxidation-reduction processes
and apoptosis. KEGG pathway enrichment of DEGs in the cisplatin vs.
NC group and cisplatin + YSJZD vs. cisplatin group was also related
to the inflammatory response, oxidative stress and apoptotic
pathways. The raw RNA-seq data that support the findings were
deposited in the gene expression omnibus (GEO) repository with an
accession number GSE262792 (https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE262792.
Target genes of YSJZD in the treatment
of cisplatin-induced AKI
GO-BP and KEGG pathway enrichment analysis of DEGs
revealed the importance of pathways related inflammation, oxidative
stress and apoptosis. Therefore, the signaling pathways involved
were examined to identify the target genes that were affected by
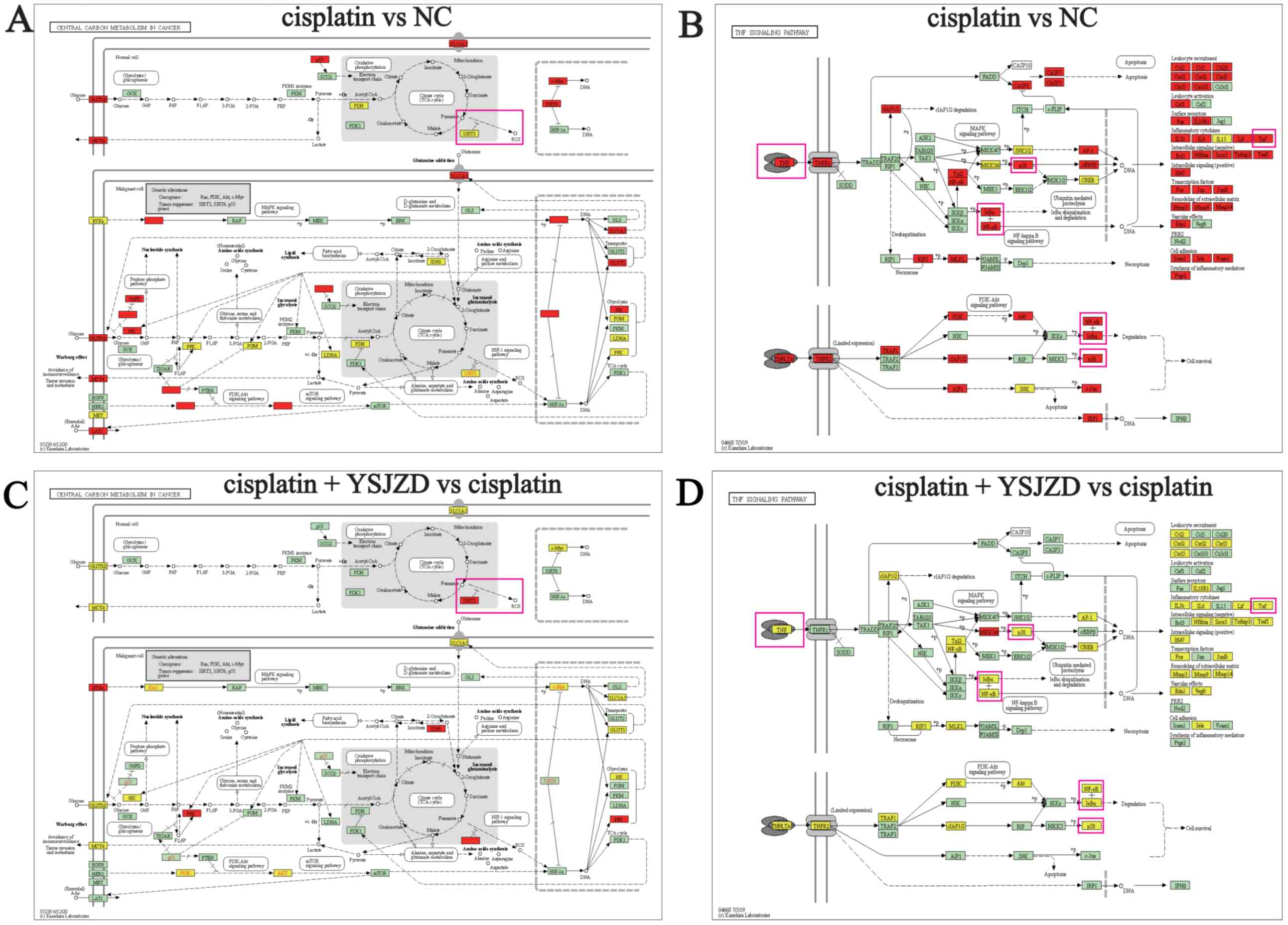
YSJZD therapy. As shown in Fig. 4A
and C, the signaling pathway
involved in central carbon metabolism in cancer involves the
oxidative stress index, NAD-dependent protein deacetylase sirtuin-3
(SIRT3). It was observed that SIRT3 expression was downregulated in
the model group and was upregulated following YSJZD treatment.
