Introduction
Atherosclerosis (AS) is a prevalent cardiovascular
disease characterized by the accumulation of lipid deposits in the
arterial walls (1). Endothelial
cells (ECs) form a layer of flat cells distributed along the inner
walls of blood vessels. The development of AS is closely associated
with EC impairment, with programmed cell death emerging as the
central mechanism responsible for EC injury, encompassing necrosis,
apoptosis and ferroptosis (2,3).
Ferroptosis, a form of programmed cell death, is
distinctive due to its iron-dependent lipid peroxidation (4,5).
Fe2+ oxidizes lipids, leading to the accumulation of
reactive oxygen species (ROS), ultimately culminating in oxidative
cell death (6,7). Modulating the ferroptosis pathway
exhibits potential as a therapeutic strategy for mitigating the
progression of various diseases (8). Results of previous studies suggested
that ferroptosis plays a significant role in the pathogenesis of
AS, through processes involving iron overload, oxidative stress and
lipid peroxidation (9-12).
Metabolism disorders of iron and lipids are implicated in
endothelial cell ferroptosis and vascular toxicity (13). However, the molecular mechanisms
underlying ferroptosis in ECs in AS remain to be fully
elucidated.
Acyl-CoA synthetase long chain family member 4
(ACSL4), a member of the ACSL family, is predominantly expressed in
the endoplasmic reticulum and mitochondrial membranes, catalyzing
the metabolism of long-chain free fatty acids into acyl-CoA
(14). Results of a previous study
revealed that ACSL4 plays a crucial role in promoting ferroptosis
through the synthesis of membrane phospholipids. This leads to an
increased susceptibility of cells to ferroptosis inducers, such as
RSL3 [(1S,3R)-RSL3] (15).
However, the regulation of ACSL4 in the pathogenesis of AS remains
to be fully elucidated. Zinc finger translocation-associated
protein (ZFTA) is a Cys2-His2 (C2H2) zinc finger protein containing
678 amino acids. Results of previous studies demonstrated that ZFTA
interacts with various transcriptional coactivators in
translocations and is involved in transcriptional regulation
(16,17). However, the potential role of ZFTA
in the formation of atherosclerotic plaques remains unclear. The
present study hypothesized that ACSL4 and ZFTA may modulate
ferroptosis in endothelial cells, thereby influencing the
pathogenesis of AS.
Materials and methods
Human sample
The patient samples and the control samples for this
study were collected from the Department of Vascular Surgery,
Nanfang Hospital, Southern Medical University, Guangzhou, China,
between May 2013 and May 2015. A total of 20 human atherosclerotic
plaque samples were obtained from patients undergoing carotid
endarterectomy. Control samples were arteries without macroscopic
evidence of atherosclerosis, obtained from individuals who died
from either a traffic accident or cerebral edema. The plaque
tissues were pathologically confirmed as primary atherosclerosis
and immediately frozen in liquid nitrogen at -196˚C. Exclusion
criteria included diabetes, cancer, congestive heart failure,
valvular heart disease, hematological system diseases, autoimmune
disease and/or infections. The basic information of the patients
included name, age and sex. Ethics approval was obtained from the
Ethics Committee of The Women and Children's Medical Center
(approval no. 286B01). The present study adhered to the principles
outlined in the Declaration of Helsinki. All patients or relatives
of deceased individuals provided written informed consent prior to
the study.
Cell culture
HUVECs and 293T cells were acquired from the ATCC
and cultivated in conditioned high-glucose DMEM (Gibco; Thermo
Fisher Scientific, Inc.) with added 10% fetal bovine serum and 100
U/ml penicillin/streptomycin (Invitrogen; Thermo Fisher Scientific,
Inc.). HUVECs-LV-ZFTA cells and HUVECs-LV-Mock cells by were
isolated by inoculating lentiviruses LV-ZFTA and LV-Mock and
selecting with puromycin. All cell lines were sustained in a
controlled environment at 37˚C with 5% CO2.
Lentivirus production and cell line
infection
To construct a vector overexpression ZFTA (LV-ZFTA),
the ZFTA gene (Gene ID: 65998) was amplified by PCR (Primer
forward: 5'-TTGAATTCATGGAGCCCGGCGGGGA-3', reverse:
5'-TTGGATCCCTACGCCCGACACACAGCG-3') and inserted into the
EcoRI and BamHI restriction enzyme sites of the
lentiviral vector CSII-EF-MCS-IRES2-Venus (Riken; cat. no.
RDB04384). The CSII-EF-MCS-IRES2-Venus was used as LV-Mock control.
For lentivirus production, the generation system used 2nd
generation lentiviral system. 293T cells were cultured with DMEM
medium and 4x106 cells were seeded per 10-cm dish. On
the next day, the cells were transfected with lentivirus plasmids
(13 µg), an encoding vesicular stomatitis virus (VSV-G) envelope
protein plasmid pMD.2G (6 µg) and a packaging plasmid psPAX2 (12
µg) using the Lipofectamine® 3000 reagent (Invitrogen;
Thermo Fisher Scientific, Inc.). Transfection was performed at 37˚C
for 6 h. The culturing medium was changed at 6 h post-transfection,
and the supernatants were harvested 48 h later. Cell line were
established by infecting human HUVECs cells with lentiviruses
supernatants at an MOI of 10 with 8 µg/ml polybrene for 24 h,
Venus-positive cells were sorted by flow cytometry. All cell lines
were maintained in a humidified atmosphere containing 37˚C and 5%
CO2. Efficient ZFTA overexpression was verified by
quantitative RT-PCR (Fig.
S1).
CRISPR/Cas9 knockout cells
The CRISPR/Cas9 system was used to generate clones
of cells with ACSL4 gene disruptions. ACSL4 single guide (sg)RNAs
(5'-aatcgcagagtgaataactt-3' and 5'-tcatgggctaaatgaatctg-3') were
designed using CHOPCHOP online tool (http://chopchop.cbu.uib.no/). These sgRNAs were cloned
into the lentiCRIPSR v2 vector, which contains the Cas9 gene (cat.
no. 52961; Addgene, Inc.). The targeted exon was exon 3 of the
ACSL4 gene, which affects the acyl-CoA synthetase domain. 293T
cells were used to package the lentivirus. FuGENE HD Reagent
(Promega Corporation) was used to introduce 400 ng of lentiCRISPR
v2 containing sgRNA, 400 ng of pSPAX2 (cat. no. 12260; Addgene,
Inc.) and 200 ng of pMD2G (cat. no. 12259; Addgene, Inc.) into 293T
cells (12-well plate). Culture supernatants were filtered through a
0.45 m filter after two days and then used for gene transduction. A
lentivirus carrying sgRNA was transduced into HUVEC cells and
selected with puromycin (2 µg/ml). Single clones were selected and
expanded with mutations characterized as small insertions or
deletions (indels), resulting in frameshift mutations that likely
disrupt the ACSL4 protein function. Detecting ACSL4 expression by
immunoblotting were performed. Cells expressing non-targeting
sgRNAs (5'-tgcgaatacgcccacgcgat-3' and 5'-atcgcgtgggcgtattcgca-3')
served as a negative control.
