Introduction
By targeting programmed death receptor 1 (PD-1),
programmed cell death ligand 1 (PD-L1) or cytotoxic
T-lymphocyte-associated protein 4 (CTLA-4), immune checkpoint
inhibitors (ICIs) enhance the cytotoxicity of T cells against tumor
cells and prevent their escape, effectively controlling tumor cell
proliferation (1-3).
Despite the important role of ICIs in tumor therapy, the
immune-related adverse events (irAEs) caused by them should not be
ignored. During clinical treatment with ICIs, irAEs have been
observed in >70% of patients (4-6).
Among all irAEs, ~10% of patients experience
cardiovascular-related adverse events. These events can include
arrhythmias, pericardial disease, myocarditis and other cardiac
symptoms (7,8). One of the most serious conditions is
myocarditis, which is frequently triggered by viral infections but
is also linked to autoimmune diseases, viral infections, and drug
use (9). The use of immune
checkpoint inhibitors is a known trigger for myocarditis. Data from
VigiBase, the WHO's global international pharmacovigilance
database, indicate that the incidence of myocarditis with single
ICI treatment is 0.54%, while the incidence of myocarditis with ICI
combination therapy is 1.22% (10). The main treatment option for immune
checkpoint inhibitor (ICI)-induced myocarditis is immunosuppressive
therapy (11). Corticosteroids,
cyclosporine, and azathioprine are drugs that have been shown to be
effective for myocarditis (12).
Glucocorticosteroids, like prednisone, have been proven effective
in alleviating immunotherapy-induced myocarditis (13). The current report presents a case
with an organized treatment plan of a breast cancer patient who
experienced symptoms, such as palpitations and increased cardiac
enzyme levels, 1 day after ICI therapy. The patient underwent
cardiac angiography to confirm the diagnosis of immune myocarditis
and received hormone shock therapy. The present report provides a
foundation for the prevention and diagnosis of immune myocarditis
caused by ICI therapy.
Case report
In August 2020, a 66-year-old female patient was
admitted to Weifang Second People's Hospital (Weifang, China) for a
biopsy of a left breast mass to determine the nature of the
pathology. The immunohistochemistry results confirmed invasive
breast cancer. The patient had type II diabetes but did not have a
history of heart disease or any other autoimmune diseases. There
was also no previous family history of heart disease or any other
autoimmune diseases.
The patient underwent a left breast mass aspiration
biopsy in August 2020 under color ultrasound guidance and was
assessed at the Weifang Second People's Hospital due to redness and
swelling of the left breast mass for 1 month. The patient had been
diagnosed with a breast nodule 1 year earlier in Weifang Second
People's Hospital (Fig. S1A). The
ultrasound results revealed heterogeneous cellular streak-like
infiltration in the tissues, with local necrosis observed in the
ducts, consistent with the features of invasive breast cancer
(Fig. S1B and C). This was further confirmed by
magnetic resonance imaging (MRI) and computed tomography (CT)
(Fig. S1D-G). The diagnosis of
stage III inflammatory breast cancer (T4N0M0) of the left breast
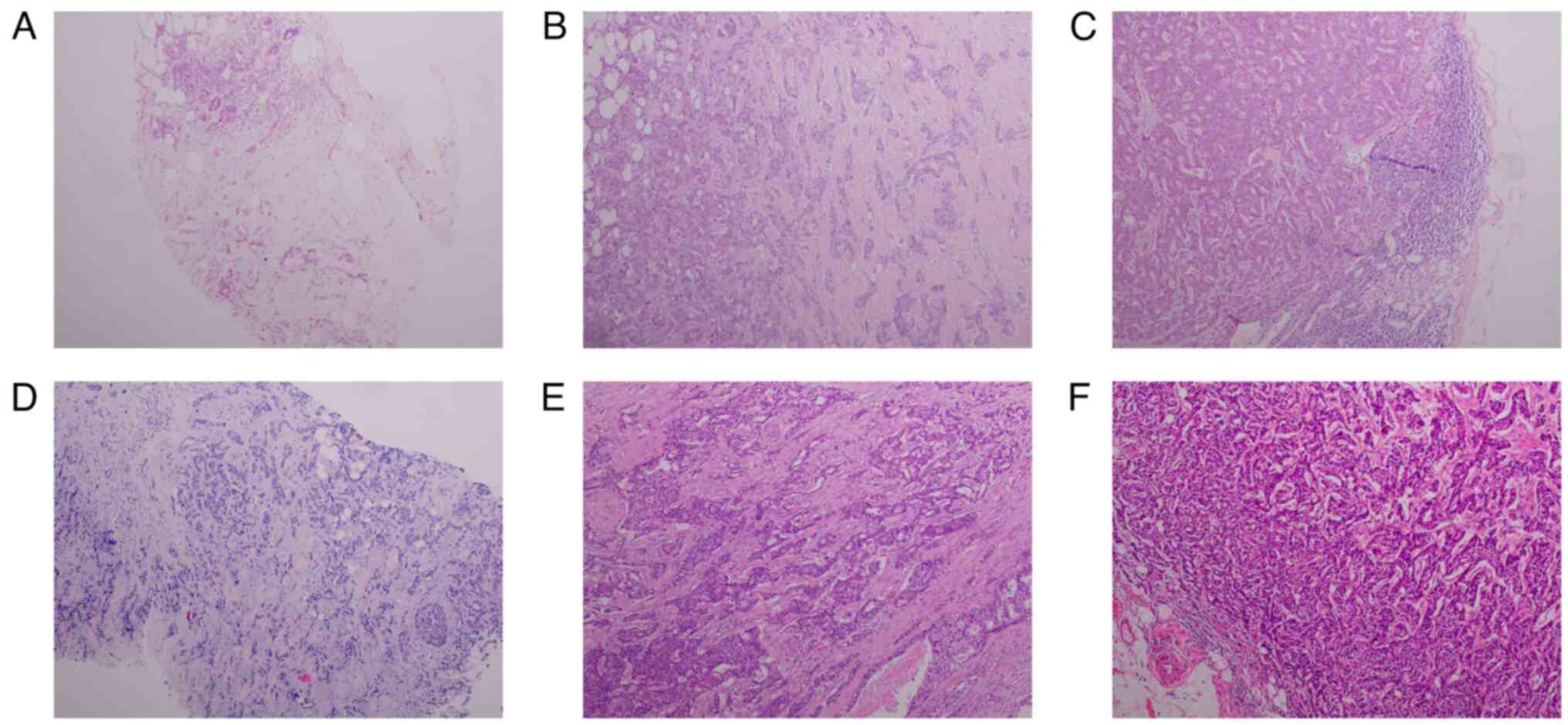
was confirmed on the basis of imaging and pathology tests (Fig. 1A) (14). Pathological testing was performed
by HE staining, tissues were removed and immediately immersed in 4%
PFA for 6 h for fixation at room temperature (RT). After
dehydration in a gradient of 70-100% alcohol, it was transparent
using xylene and finally embedded in solid paraffin. Tissue wax
blocks were finally cut into 4 µm thick sections. After staining
with hematoxylin for 7 min at room temperature to show the nuclei,
the cytoplasm was stained with eosin for 1 min at room temperature.
After gradient dehydration and xylene transparency, the sections
were sealed using neutral resin and then visualized using an
Olympus orthostatic microscope (Light microscope, objective
magnification 10X).
Considering the large size of the tumor in the left
breast of the patient, neoadjuvant chemotherapy (epirubicin +
paclitaxel regimen, i.v. over a period of 2 days, with 50 mg of
epirubicin given on the first day and 120 mg of paclitaxel given on
the second day. Dosing was started once 5 days before surgery and
once/month after surgery.) was initially administered to shrink the
tumor before the patient could undergo radical resection surgery.
