Introduction
Light chain deposition disease (LCDD) is
characterized by tissue deposition of nonamyloid immunoglobulin
light chains produced by clonal proliferation of CD38+ plasma
cells. CD38 is involved in various enzymatic activities that
control NAD+ levels in the bone marrow niche where the malignant
plasma cells grow (1). CD38 is
highly and uniformly expressed on human plasma cells and at
relatively low levels on normal lymphoid and myeloid cells, and in
certain tissues of nonhematopoietic origin, which is closely
related to the number and function of T cells through the CD38-NAD+
axis. A small number of cases of LCDD were reported to be secondary
to autoimmune diseases, such as Sjogren's syndrome (2,3). To
date, the incidence of this disorder has remained undetermined. The
diagnosis of LCDD is often missed because of its rarity and
morphological similarities. Repeated renal biopsies are essential
to establish the diagnosis (4).
There is no generally accepted standard treatment for LCDD because
of its rarity and the lack of randomized clinical trials. Our
patient was initially started on bortezomib combined with
dexamethasone (Vd) chemotherapy, and anemia and renal failure
developed progressively in the second cycle. The patient was
considered to be less responsive to this Vd approach. Certain
studies have revealed the importance of CD38 in the inflammatory
process during autoimmunity (5-7),
and CD38 is expected to serve as a promising therapeutic agent for
autoimmune diseases (6,7). CD38 is highly and uniformly expressed
on human plasma cells, making anti-CD38 antibody an ideal
therapeutic approach against CD38+ aberrant plasma cells and
immunomodulatory dynamics for clonal elimination by inducing
changes in the bone marrow microenvironment (8). To our knowledge, only a small number
of studies have reported on the use of anti-CD38 antibody therapy
in patients with LCDD without symptomatic myeloma. Its activity was
demonstrated in several studies with high overall response rates,
including a small series of 6 patients with LCDD treated with a
short course of daratumumab monotherapy (response rate, 100%)
(9), a study reporting on the use
of anti-CD38 antibody-based therapy in 25 patients with Monoclonal
gammopathy of renal significance (LCDD in 20) with a 74% overall
response rate (5), and another
report indicating a profound hematologic response and favorable
renal outcome in 6 out of 7 patients with previously treated LCDD
(10). Overall, daratumumab is an
effective option for patients with LCDD. The present study reported
on the case of a patient who developed LCCD after successful renal
transplantation and the use of anti-CD38 antibody.
Case report
A 49-year-old male patient first visited the
nephrology unit of Mianyang Central Hospital (Mianyang, China) due
to nocturia and lower extremity edema in January 2014. The patient
had a history of hypertension and lipoma. On admission, slight
pitting edema of the lower extremities was observed during physical
examination. Laboratory findings showed severe proteinuria (24-h
urine test, 15.98 g/24 h; normal, 0-0.14 g/24 h), hypoproteinemia
(serum albumin, 30.81 g/l; normal, 35-55 g/l), hyperlipidemia
(triglycerides, 1.76 mmol/l; normal, <1.70 mmol/l),
hypercholesterolemia (8.33 mmol/l; normal, <5.2 mmol/l) and
renal insufficiency (serum creatinine, 3.6 mg/dl; normal, 0.6-1.4
mg/dl). Urinary ultrasonography revealed that both kidneys had a
normal size. Bone marrow smear showed a normal number of plasma
cells. Serum or urine immunofixation electrophoresis was not
performed at this stage. Ultrasound-guided percutaneous right
kidney biopsy was performed in February 2014 and the tissue
specimens were sent to KingMed Diagnostics for analysis.