Furthermore, in the TNF signaling pathway, TNF-α, NFκB and p38 MAPK
were upregulated in the cisplatin group and downregulated following
YSJZD treatment (Fig. 4B and
D).
Effects of YSJZD on renal inflammatory
indices in cisplatin-induced AKI mice
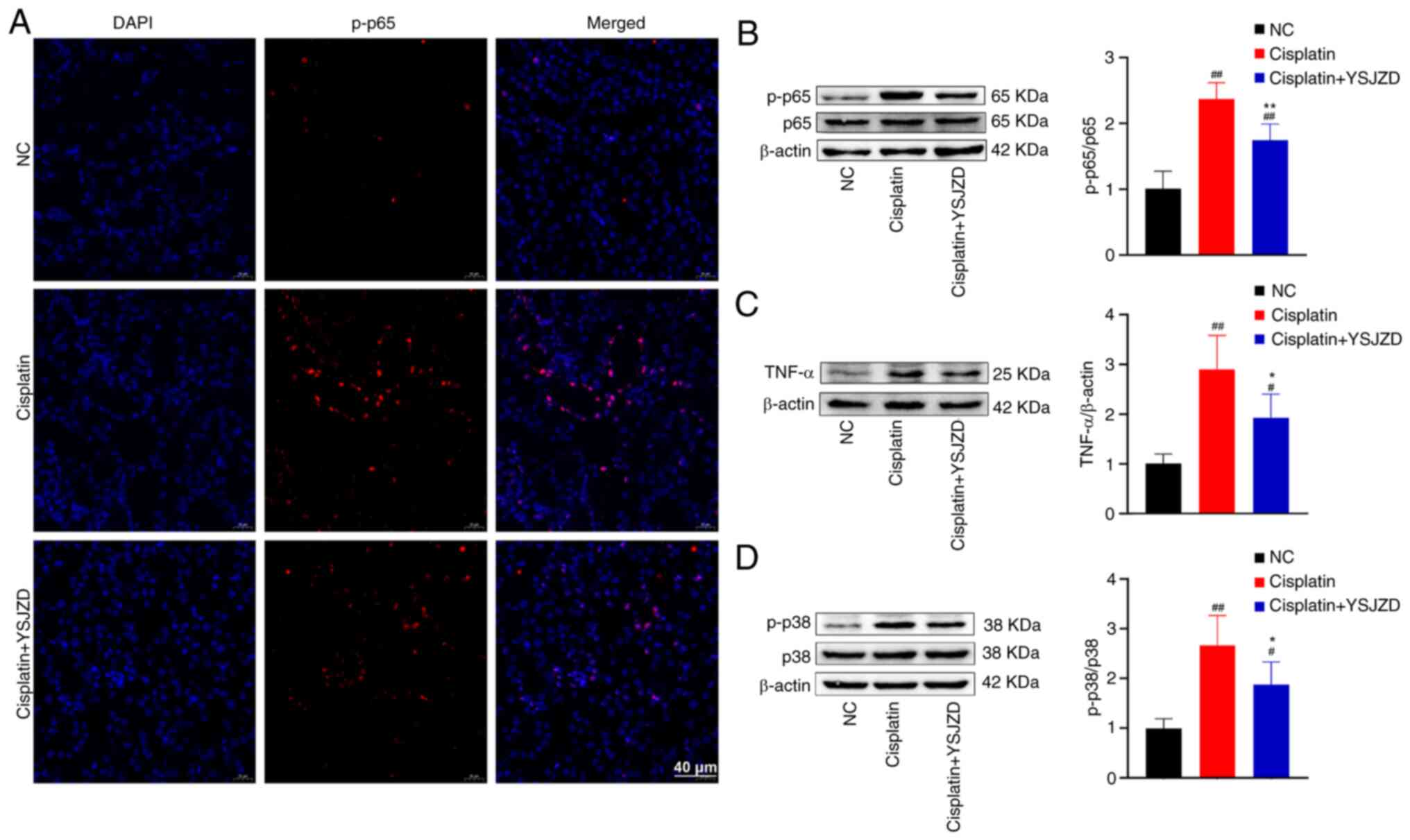
Compared with the NC group, a large amount of
phosphorylated (p-)65 red fluorescence was observed in the nucleus
of renal tubular epithelial cells in the cisplatin group and the
p-p65 immunofluorescence intensity was significantly reduced
following YSJZD treatment (Fig.
5A).
