Introduction
Postoperative (1,2) and
post-extraction bleeding in dental practice (3) are common complications in surgery.
Such bleeding is easily controlled in most cases. However, the risk
of bleeding during and after surgical procedures is notably
increased in patients treated with antithrombotic agents (4). The application of topical hemostatic
agents typically increases in clinical cases with a higher
incidence of bleeding (5,6). In dentistry and medicine, Ankaferd
BloodStopper (ABS; Ankaferd İlaç Kozmetik A.Ş.) has been
recommended as an effective and safe alternative hemostatic agent
for patients with hemostatic disorders (7-9).
ABS has been licensed as a medication to stop
bleeding by the Ministry of Health of the Republic of Türkiye. ABS
has been used as a topical hemostatic agent in medicine and
dentistry in numerous countries, based on safety and efficacy
reports indicating its sterility and non-toxicity (7). This product prevents hemorrhage in
individuals with normal hemostatic parameters and patients with
primary and/or secondary hemostasis (1,10).
ABS is composed of five plant extracts, specifically 5 Thymus
vulgaris, 6 Urtica dioica, 7 Alpinia officinarum,
8 Vitis vinifera and 9 mg Glycyrrhiza glabra in 100
ml Ankaferd solution (11). Each
of these constituents has various effects on blood cells,
angiogenesis, endothelium, cell proliferation, cell mediators and
vascular dynamics. The primary action mechanism of ABS is rapidly
forming an encapsulated protein network in the plasma (12). ABS has been used in dentistry
(5,7) and medicine (9,13-15).
To the best of our knowledge, no bleeding has been reported in
patients treated with ABS.
ABS has also been used as a hemostatic agent during
dental extractions in patients undergoing antithrombotic treatment
with warfarin sodium and acetylsalicylic acid without interrupting
anticoagulant management (16).
Biological stability is essential for local hemostatic agents
because they are in direct contact with periapical tissue,
including soft tissue flaps, cortical and cancellous bone and
peripheral nerves. Inappropriate administration of local hemostatic
agents in such areas could result in adverse local tissue response
and systemic complications. Therefore, the biological
characteristics of local hemostatic agents must be considered, as
well as their hemostatic efficacy (17).
A neuron develops a single axon that transmits
electrical signals from the cell body to the target tissue over
long distances (18). Peripheral
nerves comprise myelinated and unmyelinated axons (19). Myelin is formed around an axon by
specialized Schwann cells in the peripheral nervous system. It
provides the structural basis for saltatory action potential
propagation, which accelerates nerve conduction 20-100-fold
compared with non-myelinated axons of the same diameter (20).
Drug toxicity is known to lead to structural and
functional impairments in peripheral nerves (21). Peripheral nerve damage can impair
axonal connectivity by hindering cargo transport along axons or
dismantling cytoskeletal structures, leading to axon loss (18). ABS is directly applied without
dilution in clinical settings and the neighboring tissues are
exposed to ABS for a long time (10,22).
It was reported ABS causes neurotoxicity, inflammation,
neurodegeneration and functional impairment in animal studies
(23). Therefore, peripheral
nerves exposed to ABS in clinical settings may also be at risk of
degeneration.
The present study aimed to investigate the
degenerative effects of ABS in a mouse sciatic nerve model.
Horizontal ladder rung walking task (LRWT) was used to evaluate
gait disturbance caused by nerve damage and immunohistochemistry
(IHC) techniques were performed to assess pathological changes.
Materials and methods
Ethics statement
This study was conducted in compliance with Animal
Research: Reporting of In Vivo Experiments (ARRIVE)
guidelines and the relevant guidelines and regulations performed in
all of the methods (24). The
animal experiments were reviewed and approved by the Ethics
Committee of Van Yuzuncu Yil University Laboratory Animal Center
(Van, Türkiye, approval no.
27552122-604.01.02-E.69647-2017/09).
Experimental design
A total of 30 male BALB/c mice, weighing 27-33 g and
aged 6 to 8 weeks, were purchased from the Yüzüncü Yıl
University Experimental Animal Production and Research Center. Mice
were distributed randomly (n=10/group) into control group (no
treatment), sham group (treated with saline) and experimental group
(treated with ABS). The animals were housed in an air-conditioned
room (21±2˚C), with a relative humidity of 45-55% and a 12:12 h
light/dark cycle, with free access to food and water. All efforts
were made to minimize animal suffering and reduce the number of
animals used. The animals never reached the humane endpoints.
Drugs
The following drugs were used in this study:
Ketamine (100 mg/ml; Hexal AG; Sandoz International GmbH), xylazine
(2%; Bayer AG), saline (3 ml 0.9% sterile saline solution; Hudson
RCI), Ankaferd Blood Stopper (2 ml ampoule; Trend Teknoloji İlaç
AŞ).