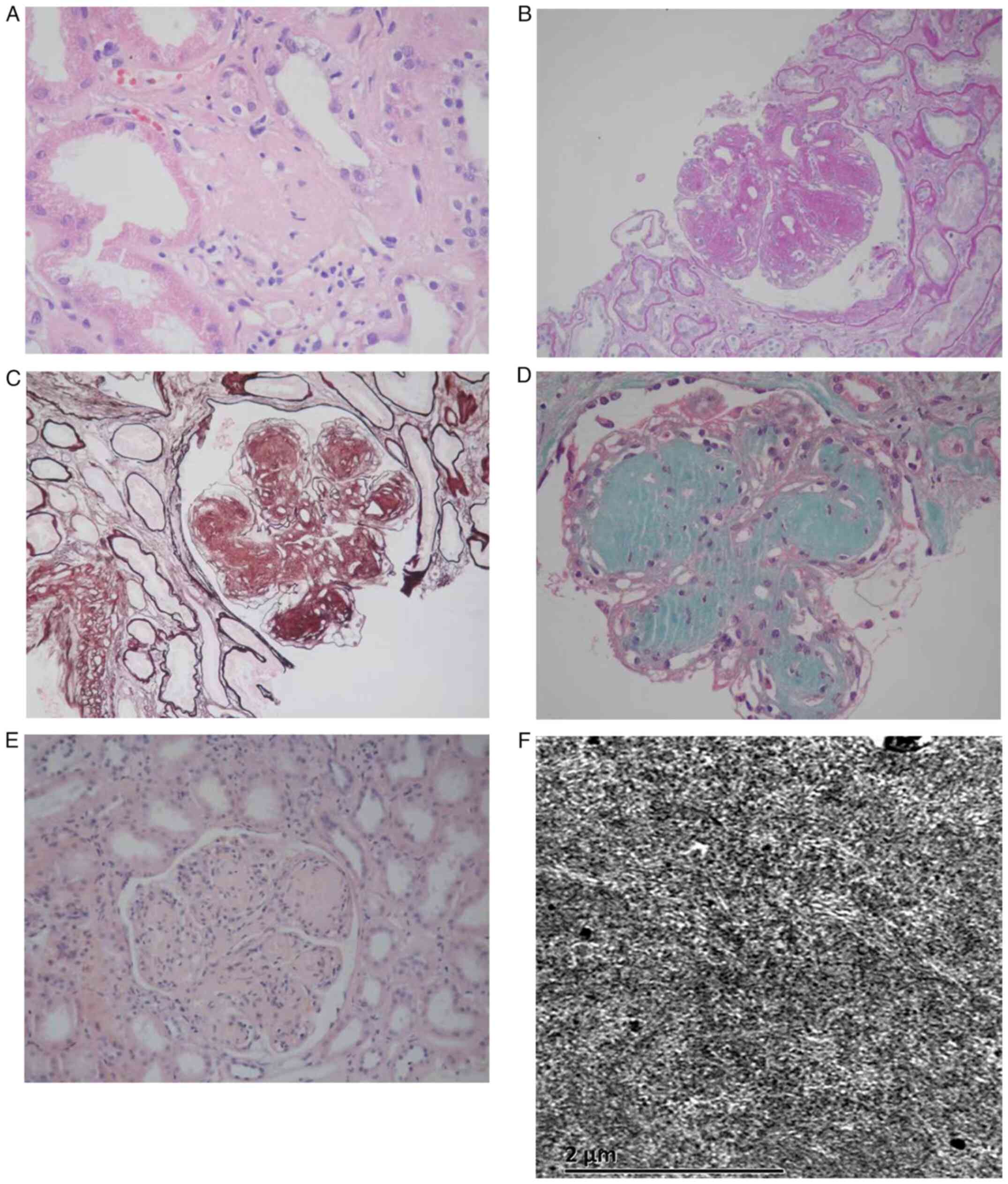
Hematoxylin-eosin staining revealed sclerosis in one glomerulus
(Fig. 1A); periodic acid-Schiff
staining showed nodular changes in the mesangial region (Fig. 1B); periodic acid silver methenamine
staining revealed that capillary loops were lobulated (Fig. 1C); Masson trichrome staining showed
no obvious fuerythrophilin deposition within the glomeruli
(Fig. 1D); and Congo red staining
was negative (Fig. 1E). Electron
microscopy showed fibrillary deposits along the glomerular basement
membrane and mesangial area (Fig.
1F). The biopsy results suggested fibrillary
glomerulonephritis. Finally, based on the above, a diagnosis of
fibrillary glomerulonephritis was reached. The patient commenced
treatment with prednisolone (50 mg once daily) for 8 weeks and
tapered by 5 mg every 2 weeks, and reduced to 7.5 mg/day for 3
months. After that, the patient did not receive any treatment and
remained in a stable condition. In June 2018, the patient was
readmitted to the nephrology unit of Mianyang Central Hospital
(Mianyang, China) due to repeated nocturia and lower extremity
edema. The patient had serum creatinine levels of 6.6 mg/dl, 24-h
urinary protein excretion of 8.65 g/day, serum albumin of 30.78 g/l
and total cholesterol of 9.13 mmol/l. The patient was started on
prednisolone again for increased serum creatinine and proteinuria.
In March 2019, the patient was readmitted to hospital due to an
increase in creatinine to 18.9 mg/dl. In March 2019, the patient
underwent surgery to create an internal arteriovenous fistula and
then began maintenance hemodialysis three times a week. Serum
creatinine levels ranged between 5.8 and 12 mg/dl. In April 2020,
the patient received a renal allograft transplantation at the West
China Hospital of Sichuan University (Chengdu, China).
Subsequently, the patient began a triple immunosuppressive regimen
consisting of prednisone, tacrolimus and mycophenolate mofetil, and
hemodialysis was stopped. In October 2021, the patient visited the
Department of Urological Surgery at West China Hospital of Sichuan
University (Chengdu, China) due to worsening serum creatinine
levels after renal transplantation. The patient underwent allograft
kidney biopsy. The allograft kidney specimens were sent to Tongji
Hospital, Tongji Medical College (Wuhan, China), and the report
indicated that all of the glomeruli showed focal mesangial matrix
hyperplasia, nodular enhancement and no evidence of rejection.