The WB results of p-p65, TNF-α and p-p38 MAPK in the
renal tissue demonstrated that the p-p65/p65, TNF-α/β-actin and
p-p38/p38 ratios of the cisplatin group were significantly greater
than those of the NC group (P<0.01). The ratios of p-p65/p65,
TNF-α/β-actin and p-p38/p38 in the cisplatin + YSJZD group were
significantly lower than those in the cisplatin group (P<0.05;
Fig. 5B-D). These findings
demonstrate that YSJZD markedly reduced the expression levels of
p-p65, TNF-α and p-p38 in the renal tissue of mice with
cisplatin-induced AKI.
Effects of YSJZD on oxidative stress
and apoptosis indices in cisplatin-induced AKI mice
The SIRT3/β-actin ratio in the cisplatin group was
significantly lower than that of the NC group on WB data of renal
tissue (P<0.01; Fig. 6A).
Conversely, the SIRT3/β-actin ratio in the cisplatin + YSJZD group
was significantly higher than that of the cisplatin group
(P<0.05). These findings demonstrated that YSJZD substantially
enhanced SIRT3 expression in the renal tissue of mice with
cisplatin-induced AKI.
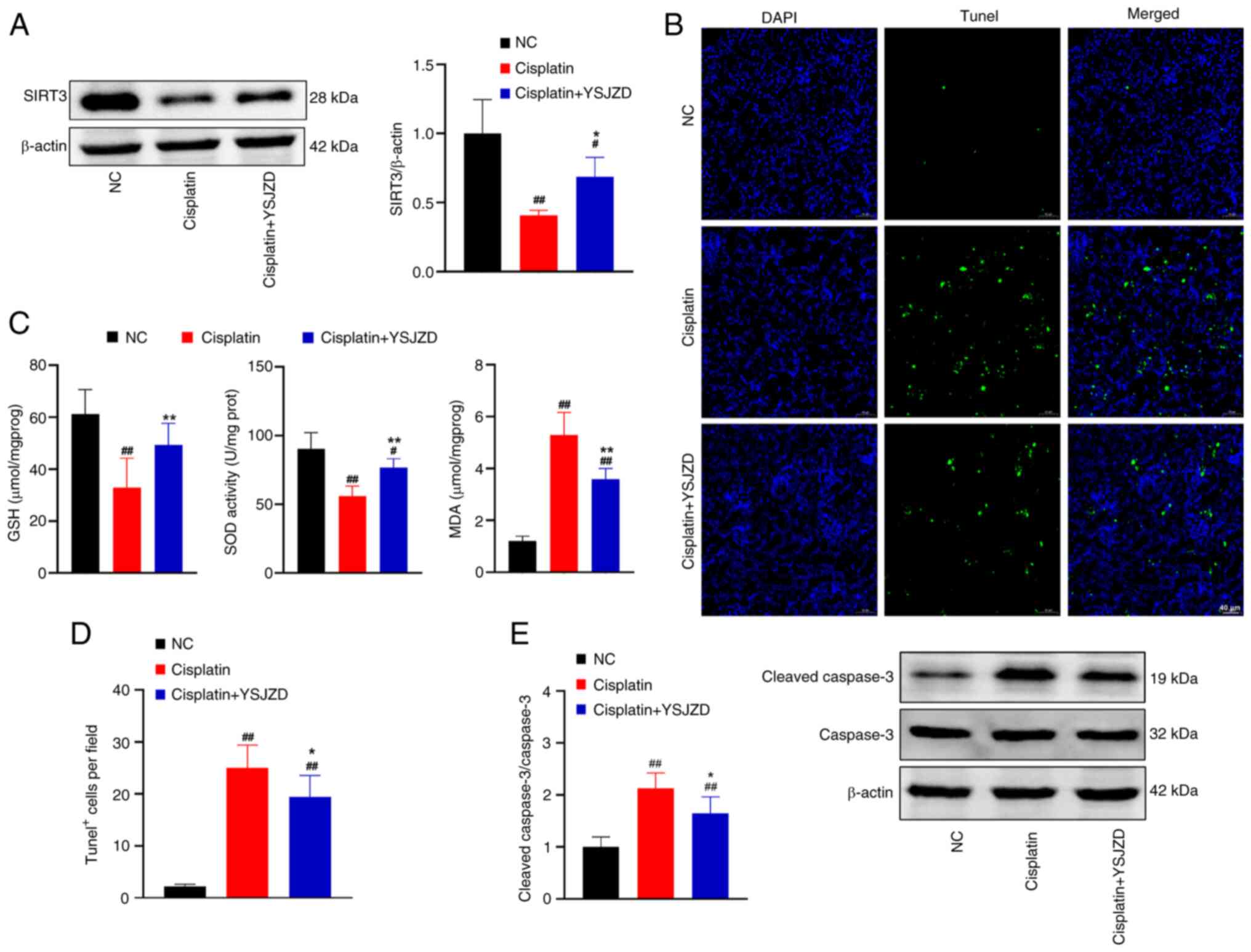 | Figure 6Effects of YSJZD on oxidative stress
and apoptosis indices in cisplatin-induced AKI mice. (A) Effect of
YSJZD on levels of SIRT3 in the renal tissue of cisplatin-induced
AKI mice. The bar chart shows gray value ratio of SIRT3/β-actin in
renal tissue of each group (n=5). (B) TUNEL fluorescence images of
renal tissue in each group (magnification, x200; scale bar, 40 µm).