Surgical procedure
The mice were anesthetized using 10 mg/kg xylazine
intraperitoneally (i.p.) and 100 mg/kg ketamine i.p. to minimize
their pain. Surgery was performed unilaterally in the right
hindlimb. The sciatic nerves were exposed by making an incision in
the skin and carefully separating the underlying muscles of the
upper thigh. In the experimental group, 0.3 ml ABS was administered
directly onto the sciatic nerve using a sterile insulin syringe.
ABS was applied for 5 min and then removed by aspiration with the
syringe (23). The ampoule form of
ABS is used in clinical practice with 100% purity (25). Therefore, the sciatic nerves of
mice were exposed to 100% ABS to mimic clinical practice. In the
sham group, 0.3 ml saline was administered instead. In the control
group, no surgery was performed.
LRWT
The horizontal LRWT evaluates the walking ability of
mice. It measures both forelimb and hindlimb placing, stepping and
inter-limb coordination (26). The
present study aimed to characterize the gait impairment of the
sciatic nerve in the ABS group using the LRWT. The LRWT apparatus
was adapted from the LRWT previously used in rats (26). The apparatus was composed of 2
plexiglass walls (70x16 cm). Metal rungs (2 mm diameter) were
inserted to create a floor with a minimum distance of 7 mm between
rungs. The ladder was elevated 11 cm above the ground. The width
was 4 cm. The animals were tested on an irregular ladder pattern
(random). In the irregular pattern, the distance of the rungs
ranged from 0.7 to 14.0 mm. Tests were performed pre-treatment (day
0) and post-treatment (day 7). Each animal had 2 runs per session.
The foot placement and walking were video-recorded from the side
portion of the ladder, with a Canon EOS 750D camera (Canon Inc.)
positioned at a slight ventral angle to record the experimental
limb (27).
The qualitative assessment of the rear limb
placement was carried out using a foot fault scoring system, as
described previously (26). All
video recordings were analyzed frame-by-frame, by an observer
blinded to the experiments. Each step was scored according to the
quality of limb placement. The following scale was used, adapted
from that of Metz and Whishaw (26): 0, total miss (the limb did not
touch a rung, which induced a fall; the fall disturbed the body
posture and balance of the animal and stepping cycle was
interrupted); 1, deep slip (limb was initially placed on a rung,
then slipped off when weight-bearing, which caused the animal to
fall); 2, slight slip (limb was placed on a rung and slipped;
however, no fall was observed and the animal continued walking); 3,
replacement (limb was placed on a rung; however, it subsequently
moved to another rung); 4, correction (limb aimed for one rung;
however, it was then placed on another rung without touching the
first one); 5, partial placement (limb was placed on a rung with
either the heel or toes; limb was fully weight bearing) and 6,
correct placement (limb was placed on a rung with the palm and toes
closed around it; limb was fully weight bearing) (27).
The scores and step count in each trial were
calculated, and then the sum of the scores was divided by the step
count. The scores of two trials were averaged for the analysis. As
a result, the average gait scores of the animals were
calculated.
Immunofluorescent staining
At 7 days post-treatment, animals were euthanized
via decapitation under deep anesthesia by i.p. injections of
xylazine (10 mg/kg) and ketamine (100 mg/kg). The sciatic nerve
segment between the sciatic notch and the tibial nerve was exposed
to ABS. The sciatic nerves were dissected and immediately fixed in
4% cold paraformaldehyde for 4 h at room temperature (28), then cryoprotected in 30% sucrose.
The tissues were then rinsed in 0.1 M PBS three times. Next,
15-µm-thick frozen sagittal sections were cut serially at -25˚C on
a Leica CM1520 cryostat (Leica Microsystems), and placed on glass
slides covered with poly-L-lysine (MilliporeSigma). The sections
were blocked with blocking buffer (2% BSA and 0.2% Triton-X100 in
PBS; MilliporeSigma) for 1 h at room temperature. Sections were
washed in PBS three times. Next, primary antibody incubation was
performed overnight at 4˚C. The primary antibodies used were as
follows: Anti-neurofilament heavy-chain 200 kDa (NF200; 1:100;
mouse monoclonal; cat. no. NE14; Sigma-Aldrich; Merck KGaA),
anti-class III β-tubulin (βIII-Tub; 1:100; mouse monoclonal; cat.
no. TU-20; MilliporeSigma), anti-S100 (1:100; rabbit polyclonal;
cat. no. Z0311; Dako; Agilent Technologies, Inc.), anti-myelin
basic protein (MBP; 1:50; goat polyclonal; cat. no. sc-13912; Santa
Cruz Biotechnology, Inc.) and anti-myelin protein 0 or peripheral
myelin (P0; 1:50; rabbit polyclonal; cat. no. ABN363;
MilliporeSigma). The tissue sections were washed three times in PBS
and incubated with secondary antibodies for 2 h at 4˚C in the dark.