There was no obvious matrix increase and tubular atrophy in the
renal interstitium [no high-definition images were acquired, as
Tongji Hospital is an external hospital. The analyses were
performed according to the protocols of Tongji Hospital (Tongji
Medical College, Wuhan, China)]. Immunofluorescence staining, also
performed at Tongji Hospital according to their in-house
procedures, revealed diffuse linear deposition of κ light chain
along the glomerular basement membrane, tubular basement membranes
and small vascular intima. Electron microscopy showed
electron-dense deposits in the outer aspect of the tubular basement
membrane, mesangium and basement membrane of the capillary
endothelium, confirming the pathological diagnosis of LCDD (only a
pathological copy report with a small number of unclear images was
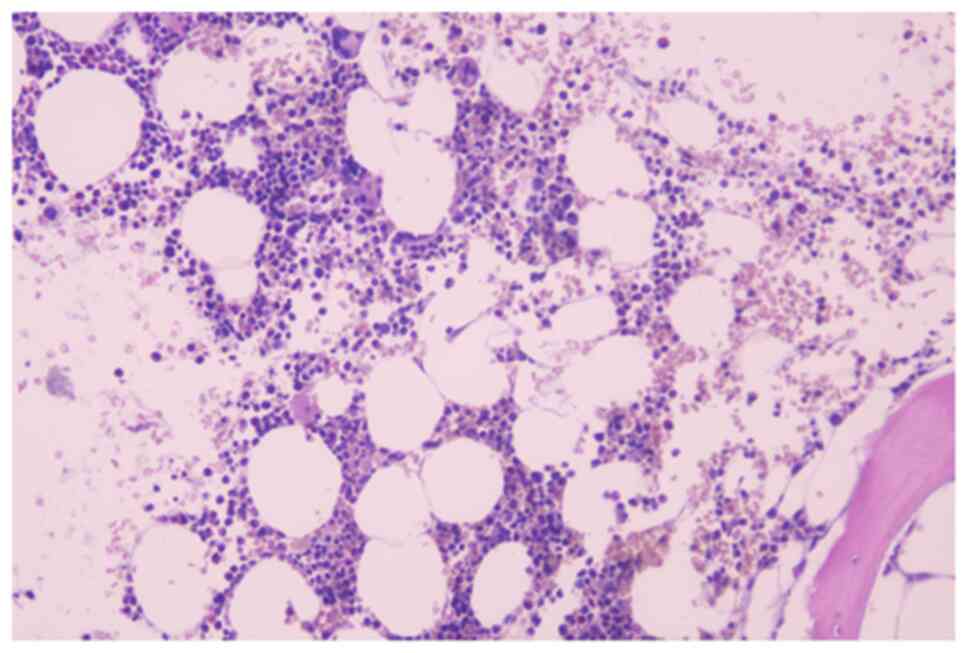
available; Fig. S1A and B). Bone marrow biopsy showed normal
numbers of plasma cells (Fig. 2)
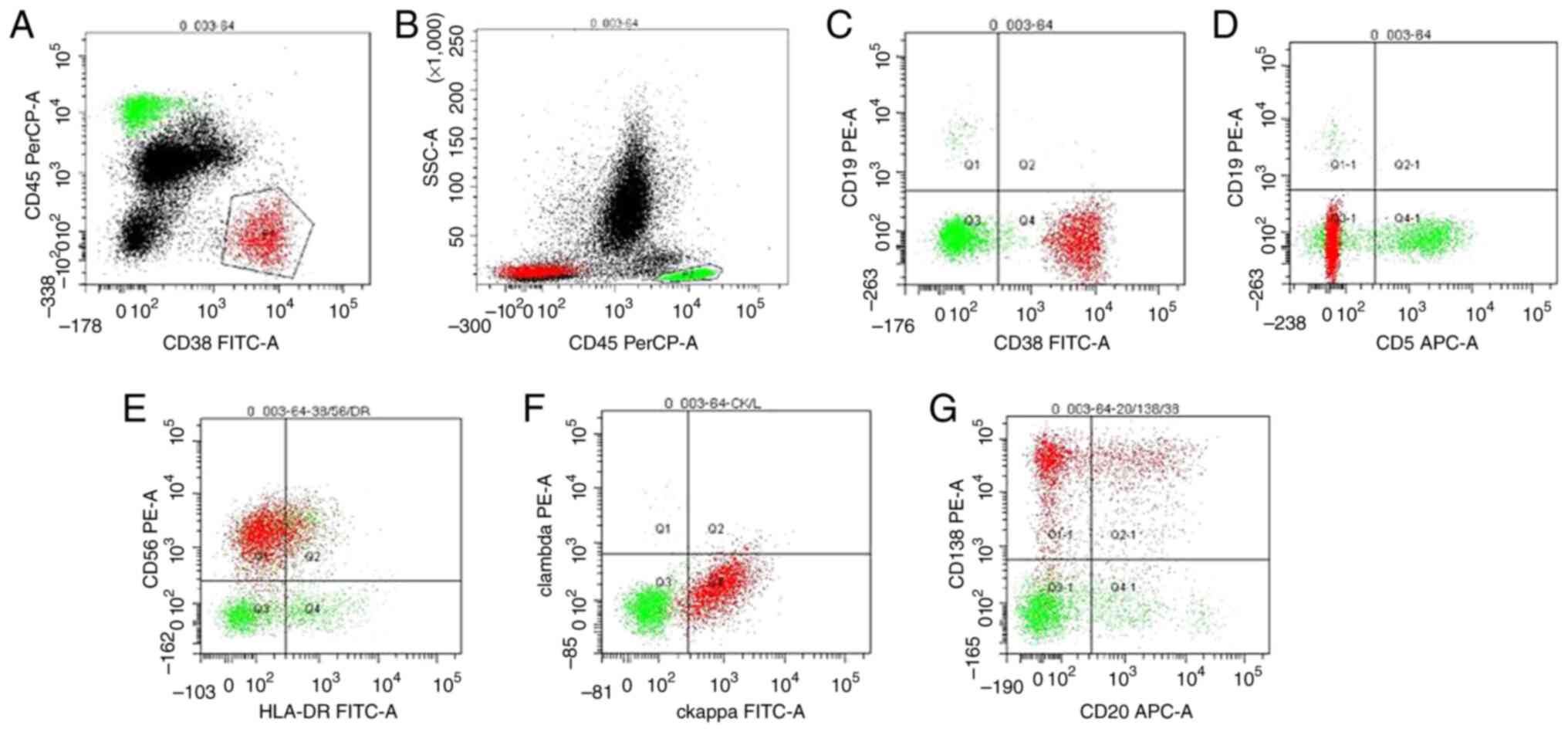
and flow cytometry revealed 7.1% clonal plasma cells (Fig. 3). Serum immunofixation
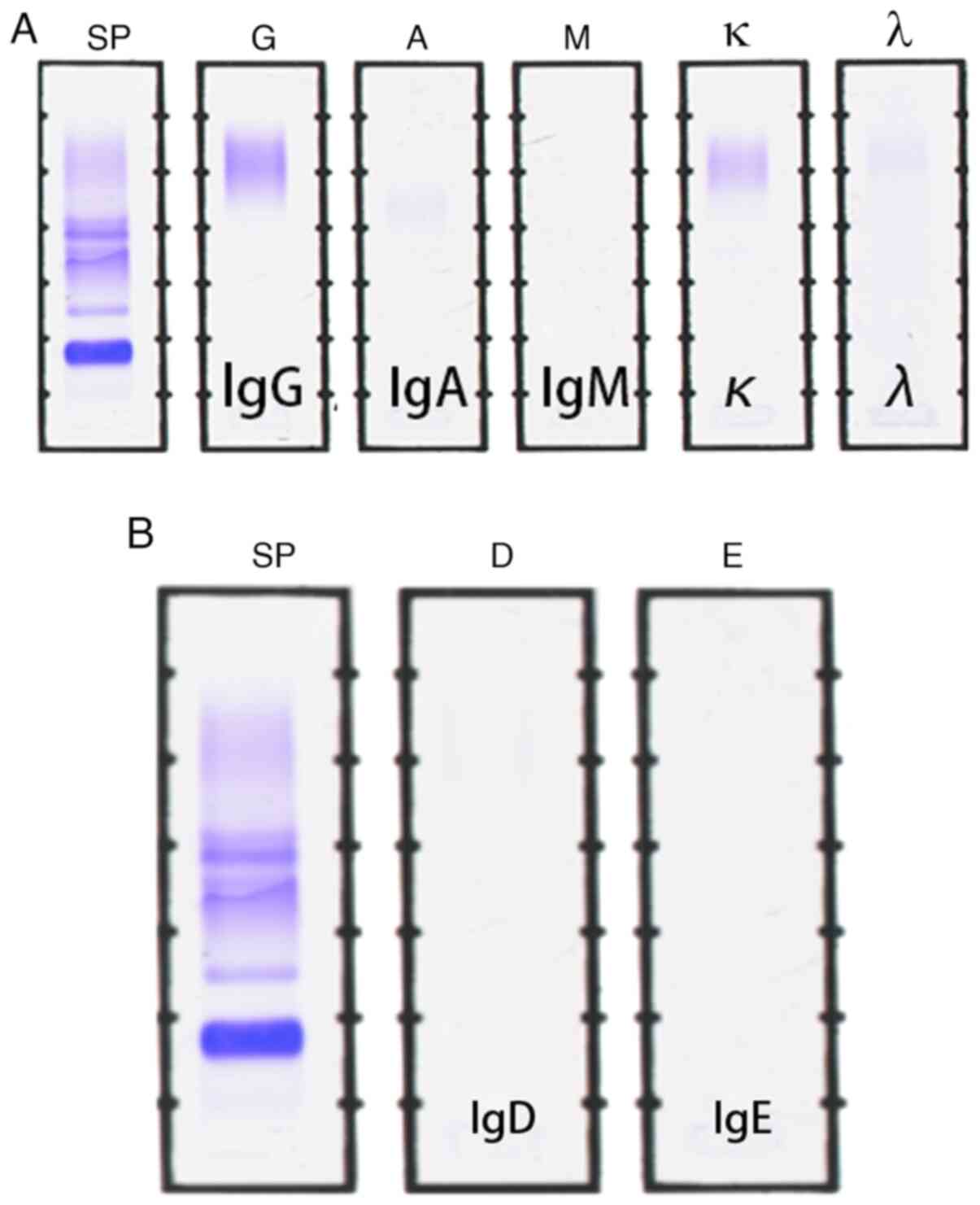
electrophoresis was negative (Fig.