(C) Effect of YSJZD on the GSH, SOD and MDA detection level in the
renal tissue of cisplatin-induced AKI (n=8). (D) Statistical bar
chart of TUNEL-positive cells per field of renal tissue for each
group (n=8). (E) Effect of YSJZD on cleaved-caspase-3 in the renal
tissue of cisplatin-induced AKI mice. Bar chart is gray value ratio
of cleaved-caspase-3/caspase-3 in renal tissue of each group (n=5).
#P<0.05, ##P<0.01 vs. the NC group;
*P<0.05, **P<0.01 vs. the cisplatin
group. YSJZD, Yishen Jiangzhuo decoction; AKI, acute kidney injury;
SIRT3, NAD-dependent deacetylase sirtuin-3; GSH, glutathione; SOD,
superoxide dismutase; MDA, malondialdehyde TUNEL, TdT-mediated dUTP
nick end labeling; NC, normal control; cl, cleaved. |
As shown in Fig.
6C, GSH and SOD activity levels in the cisplatin group were
significantly lower than those in the NC group (P<0.01). MDA
levels significantly increased in the cisplatin group (P<0.01).
Similarly, GSH and SOD activity levels in the cisplatin + YSJZD
group were significantly higher (P<0.01) than those in the
cisplatin group, whereas MDA levels were significantly lower
(P<0.01) in the cisplatin + YSJZD group. These findings
demonstrated that YSJZD therapy greatly increased GSH and SOD
levels in the renal tissue of cisplatin-induced AKI mice and
markedly reduced MDA levels.
Compared with the NC group, the number of
TUNEL-positive cells per field of renal tissue was significantly
higher in the cisplatin group (P<0.01). Conversely, the number
of TUNEL-positive cells per field in the cisplatin + YSJZD group
was considerably lower than that in the cisplatin group (P<0.05)
(Fig. 6B and D). As shown in Fig. 6E, the ratio of
cle-caspase-3/caspase-3 in the cisplatin group was significantly
higher (P<0.01) than that in the NC group, according to the WB
findings of cle-caspase-3 in the renal tissue. The
cle-caspase-3/caspase-3 ratio in the cisplatin + YSJZD group was
significantly lower than that in the cisplatin group (P<0.05).
These results revealed that YSJZD considerably reduced
cisplatin-induced apoptosis of renal tubular epithelial cells in
AKI mice.
Discussion
The clinical application of cisplatin or similar
platinum-based treatments is commonly limited by the application of
cisplatin-induced AKI. Complex processes underlie cisplatin-induced
AKI, including the accumulation of cisplatin in renal tissue and
the activation of inflammatory, oxidative and apoptotic pathways
(5,14-16).
The present study found that the mortality rate of
cisplatin-induced AKI mice was dose-dependent and that the 20 mg/kg
cisplatin-induced mouse model had a high mortality rate. The
mortality rate observed in the 20 mg/kg cisplatin-induced mouse
model was similar to that observed by Linkermann et al
(17). Therefore, the dose of
cisplatin was reduced to 18.75 mg/kg. It was found that YSJZD
reduces mortality in cisplatin-induced AKI mice. However, the YSJZD
concentration of 28 g/kg was no improvement on 14 and 7 g/kg in
terms of improving the survival rate, with the survival rate in the
14 g/kg group being the highest. Therefore, the optimal
concentration of YSJZD was determined to be 14 g/kg. Furthermore,
compared with the cisplatin group on the second day, the serum BUN
and SCr levels in the cisplatin + YSJZD group were reduced;
however, the difference was not statistically significant. This may
be because the efficacy of YSJZD was time-dependent. YSJZD
treatment for 4 days enhanced renal function and reduced
pathological and renal tubular injury scores. YSJZD also protected
RTECs against cisplatin-induced ultrastructural damage,
particularly mitochondrial dysfunction. Overall, these results
indicated that YSJZD was an effective drug for the treatment of
cisplatin-induced AKI in mice.