Briefly, the patient underwent in September 2020 the patient
underwent 50 mg (dissolved in 250 ml of saline) of epirubicin
intravenous drip chemotherapy. The following day, 120 mg (dissolved
in 500 ml saline) paclitaxel IV drip chemotherapy was administered
and preoperative preparations were completed, with surgery
scheduled for day 4 after the completion of chemotherapy. After 4
days, the patient underwent a modified radical left breast cancer
surgery, taking into account the relatively small size of the tumor
and its location in a non-central area of the breast. Pathology and
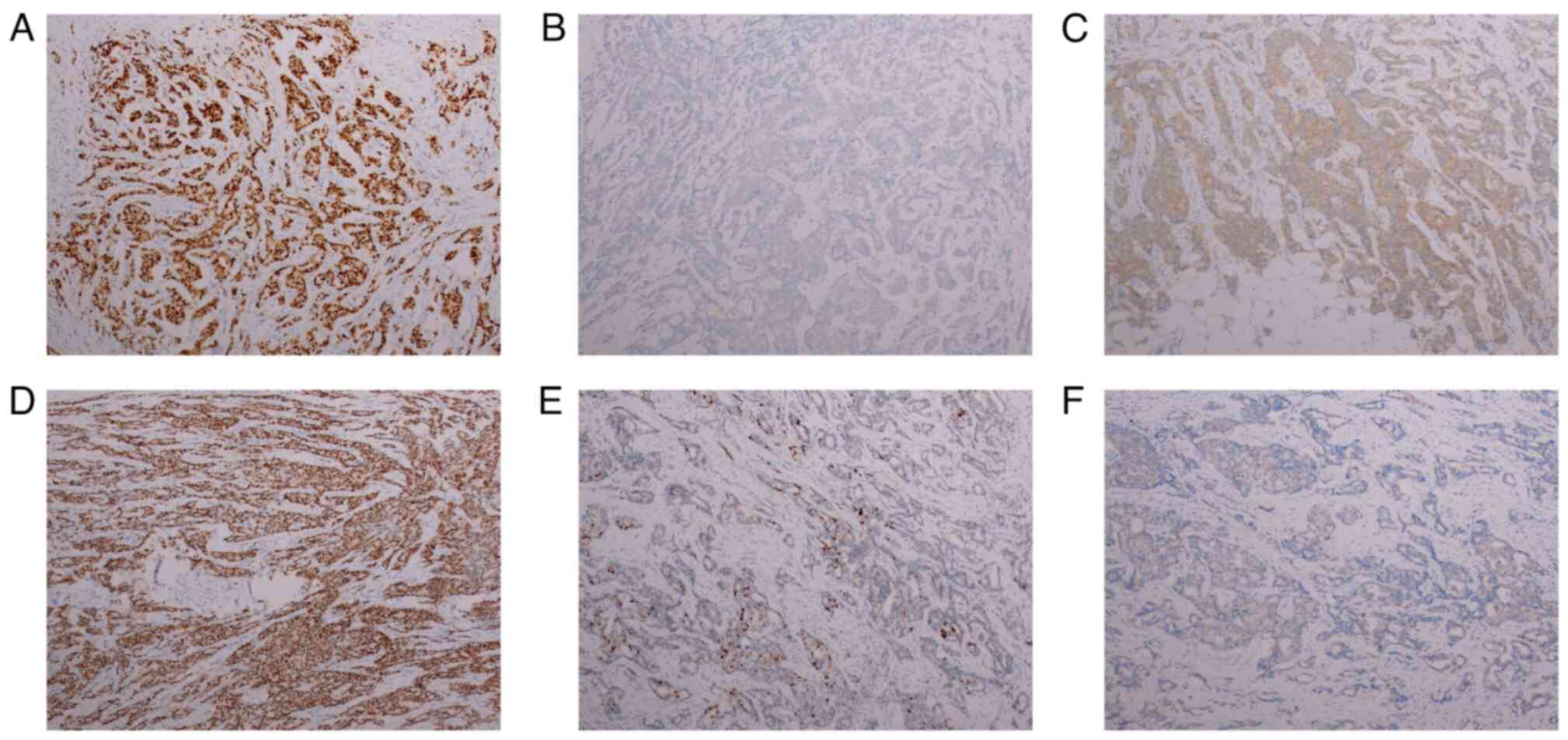
immunohistochemistry (IHC) results confirmed a diagnosis of
invasive ductal carcinoma, grade II, with metastases found in 28/35
axillary lymph nodes (Figs. 1B and
C, and 2A-C), 13 days after admission, the
patient was discharged. IHC was performed using paraffin-embedded
tissues; briefly, tissues were removed and immediately immersed in
4% PFA for 6 h for fixation (Room temperature, RT). After
dehydration in a gradient of 70-100% alcohol, it was transparent
using xylene and finally embedded in solid paraffin. Tissue wax
blocks were finally cut into 4 µm thick sections. Tissue processing
and interpretation of results were performed using Roche's
Benchmark GX. EDTAVEGTA pH 8 was selected for antigen repair and
was used for repair using machine parameters (100̊C, 30 min). And
endogenous catalase was closed with 3% hydrogen peroxide for 5 min
(RT). HER2, PR and ER monoclonal antibodies were used to incubate
at 37̊C for 30 min (ready-to-use antibody, Maixin, Fuzhou, China,
HER2: Kit-0043, 0013; ER: Kit-0012), respectively. Secondary
antibodies were incubated with ready-to-use HRP-labeled secondary
antibodies from Roche (Roche, Cat. No 760-500) for 8 min at room
temperature. This was followed by color development using DAB color
solution at room temperature for 8 min (Roche, Cas. No 760-500),
and hematoxylin staining was used for 7 min to show the nuclei
(RT). After gradient dehydration and xylene transparency, the
slices were sealed using neutral resin and observed using an
Olympus ortho-microscope (Light microscope, objective magnification
10X). The patient was admitted to the hospital for chemotherapy
with a 50 mg (dissolved in 250 ml saline) epirubicin IV drip and a
120 mg (dissolved in 500 ml saline) paclitaxel IV drip regimen the
following day, 21 days after left breast surgery (September 2020).
The patient received chemotherapy on day 54 (October 2020), day 80
(November 2020), day 107 (December 2020), day 134 (January 2021)
and day 162 (February 2021). Chemotherapy was administered
according to the same regimen for a total of 6 cycles. At 187 days
postoperatively (March 2021) the patient was readmitted to the
hospital and discharged after receiving 5 days of adaptive
intensity-modulated radiotherapy to the axillary region of the left
breast. This treatment was administered after consultation with a
radiologist, who determined that the patient met the criteria for
radiotherapy. After surgical treatment, the patient experienced a
progression-free survival of ~2 years.