4A and B). The above-mentioned
analyses were performed at West China Hospital, Sichuan University
(Chengdu, China) according to standard protocols. In November 2021,
the patient first visited the hematology department at West China
Hospital of Sichuan University (Chengdu, China). Serum and urine
immunofixation electrophoresis was negative. Serum free κ light
chain was 317.81 mg/l (normal, 3.3-19.4 mg/l) and free λ light
chain was 23.05 mg/l (normal, 5.7-26.3 mg/l), with a κ/λ a light
chain ratio of 13.79 (normal, 0.26-1.65). The patient's serum
creatinine level was 3.6 mg/dl and hemoglobin was 115 g/l (normal,
130-175 g/l). Finally, he was diagnosed with LCDD. He was
transferred to our hospital and subsequently started on Vd
chemotherapy [bortezomib (Shandong Qilu) 1.3 mg/m2
subcutaneously combined with dexamethasone 20 mg intravenously
weekly, in a 35-day cycle]. In December 2021, during the second
cycle of the Vd regimen, his creatinine level was increased to 7.6
mg/dl and hemoglobin was decreased to 66 g/l. The patient was
considered to be less responsive to the Vd regimen and anti-CD38
antibody-based chemotherapy was initiated in January 2022.
Daratumumab (Cilag AG) is an anti-CD38 monoclonal antibody, which
was administered intravenously at 16 mg/kg weekly for 8 weeks,
followed by every 2 weeks for 16 weeks, and then every 4 weeks
thereafter. Thrice-a-week dialysis was maintained. The patient's
hemoglobin level improved to 125 g/l; furthermore, serum creatinine
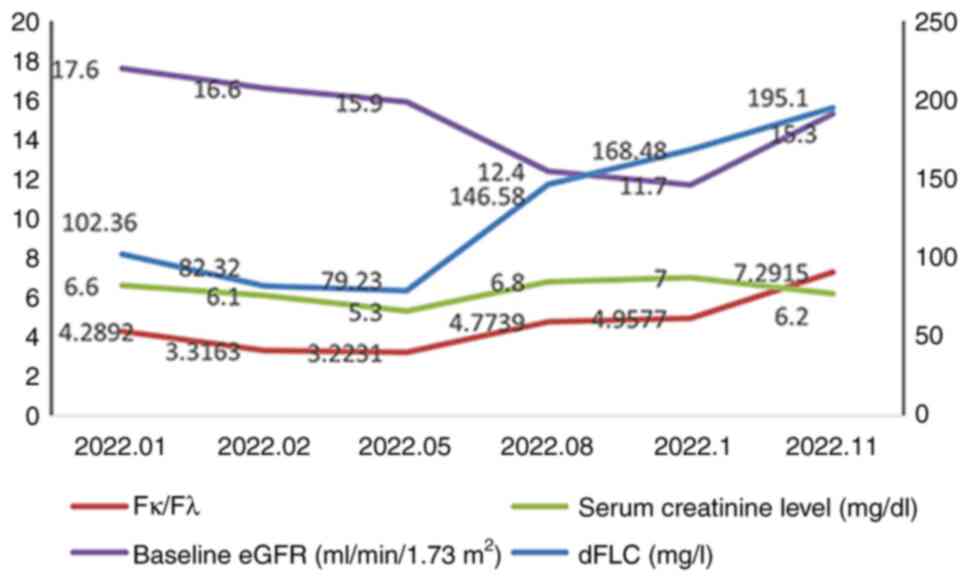
levels ranged between 5.3 and 7 mg/dl and serum free κ light chain
ranged between 114.87 and 226.11 mg/l during the use of Daratumumab
(Fig. 5). In this period, the best
response achieved was hematological partial response (>50%
reduction in dFLC); the patient's renal function did not improve
and he remained in end-stage kidney disease. The patient died of
coronavirus disease complications in June 2023, which was unrelated
to his condition.