Cell viability assays
CCK8 (Cell Counting Kit-8) was purchased from MC
Express to measure cell viability. In brief, cells were seeded into
96-well plates and cultured overnight. A glutathione peroxidase 4
inhibitor, RSL-3 (Selleck Chemicals), was then applied to the cells
for 24 h. Following treatment for the indicated time points, 10 µl
CCK-8 reagent were introduced to each well of the plate, followed
by an incubation period at 37˚C for 1 h. Subsequently, the optical
density (OD) value was determined using a BioTek Instrument (BioTek
China) at 450 nm.
Reverse transcription-quantitative
(RT-q) PCR
Total RNA from patient atherosclerotic plaques and
HUVECs (1x106/well in 6-well plates) was extracted using
TRIzol® reagent (Invitrogen; Thermo Fisher Scientific,
Inc.) according to the manufacturer's protocol. Additionally, 1 µg
RNA was reverse transcribed into cDNA using the HI Script Q RT
SuperMix (Vazyme Biotech Co., Ltd.) according to the manufacturer's
instructions. RT-qPCR was performed using the cDNA templates and
SYBR (Takara Bio, Inc.) in a Bio-Rad CFX96 system (Bio-Rad
Laboratories, Inc.). The PCR cycling conditions were: denaturation
at 95˚C for 30 sec, annealing at 60˚C for 30 sec, and extension at
72˚C for 30 sec, for a total of 40 cycles. Gene expression levels
were calculated using the 2-ΔΔCq method, normalized to β-actin, as
described by Livak and Schmittgen (18). The experiments were replicated
three times. The primers were used in RT qPCR as follows: ACSL4
forward, 5'-CATCCCTGGAGCAGATACTCT-3'; ACSL4 reverse,
5'-TCACTTAGGATTTCCCTGGTCC-3'. ZFTA forward,
5'-CTCAAGGTGAGCACCATCAAG-3'; ZFTA reverse,
5'-GCTCCTCAGGCGTGAAGTC-3'. β-actin forward,
5'-CCAACCGCGAGAAGATGA-3'; β-actin reverse,
5'-CCAGAGGCGTACAGGGATAG-3'.
Iron content assay
Plaques from human atherosclerosis and pellets from
cultured HUVECs were homogenized in saline and PBS, and centrifuged
following homogenization. The centrifugation conditions were 10,000
x g, 4˚C and a duration of 15 min. Based on the manufacturer's
instructions, an Iron Assay Kit (cat. no. TC1015; Leagene; Beijing
Regen Biotechnology Co., Ltd.) was used to determine the iron
content. The OD value was determined using a BioTek instrument
(BioTek China) at 562 nm.
Measurement of lipid peroxidation
Lipid peroxidation was assessed by quantifying MDA
concentrations using the thiobarbituric acid (TBA) method, as per
commercial recommendations (Nanjing Jiancheng Bioengineering
Institute). This method relies on the spectrophotometric
measurement of the color generated in the reaction between TBA and
malondialdehyde (MDA). MDA concentrations were determined based on
the absorbance of TBA reactive substances at 532 nm, measured using
a microplate reader (BioTek China).
Immunofluorescence staining
The atherosclerotic plaques derived from the
patients were fixed in 4% paraformaldehyde (PFA), dehydrated
through ascending ethanol series (70, 80, 95, and 100%), followed
by xylene, and then embedded in paraffin wax and sectioned into
five µm sections. For tissue cell death detection, In situ Cell
Death Detection Kit was used to stain tissue sections; fluorescein
was added according to the manufacturer's instructions (cat. no.
11684795910; Roche Diagnostics) at 37˚C for 60 min, and DAPI
(Thermo Fisher Scientific, Inc.) was used to stain the nucleus at
room temperature for 5 min. In order to determine the expression
levels of CD31 and ACSL4 in atherosclerotic plaques, antibodies
were used as follows: Mouse antihuman CD31 antibody (clone JC/70A;
cat. no. ab9498; Abcam) and rabbit antihuman ACSL4 antibody (clone
EPR8640; cat. no. ab155282; Abcam). As secondary antibodies, Alexa
Fluor 594-conjugated goat anti-rabbit IgG H&L (A-11012, Thermo
Fisher Scientific) and Alexa Fluor 488-conjugated goat anti-mouse
IgG H&L (A-11001, Thermo Fisher Scientific, Inc.) were used.
Images were captured using a fluorescence microscope (DM6000B;
Leica Microsystems GmbH).
Western blotting
In order to prepare the whole cell extract, 1 mM
PMSF, 1% protease inhibitor cocktail (MilliporeSigma) and
dithiothreitol were used. Protein concentration was determined
using the Bradford assay, 30 µg of protein was loaded per lane.
Proteins were separated by 10% SDS-PAGE and transferred onto
nitrocellulose membranes, followed by blocking in 0.1% PBST (PBS
with 0.1% Tween 20) with 5% bovine serum albumin (New England
BioLabs, Inc.) at room temperature for 1 h. The membranes were then
incubated with primary antibodies at 4˚C overnight. Primary
antibodies included anti-tubulin antibody (1:5,000; cat. no.
RM2007; BBI Life Sciences) and anti-ACSL4 antibody (1:10,000; cat.
no. EPR8640; Abcam). Membranes were incubated with IRDye 800
CW-conjugated anti-rabbit-IgG and IRDye 680 LT-conjugated
anti-mouse-IgG secondary antibodies (LI-COR Biosciences) at room
temperature for 1 h. Immunoreactive bands were visualized using an
Odyssey infrared imaging system (LI-COR Biosciences). Densitometry
was performed using Image Studio software, version 5.2 (LI-COR
Biosciences).
RNA interference
The small interfering RNAs (siRNAs) were sourced
from Guangzhou RiboBio Co., Ltd. The sequences of siRNAs targeting
human ZFTA mRNA and ACSL4 mRNA were 5'-GCACAGUUAUGCUGUACAACU-3' and
5'-GAGCGAUUUGAAAUUCCAA-3', respectively. A control siRNA with
scrambled sequence was used as a negative control (cat. no.
siN0000001-1-5). Cells were transfected with siRNAs or a negative
control using Lipofectamine® 3000 reagent (cat. no.
L3000015; Invitrogen; Thermo Fisher Scientific, Inc.), following
the manufacturer's instructions. Transfection was performed at 37˚C
for 48 h. Following transfection, a 48-h interval was allowed
before subsequent experimentation. Subsequently, RT-qPCR was
conducted to assess knockdown efficiencies as above.
Detection of reactive oxygen species
(ROS)
Lipid ROS levels in HUVECs were assessed using
C11-BODIPY581/591 (cat. no. D3861; Gibco; Thermo Fisher Scientific,
Inc.). Following treatment and culture, cells were washed with PBS
and labeled with C11-BODIPY581/591 (5 mM) for 15 min at 37˚C in the
dark. The lipid ROS levels were then observed under a fluorescence
microscope.