A total of 2 years after the radical resection
surgery, in April 2022, the patient was admitted to the Weifang
Second People's Hospital after experiencing redness and swelling of
the right breast mass for 1 month. Considering the medical history
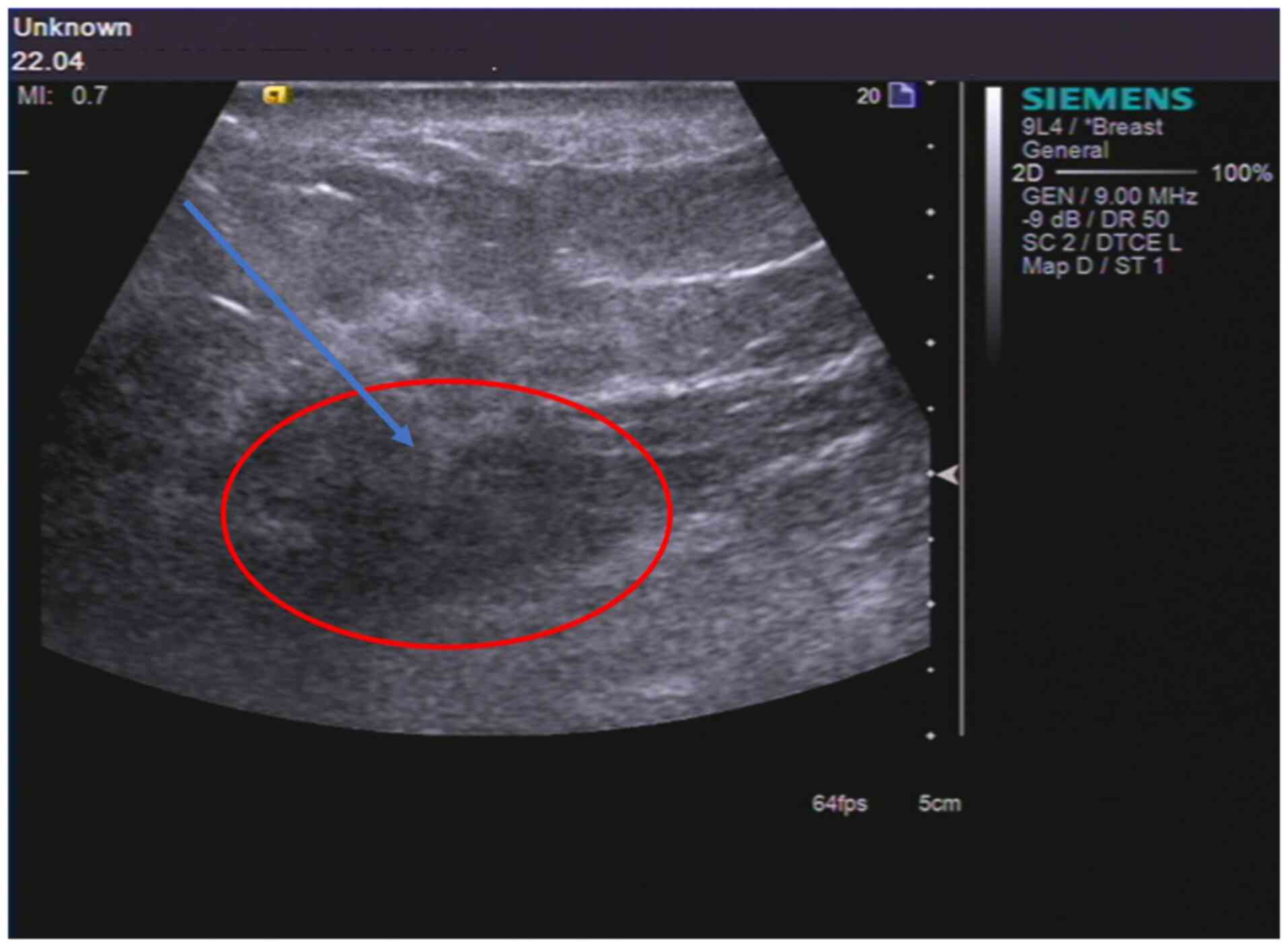
of the patient, an ultrasound-guided aspiration biopsy of the right
breast mass was performed (Fig.
3). The pathology results revealed stage III inflammatory
breast carcinoma (T4N0M1) in the right breast (Fig. 1D). The patient was treated with
chemotherapy (preoperative chemotherapy twice, postoperative
chemotherapy once a month) using an IV drip regimen of epirubicin
(80 mg dissolved in 250 ml saline) + paclitaxel (120 mg dissolved
in 500 ml saline). The patient received two rounds of chemotherapy
in April and May, respectively, and underwent a modified radical
surgery for right breast cancer 20 days after the end of
chemotherapy in May. In May 2022, the updated pathology report of
the right breast revealed grade III invasive carcinoma, with
visible vascular embolus and nerve invasion. No cancerous tissues
were found in the nipple or the base margin. Carcinoma metastasis
was identified in all 23 axillary lymph nodes and in all 3 of the
other lymph nodes assessed (Figs.
1E and F, and 2D-F). CT scan showed multiple microscopic
nodules in the lung, which raised concerns about metastatic tumors
(Fig. S2A). The patient was
discharged from the hospital 14 days after undergoing surgery on
the right breast and following chemotherapy treatment with an
epirubicin (90 mg dissolved in 250 ml saline) + cyclic AMP (0.8 g).
The chemotherapy was administered intravenous injection for 2 days,
with epirubicin injected on the first day and cyclophosphamide on
the second day, administered once a month.
A total of 34 days after the right breast surgery
(June 2022), a CT review revealed multiple microscopic nodules in
both lungs and MRI demonstrated possible secondary metastases in
the lumbosacral spine (Fig. S2B
and C). In conjunction with the
self-reported pain of the patient, zoledronic acid (4 mg in 100 ml
saline intravenous injection) was administered to alleviate the
pain from the bone metastasis. Additionally, chemotherapy with an
epirubicin (90 mg dissolved in 250 ml saline) + cyclic AMP (0.8 g
dissolved in 250 ml saline) regimen was initiated 1 day after
admission (July 2022). A total of 60 days after the right breast
surgery (July 2022), chemotherapy with epirubicin (90 mg dissolved
in 250 ml saline) + cyclic AMP (0.8 g dissolved in 250 ml saline)
regimen was administered.
The patient was admitted again in August 2022 for
the fourth time post- right breast surgery (The date of the
patient's current admission was noted as Day 0), and the patient
denied any recent gastrointestinal illness before admission. On
admission, the temperature of patient was 36.5˚C, heart rate was 70
beats/min, respiratory rate was 20 per min, and blood pressure was
139/78 mmHg. The patient presented with a cough and wheezing, and a
complete chest CT was performed to assess any postradiotherapy
changes in the left lung (Fig.
S2D). Additionally, nasopharyngeal swabs were taken and tested
negative for coronavirus disease 2019 (COVID-19). The patient was
initially diagnosed with secondary malignant tumors in both lungs,
secondary metastases in the ribs and secondary metastases in the
lumbosacral spine based on the lumbar spine MRI and thoracic spiral
CT scans.
The patient was treated with antitumor immunotherapy
with an IV drip of sintilimab (self-contained; cat. no. Innovent
Co. Suzhou China; DP2201036; 200 mg dissolved in 100 ml saline).
The patient experienced panic and palpitations. Although the
patient developed this irAE within 1 day of administration, the
symptoms did not develop within 1-3 h of the start of the infusion,
which indicated that it was not an infusion reaction. In Day 6,
Creatine kinase (CK) was 2263.2 U/l (reference values, 40-200 U/l),
cardiac troponin I (cTn I) was 18.01 ng/ml (reference values,
0-0.028 ng/ml), CK-isoenzymes (CK-MB) was 172 U/l (reference
values, 0-20 U/l) and N-terminal pro-brain natriuretic peptide was
9,121 pg/ml (reference values, 0-300 pg/ml), as presented in
Table I, indication of changes in
cardiac enzyme profiles. An electrocardiogram (Fig. 4) revealed sinus rhythm,
first-degree atrioventricular block, complete right bundle branch
block, frequent premature ventricular contractions and myocardial
ischemia. Acute coronary syndrome or immune-related myocarditis was
considered. After transfer to the cardiology department, a review
of the myocardial enzyme profile was performed (Fig. 5). The N-terminal brain-lysergic
peptide precursor was detected, and all the indices were elevated
compared with Day 0. This led to a preliminary diagnosis of acute
coronary syndrome and was treated with 0.5 g methylprednisolone
(IV) to prevent further deterioration of the patient condition.