Discussion
LCDD is a rare monoclonal gammopathy that affects
the kidneys (11,12), and represents a diagnostic and
therapeutic challenge. Only a small number of studies have reported
LCDD as a recurrent disease or a de novo entity after renal
transplantation. While the exact mechanism remains elusive, the
condition may arise from the current immunosuppressive status and
minor plasma cell clone escaping from the tumor immunity
surveillance, finally resulting in light chain deposition (13). To date, no evidence-based consensus
has been reached for the treatment of LCDD. Treatment strategies
comprise systemic chemotherapy with or without autologous stem cell
transplantation to eliminate the plasma cell burden in the bone
marrow that produces light chains. The bortezomib-based regimen is
the most common chemotherapy protocol (14), and anti-CD38 antibody is also an
effective option for patients with LCDD due to its direct antitumor
and immunomodulatory mechanism (5,9,10,15,16).
As part of the present study, 12 previously reported
cases of LCDD diagnosed after kidney transplantation were reviewed
and summarized (Table I) (4,17-25).
These 12 cases comprised 8 males and 1 female, and the sex of the
other 3 cases was unknown. This suggests greater male
susceptibility. The median age was 52 years (range, 28-72 years).
Serum immunofixation electrophoresis was available in 10 of 12
patients and was negative in 5. Serum immunoglobulin types seemed
to be nonspecific. Urine immunofixation electrophoresis was
available in 8 of 12 patients and negative in 2. Five of 10
patients had negative serum immunofixation electrophoresis for
monoclonal proteins, and 6 of 12 patients were confirmed to have
de novo LCDD after review of their original kidney biopsies.
Unfortunately, serum and urine monoclonal light chain could not be
detected in the patient of the present study. Retrospective
analysis of the patient's first renal biopsy specimen was not
possible due to it being unavailable, and thus, it remains
undetermined whether LCDD was recurrent or de novo. Most
patients with delayed diagnosis had negative serum immunofixation
electrophoresis. Serum and urine immunofixation electrophoresis was
negative in 3 of 9 patients. The positive rate of blood M protein
(5/10 patients) was lower than corresponding urine M protein (6/8
patients). As for free light chain assays, increased involved serum
free light chains (4/4), an abnormal κ/λ ratio (4/4) and difference
between involved and uninvolved free light chains (dFLC) (3/3) were
reported. The determination of serum free light chains may be
crucial for identifying LCDD in clinical practice.
 | Table IPrevious reports of light-chain
deposition disease after renal transplantation. |
Table I
Previous reports of light-chain
deposition disease after renal transplantation.
| First author,
year | Age, years; sex | Number of
patients | Country | BM plasma cells,
% | Retrospective
diagnosis | Serum IFE | Urine IFE | Involved FLC; κ/λ
ratio; dFLC, mg/l | Clone-directed
therapy before RT | Transplant
number | Kidney biopsy side at
diagnosis | (Refs.) |
|---|
| Aoudia et al,
2020 | 28; male | 1 | Algeria | 5 | No | κ | κ | 480; 18; 453.4 | No | 1 | Allograft kidney | (4) |
| Heybeli et al,
2021 | NA | 3 | USA | ≤5 | Yes (n=3) | λ (n=١); negative
(n=٢) | λ (١); negative
(n=1); κ (n=١) | NA; NA; NA | No | NA | Native kidney | (17) |
| Nambirajan et
al, 2015 | 65; male | 1 | India | 5 | No | Negative | Negative | NA; 4.8; NA | No | 1 | Allograft kidney | (18) |
| Kuppachi et
al, 2015 | 51; male | 1 | USA | <2 | No | IgG-k | ND | 723; 54.9; 709.8 | No | 1 | Na | (19) |
| Jimenez-Zepeda et
al, 2012 | 61; male | 1 | Canada | 3 | No | Negative | ND | 1190; 30.99;
1151.6 | No | 1 | Allograft kidney | (20) |
| Tanenbaum et
al, 2005 | 72; male | 1 | USA | <2 | No | IgG-λ | IgG-λ | ND; ND; ND | No | 1 | Allograft kidney | (21) |
| Larsen et al,
2008 | 51; male | 1 | Denmark | <2 | Yes | ND | ND | 320; ND; ND | No | 1 | Native kidney | (22) |
| Ecder et al,
1996 | 30; male | 1 | USA | 4 | Yes | ND | ND | ND; ND; ND | No | 1 | Allograft
kidney | (23) |
| Horike et
al, 2012 | 53; female | 1 | Japan | <2 | Yes | IgA-λ | λ | NA; NA; NA | No | 1 | Allograft
kidney | (24) |
| Taneda et
al, 2008 | 61; male | 1 | USA | 11.6 | No | Negative | κ | NA; NA; NA | No | 1 | NA | (25) |
| Current case | 49; male | 1 | China | 0.5 | No | Negative | Negative | 317.81; 13.79;
294.76 | No | 1 | Allograft
kidney | - |
There is no standard clinical treatment for LCDD due
to its rarity. Currently, treatment is based on regimens used in
multiple myeloma and amyloid light chain amyloidosis, and it is
generally accepted that the bortezomib-based regimen represents a
successful first-line therapy (14,26).