Transmission electron microscopy (TEM)
analysis
The atherosclerotic plaques derived from the
patients were cut into 1 mm³ blocks and fixed in 4%
paraformaldehyde. After washing with 0.1 M phosphate buffer (PB, pH
7.4), samples were post-fixed in 1% osmium tetroxide for 2 h at
room temperature. Dehydration was carried out through graded
ethanol (30, 50, 70, 80, 95, and 100%) and acetone. Samples were
then infiltrated with acetone and EMBed 812 resin mixtures,
embedded in pure resin and polymerized at 65˚C for 48 h. Ultrathin
sections (60-80 nm) were cut using an ultra-microtome (Leica UC7)
and placed on 150 mesh copper grids. Sections were stained with 2%
uranyl acetate and 2.6% lead citrate, then observed under a HITACHI
HT7800 TEM.
Fluorescence in situ
hybridization
HUVECs cells were cultured on glass coverslips until
reaching 70-80% confluence, then fixed in 4% paraformaldehyde at
room temperature. The cells were permeabilized with 0.5% Triton
X-100 for 10 min. A FAM-labeled probe specific for ZFTA was
denatured at 75˚C for 5 min and hybridized to the cells at 37˚C for
16 h. After hybridization, cells were washed with 2x SSC at 42˚C
for 10 min, 1x SSC at 42˚C for 10 min, and 0.5x SSC at room
temperature for 5 min. Nuclei were counterstained with DAPI at room
temperature for 5 min, then examined under a fluorescence
microscope (cat. no. DM6000B; Leica Microsystems GmbH).
TUNEL staining
Tissues were fixed in 4% paraformaldehyde in PBS for
15 min and washed with PBS. Samples were permeabilized with 20
µg/ml Proteinase K for 10 min. TUNEL reaction mix (cat. no.
11684795910; Roche Diagnostics) was added according to the kit
instructions and incubated at 37˚C in the dark for 60 min. After
PBS washes, samples were counterstained with 1 µg/ml DAPI for 5
min. The sections were then washed, mounted, and examined under a
fluorescence microscope (DM6000B; Leica Microsystems GmbH) to
identify death cells.
Spearman correlation analysis based on
TCGA database data
First, input files containing gene expression data
from different cancer types were prepared, selecting the gene pair
ZFTA and ACSL4. The working directory was set, and the input files
were read to extract the gene expression data. The Spearman method
was then used to calculate the correlation coefficient and P-value
between the gene expression data. Finally, the ggplot2 package was
used to draw a scatter plot, adding regression lines and
correlation coefficients for visualization.
Statistical analysis
All experiments were independently replicated a
minimum of three times. Data analysis was carried out using
GraphPad Prism 7.0 (Dotmatics) and the results were expressed as
mean ± SEM. Statistical significance was determined through a
one-way ANOVA with Dunnett's test or a two-way ANOVA analysis.
Significance levels were denoted as follows: *P<0.05,
**P<0.01, ***P<0.001,
****P<0.0001. P<0.05 was considered to indicate a
statistically significant difference.
Results
The present study investigated the role of ACSL4 and
ZFTA in regulating endothelial cell ferroptosis in AS. It was
hypothesized that ACSL4 and ZFTA may modulate ferroptosis in
endothelial cells and contribute to AS pathogenesis. Results of the
present study demonstrated that ACSL4 is associated with
ferroptosis in human AS plaques, as ACSL4 knockdown significantly
inhibited the ferroptosis of HUVECs. In addition, ZFTA expression
was increased in atherosclerotic plaques, and the ferroptosis of
HUVECs was increased following increased ACSL4 expression.
Collectively, the findings of the present study revealed that ZFTA
may initiate endothelial cell ferroptosis through the regulation of
ACSL4, thus, leading to the occurrence and further development of
AS.
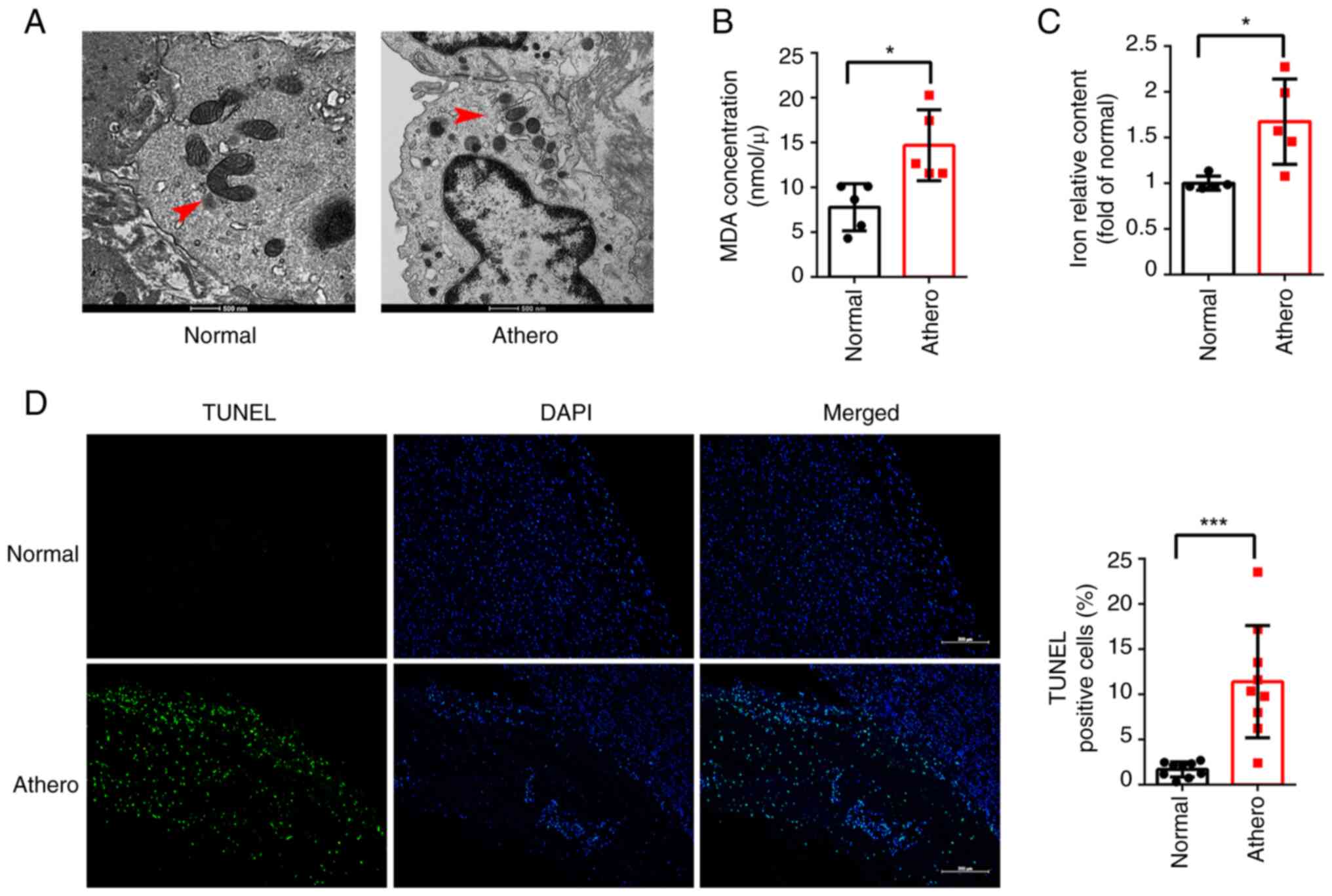
Ferroptosis in human atherosclerotic
plaques
Mitochondrial damage is indicative of ferroptosis
(19). Results of transmission
electron microscopy (TEM) demonstrated consistent morphological
changes in the mitochondria during ferroptosis in human
atherosclerotic plaques, including mitochondria shrinkage, cristae
lysis and the increased density of the mitochondrial outer membrane
(Fig. 1A). To further demonstrate
the presence of ferroptosis in human atherosclerotic plaques, lipid
peroxidation and iron content was compared between five human
atherosclerotic plaques and five human healthy tissues. The results
revealed a substantial increase in both MDA and iron contents
within human atherosclerotic plaques compared with healthy tissues
(Fig. 1B and C). Subsequently, cell death was examined
in eight human atherosclerotic plaque and eight human healthy
control tissues using TUNEL staining, and the results demonstrated
a significant increase in cell death in atherosclerotic plaques
(Fig. 1D). These findings
highlight the role of ferroptosis in the development and
progression of AS.