Coronary angiography combined with stenting was proposed on Day 4
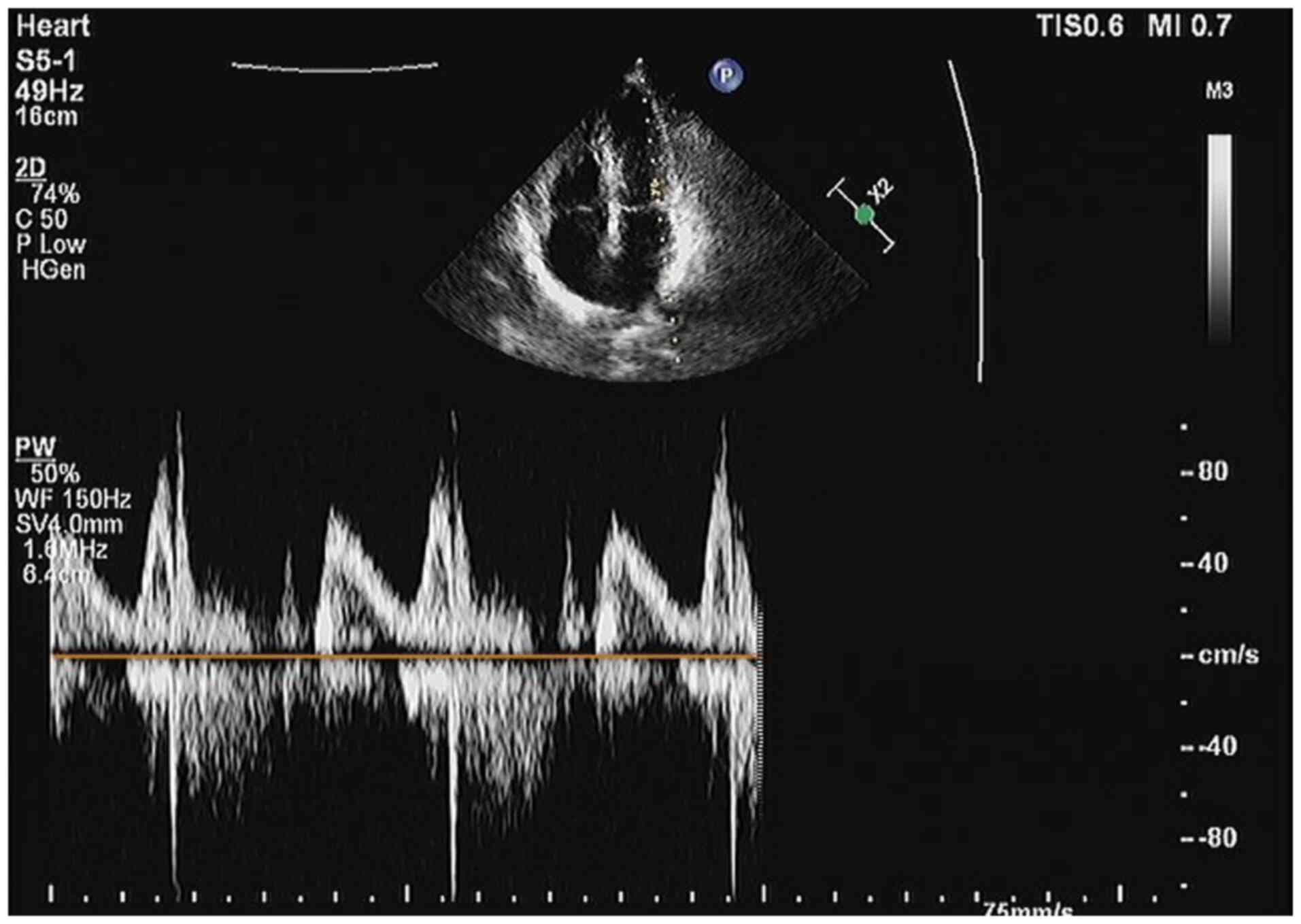
to determine the cause of the patient's condition. A cardiac
ultrasound determined that the patient did not have a medically
significant myocardial infarction (Fig. 6). Cardiac angiography showed that
there was no stenosis in the left main stem, 40% stenosis in the
middle of the left anterior descending branch, and no significant
stenosis in the echogenic branch (Fig.
7). The right crown was thick and smooth with no narrowing.
Based on the results of the electrocardiogram, cardiac enzyme
levels and a history of immunosuppressant medication, immune
myocarditis was considered. The patient was treated with hormone
shock therapy (methylprednisolone sodium succinate, 0.5 g dissolved
in 100 ml saline IV drip) specifically for this condition, which
was supplemented with pro-immunoglobulin (10 g daily by intravenous
injection until Day 11). As a result, the cTn I level decreased to
11.5 pg/ml (reference values, 0-300 pg/ml). On Day 6, paroxysmal
ventricular tachycardia was observed on monitoring. The
electrocardiogram indicated frequent ventricular premature beats
and a high risk of sudden cardiac death due to cardiac arrest
(Fig. 4B marked with red line).
Therefore, the patient was immediately placed on a single dose of
hormonal therapy with a single dose of the antiarrhythmic drug
esmolol administered intravenously along with 0.5 g
methylprednisolone, a hormonal shock treatment drug. The cardiac
enzyme spectra review on Day 10 demonstrated that the patient's
CK-MB levels had decreased compared with the previous test (116.6
U/l; reference values, 0-20 U/l), which suggested an improvement in
myocardial damage. Maintenance of hormone shock therapy and regular
review of the myocardial enzyme spectrum were performed until Day
22. The cardiac enzyme markers improved significantly, indicating a
good treatment response. The patient's general condition was
stabilized and the level of care was changed from level 1 to level
2, and the patient was observed every two hours for changes in
condition and vital signs. A new coronary nucleic acid test was
negative. It was agreed that the patient could be discharged. The
patient was discharged from the hospital on Day 22 and after
discharge the patient took the medicine of Spironolactone (20 mg),
tachycardia (20 mg) and potassium chloride extended-release tablets
(0.5 g) were administered to enhance the cardiac function of the
patient and to regulate blood potassium levels. Hormonal therapy
was continued until the patient was discharged from the hospital
after 10 days. The patient received 20 mg/day prednisone acetate
tablets in the morning from Days 1 to 5, which was then reduced to
10 mg/day after Day 5 and discontinued on Day 10. It was suggested
that the patient should improve their lifestyle after
hospitalization, including paying attention to rest, avoiding
exertion and emotional excitement, taking medication on time,
coming to the hospital regularly for review, and promptly
consulting a doctor if they felt unwell. One month after discharged
from the hospital, the patient was followed up by telephone and
there was no recurrence of myocarditis or other specific conditions
such as chest tightness and palpitations.