The secondary antibodies used in were as follows: Alexa Fluor 568
goat anti-rabbit IgG and Alexa Fluor 488-goat anti-mouse IgM (both
1:200; cat. nos. A11036 and A11029, respectively; both Invitrogen;
Thermo Fisher Scientific, Inc.) After the reaction, the sections
were rinsed once with PBS. The slides were coverslipped and imaged
using a Zeiss LSM 510 meta laser scanning confocal microscope (Carl
Zeiss NTS GmbH) on an AxioVert 200M fluorescent microscope (Carl
Zeiss NTS GmbH). The images were obtained using a 20X objective
(28). NF200 and βIII-Tub are
cytoskeleton proteins that are expressed by neurons (29). Axons were visualized employing
NF200 and βIII-Tub immunofluorescence staining. S100 is a protein
that is expressed by myelin-producing Schwann cells (30). Schwann cells were visualized with
S100 immunofluorescence staining. P0 (a myelin marker) is a
critical protein for myelin compaction and biogenesis. This protein
can interact with signaling molecules, which is vital for
myelination (31). The myelin was
visualized by P0 and MBP immunofluorescence staining.
Image analysis was performed using Image J software
(version 1.53; National Institutes of Health). Fluorescence
intensity was measured for all of the biomarkers (NF200, S100,
βIII-Tub, MBP, and P0) individually on the same day, choosing an
intensity threshold in the NF200 channel so the axons and myelin
were highlighted, corresponding to the regions of interest and
immunoreactivity was quantified by their integrated intensity
(28).
Statistical analysis
Descriptive statistics for the step scores are
presented as the mean, standard deviation, minimum and maximum
values. Two-way repeated measures analysis of variance (ANOVA) was
used to compare the groups and pre/post treatments. IBM SPSS
Statistics for Windows 23.0 (IBM Corp.) was used for all
statistical computations. P<0.05 was considered to indicate a
statistically significant difference
Results
Foot fault score
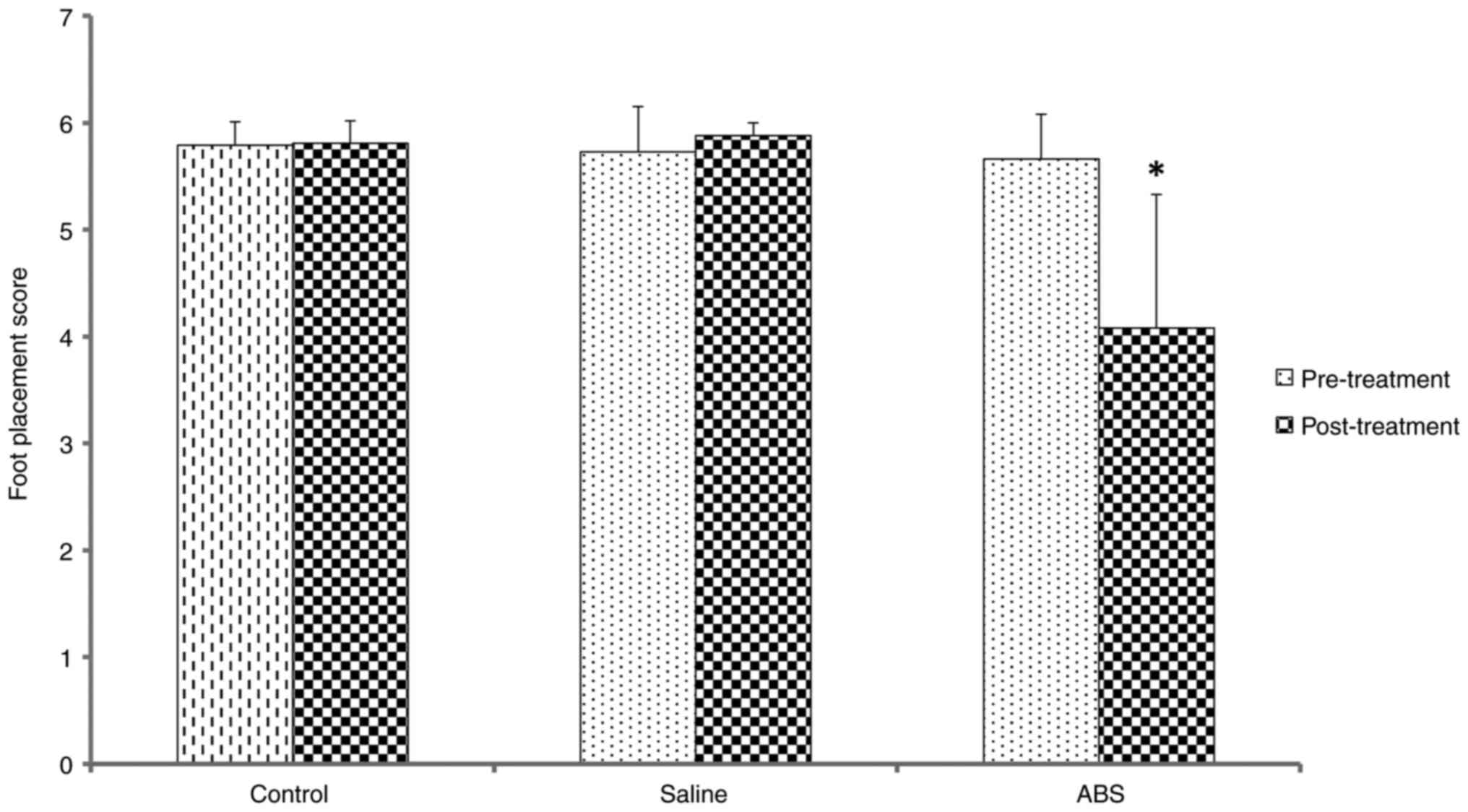
The results of the foot placement score analyses are
shown in Fig. 1. Pre-treatment,
the mean foot placement scores indicated that the mice carried out
primarily correct placements, resulting in high scores. All animals
had a healthy gait pattern. There was no significant difference
between groups. Post-treatment, the control and saline group scores
showed that the mice performed primarily correct placements
(Videos S1 and S2, respectively). The post-treatment
foot placement scores were compared with those pre-treatment for
all groups. There was no significant difference between the control
and saline groups. However, the ABS group made more errors in
placement, resulting in low scores (Video S3). In the ABS group, mean foot
placement score was significantly decreased post-treatment. In
summary, ABS produced more foot placement deficit or gait
impairment in the experimental limb.