Elevation of ACSL4 in human
atherosclerotic plaques and inhibition of ferroptosis of HUVECs by
knockout of ACSL4
Results of the present study revealed that ACSL4
plays a role in ferroptosis. ACSL4 mRNA expression was compared
between six atherosclerotic plaques and six healthy controls
obtained from human samples. Results revealed that ACSL4 mRNA
expression levels were significantly increased in human
atherosclerotic plaques compared with healthy tissues (Fig. 2A). Immunofluorescence was
subsequently carried out to determine the localization of ACSL4 in
atherosclerotic plaques, and the results demonstrated that ACSL4
was predominantly expressed within the HUVECs (Fig. 2B).
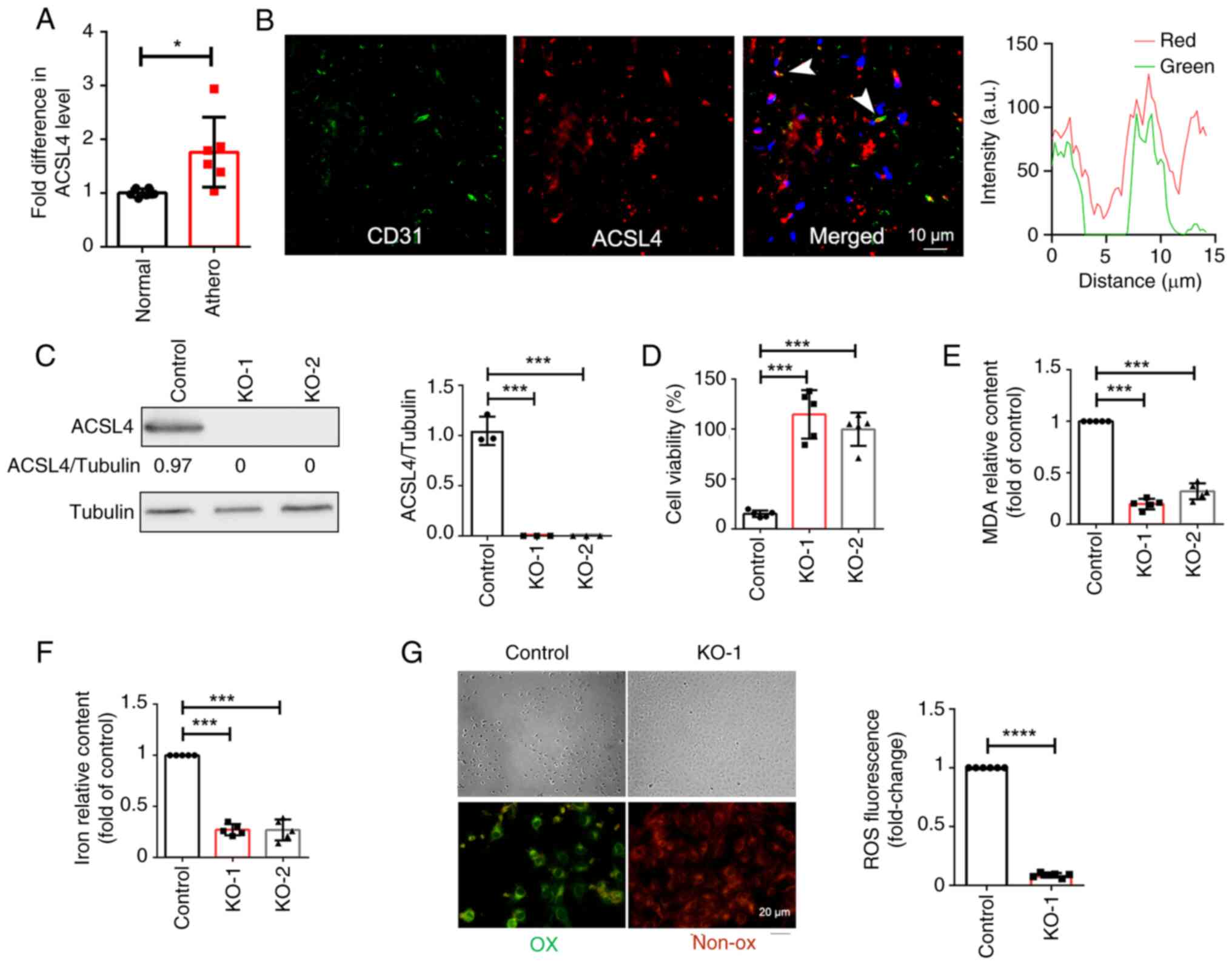 | Figure 2ACSL4 promotes the ferroptosis of
endothelial cells. (A) The mRNA levels of ACSL4 were measured using
RT-qPCR in human atherosclerotic plaques (n=6) and healthy arterial
tissues (n=6). (B) Immunofluorescence staining revealed the
co-localization of ACSL4 and the endothelial cell marker CD31 in
human atherosclerotic plaques. (C) ACSL4 expression was determined
in knockout and control cells using western blot analysis (D) Cell
viability was determined in knockout and control cells using a
CCK-8 assay. (E) MDA levels and (F) Iron content were significantly
reduced in knockout cells, compared with controls. (G) Control and
knockout cells were treated with 2 µM RSL3 for 75 min, and active
oxygen was labeled with BODIPY 581/591 C11 before imaging (green,
oxidized; red, non-oxidized). *P<0.05,
***P<0.001, ****P<0.0001. ACSL4,
acyl-CoA synthetase long chain family member 4; RT-qPCR, reverse
transcription-quantitative PCR; KO, knockout; CCK-8, Cell Counting
Kit-8; RSL3, (1S,3R)-RSL3; athero, atherosclerotic plaques. |
To verify the role of ACSL4 in the ferroptosis of
HUVECs, CRISPR/Cas9 was used for ACSL4 knockout in HUVECs and two
monoclonal cell lines with ACSL4 knockout were screened. ACSL4
knockout in HUVECs was confirmed via western blot analysis
(Fig. 2C). Ferroptosis was induced
in HUVECs using RSL3, and cell survival, MDA content, iron content
and OS) levels were compared between ACSL4 knockout cells and
controls. The results of the present study demonstrated that ACSL4
knockout significantly improved cell survival (Fig. 2D), reduced the levels of MDA, an
end product of lipid peroxidation (Fig. 2E), reduced iron content (Fig. 2F) and decreased the levels of ROS
(Fig. 2G), compared with control
cells. Collectively, these findings indicated that ACSL4 knockout
effectively inhibited the ferroptosis of HUVECs.