YSJZD, a TCM compound with complex components, has
multiple targets (11). Therefore,
to elucidate its underlying mechanism of action, transcriptomic
analysis was applied to analyze the effects of YSJZD. Subsequent
GO-BP and KEGG pathway enrichment showed that the mechanisms of
action of YSJZD may be related to inflammation, oxidation-reduction
processes, apoptosis and the TNF signal pathway.
Cisplatin-induced AKI is strongly associated with
the inflammatory response (8,18).
Research has found that the activation of the NF-κB signaling
pathway may be one of the primary mechanisms underlying
cisplatin-induced AKI. Proximal tubular epithelial cells and immune
cells infiltrating the kidney produce inflammatory cytokines (such
as TNF-α) due to activation of the p65 pathway by cisplatin
(2). Cisplatin causes the
phosphorylation of p-p65 and its translocation from the cytosol to
the nucleus (7). Inhibition of p65
transcriptional activity by an p65 inhibitor ameliorates
cisplatin-induced AKI (19).
Cisplatin nephrotoxicity is also significantly influenced by the
p38 MAPK signaling pathway. The role of p38 MAPK in
cisplatin-induced nephrotoxicity has been demonstrated both in
vitro and in vivo. Pharmacological inhibitors of p38
(SB203580 and SKF-86002) were found to exert renoprotective effects
in these models (7,20,21).
The p38 MAPK pathway modulates TNF-α expression in renal tubular
cells and the subsequent inflammatory response during cisplatin
nephrotoxicity rather than directly controlling tubular cell damage
and death (22). Thus, TNF-α plays
a significant role in the pathophysiology of cisplatin-induced AKI
(23). In the context of cisplatin
nephrotoxicity, indigenous kidney cells, rather than invading
inflammatory cells, create the majority of the TNF-α (24), Moreover, during cisplatin
nephrotoxicity, renal tubular cells considerably contribute to the
generation of TNF-α (25). These
inflammatory factors further induce inflammatory in renal tubular
epithelial cells, leading to cell death and shedding, thus causing
the onset and progression of AKI.
In the cisplatin-induced AKI mice in the present
study, p-p65 red fluorescence was observed in the nuclei of renal
tubular epithelial cells. The intensity of immunofluorescence was
substantially diminished after treatment with YSJZD, thus
indicating that YSJZD significantly reduced the expression level of
p-p65 and TNF-α in the renal tissue of cisplatin-induced AKI mice.
According to the results of WB for p-p65 and TNF-α in renal tissue,
it was hypothesized that YSJZD decreased the renal inflammatory
response in mice with cisplatin-induced AKI by reducing the
phosphorylation and translocation of p65 into the nucleus and
decreasing the expression level of TNF-α. Additionally, YSJZD
significantly reduced the expression of p-p38 in the renal tissues
of mice with cisplatin-induced AKI. The inhibition of p38 MAPK
could also reduce the production of TNF-α, thus effectively
protecting against cisplatin-induced kidney damage. These findings
suggest that the anti-inflammatory activity of YSJZD is one of the
mechanisms by which it alleviates cisplatin-induced AKI in
mice.
Oxidative stress contributes significantly to
cisplatin-induced nephrotoxicity (8). The increase in the endogenous
antioxidant enzymes, GSH and SOD, in the renal tissue can reduce
ROS accumulation in the kidneys (26,27).
SOD, GSH and catalase production decrease when cisplatin enters
renal tubular cells, eventually causing a build-up of ROS and an
increase in MDA within the cells (5,6,8). The
accumulation of cisplatin in the mitochondria of renal cells
results in malfunction and damage, mostly manifesting as increased
ROS generation (2,7,28).
As the mitochondria are the main generators of ROS, SIRT3, a member
of the NAD+-dependent deacetylase family, may reduce ROS
generation (29). The
renoprotective benefits of the SIRT3-ROS pathway have also been
demonstrated (30-33).
YSJZD can downregulate TNF-α levels and upregulate the levels of
SIRT3, GSH and SOD in the renal tissue, thus reducing ROS
production. The mechanistic study was based on target genes
screened using transcriptome sequencing; therefore, ROS detection
in frozen sections of renal tissue could not be performed. YSJZD
can significantly reduce MDA levels and alleviate mitochondrial
damage in RTECs and may be able to reduce cisplatin-induced AKI
induced by cisplatin in mice by preventing oxidative stress.
Decreased ROS production leads to the downregulation of P38 MAPK
phosphorylation and ultimately to decreased TNF-α production
(5,22).