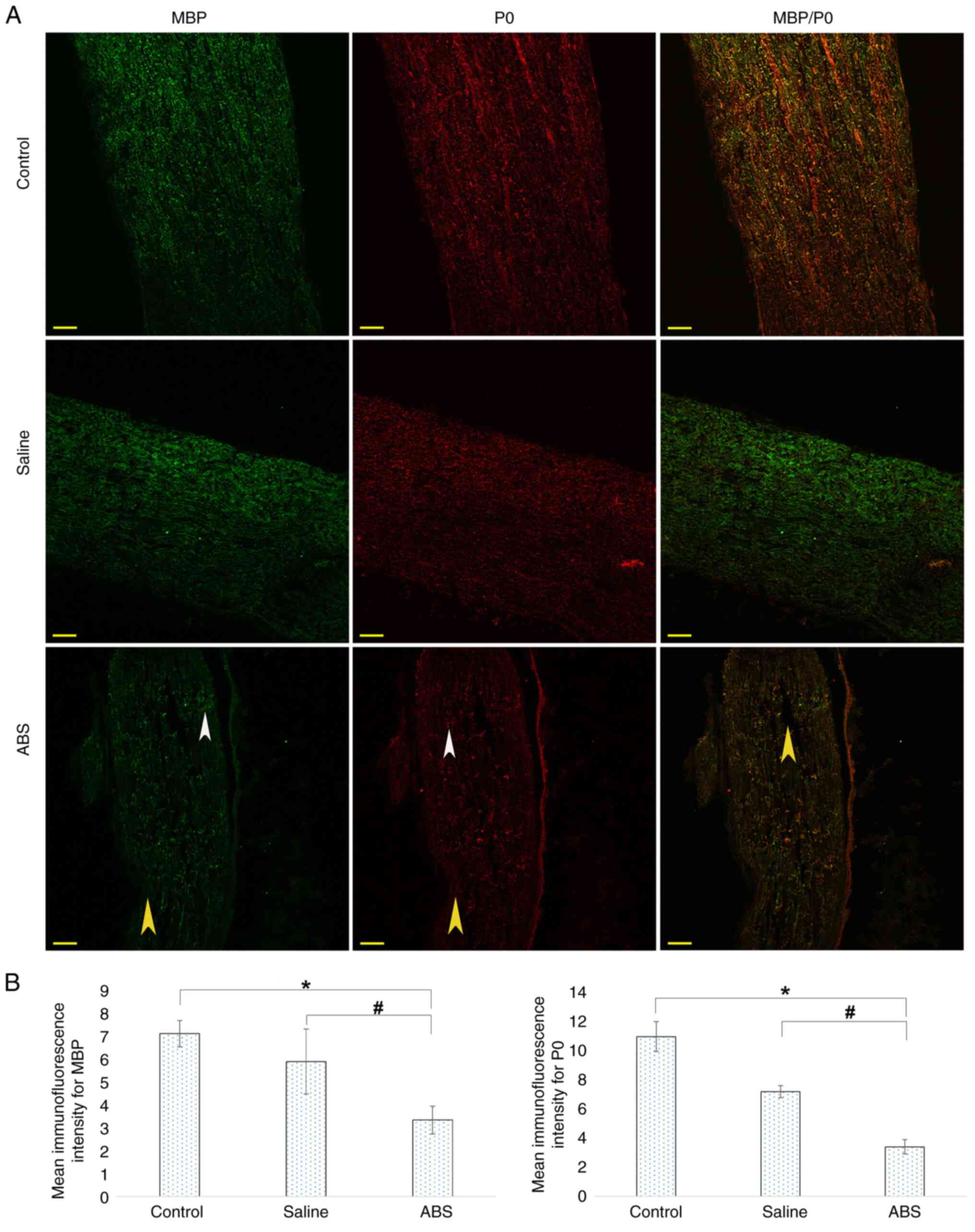
Topical ABS treatment damages NF200
and S100
Axonal degeneration and Schwann cell destruction
were evaluated by IHC. For this assay, longitudinal sections of the
sciatic nerve were stained with fluorescently labeled antibodies
against NF200 and S100. The representative IHC images are shown in
Fig. 2A. In both the control and
saline groups, NF200 (green) and S100 (red) were strong, intact and
displayed regular and compact structures. Brightly labeled NF200
and S100 were observed through the longitudinal sections of the
sciatic nerves. By contrast, in sciatic nerves from the ABS group,
the integrity of the NF200 degraded. The NF200 displayed an
irregular and disconnected structure. In addition, cytoskeletal
structure was wholly disorganized and displayed extensive cavities
across the long sections. Weak red fluorescent protein (S100)
showed that the Schwan cells were degenerated and decreased in
number. Moreover, ABS-induced axonal damage was evaluated by
assessing the immunostaining intensity (Fig. 2B). There was a significant decrease
in the immunofluorescence intensities of the NF200 and S100 when
the ABS group was compared with control and saline groups.