Increased ZFTA in human
atherosclerotic plaques and ZFTA silencing suppresses ferroptosis
of HUVECs
ZFTA (C11orf95) is a functionally unknown gene that
binds to various transcriptional coactivators in translocations.
RT-qPCR was performed using six atherosclerotic plaques and six
healthy control tissues. Results of the present study indicated a
significant upregulation in ZFTA RNA expression levels in
atherosclerotic plaques, compared with healthy arterial tissues
(Fig. 3A). ZFTA localization was
determined using fluorescence in situ hybridization (FISH)
analysis and the results demonstrated that ZFTA was expressed in
both the cytoplasm and the nucleus of HUVECs (Fig. 3B). Cells were transfected with ZFTA
siRNA (siZFTA) and viability, MDA content, iron content and ROS
levels were compared between siZFTA and negative control (siNC)
cells, following the RSL3-mediated induction of ferroptosis.
Compared with siNC cells, transfection with siZFTA significantly
increased cell viability, reduced MDA content, decreased iron
content and reduced ROS levels, indicating that ZFTA knockdown may
suppress ferroptosis in HUVECs (Fig.
3C-G).
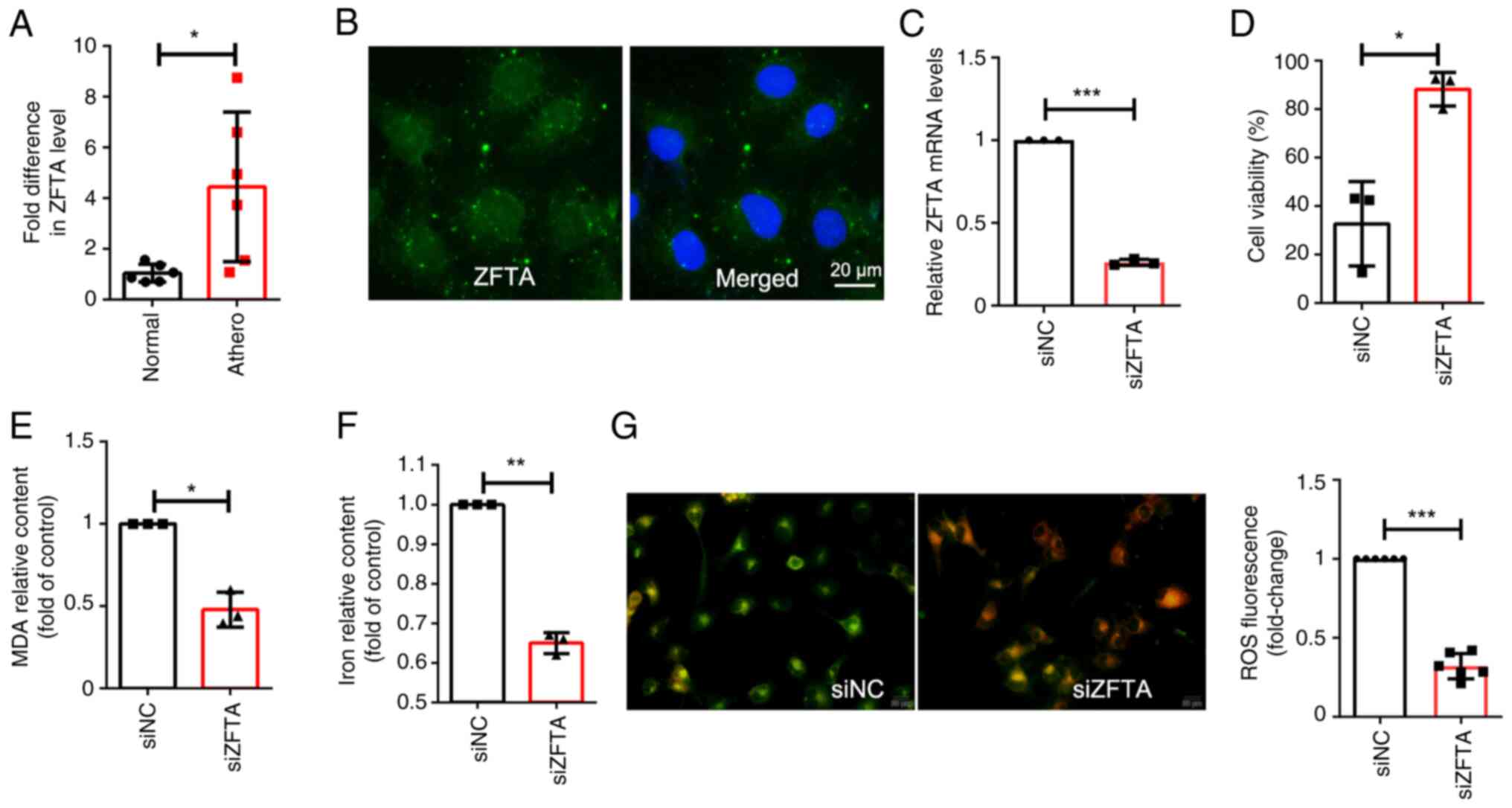 | Figure 3ZFTA promotes the ferroptosis of
endothelial cells. (A) ZFTA mRNA expression levels were measured
using RT-qPCR in human atherosclerotic plaques (n=6) and healthy
arterial tissues (n=6). (B) Fluorescence in situ
hybridization was used to determine the localization of ZFTA in
HUVECs. (C) Cells were transfected with siRNA with scrambled
sequence (siNC), or siRNAs against ZFTA (siZFTA), mRNA expression
levels of ZFTA were measured using RT-qPCR. (D) Cell viability was
determined using a CCK-8 assay following ZFTA knockdown and RSL3
treatment in endothelial cells. (E) The levels of MDA were
significantly reduced in cells following transfection with siZFTA,
compared with cells transfected with siNC. (F) Iron content was
significantly reduced in cells following transfection with siZFTA,
compared with cells transfected with siNC. (G) siNC and siZFTA
cells were treated with 2 µM RSL3 for 75 min, and active oxygen was
labeled with BODIPY 581/591 C11 before imaging. (Green, oxidized;
red, non-oxidized). *P<0.05, **P<0.01
and ***P<0.001. ZFTA, zinc finger
translocation-associated protein; RT-qPCR, reverse
transcription-quantitative PCR; HUVECs, human umbilical vein
endothelial cells; CCK-8, Cell Counting Kit-8; RSL3,; siRNA, small
interfering RNA; NC, negative control. |
ACSL4 knockdown reverses the promoting
effect of ZFTA overexpression on endothelial cell ferroptosis
TCGA database revealed a significant positive
correlation between the expression of ZFTA-ACSL4 in various tumor
types, including prostate adenocarcinoma and pancreatic
adenocarcinoma (Fig. S2). To
further verify the role of ZFTA in the regulation of ACSL4
expression, cells were transfected with siZFTA. Results revealed
that ACSL4 mRNA and protein expression levels were decreased
following ZFTA knockdown (Fig. 4A
and B). The present study also
demonstrated that ZFTA overexpression significantly increased the
mRNA and protein expression levels of ACSL4 (Fig. 4C and D), indicating that ZFTA may promote the
expression of ACSL4.