Renal tubular cell death is a common
histopathological feature of cisplatin-induced nephrotoxicity
(34). Cisplatin induces cell
death via two primary mechanisms: Necrosis and apoptosis (35). Several apoptotic pathways have been
implicated in the cisplatin-induced death of renal epithelial
cells, including the endoplasmic reticulum stress-driven apoptosis
and the intrinsic (mitochondrial) and extrinsic death receptor
pathways through TNF-α generation (5,36).
The activation of one or more of the three apoptotic pathways
causes caspase-3 cleavage. The present study found that the
cleaved-caspase-3/caspase-3 ratio and the number of TUNEL-positive
cells per field were significantly lower in the cisplatin + YSJZD
group than in the cisplatin group. The NF-κB signaling pathway can
regulate the apoptotic pathways in renal tubular epithelial cells,
enhancing the expression of downstream apoptosis-related genes,
inducing apoptosis in these cells, thereby accelerating cell death
and shedding and contributing to the development of AKI. YSJZD
significantly decreased the expression level of TNF-α, thus
reducing the apoptosis of cisplatin-induced RTECs. Decreased p38
activation reduces activation of downstream proteins and
caspase-3(36). YSJZD demonstrated
a protective effect by decreasing oxidative stress and downstream
consequences, such as RTECs apoptosis in cisplatin-induced AKI.
These results showed that YSJZD considerably reduced
cisplatin-induced apoptosis of renal tubular epithelial cells in
AKI mice.
There are some limitations in this study. First,
only WB is used to detect inflammatory factors. In subsequent
experiments, newer and more targeted experimental techniques will
be used to detect inflammatory factors to increase the reliability
of data. Second, there was no liver toxicity in short-term
application of YSJZD in this study. However, data on long-term
hepatotoxicity are not available, so the long-term side effects of
drugs will be monitored carefully in the subsequent studies. Third,
because the verification is based on differentially expressed genes
in transcriptomics, the present study did not detect JNK and ERK in
MAPK signal pathway. The MAPK pathway will be the focus of
subsequent studies to investigate the pharmacodynamic mechanism.
The pathogenesis of cisplatin-induced AKI is complex and compound
Chinese medicine has multiple targets; therefore, the core
pharmacodynamic target gene pathway of YSJZD has not be completely
elucidated in the present study.
Overall, the results of the present study showed
that YSJZD was an effective drug for the treatment of
cisplatin-induced AKI. The main target genes of YSJZD include
markers of oxidative stress, such as SIRT3, and markers of
inflammation and apoptosis, such as TNF-α, p65 and p38 MAPK. These
findings provide a theoretical and experimental foundation for the
use of YSJZD to prevent cisplatin-induced AKI.
Supplementary Material
Flow chart of the experiments. (A)
Mortality rates of mice induced by different doses of cisplatin
(n=8). (B) Effects of YSJZD on the survival rate of cisplatin (20
mg/kg)-induced AKI mice (n=10). (C) Effects of YSJZD on the
survival rate of cisplatin (18.75 mg/kg)-induced AKI mice (n=15).
(D) Short-term effects of YSJZD on hepatotoxicity in mice (n=8).
(E) Effects of YSJZD therapy in cisplatin (18.75 mg/kg) induced AKI
mice. YSJZD,Yishen Jiangzhuo decoction; AKI, acute kidney
injury.
Acknowledgements
Not applicable.
Funding
Funding: The present study was supported by the Fujian
Provincial Natural Science Foundation (grant nos. 2022J01828,
2016J01468 and 2016J01559).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author. The raw RNA-seq data that
support the findings were deposited in the gene expression omnibus
(GEO) repository with an accession no. GSE262792 (https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE262792).
Authors' contributions
DZ and SR contributed to the conception of the study
and design of the experiments. DZ, XR and YQ performed the
experiments. DZ, XR, QW and YQ analyzed and interpreted the data.
DZ, XR, QW and SR wrote and revised the manuscript. DZ and SR
confirm the authenticity of all the raw data. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
The protocol of the present study was reviewed and
approved by the Fujian University of Traditional Chinese Medicine
Laboratory Animal Welfare and Ethics Committee, approval no. FJ-TCM
IACUC2020021.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Zhang Q, Qi J, Luo Q, Wu M, Zhang L, Qin L
and Nie X: Yishen Xiezhuo formula ameliorates the development of
cisplatin-induced acute kidney injury by attenuating renal tubular
epithelial cell senescence. Ann Transl Med. 10(1392)2022.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Volarevic V, Djokovic B, Jankovic MG,
Harrell CR, Fellabaum C, Djonov V and Arsenijevic N: Molecular
mechanisms of cisplatin-induced nephrotoxicity: a balance on the
knife edge between renoprotection and tumor toxicity. J Biomed Sci.