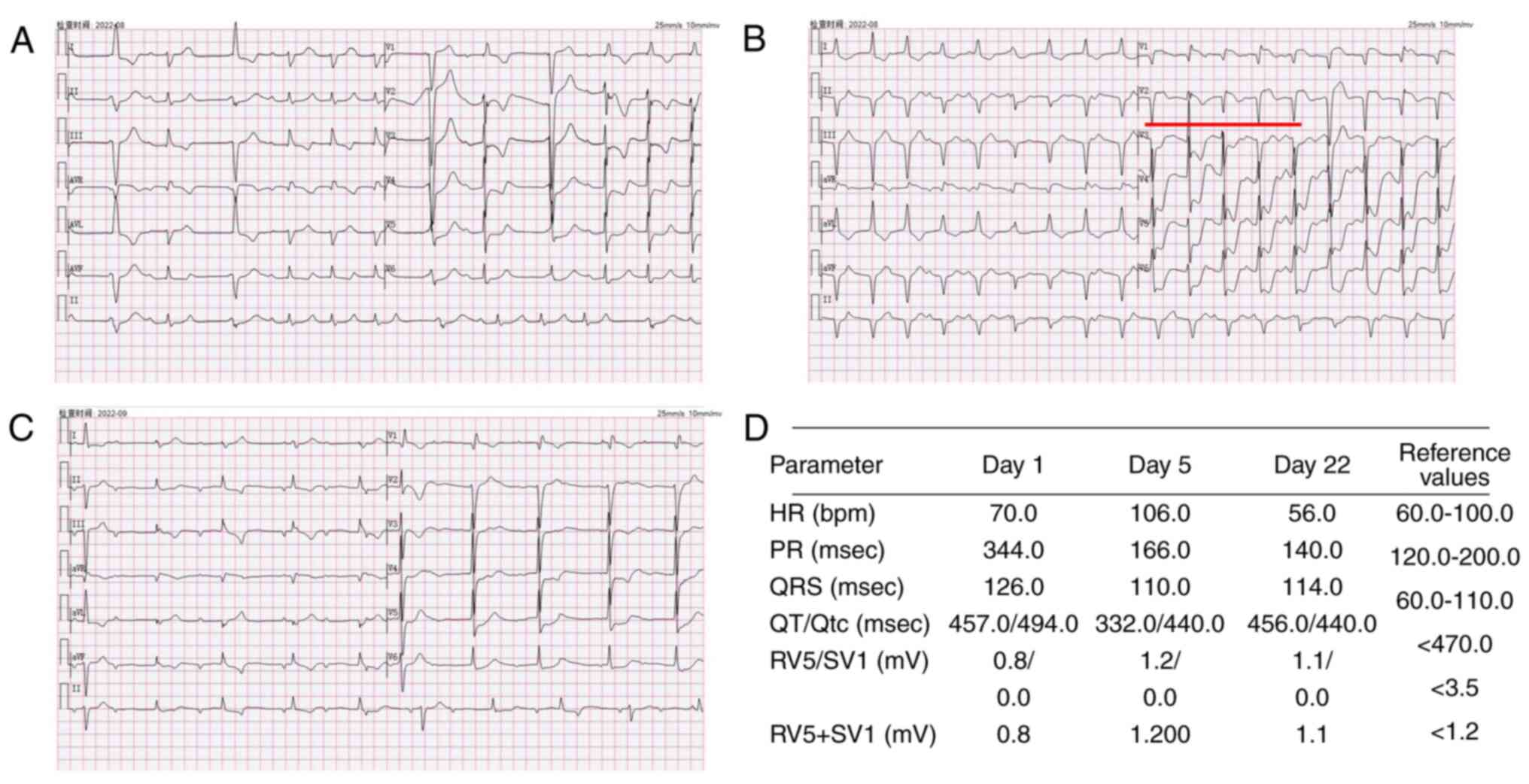 | Figure 4Electrocardiograms of the patient.
Electrocardiogram performed (A) after the patient developed
palpitations (B) prior to hormonal shock therapy and (C) prior to
discharge from the hospital, the areas marked in red indicate
frequent premature ventricular contractions. (D) Parameters related
to the electrocardiograms performed and their reference ranges.HR,
Heart rate; PR, P-R interval; QRS, Total time from Q-wave, R-wave,
and S-wave on the ECG; QT, Q wave) to the end of the T wave; Qtc,
The corrected QT interval, RV5 Amplitude of the R-wave recorded on
lead V5 in the fifth intercostal space in the left anterior
axillary line; SV1, Amplitude of the S-wave recorded on the V1 lead
in the fourth intercostal space next to the right sternum. |
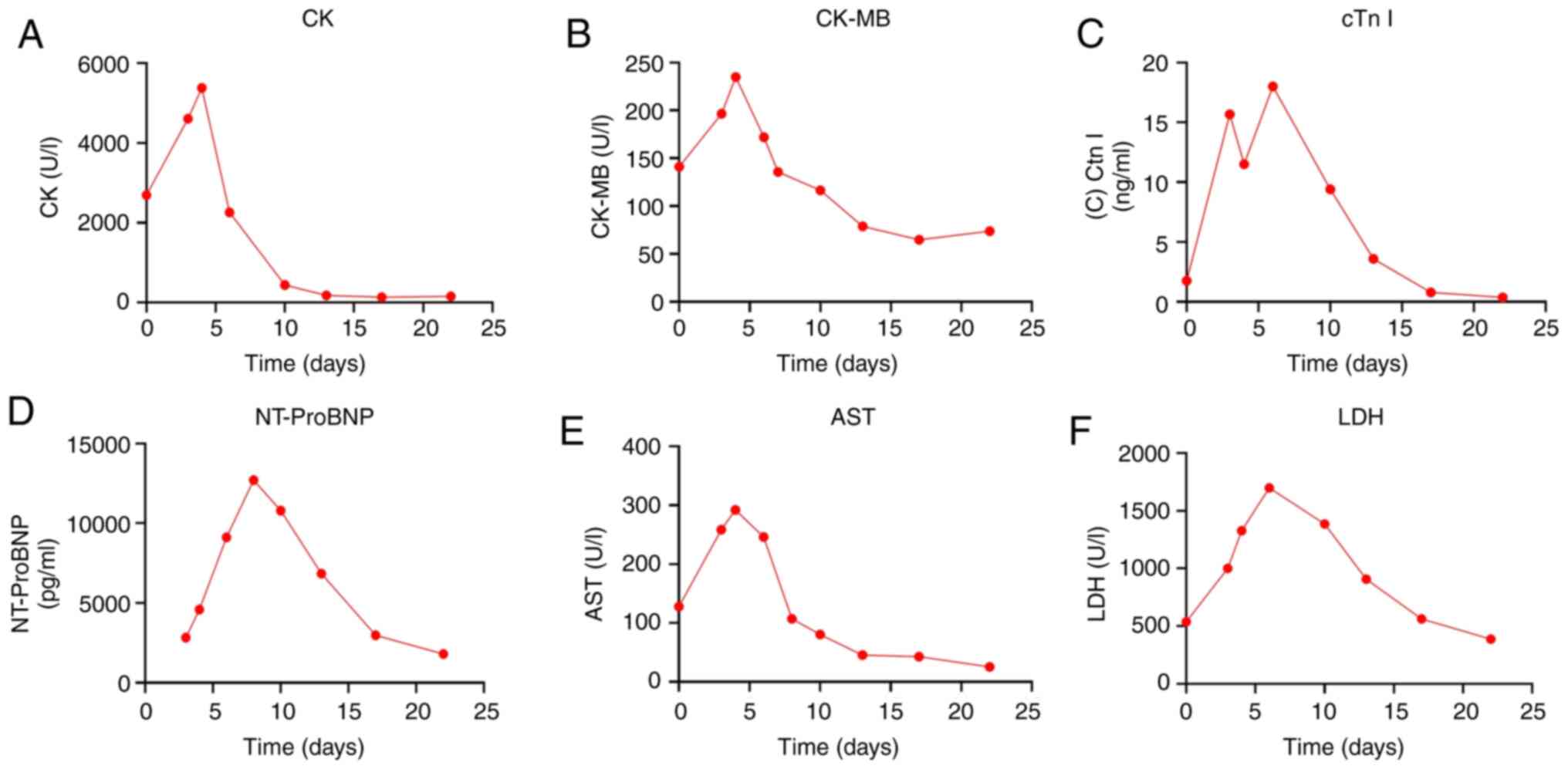 | Figure 5Changes in myocarditis-related
indicators during hospitalization. Changes in (A) CK, (B) CK-MB,
(C) cTn I, (D) NT-proBNP, (E) AST and (F) LDH. CK, creatine kinase;
CK-MB, creatine kinase isoenzymes; cTn I, cardiac troponin I;
NT-proBNP, N-terminal pro-brain natriuretic peptide; AST, aspartate
aminotransferase; LDH, lactate dehydrogenase. |
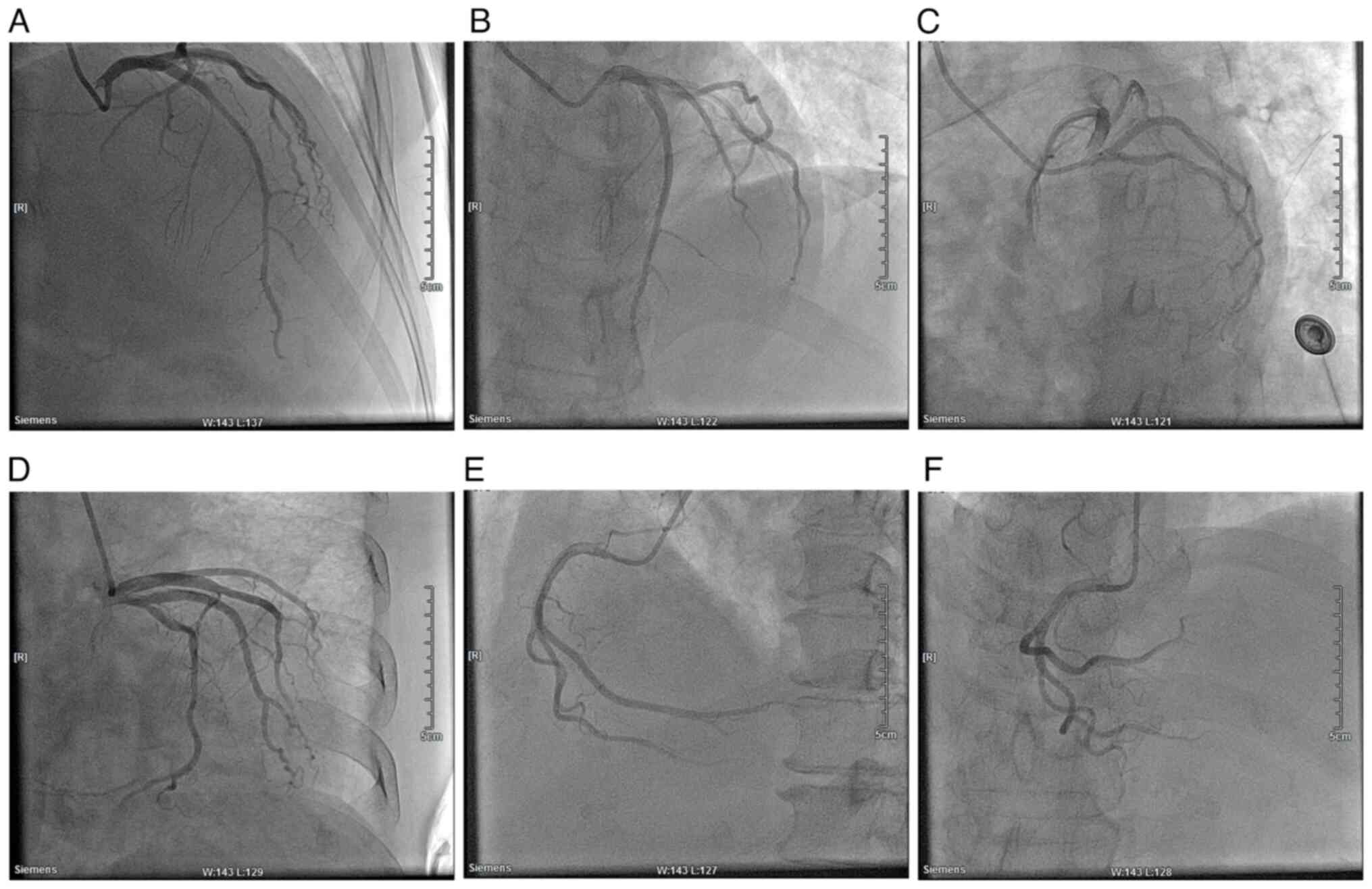 | Figure 7Cardiac angiography of the patient.
(A) RAO, 30˚ and CRA, 30˚. (B) LAO, 30˚ and CRA, 20˚. (C) LAO, 45˚
and CRU, 20˚. (D) RAO, 30˚ and CAU, 20˚. (E) LAO, 45˚. (F) CRA,
30˚. RAO, right anterior oblique; CRA, cranial; LAO, left anterior
oblique; CRU, caudal. |
 | Table IChanges in myocardial enzyme
profiles. |
Table I
Changes in myocardial enzyme
profiles.
| | Day | |
|---|
| Myocardial
enzyme | 0 | 6 | 10 | 13 | 22 | Reference
values |
|---|
| cTn I, ng/ml | 1.761 | 18.010 | 9.401 | 3.598 | 0.383 | 0.000-0.028 |
| LDH, U/l | 537.1 | 1,699.4 | 1,385.7 | 906.7 | 388.2 | 120.0-250.0 |
| CK, U/l | 2,693.2 | 2,263.2 | 443.7 | 183.5 | 158.1 | 40.0-200.0 |
| CK-MB, Ul | 141.2 | 172.0 | 116.6 | 78.9 | 73.9 | 0.0-25.0 |
| NT-proBNP,
pg/ml | 2,834 | 9,121 | 10,796 | 6,849 | 1,808 | 0-300 |
Discussion
Myocarditis is the clinical and histologic
manifestation of a widespread pathologic immune process in the
heart, which is divided into acute myocarditis and chronic
myocarditis (15). The common
causes of myocarditis include viral infections or immune responses
to viral infections (16). The
wide range of clinical manifestations makes the diagnosis of the
disease challenging. An attack of myocarditis is typically
characterized by the acute onset of chest pain, dyspnea and
palpitations (17). The diagnosis
of acute myocarditis is typically made by considering clinical
symptoms, electrocardiographic findings, echocardiographic results
and troponin levels; however, the gold standard for confirming the
diagnosis and distinguishing it from other conditions is an
endomyocardial myocardial biopsy, despite its limitations due to