The patient of the present study was started on a Vd regimen and
then switched to the anti-CD38 antibody regimen. Anti-CD38 antibody
can improve the depth of response and prevent the progression of
renal failure in treated LCDD (9,10).
No severe infusion-related reaction and no severe grade 4 adverse
event occurred during the course of anti-CD38 antibody therapy. The
daratumumab-based regimen does not belong to the experimental
treatment, which is an effective option for patients with LCDD
(9,10). The best response achieved by the
patient of the present study was hematological partial response
(>50% reduction in dFLC). However, the patient's renal function
did not improve and he remained in end-stage kidney disease. Milani
et al (10) also reported
that two patients progressed to end-stage renal disease and one of
them did not respond to anti-CD38 antibody, and the other
experienced further deterioration of renal dysfunction. Thus,
anti-CD38 antibody is effective in treated LCDD but it may not
overcome the marked deterioration of renal function. Further large
multicenter retrospective and prospective studies are required to
assess the role of anti-CD38 antibody.
A limitation of the present study was that for the
allograft kidney biopsy specimens, which were sent to an external
hospital for analysis, no high-definition images were acquired and
scale bar/magnification details of light microscopy,
immunofluorescence and electron microscopy of the biopsy were not
available.
In conclusion, a panel of serum immunofixation
electrophoresis, urine immunofixation electrophoresis and serum
free light chain assays can improve the early recognition of LCDD.
Early clone-directed therapy can result in a deeper hematological
response and better renal outcome. Anti-CD38 antibody may help
patients with LCDD obtain a rapid and profound hematological
response that preserves kidney function. However, it may not
overcome the marked deterioration of renal function in LCDD.
Supplementary Material
Pathological findings of the allograft
renal biopsy. (A) Immunofluorescence staining revealed diffuse
linear deposition of κ light chain along the glomerular basement
membrane, tubular basement membranes and small vascular intima. (B)
Electron microscopy showed electron-dense deposits in the outer
aspect of the tubular basement membrane, mesangium and basement
membrane of the capillary endothelium (magnification, x2,500).
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
YZ, FX and JY contributed to the conception and
design of the work. JY and JW contributed to the acquisition,
analysis and interpretation of data. JY, YZ and QZ researched the
literature and drafted the manuscript. JY and FX checked and
confirm the authenticity of all the raw data. JY and FX reviewed
and edited the final manuscript. All authors read and approved the
final version of the manuscript and agree to be accountable for all
aspects of the work in ensuring that questions related to the
accuracy or integrity of any part of the work are appropriately
investigated and resolved.
Ethics approval and consent to
participate
Written informed consent prior to and regarding the
treatment protocol was obtained from the patient analyzed in the
present study. The study was approved by the Ethics Committee of
Mianyang Central Hospital (Mianyang, China; approval no.
S2021080).
Patient consent for publication
Written informed consent for publication was
obtained from the individual participant included in the study.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Bisht K, Fukao T, Chiron M, Richardson P,
Atanackovic D, Chini E, Chng WJ, Van De Velde H and Malavasi F:
Immunomodulatory properties of CD38 antibodies and their effect on
anticancer efficacy in multiple myeloma. Cancer Med.
12:20332–20352. 2023.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Arrossi AV, Merzianu M, Farver C, Yuan C,
Wang SH, Nakashima MO and Cotta CV: Nodular pulmonary light chain
deposition disease: an entity associated with Sjögren syndrome or
marginal zone lymphoma. J Clin Pathol. 69:490–496. 2016.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Steward M, Yu JH and Gibbons MA: Sjögren's
syndrome as a cause of both lymphoid interstitial pneumonia and
light chain deposition disease in a single patient. BMJ Case Rep.
15(e249747)2022.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Aoudia R, Bacha MM, Ounissi M, Gaied H,
Jerbi M, Abderrahim E, Abdallah TB and Goucha R: Monoclonal
gammopathy of renal significance with light-chain deposition
disease in kidney transplantation. Saudi J Kidney Dis Transpl.
30:1161–1165. 2019.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Li W, Liang L, Liao Q, Li Y and Zhou Y:
CD38: An important regulator of T cell function. Biomed
Pharmacother. 153(113395)2022.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Ye X, Zhao Y, Ma W, Ares I, Martínez M,
Lopez-Torres B, Martínez-Larrañaga MR, Wang X, Anadón A and
Martínez MA: The potential of CD38 protein as a target for
autoimmune diseases. Autoimmun Rev. 22(103289)2023.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Holzer MT, Ruffer N, Huber TB, Kötter I,
Ostendorf L and Krusche M: Daratumumab for autoimmune diseases: A
systematic review. RMD Open. 9(e003604)2023.PubMed/NCBI View Article : Google Scholar
|
|
8
|
De Novellis D, Fontana R, Giudice V, Serio
B and Selleri C: Innovative anti-CD38 and anti-BCMA targeted
therapies in multiple myeloma: Mechanisms of action and resistance.