 | Figure 2ABS treatment damages neuronal
cytoskeleton marker NF200 and myelinating Schwann cell marker S100.
(A) Longitudinal nerve sections were prepared from sciatic nerves
on day 7 post-treatment. Axons were labeled with NF200 (green), and
Schwann cells were marked with S100 (red). In the control and the
saline groups, the NF200 and S100 were expressed at high levels by
neurons and Schwann cells, respectively. However, in the ABS group,
the NF200 and S100 were expressed at low levels. Laser-scanned
confocal images showed that the ABS caused degeneration,
deformation, degradation and fragmentation of the NF200 and S100
proteins (white and blue arrowheads, respectively). There were
large cavities along the sections (yellow arrowhead). Scale bar, 50
µm. (B) Mean immunofluorescence intensities of the NF200 and S100.
The ABS group showed lower fluorescence intensity than the control
and saline groups. *P<0.05, ABS group vs. Control
group; #P<0.05, ABS group vs. Saline group). ABS,
Ankaferd BloodStopper®; NF, neurofilament. |
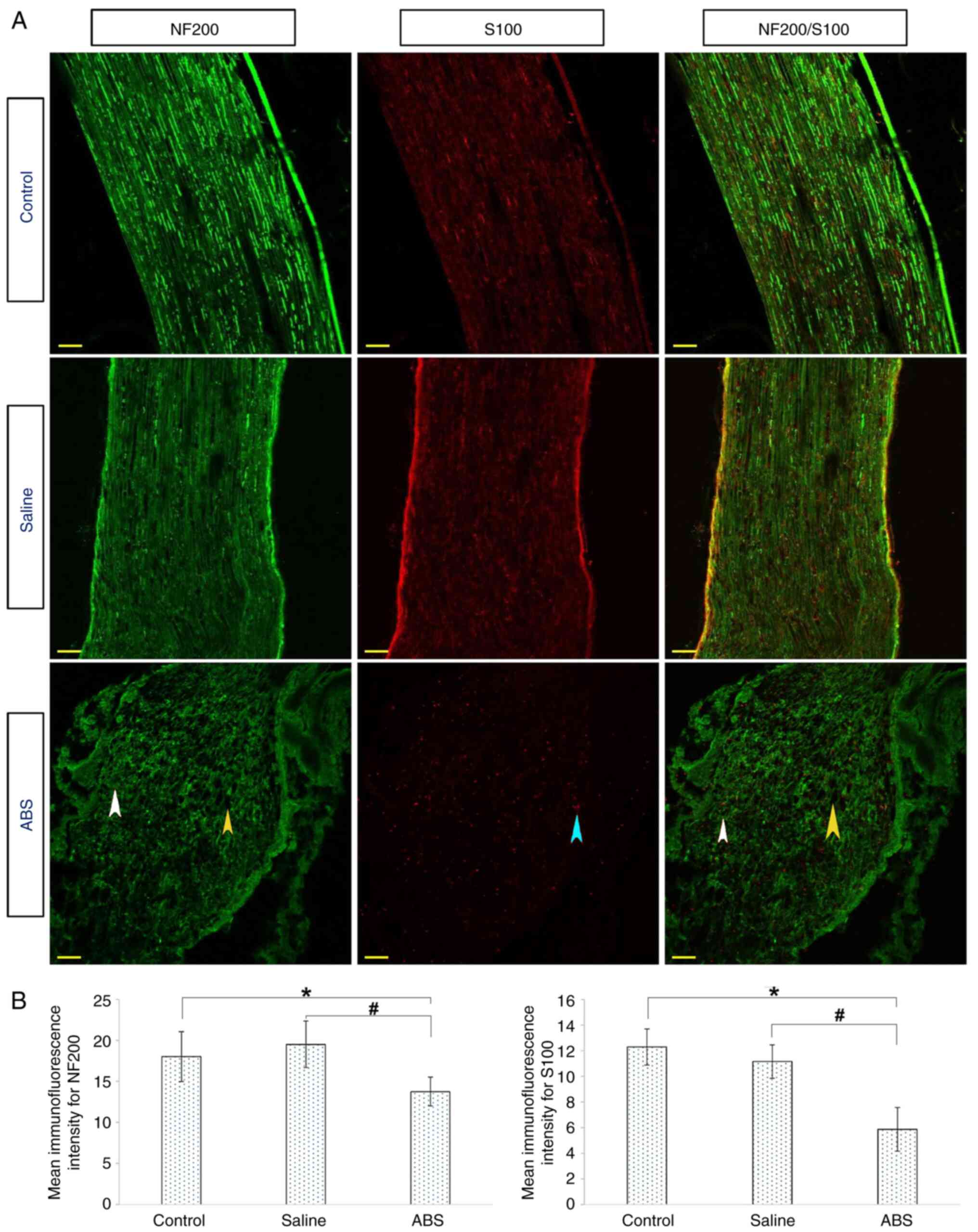
Topical ABS treatment deforms
microtubules and alters expression of β-III-Tub
Nerve sections were stained with fluorescently
labeled antibodies against βIII-Tub (green) and S100 (red). IHC
images are shown in Fig. 3A.