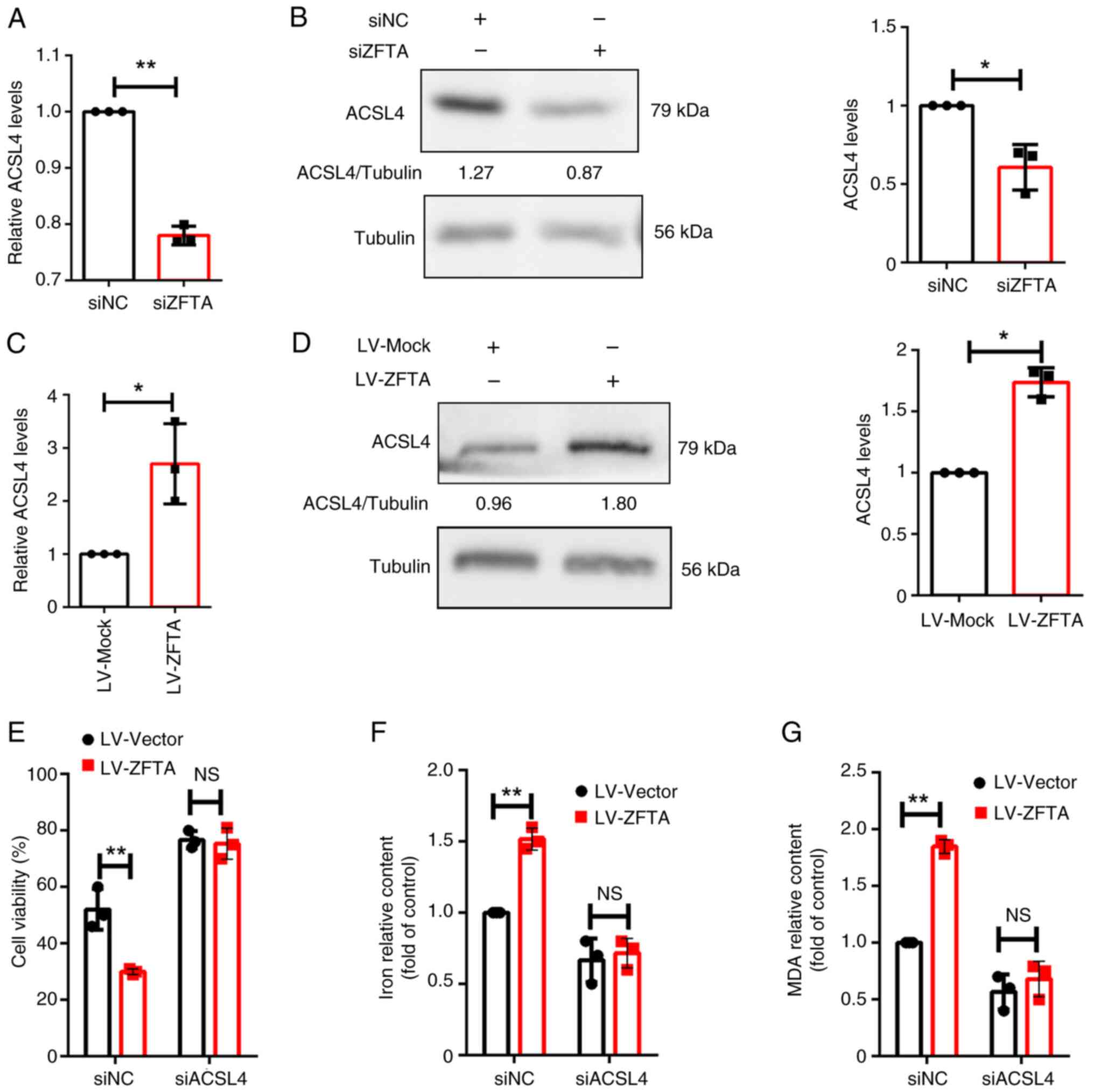 | Figure 4Regulation of ZFTA and ACSL4 in
ferroptosis. (A) Following ZFTA knockdown, ACSL4 mRNA levels were
measured using RT-qPCR. (B) Following ZFTA knockdown, western blot
analysis was performed to assess the protein expression levels of
ACSL4, with Tubulin as the internal reference protein. (C)
Following ZFTA overexpression, ACSL4 mRNA levels were measured
using RT-qPCR. (D) Following ZFTA overexpression, western blot
analysis was performed to assess the protein expression levels of
ACSL4, with Tubulin as the internal reference protein. Cells with
ACSL4 knockdown and ZFTA overexpression were treated with 2 µM RSL3
for 24 h. (E) Cell viability, (F) iron content and (G) MDA levels
were measured. *P<0.05, **P<0.01. ZFTA,
zinc finger translocation-associated protein; ACSL4, acyl-CoA
synthetase long chain family member 4; RT-qPCR, reverse
transcription-quantitative PCR; RSL3; MDA, malondialdehyde; siRNA,
small interfering RNA; NC, negative control. |
To explore the potential association between ACSL4
and ZFTA in the ferroptosis of ECs, rescue experiments were
conducted following the co-transfection of LV-ZFTA and si-ACSL4 in
HUVECs. Results of the present study revealed that ACSL4 knockdown
reversed ZFTA overexpression-mediated ferroptosis (Fig. 4E-G). Therefore, ACSL4 may mediate
the regulation of ZFTA in the ferroptosis of HUVECs.
Discussion
Ferroptosis, a form of programmed cell death, is
distinctive due to its iron-dependent lipid peroxidation.
Manifestation of ferroptosis includes mitochondrial structural
abnormalities and increased levels of iron and lipid peroxides
(19). AS is associated with the
accumulation of lipids within the walls of medium and large-sized
arteries. Results of previous studies suggest a potential
association between ferroptosis and various cellular pathological
processes that influence the initiation and advancement of AS.
These processes encompass disruptions in lipid and iron metabolism,
oxidative stress and inflammatory responses (9,20-22).