the invasive nature of the procedure (18). Here, the patient developed symptoms
related to myocarditis at an early stage and was treated
appropriately, which can provide a clinical reference for the use
of chemotherapeutic agents in the PD-1 class resulting in
myocarditis.
Worldwide, viral infections are the most common
cause of myocarditis, the incidence of myocarditis is estimated to
be about 10 to 22 cases per 100,000 people (19). Although myocarditis caused by other
factors is less common, it still requires attention. For example,
myocarditis caused by autoimmune diseases, such as systemic lupus
erythematosus, accounts for ~7% of patients with myocarditis
(20). Additionally, drug-induced
myocarditis primarily occurs in patients who are vaccinated,
particularly following smallpox vaccination, with 13.2 cases per
100,000 doses. Following COVID-19 vaccination, the median incidence
was 1.9 cases per 100,000 people for the first two doses of the
BNT162b2 vaccine and 3.5 cases for the mRNA-1273 vaccine. Influenza
vaccination had an incidence of 0.13 cases per 100,000 doses, while
other non-smallpox vaccines had an incidence of 5.7 cases per
100,000 doses (21).
The patient in the present study developed
palpitations after treatment with Sintilimab. Myocardial enzyme
profiling revealed increased levels of markers of myocardial
injury, and cardiography indicated no significant stenotic lesions.
Considering the temporal association between the administration of
the ICI and the onset of clinical symptoms, as well as the
exclusion of other potential causes (such as normal coronary
arteries, no history of rheumatoid virus infections, no chronic
medications that could be linked to myocarditis, no history of
autoimmune disease and negative swabs for COVID-19 infection), it
was determined that the patient experienced immune myocarditis
caused by ICIs.
ICIs are commonly used as the initial treatment for
numerous types of cancer. These inhibitors work by blocking either
CTLA-4 or PD-1, which helps to reactivate the T-cells and enhance
their ability to eliminate tumors (22-24).
Sintilizumab is a recombinant, fully human IgG4-type anti-PD-1
monoclonal antibody that blocks the interaction between PD-1 and
its ligands, thereby helping T cells to regain their anti-tumor
effects (25-27).
Typically, the heart is susceptible to immune-mediated injury due
to its dense vascular system providing access to immune cells and
antibodies. Normally, the presence of cardiomyocytes, including Th1
cells that secrete the cytokine IFN-γ, causes cardiac endothelial
cells to upregulate PD-L1 to suppress effector T cells. Sintilimab
was able to block the interaction between PD-1 and its ligand,
which may lead to effector T cell attack on cardiac endothelial
cells (28). According to its
antitumor mechanism, this drug may cause irAEs.