Int J Mol Sci. 24(645)2022.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Kastritis E, Rousakis P, Kostopoulos IV,
Gavriatopoulou M, Theodorakakou F, Fotiou D, Dialoupi I, Migkou M,
Roussou M, Kanellias N, et al: Consolidation with a short course of
daratumumab in patients with AL amyloidosis or light chain
deposition disease. Amyloid. 28:259–266. 2021.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Milani P, Basset M, Curci P, Foli A, Rizzi
R, Nuvolone M, Guido R, Gesualdo L, Specchia G, Merlini G, et al:
Daratumumab in light chain deposition disease: Rapid and profound
hematologic response preserves kidney function. Blood Adv.
4:1321–1324. 2020.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Leung N, Bridoux F, Batuman V, Chaidos A,
Cockwell P, D'Agati VD, Dispenzieri A, Fervenza FC, Fermand JP,
Gibbs S, et al: The evaluation of monoclonal gammopathy of renal
significance: A consensus report of the International Kidney and
Monoclonal Gammopathy Research Group. Nat Rev Nephrol. 15:45–59.
2019.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Kobayashi A, Takeda A, Shinjo H, Iguchi D,
Ito C, Okada E, Goto N, Futamura K, Okada M, Hiramitsu T, et al:
Light chain deposition disease recurrence in renal allograft after
long-term remission. Nephron. 147 (Suppl 1):96–100. 2023.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Li J, Koerner J, Basler M, Brunner T, Kirk
CJ and Groettrup M: Immunoproteasome inhibition induces plasma cell
apoptosis and preserves kidney allografts by activating the
unfolded protein response and suppressing plasma cell survival
factors. Kidney Int. 95:611–623. 2019.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Masood A, Ehsan H, Iqbal Q, Salman A and
Hashmi H: Treatment of light chain deposition disease: A systematic
review. J Hematol. 11:123–130. 2022.PubMed/NCBI View
Article : Google Scholar
|
|
15
|
Shimamura Y, Ogawa Y, Takizawa H, Hayashi
T and Sakurai Y: Light chain deposition disease diagnosed using
computed tomography-guided kidney biopsy. Cureus.
13(e15102)2021.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Tsushima T, Suzuki T, Terao T, Miura D,
Narita K, Takeuchi M, Shimuzu A and Matsue K: Light chain
deposition disease involving kidney and liver in a patient with IgD
myeloma. BMC Nephrol. 22(40)2021.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Heybeli C, Alexander MP, Bentall AJ, Amer
H, Buadi FK, Dean PG, Dingli D, Dispenzieri A, El Ters M, Gertz MA,
et al: Kidney transplantation in patients with monoclonal
gammopathy of renal significance (MGRS)-associated lesions: A case
series. Am J Kidney Dis. 79:202–216. 2022.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Nambirajan A, Bhowmik D, Singh G, Agarwal
SK and Dinda AK: Monoclonal gammopathy of renal significance with
light-chain deposition disease diagnosed postrenal transplant: A
diagnostic and therapeutic challenge. Transpl Int. 28:375–379.
2015.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Kuppachi S, Holanda D and Thomas CP: Light
chain deposition disease after kidney transplantation with long
graft survival: Case report. Transplant Proc. 48:255–258.
2016.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Jimenez-Zepeda VH, Vajpeyi R, John R and
Trudel S: Light chain deposition disease affecting the
gastrointestinal tract in the setting of post-living donor kidney
transplantation. Int J Hematol. 96:125–131. 2012.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Tanenbaum ND, Howell DN, Middleton JP and
Spurney RF: Lambda light chain deposition disease in a renal
allograft. Transplant Proc. 37:4289–4292. 2005.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Larsen T, Hammer A and Jørgensen KA:
Recurrence of light-chain deposition disease after renal
transplantation. Scand J Urol Nephrol. 42:187–188. 2008.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Ecder T, Tbakhi A, Braun WE, Tubbs RR,
Myles J and McMahon JT: De novo light-chain deposition disease in a
cadaver renal allograft. Am J Kidney Dis. 28:461–465.
1996.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Horike K, Takeda A, Otsuka Y, Inaguma D,
Goto N, Watarai Y, Uchida K and Morozumi K: A case of recurrent
light chain deposition disease after living-related renal
transplantation-Detailed process of the recurrence. Clin
Transplant. 26 (Suppl 24):64–69. 2012.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Taneda S, Honda K, Horita S, Koyama I,
Teraoka S, Oda H and Yamaguchi Y: Light chain deposition disease
after renal transplantation. Am J Kidney Dis. 52:621–625.
2008.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Ziogas DC, Kastritis E, Terpos E, Roussou
M, Migkou M, Gavriatopoulou M, Spanomichou D,
Eleutherakis-Papaiakovou E, Fotiou D, Panagiotidis I, et al:
Hematologic and renal improvement of monoclonal immunoglobulin
deposition disease after treatment with bortezomib-based regimens.
Leuk Lymphoma. 58:1832–1839. 2017.PubMed/NCBI View Article : Google Scholar
|