Zhou et al (23) revealed
the differential expression of ferroptosis-associated genes within
human coronary arteries, which was positively correlated with the
severity of AS (23). The present
study provided evidence for the role of ferroptosis in human AS.
Morphological changes in mitochondria were observed within human
atherosclerotic plaques and these results were indicative of
ferroptosis, including mitochondrial shrinkage, cristae lysis and
increased mitochondrial outer membrane density. In addition, the
results of the present study demonstrated elevated levels of MDA
and iron content in human atherosclerotic plaques. These results
suggested that ferroptosis may play a crucial role in the
development and progression of AS. However, the underlying
molecular mechanisms of ferroptosis in AS remain to be
elucidated.
ECs form the inner lining of blood vessels, and
ferroptosis of these cells may promote endothelial dysfunction,
leading to lipid accumulation within arterial vessel walls,
ultimately contributing to AS (13,24,25).
The administration of iron chelators in mice inhibits EC
ferroptosis, mitigates EC dysfunction and consequently slows the
progression of AS (26). Thus,
targeting the regulation of endothelial ferroptosis may exhibit
potential in the treatment of AS. ACSL4 is a biomarker of
pathological ferroptosis (27,28).
Previous studies on the involvement of ACSL4 in AS primarily
focused on ACSL4 expression profiling and functional analysis
(23,29,30).
However, the regulatory functions of ACSL4 remain to be fully
elucidated. The present study highlighted the key role of ACSL4 in
both human atherosclerotic plaques and the ferroptosis of ECs. The
results revealed a significant increase in the ACSL4 mRNA
expression levels in human atherosclerotic plaques and ACSL4
localization was further confirmed using immunofluorescence.
Moreover, using CRISPR/Cas9-technology in HUVECs, results of the
present study demonstrated that ACSL4 knockout increased cell
survival rates, reduced the levels of MDA, decreased iron content
and reduced ROS levels following the induction of ferroptosis.
Collectively, the results of the present study demonstrated that
ACSL4 expression may be associated with the initiation and
development of ferroptotic cell death. Monitoring ACSL4 protein
levels may be a suitable diagnostic approach for AS.
ZFTA is a zinc finger protein, and the regulatory
molecular mechanism remains elusive. Previous studies demonstrated
that ZFTA expression leads to continual nuclear translocation,
potentially playing a role in unique DNA binding and
transcriptional regulation (16,17).
In addition, ZFTA collaborates with diverse transcriptional
coactivators in various translocation events (31). The results of the present study
revealed that ZFTA was significantly upregulated in atherosclerotic
plaques, compared with healthy arterial tissues. FISH analysis
revealed that ZFTA was expressed in both the cytoplasm and nucleus
of ECs. In addition, ZFTA knockdown led to increased cell
viability, decreased MDA content, reduced iron content and reduced
ROS levels in ECs following the induction of ferroptosis. These
results highlight the role of ZFTA in promoting ferroptosis in
ECs.
The present study revealed the association between
ZFTA and ACSL4 and highlighted their interaction in the regulation
of ferroptosis. ZFTA knockdown resulted in a decrease in ACSL4 mRNA
and protein expression levels, suggesting that ZFTA positively
modulated the expression of ACSL4. Conversely, overexpression of
ZFTA led to an increase in ACSL4 mRNA and protein expression
levels. To investigate whether ACSL4 plays a role in the
ZFTA-mediated regulation of ferroptosis of ECs, rescue experiments
were conducted. Co-transfection of ZFTA overexpression vectors and
siACSL4 in ECs demonstrated that ACSL4 knockdown reversed the
ferroptosis-inducing effects of ZFTA overexpression. These results
demonstrated that ACSL4 may act as a mediator in the ZFTA-mediated
regulation of ferroptosis in ECs.
The findings of the present study have to be seen in
light of some limitations. The first is the absence of further
investigation into the underlying mechanism of the interaction
between ZFTA and ACSL4 proteins. Future studies employing
techniques such as co-immunoprecipitation could provide insights
into the nature of this interaction, including the binding domains
involved. Secondly, due to the small sample size, the reliability
and accuracy of the study will be affected. In future studies, more
specimens will be collected for further verification.
In conclusion, results of the present study
preliminarily revealed the complex molecular mechanism underlying
ferroptosis in human atherosclerotic plaques. The pivotal roles of
ACSL4 and ZFTA in the ferroptosis of ECs may uncover potential
therapeutic targets for managing AS and associated cardiovascular
diseases. Further investigations are required to explore the
specific mechanisms and potential targets of ferroptosis, which may
aid in the development of innovative clinical treatment
strategies.
Supplementary Material
Verification of ZFTA expression in
cells transduced with an ZFTA expressing lentivirus. Cultured human
endothelial cells (EC) were transduced with either an LV-ZFTA or
LV-Mock, followed by RT-qPCR analysis of ZFTA mRNA levels.
***P<0.001. ZFTA, zinc finger
translocation-associated protein; LV, lentivirus; Mock, LV vector
serving as a control; RT-qPCR, reverse transcription-quantitative
PCR.
Spearman analysis on data from the
TCGA database. Bioinformatics analysis revealed correlations
between ZFTA and ACSL4 expression in prostate adenocarcinoma,
pancreatic cancer, kidney renal papillary cell carcinoma, bladder
cancer, head and neck squamous cell carcinoma and colon
adenocarcinoma. TCGA The Cancer Genome Atlas; ZFTA, zinc finger
translocation-associated protein; ACSL4, acyl-CoA synthetase long
chain family member 4.
Acknowledgements
Not applicable.
Funding
Funding: The present study was supported by grants from The
National Natural Science Foundation of China (grant no. 82372304),
Scientific and Technological Planning Project of Guangzhou City
(grant nos. 202201010886 and 2023A03J0926) and Guangzhou Women and
Childrens Medical Center (grant no. 2020BS024).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
YWH designed the study. HXG, JJ, CY, JFX and QH
conducted the experiments and acquired and analyzed the data. HXG
wrote the manuscript. YWH and HXG revised the manuscript. All
authors read and approved the final manuscript. HXG and JJ confirm
the authenticity of all the raw data.
Ethics approval and consent to
participate
Ethics approval was obtained from the Ethics
Committee of The Women and Children's Medical Center (approval no.
286B01). The present study adhered to the principles outlined in
the Declaration of Helsinki. All patients or relatives of deceased
individuals provided written informed consent prior to the
study.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Libby P: The changing landscape of
atherosclerosis. Nature. 592:524–533. 2021.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Gimbrone MA Jr and García-Cardeña G:
Endothelial cell dysfunction and the pathobiology of
atherosclerosis. Circ Res. 118:620–636. 2016.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Lin L, Zhang MX, Zhang L, Zhang D, Li C
and Li YL: Autophagy, pyroptosis, and ferroptosis: New regulatory
mechanisms for atherosclerosis. Front Cell Dev Biol.