Immune myocarditis is a rare but highly lethal
complication, and the mechanism underlying this condition has not
yet been fully elucidated, the incidence ranges from 0.06% in
patients treated with single-agent anti-PD-1 therapy to 0.27% in
patients treated with a combination of anti-CTLA-4 and anti-PD-1
therapy (29). A retrospective
analysis of the World Health Organization Pharmacovigilance
Database revealed that immune-associated myocarditis had the
highest mortality rate among all immune-related adverse events
(irAEs), with fatalities reported in ~ 40% of the 131 cases
(30); however, previous studies
have reported that the presence of high-frequency T-cell receptor
sequences in cardiomyocytes suggests that they may share targets
with tumor cells (31,32). Animal model studies have reported
that CTLA-4, PD-1 and PD-L1 have cardioprotective effects against
immune-mediated injury following stress (28,33,34).
This suggests a potential mechanism for the development of
myocarditis in ICIs. However, since the median time to onset of
immune myocarditis due to ICIs is 34 days after initiation of ICI
therapy, early symptoms can be easily dismissed as other
cardiovascular diseases, leading to delayed treatment (29).
The present study reports a case of immune
myocarditis induced by ICIs. The cardiac enzyme profiles throughout
the course of the disease were recorded and detailed. Although the
incidence of immune myocarditis due to ICIs is relatively low
(about 0.06-0.27%), the median time of onset of the disease is
typically ~1 month after starting ICI therapy (35). However, in the present study, the
patient developed palpitations 2 days after receiving the drug.
Additionally, there was a marked increase in the levels of cardiac
enzyme-related markers within a short period of time. It was
hypothesize that this phenomenon could be due to several reasons:
First, the patient had diabetes mellitus. A retrospective study by
Mahmood et al (36),found
that a higher proportion of patients who developed myocarditis had
a higher prevalence of diabetes mellitus (both type I and type II)
compared with those who did not, suggesting that diabetes mellitus
may be an unfavorable factor for the elicitation of myocarditis
while undergoing ICIs (37).
Second, the patient received the EC-T regimen (Epirubicin,
Cyclophosphamide and Paclitaxel) of chemotherapy before ICIs. The
cardiotoxicity of epirubicin accumulated and resulted in a notable
increase in cardioactive enzymes in the patient prior to treatment
with ICIs. Certain patients may exhibit myocardial damage prior to
treatment with ICIs, which could also contribute to the early onset
of immune myocarditis (38).
Notably, when considering myocarditis caused by ICIs, it is
important to also consider other potential diagnoses, which may
include acute coronary syndrome, stress cardiomyopathy and viral
myocarditis. These conditions can be challenging to accurately
diagnose based solely on clinical presentation (39-41).
Comprehensive coronary CT angiography, invasive coronary
angiography, electrocardiogram, infection indicators and patient
history can help clinicians make a differential diagnosis.
Finally, there are certain limitations in the
present study. Detection of anti-myosin antibodies and anti-cardiac
troponin antibodies by flow cytometry could have provided more
accurate data to help clinicians make a diagnosis; however, the
Weifang Second People's Hospital did not have this kind of testing
equipment to carry out anti-myosin antibody and anti-cardiac
troponin antibody testing, and there was insufficient time to send
patient samples to a third-party testing organization with relevant
capabilities for testing or to carry out this kind of testing, due
to the patient's condition deteriorating rapidly and the need to
carry out rescue as soon as possible. There was also insufficient
time to send samples to a competent third-party testing
organization for testing.
In summary, although immune myocarditis is a rare
side effect of ICI therapy and has a delayed onset, it is important
to closely monitor patients for any signs of myocarditis.
Additionally, timely detection of myocardial enzyme profiles should
be performed after the drug is administered clinically. For
patients with underlying medical conditions such as diabetes
mellitus, and patients who have received chemotherapy using
anthracyclines prior to dosing, it may be advisable to administer a
specific dosage of hormones beforehand to mitigate potential
adverse effects, if deemed necessary.
Supplementary Material
Color chest ultrasound, MRI and CT
scan images. The areas circled in red are suspected tumor areas).
Color chest ultrasound of the (A) left intramammary nodule, (B)
Pre-operative changes in the left breast and (C) left breast
lesion. (D) MIR images of the breast (E) Magnetic resonance dynamic
enhancement silhouette of the patient. (F) Magnetic resonance
dynamic enhancement time-one signal intensity curves in patients.
(G) Chest CT scan. MRI, magnetic resonance imaging; CT, computed
tomography.
Chest CT and MRI results. (A) Multiple
microscopic nodules in the lungs. (B) Review of multiple
microscopic nodules in the lungs. (C) Lumbar spine MRI. (D) Chest
CT. Nodes circled in red are suspected secondary metastases. MRI,
magnetic resonance imaging; CT, computed tomography.
Acknowledgements
Not applicable.
Funding
Funding: The present study was supported by the Shandong
Province School Health Association (grant no. SDWS2023179).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
YL was the primary care physician of the patient and
developed and implemented the treatment plan. YW and BW performed
the literature review and analyzed and interpreted the data in the
paper. BZ and BY organized the patient's treatment process and
analysis of the data. MQ and YT drafted the work and critically
revised important intellectual content. MQ and YT confirm the
authenticity of all the raw data MQ completed immunohistochemical
examination and imaging analysis. YT performed the histological
examination of the tumor and wrote the manuscript. All authors
commented on the manuscript and agreed with the conclusions of the
manuscript. All authors have read and approved the final
manuscript.
Ethics approval and consent to
participate
This study was reviewed by the Ethics Committee of
the Weifang Second People's Hospital (approval no. YX2020-001-01)
and was performed in accordance with the Declaration of
Helsinki.
Patient consent for publication
The patient provided written informed consent for
the publication of the manuscript including any identifying images
or data.
Competing interests
The authors declare that they have no competing
interests.
Use of artificial intelligence tools
During the preparation of this work, artificial
intelligence tools were used to improve the readability and
language of the manuscript or to generate images, and subsequently,
the authors revised and edited the content produced by the
artificial intelligence tools as necessary, taking full
responsibility for the ultimate content of the present
manuscript.
References
|
1
|
Chen W, Huang Y, Pan W, Xu M and Chen L:
Strategies for developing PD-1 inhibitors and future directions.
Biochem Pharmacol. 202(115113)2022.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Yi M, Zheng X, Niu M, Zhu S, Ge H and Wu
K: Combination strategies with PD-1/PD-L1 blockade: Current
advances and future directions. Mol Cancer. 21(28)2022.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Ghahremani Dehbokri S, Alizadeh N,
Isazadeh A, Baghbanzadeh A, Abbaspour-Ravasjani S, Hajiasgharzadeh
K and Baradaran B: CTLA-4: As an immunosuppressive immune
checkpoint in breast cancer. Curr Mol Med. 23:521–526.
2023.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Chennamadhavuni A, Abushahin L, Jin N,
Presley CJ and Manne A: Risk factors and biomarkers for
immune-related adverse events: A practical guide to identifying
high-risk patients and rechallenging immune checkpoint inhibitors.
Front Immunol. 13(779691)2022.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Darnell EP, Mooradian MJ, Baruch EN,
Yilmaz M and Reynolds KL: Immune-related adverse events (irAEs):
Diagnosis, management, and clinical pearls. Curr Oncol Rep.
22(39)2020.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Das S and Johnson DB: Immune-related
adverse events and anti-tumor efficacy of immune checkpoint
inhibitors. J Immunother Cancer. 7(306)2019.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Cozma A, Sporis ND, Lazar AL, Buruiana A,
Ganea AM, Malinescu TV, Berechet BM, Fodor A, Sitar-Taut AV, Vlad
VC, et al: Cardiac toxicity associated with immune checkpoint
inhibitors: A systematic review. Int J Mol Sci.
23(10948)2022.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Dolladille C, Ederhy S, Sassier M, Cautela
J, Thuny F, Cohen AA, Fedrizzi S, Chrétien B, Da-Silva A, Plane AF,
et al: Immune checkpoint inhibitor rechallenge after immune-related
adverse events in patients with cancer. JAMA Oncol. 6:865–871.