βIII-Tub in the control and saline groups showed brightly labeled
regular, continuous and tight cytoskeletal structures along the
axons. The S100 fluorescence indicated normal myelin-producing
Schwann cell distribution. By contrast, markedly increased
deformity of the βIII-Tub in the ABS group indicated disorganized
and disconnected axons. Furthermore, there was a loss of density
and integrity of microtubules compared with axons in the control
and saline groups. There were also deep and widespread pores.
ABS-induced axonal damage was evaluated by assessing the
immunostaining intensity of βIII-Tub and S100 (Fig. 3B). There was a significant decrease
in the βIII-Tub and S100 immunofluorescence intensities when ABS
group was compared with the control and saline groups. The βIII-Tub
immunofluorescence intensity significantly decreased in the saline
group compared with that in the control group.
 | Figure 3ABS treatment causes axonal marker
βIII-Tub degeneration and deformation. (A) Axons were labeled with
βIII-Tub (green) and Schwann cells were marked with S100 (red).
βIII-Tub and S100 were highly expressed in the control and saline
groups compared with the ABS group. However, both the βIII-Tub and
S100 were expressed at low levels by the neurons in the ABS group.
Control and saline groups showed regular, intact and tight
cytoskeletal structures that continued along the axon. In the ABS
group, however, markedly increased deformity of the βIII-Tub were
observed. Furthermore, there was a loss of density and integrity of
βIII-Tub (pink arrowheads). There were also large cavities (yellow
arrowhead). Scale bar, 50 µm. (B) Mean immunofluorescence intensity
of the βIII-Tub and S100. The ABS group showed lower fluorescence
intensity than the control and saline groups.
*P<0.05, ABS group vs. Control group;
#P<0.05, ABS group vs. Saline group. ABS, Ankaferd
BloodStopper®; βIII-Tub, Class III β-tubulin. |
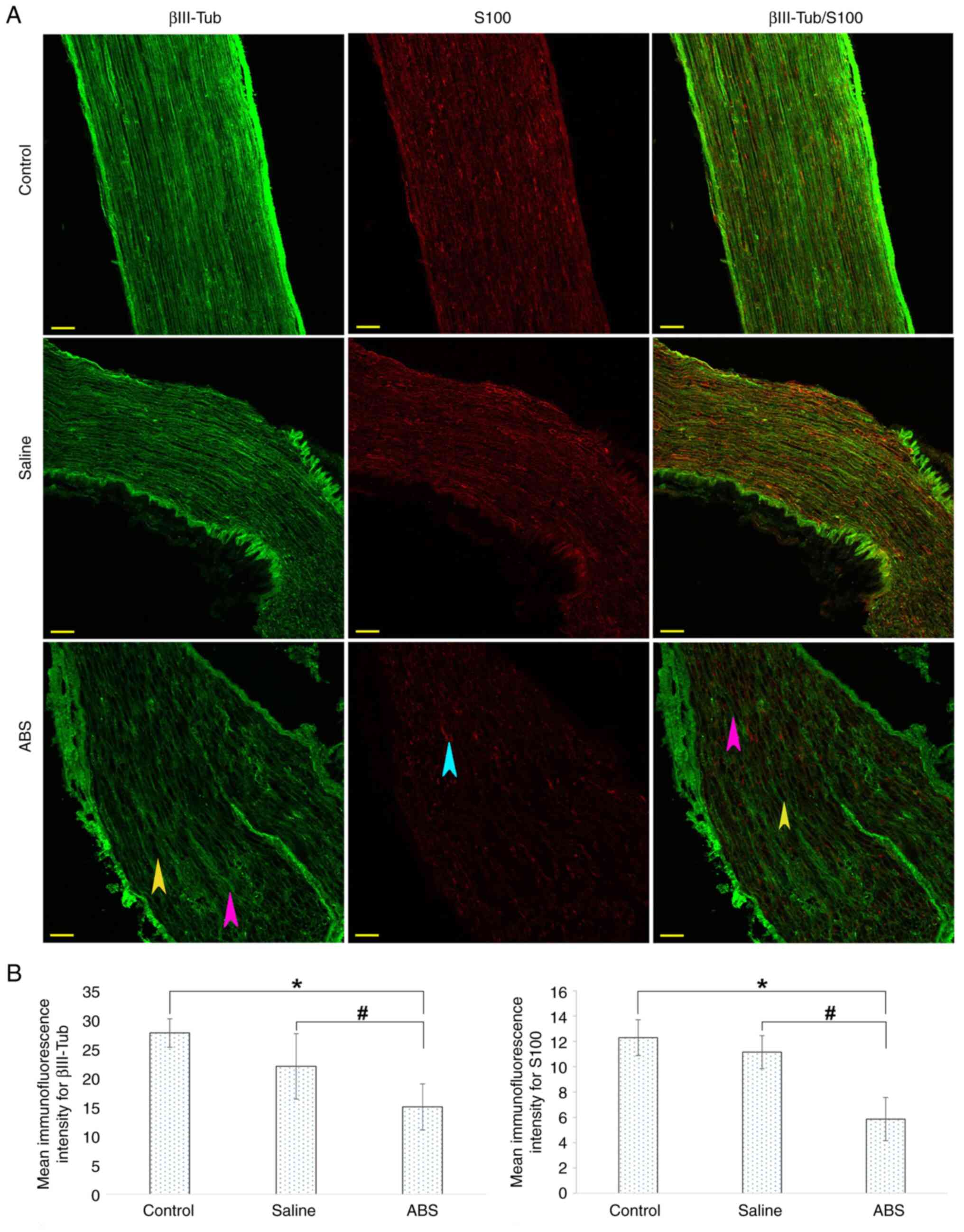
Topical ABS treatment disrupts myelin
protein
The low fluorescence intensity of the S100 suggested
a substantial degeneration and loss of Schwann cells as a result of
the ABS treatment. Therefore, it was investigated whether the
myelin sheaths were also affected by the ABS treatment. To
determine the extent of damage to the myelin sheaths, IHC was
performed using antibodies against myelin-specific proteins P0 and
MBP. Representative IHC images are shown in Fig. 4A. In the control and saline groups,
MBP and P0 expression was evident and regularly distributed along