9(809955)2021.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Yu H, Guo P, Xie X, Wang Y and Chen G:
Ferroptosis, a new form of cell death, and its relationships with
tumourous diseases. J Cell Mol Med. 21:648–657. 2017.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Latunde-Dada GO: Ferroptosis: Role of
lipid peroxidation, iron and ferritinophagy. Biochim Biophys Acta
Gen Subj. 1861:1893–1900. 2017.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Stockwell BR, Friedmann Angeli JP, Bayir
H, Bush AI, Conrad M, Dixon SJ, Fulda S, Gascón S, Hatzios SK,
Kagan VE, et al: Ferroptosis: A regulated cell death nexus linking
metabolism, redox biology, and disease. Cell. 171:273–285.
2017.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Xu S, Min J and Wang F: Ferroptosis: An
emerging player in immune cells. Sci Bulletin (Beijing).
66:2257–2260. 2021.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Li J, Cao F, Yin HL, Huang ZJ, Lin ZT, Mao
N, Sun B and Wang G: Ferroptosis: Past, present and future. Cell
Death Dis. 11(88)2020.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Wang Y, Zhao Y, Ye T, Yang L, Shen Y and
Li H: Ferroptosis signaling and regulators in atherosclerosis.
Front Cell Dev Biol. 9(809457)2021.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Yu Y, Yan Y, Niu F, Wang Y, Chen X, Su G,
Liu Y, Zhao X, Qian L, Liu P and Xiong Y: Ferroptosis: A cell death
connecting oxidative stress, inflammation and cardiovascular
diseases. Cell Death Discov. 7(193)2021.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Cornelissen A, Guo L, Sakamoto A, Virmani
R and Finn AV: New insights into the role of iron in inflammation
and atherosclerosis. EBioMedicine. 47:598–606. 2019.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Fang X, Ardehali H, Min J and Wang F: The
molecular and metabolic landscape of iron and ferroptosis in
cardiovascular disease. Nat Rev Cardiol. 20:7–23. 2023.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Lin X, Ouyang S, Zhi C, Li P, Tan X, Ma W,
Yu J, Peng T, Chen X, Li L and Xie W: Focus on ferroptosis,
pyroptosis, apoptosis and autophagy of vascular endothelial cells
to the strategic targets for the treatment of atherosclerosis. Arch
Biochem Biophys. 715(109098)2022.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Kuwata H and Hara S: Role of acyl-CoA
synthetase ACSL4 in arachidonic acid metabolism. Prostaglandins
Other Lipid Mediat. 144(106363)2019.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Lin Z, Liu J, Long F, Kang R, Kroemer G,
Tang D and Yang M: The lipid flippase SLC47A1 blocks metabolic
vulnerability to ferroptosis. Nat Commun. 13(7965)2022.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Ozawa T, Kaneko S, Szulzewsky F, Qiao Z,
Takadera M, Narita Y, Kondo T, Holland EC, Hamamoto R and Ichimura
K: C11orf95-RELA fusion drives aberrant gene expression through the
unique epigenetic regulation for ependymoma formation. Acta
Neuropathol Commun. 9(36)2021.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Zhu JJ, Jillette N, Li XN, Cheng AW and
Lau CC: C11orf95-RELA reprograms 3D epigenome in supratentorial
ependymoma. Acta Neuropathol. 140:951–960. 2020.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Dixon SJ, Lemberg KM, Lamprecht MR, Skouta
R, Zaitsev EM, Gleason CE, Patel DN, Bauer AJ, Cantley AM, Yang WS,
et al: Ferroptosis: An iron-dependent form of nonapoptotic cell
death. Cell. 149:1060–1072. 2012.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Liu W, Östberg N, Yalcinkaya M, Dou H,
Endo-Umeda K, Tang Y, Hou X, Xiao T, Fidler TP, Abramowicz S, et
al: Erythroid lineage Jak2V617F expression promotes atherosclerosis
through erythrophagocytosis and macrophage ferroptosis. J Clin
Invest. 132(e155724)2022.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Zhang J, Wang X, Guan B, Wang X, An X,
Wang T, Chen X, Zhao L, Jia J, Song L, et al: Qing-Xin-Jie-Yu
Granule inhibits ferroptosis and stabilizes atherosclerotic plaques
by regulating the GPX4/xCT signaling pathway. J Ethnopharmacol.
301(115852)2023.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Su G, Yang W, Wang S, Geng C and Guan X:
SIRT1-autophagy axis inhibits excess iron-induced ferroptosis of
foam cells and subsequently increases IL-1Β and IL-18. Biochem
Biophys Res Commun. 561:33–39. 2021.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Zhou Y, Zhou H, Hua L, Hou C, Jia Q, Chen
J, Zhang S, Wang Y, He S and Jia E: Verification of ferroptosis and
pyroptosis and identification of PTGS2 as the hub gene in human
coronary artery atherosclerosis. Free Radic Biol Med. 171:55–68.
2021.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Zheng D, Liu J, Piao H, Zhu Z, Wei R and
Liu K: ROS-triggered endothelial cell death mechanisms: Focus on
pyroptosis, parthanatos, and ferroptosis. Front Immunol.
13(1039241)2022.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Yang K, Song H and Yin D: PDSS2 inhibits
the ferroptosis of vascular endothelial cells in atherosclerosis by
activating Nrf2. J Cardiovasc Pharmacol. 77:767–776.
2021.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Bai T, Li M, Liu Y, Qiao Z and Wang ZJ:
Inhibition of ferroptosis alleviates atherosclerosis through
attenuating lipid peroxidation and endothelial dysfunction in mouse
aortic endothelial cell. Free Radic Biol Med. 160:92–102.
2020.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Doll S, Proneth B, Tyurina YY, Panzilius
E, Kobayashi S, Ingold I, Irmler M, Beckers J, Aichler M, Walch A,
et al: ACSL4 dictates ferroptosis sensitivity by shaping cellular
lipid composition. Nat Chem Biol. 13:91–98. 2017.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Dixon SJ, Winter GE, Musavi LS, Lee ED,
Snijder B, Rebsamen M, Superti-Furga G and Stockwell BR: Human
haploid cell genetics reveals roles for lipid metabolism genes in
nonapoptotic cell death. ACS Chem Biol. 10:1604–1609.
2015.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Xiao FJ, Zhang D, Wu Y, Jia QH, Zhang L,
Li YX, Yang YF, Wang H, Wu CT and Wang LS: miRNA-17-92 protects
endothelial cells from Erastin-induced ferroptosis through
targeting the A20-ACSL4 axis. Biochem Biophys Res Commun.
515:448–454. 2019.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Zhang M, Yu Z, Zhao L and Luo H: Long
non-coding RNA PVT1 regulates atherosclerosis progression via the
microRNA-106b-5p/ACSL4 axis. Biochem Biophys Res Commun.
667:170–179. 2023.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Parker M, Mohankumar KM, Punchihewa C,
Weinlich R, Dalton JD, Li Y, Lee R, Tatevossian RG, Phoenix TN,
Thiruvenkatam R, et al: C11orf95-RELA fusions drive oncogenic NF-κB
signalling in ependymoma. Nature. 506:451–455. 2014.PubMed/NCBI View Article : Google Scholar
|