26(25)2019.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Oliveira BM, de Almeida LF, Deluque AL,
Souza CS, Maciel ALD, Francescato HDC, Costa RS, Giovanini C, de
Paula FJA and Coimbra TM: Calcitriol reduces the inflammation,
endothelial damage and oxidative stress in AKI caused by cisplatin.
Int J Mol Sci. 23(15877)2022.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Lu L, Liu W, Li S, Bai M, Zhou Y, Jiang Z,
Jia Z, Huang S, Zhang A and Gong W: Flavonoid derivative DMXAA
attenuates cisplatin-induced acute kidney injury independent of
STING signaling. Clin Sci (Lond). 137:435–452. 2023.PubMed/NCBI View Article : Google Scholar
|
|
5
|
McSweeney KR, Gadanec LK, Qaradakhi T, Ali
BA, Zulli A and Apostolopoulos V: Mechanisms of cisplatin-induced
acute kidney injury: Pathological mechanisms, pharmacological
interventions and genetic mitigations. Cancers (Basel).
13(1572)2021.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Holditch SJ, Brown CN, Lombardi AM, Nguyen
KN and Edelstein CL: Recent advances in models, mechanisms,
biomarkers and interventions in cisplatin-induced acute kidney
injury. Int J Mol Sci. 20(3011)2019.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Qi L, Luo Q, Zhang Y, Jia F, Zhao Y and
Wang F: Advances in toxicological research of the anticancer drug
cisplatin. Chem Res Toxicol. 32:1469–1486. 2019.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Fang CY, Lou DY, Zhou LQ, Wang JC, Yang B,
He QJ, Wang JJ and Weng QJ: Natural products: Potential treatments
for cisplatin-induced nephrotoxicity. Acta Pharmacol Sin.
42:1951–1969. 2021.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Mapuskar KA, Steinbach EJ, Zaher A, Riley
DP, Beardsley RA, Keene JL, Holmlund JT, Anderson CM, Zepeda-Orozco
D, Buatti JM, et al: Mitochondrial superoxide dismutase in
cisplatin-induced kidney injury. Antioxidants (Basel).
10(1329)2021.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Sears SM and Siskind LJ: Potential
therapeutic targets for cisplatin-induced kidney injury: Lessons
from other models of AKI and Fibrosis. J Am Soc Nephrol.
32:1559–1567. 2021.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Xu YF, Ruan SW, Lin JM and Zhang Z: Yishen
Jiangzhuo Granules affect tubulointerstitial fibrosis via a
mitochondrion-mediated apoptotic pathway. Chin J Integr Med.
21:928–937. 2015.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Xu SY, Bian RL and Chen X: Experimental
methodology of Pharmacology. People's Medical Publishing House,
Beijing, 2002.
|
|
13
|
Zhang Y, Chang Y, Han Z, Ma K, Zeng X and
Li L: Estrogen protects against renal ischemia-reperfusion injury
by regulating Th17/Treg cell immune balance. Dis Markers.
2022(7812099)2022.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Ranasinghe R, Mathai ML and Zulli A:
Cisplatin for cancer therapy and overcoming chemoresistance.
Heliyon. 8(e10608)2022.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Xu Z, Zhang M, Wang W, Zhou S, Yu M, Qiu
X, Jiang S, Wang X, Tang C, Li S, et al: Dihydromyricetin
attenuates cisplatin-induced acute kidney injury by reducing
oxidative stress, inflammation and ferroptosis. Toxicol Appl
Pharmacol. 473(116595)2023.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Chou YN, Lee MM, Deng JS, Jiang WP, Lin JG
and Huang GJ: Water extract from brown strain of flammulina
velutipes alleviates cisplatin-induced acute kidney injury by
attenuating oxidative stress, inflammation, and autophagy via
PI3K/AKT pathway regulation. Int J Mol Sci. 24(9448)2023.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Linkermann A, Bräsen JH, Darding M, Jin
MK, Sanz AB, Heller JO, De Zen F, Weinlich R, Ortiz A, Walczak H,
et al: Two independent pathways of regulated necrosis mediate
ischemia-reperfusion injury. Proc Natl Acad Sci USA.
110:12024–12029. 2013.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Perse M and Veceric-Haler Z:
Cisplatin-induced rodent model of kidney injury: Characteristics
and challenges. Biomed Res Int. 2018(1462802)2018.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Ozkok A, Ravichandran K, Wang Q,
Ljubanovic D and Edelstein CL: NF-κB transcriptional inhibition
ameliorates cisplatin-induced acute kidney injury (AKI). Toxicol
Lett. 240:105–113. 2016.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Kim C, Kwak W, Won DH, Kim J, Hwang DB,
Kim N, Kang M, Jeon Y, Park YI, Park JW and Yun JW: Loss of Dact2
alleviates cisplatin-induced nephrotoxicity through regulation of
the Igfl-MAPK pathway axis. Cell Biol Toxicol. 39:3197–3217.