2020.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Salvi A, Di Lenarda A, Dreas L, Silvestri
F and Camerini F: Immunosuppressive treatment in myocarditis. Int J
Cardiol. 22:329–338. 1989.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Rubio-Infante N, Ramirez-Flores YA,
Castillo EC, Lozano O, Garcia-Rivas G and Torre-Amione G: A
systematic review of the mechanisms involved in immune checkpoint
inhibitors cardiotoxicity and challenges to improve clinical
safety. Front Cell Dev Biol. 10(851032)2022.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Cooper LT Jr, Berry GJ and Shabetai R:
Idiopathic giant-cell myocarditis-natural history and treatment.
Multicenter giant cell myocarditis study group investigators. N
Engl J Med. 336:1860–1866. 1997.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Moslehi J, Lichtman AH, Sharpe AH,
Galluzzi L and Kitsis RN: Immune checkpoint inhibitor-associated
myocarditis: Manifestations and mechanisms. J Clin Invest.
131(e145186)2021.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Norwood TG, Westbrook BC, Johnson DB,
Litovsky SH, Terry NL, McKee SB, Gertler AS, Moslehi JJ and Conry
RM: Smoldering myocarditis following immune checkpoint blockade. J
Immunother Cancer. 5(91)2017.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Giuliano AE, Edge SB and Hortobagyi GN:
Eighth edition of the AJCC cancer staging manual: Breast cancer.
Ann Surg Oncol. 25:1783–1785. 2018.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Ammirati E and Moslehi JJ: Diagnosis and
treatment of acute myocarditis: A review. JAMA. 329:1098–1113.
2023.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Matsumori A: Myocarditis and autoimmunity.
Expert Rev Cardiovasc Ther. 21:437–451. 2023.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Cooper LT Jr: Myocarditis. N Engl J Med.
360:1526–1538. 2009.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Lampejo T, Durkin SM, Bhatt N and Guttmann
O: Acute myocarditis: Aetiology, diagnosis and management. Clin Med
(Lond). 21:e505–e510. 2021.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Olejniczak M, Schwartz M, Webber E,
Shaffer A and Perry TE: Viral myocarditis-incidence, diagnosis and
management. J Cardiothorac Vasc Anesth. 34:1591–1601.
2020.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Rose NR: Myocarditis: Infection versus
autoimmunity. J Clin Immunol. 29:730–737. 2009.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Ling RR, Ramanathan K, Tan FL, Tai BC,
Somani J, Fisher D and MacLaren G: Myopericarditis following
COVID-19 vaccination and non-COVID-19 vaccination: A systematic
review and meta-analysis. Lancet Respir Med. 10:679–688.
2022.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Kgokolo MCM, Anderson K, Siwele SC, Steel
HC, Kwofie LLI, Sathekge MM, Meyer PWA, Rapoport BL and Anderson R:
Elevated levels of soluble CTLA-4, PD-1, PD-L1, LAG-3 and TIM-3 and
systemic inflammatory stress as potential contributors to immune
suppression and generalized tumorigenesis in a cohort of South
African xeroderma pigmentosum patients. Front Oncol.
12(819790)2022.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Im E, Sim DY, Lee HJ, Park JE, Park WY, Ko
S, Kim B, Shim BS and Kim SH: Immune functions as a ligand or a
receptor, cancer prognosis potential, clinical implication of VISTA
in cancer immunotherapy. Semin Cancer Biol. 86:1066–1075.
2022.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Zhou X, Khan S, Huang D and Li L: V-Set
and immunoglobulin domain containing (VSIG) proteins as emerging
immune checkpoint targets for cancer immunotherapy. Front Immunol.
13(938470)2022.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Dall'Olio FG, Marabelle A, Caramella C,
Garcia C, Aldea M, Chaput N, Robert C and Besse B: Tumour burden
and efficacy of immune-checkpoint inhibitors. Nat Rev Clin Oncol.
19:75–90. 2021.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Zhang L, Mai W, Jiang W and Geng Q:
Sintilimab: A promising anti-tumor PD-1 antibody. Front Oncol.
10(594558)2020.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Hoy SM: Sintilimab: First global approval.
Drugs. 79:341–346. 2019.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Grabie N, Gotsman I, DaCosta R, Pang H,
Stavrakis G, Butte MJ, Keir ME, Freeman GJ, Sharpe AH and Lichtman
AH: Endothelial programmed death-1 ligand 1 (PD-L1) regulates CD8 +
T-cell-mediated injury in the heart. Circulation. 116:2062–2071.
2007.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Inno A, Tarantini L, Parrini I,
Spallarossa P, Maurea N, Bisceglia I, Silvestris N, Russo A and
Gori S: Cardiovascular effects of immune checkpoint inhibitors:
More than just myocarditis. Curr Oncol Rep. 25:743–751.
2023.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Wang DY, Salem JE, Cohen JV, Chandra S,
Menzer C, Ye F, Zhao S, Das S, Beckermann KE, Ha L, et al: Fatal
toxic effects associated with immune checkpoint inhibitors: A
systematic review and meta-analysis. JAMA Oncol. 4:1721–1728.
2018.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Johnson DB, Balko JM, Compton ML, Chalkias
S, Gorham J, Xu Y, Hicks M, Puzanov I, Alexander MR, Bloomer TL, et
al: Fulminant myocarditis with combination immune checkpoint
blockade. N Engl J Med. 375:1749–1755. 2016.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Reuben A, Petaccia de Macedo M, McQuade J,
Joon A, Ren Z, Calderone T, Conner B, Wani K, Cooper ZA, Tawbi H,
et al: Comparative immunologic characterization of autoimmune giant
cell myocarditis with ipilimumab. Oncoimmunology.
6(e1361097)2017.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Grabie N, Lichtman AH and Padera R: T cell
checkpoint regulators in the heart. Cardiovasc Res. 115:869–877.
2019.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Ise W, Kohyama M, Nutsch KM, Lee HM, Suri
A, Unanue ER, Murphy TL and Murphy KM: CTLA-4 suppresses the
pathogenicity of self antigen-specific T cells by cell-intrinsic
and cell-extrinsic mechanisms. Nat Immunol. 11:129–135.
2010.PubMed/NCBI View
Article : Google Scholar
|
|
35
|
Zhang C, Qin S and Zuo Z: Immune-related
myocarditis in two patients receiving camrelizumab therapy and
document analysis. J Oncol Pharm Pract. 28:1350–1356.
2022.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Mahmood SS, Fradley MG, Cohen JV, Nohria
A, Reynolds KL, Heinzerling LM, Sullivan RJ, Damrongwatanasuk R,
Chen CL, Gupta D, et al: Myocarditis in patients treated with
immune checkpoint inhibitors. J Am Coll Cardiol. 71:1755–1764.
2018.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Varricchi G, Galdiero MR, Marone G,
Criscuolo G, Triassi M, Bonaduce D, Marone G and Tocchetti CG:
Cardiotoxicity of immune checkpoint inhibitors. ESMO Open.
2(e000247)2017.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Lee SH, Cho I, You SC, Cha MJ, Chang JS,
Kim WD, Go KY, Kim DY, Seo J, Shim CY, et al: Cancer
therapy-related cardiac dysfunction in patients treated with a
combination of an immune checkpoint inhibitor and doxorubicin.
Cancers (Basel). 14(2320)2022.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Wieczorkiewicz P, Supel K, Przybylak K,
Kacprzak M and Zielinska M: Acute coronary syndrome versus acute
myocarditis in young adults-value of speckle tracking
echocardiography. PLoS One. 17(e0271483)2022.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Canan A, Van Woerkom RC and Rajiah PS:
Myocarditis mimicking stress-induced (takotsubo) cardiomyopathy.
Tex Heart Inst J. 49(e207430)2022.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Pollack A, Kontorovich AR, Fuster V and
Dec GW: Viral myocarditis-diagnosis, treatment options, and current
controversies. Nat Rev Cardiol. 12:670–680. 2015.PubMed/NCBI View Article : Google Scholar
|