axons, exhibiting regular myelination. Compared with control group,
the ABS group displayed notable irregularity of the MBP and P0
expressions and marked deformation of the myelin structures along
the axons, suggesting disrupted myelination process. In addition,
there were deep and widespread pores. The ABS-induced axonal damage
was evaluated by assessing the immunostaining intensity of MBP and
P0 (Fig. 4B). There was a
significant decrease in the MBP and P0 immunofluorescence intensity
when the ABS group was compared with the control and saline groups.
The MBP and P0 immunofluorescence intensity significantly decreased
in the saline group compared with that in the control group.
Discussion
Previous experimental studies have suggested that
ABS may have cytotoxic and degenerative effects (23,32-35).
Using a sciatic nerve mouse model, the present study evaluated the
potential neurodegenerative impact of ABS on peripheral nerves. The
experimental design allowed evaluation of the functional and
structural impacts of ABS on mouse sciatic nerve using the LRWT and
IHC. The LRWT has been used as a stringent test of the sensorimotor
function of rodents (36). LRWT
was used to determine severity of nerve damage. The LRWT data
revealed that the functional performance of sciatic nerves exposed
to ABS was notably disrupted. The reduction in the foot placement
score indicated damage to axons and loss of the myelin sheath
around the axons. Thus, animals treated with ABS showed functional
impairment. The functional results were also supported by IHC
analysis.
IHC is a valuable method for demonstrating and
validating impairment/damage discovered through sensorimotor
function tests. IHC is an essential method as it specifically
visualizes distribution and the amount of a certain molecule in the
tissue using a specific antigen-antibody reaction. The use of IHC
in clinical laboratories has recently expanded as more molecules
involved in the pathogenesis, diagnosis, and treatment of diseases
are discovered (37-39).
IHC does not involve destruction of histological architecture, and
thus, assessment of an expression pattern of the molecule in the
microenvironment is possible (40).
Structural, functional and biomechanical integrity
is key for the high conduction velocity of myelinated nerve
function and axonal transport (41). The ABS group exhibited substantial
degeneration and deformation as the structures of the NF200 and
βIII-Tub were notably altered and displayed a loss of integrity.