2023.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Yuan H, Zhao Y, Li S, Qin J and Yu X:
Madecassoside ameliorates cisplatin-induced nephrotoxicity by
inhibiting activation of the mitogen activated protein kinase
pathway. Environ Toxicol. 38:1473–1483. 2023.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Ramesh G and Reeves WB: p38 MAP kinase
inhibition ameliorates cisplatin nephrotoxicity in mice. Am J
Physiol Renal Physiol. 289:F166–F174. 2005.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Ramesh G and Reeves WB: TNF-alpha mediates
chemokine and cytokine expression and renal injury in cisplatin
nephrotoxicity. J Clin Invest. 110:835–842. 2002.PubMed/NCBI View
Article : Google Scholar
|
|
24
|
Zhang B, Ramesh G, Norbury CC and Reeves
WB: Cisplatin-induced nephrotoxicity is mediated by tumor necrosis
factor-alpha produced by renal parenchymal cells. Kidney Int.
72:37–44. 2007.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Ramesh G, Kimball SR, Jefferson LS and
Reeves WB: Endotoxin and cisplatin synergistically stimulate
TNF-alpha production by renal epithelial cells. Am J Physiol Renal
Physiol. 292:F812–F819. 2007.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Pan Y, Zhang Y, Li J, Zhang Z, He Y, Zhao
Q, Yang H and Zhou P: A proteoglycan isolated from Ganoderma
lucidum attenuates diabetic kidney disease by inhibiting oxidative
stress-induced renal fibrosis both in vitro and in vivo. J
Ethnopharmacol. 310(116405)2023.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Lu XH, Zhang J and Xiong Q: Suppressive
effect erythropoietin on oxidative stress by targeting
AMPK/Nox4/ROS pathway in renal ischemia reperfusion injury. Transpl
Immunol. 72(101537)2022.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Ma Q, Xu Y, Tang L, Yang X, Chen Z, Wei Y,
Shao X, Shao X, Xin Z, Cai B, et al: Astragalus polysaccharide
attenuates cisplatin-induced acute kidney injury by suppressing
oxidative damage and mitochondrial dysfunction. Biomed Res Int.
2020(2851349)2020.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Yang L, Wang B, Guo F, Huang R, Liang Y,
Li L, Tao S, Yin T, Fu P and Ma L: FFAR4 improves the senescence of
tubular epithelial cells by AMPK/SirT3 signaling in acute kidney
injury. Signal Transduct Target Ther. 7(384)2022.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Huang Z, Li Q, Yuan Y, Zhang C, Wu L, Liu
X, Cao W, Guo H, Duan S, Xu X, et al: Renalase attenuates
mitochondrial fission in cisplatin-induced acute kidney injury via
modulating sirtuin-3. Life Sci. 222:78–87. 2019.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Li Y, Ye Z, Lai W, Rao J, Huang W, Zhang
X, Yao Z and Lou T: Activation of sirtuin 3 by silybin attenuates
mitochondrial dysfunction in cisplatin-induced acute kidney injury.
Front Pharmacol. 8(178)2017.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Morigi M, Perico L, Rota C, Longaretti L,
Conti S, Rottoli D, Novelli R, Remuzzi G and Benigni A: Sirtuin
3-dependent mitochondrial dynamic improvements protect against
acute kidney injury. J Clin Invest. 125:715–726. 2015.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Zi Y, Wang X, Zi Y, Yu H, Lan Y, Fan Y,
Ren C, Liao K and Chen H: Cigarette smoke induces the ROS
accumulation and iNOS activation through deactivation of
Nrf-2/SIRT3 axis to mediate the human bronchial epithelium
ferroptosis. Free Radic Biol Med. 200:73–86. 2023.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Wen L, Wei Q, Livingston MJ, Dong G, Li S,
Hu X, Li Y, Huo Y and Dong Z: PFKFB3 mediates tubular cell death in
cisplatin nephrotoxicity by activating CDK4. Transl Res. 253:31–40.
2023.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Lee D, Yamabe N, Lee H, Lim Lee H, Kim DW,
Wook Lee J and Sung Kang K: Necrostatins regulate apoptosis,
necroptosis, and inflammation in cisplatin-induced nephrotoxicity
in LLC-PK1 cells. Bioorg Med Chem Lett. 48(128256)2021.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Oh GS, Kim HJ, Shen A, Lee SB, Khadka D,
Pandit A and So HS: Cisplatin-induced kidney dysfunction and
perspectives on improving treatment strategies. Electrolyte Blood
Press. 12:55–65. 2014.PubMed/NCBI View Article : Google Scholar
|