There were long and broad cavities along the axons. Neurofilament
degradation may act as a trigger for other downstream degenerative
events (42). Following axonal
degeneration, Schwann cells undergo a conventional cellular program
that disintegrates the myelin sheaths, a process called
demyelination (43). In sciatic
nerves treated with topical ABS, representative confocal images of
S100 indicated that the Schwann cells were damaged and decreased in
number. The achievement of peripheral nerve reconstruction and
restoration relies on the ability of Schwann cells to proliferate
and supply trophic support for regenerating axons (44). Within 48 h following injury,
Schwann cells stop producing myelin proteins (44). In the present study, MBP and P0
were damaged and fragmented. These findings indicated myelin
degeneration and fragmentation. ABS disrupted the robust myelin
structure and led to fragmentation of the myelin sheaths. Both MBP
and P0 are key for the maintenance of the structure of the
peripheral nervous system (31).
Topical ABS administration led to axonal degeneration,
demyelination and decreased number of the Schwann cells. Myelin
breakdown and axonal damage may alter the structure and function of
peripheral nerves, which contributes to the malfunction of sensory
perception and motor function (45).
Ustun and Oguz (32) showed that ABS has a degenerative
effect on the peripheral sensory neurons of mice, depending on the
ABS density. An in vivo study by Ustun et al measured
compound muscle action potential (CMAP), motor nerve conduction
velocity (MNCV), nociceptive pain sensation and motor coordination
in a mouse sciatic nerve model (46). ABS treatment decreased CMAP and
MNCV values, diminished the nociceptive pain sensation, weakened
motor coordination and increased atrophy of the target muscles.
Thus, ABS functionally led to neuromuscular impairment in mice
(46). The results of the present
study support the aforementioned results.
In a study by Adak et al (23), the left mental nerve of rats was
exposed to 0.3 ml sterile saline for 5 min in the control group,
while the other groups were treated with ABS, tranexamic acid and
Floseal® (thrombin-containing hemostatic matrix),
respectively. Following a 28-day recovery period, the nerve tissues
were removed from the rats and placed in 10% formaldehyde solution
for histopathological (hematoxylin & eosin, Luxol fast blue)
and immunohistochemical examination. A detailed assessment was
conducted on the sciatic nerve tissue, which included an evaluation
of the axon structure, myelin thickness, Schwann cell count,
endoneurium continuity and severity of inflammation. ABS led to a
significant decrease in the number of axons and Schwann cells,
decreased the myelin thickness and increased inflammation. The
present study validates the histopathological results of the
aforementioned study.
The neurotoxic effect on medulla spinalis of the ABS
was studied histopathologically in rats by Turkoz et al
(47). Histopathological features
such as oedema, gliosis, neuronal degeneration, myelin degeneration
and inflammatory cell concentration were scored as none (0), mild
(1) and significant (2). ABS induced few neurologic toxicities for
spinal neural tissue. However, in the present study, the ABS led to
a notable degenerative effect on the peripheral nerve. In the
aforementioned study, spinal cord section was stained with
hematoxylin and eosin stain and luxury fast blue stain and
evaluated using a light microscope, which may not fully reflect
damage to the medulla spinalis. Ultrastructural microscopic
examination performed with IHC and confocal or electron microscopy,
may better reflect severity and extent of neurodegeneration
(48,49).
Pampu et al (50) performed experimental work on rat
sciatic nerve with ABS. In the ABS group, 2 ml ABS was applied to
the nerve region with sterile sponges for 3 min. The sponges were
then removed, and NCV was assessed at 30 and 120 min and 3 weeks. A
process similar to that in the ABS group was performed in the
control group with sterile saline solution. According to the
baseline values, the NCV values measured at different times were
increased by 5% in the saline group and decreased by 20% in the ABS
group. However, the difference between the ABS and saline groups
was not statistically significant. The aforementioned study
contradicted the results of the present research. Here, the sciatic
nerve was exposed directly to pure ABS liquid for 5 min. In the
aforementioned study, the sciatic nerve was in contact with an
ABS-soaked sponge for 3 min. Therefore, in the present study, the
sciatic nerve was more exposed to ABS; thus, greater degeneration,
deformation and functional impairment occurred.
The degenerative effect of ABS on cartilage tissue
and fibroblast cells has been investigated in experiments with
rabbits and rats (33-35).
The histopathological examination of cartilage tissue showed ABS
causes fibrosis and necrosis (34,35).
Mcroscopic examination of the fibroblast cells revealed decreased
survival and proliferation (33).
ABS may damage other tissues, such as cartilage and connective
tissue, as well as peripheral nerves. Collectively, the
aforementioned studies are consistent with the present findings
that the application of ABS causes neurodegenerative effects in
sciatic nerves.
Patients with bleeding in oral and maxillofacial
surgery report numbness and dysfunction after treatment with ABS
(46). The present study was
designed to investigate a mouse model of the degenerative effect of
ABS. The findings validate loss of sensation reported in dental
practice. However, the relevance of animal studies to humans is not
always clear. The present experimental data will help inform future
studies related to the actual clinical practice patterns. Clinical
trials will provide a clear picture of ABS-induced peripheral nerve
degeneration and dysfunction in humans.
The present study focused on the effect of the ABS
on the sciatic nerve. However, during ABS application, the skeletal
muscles around the nerve were also exposed to the ABS. Therefore,
the effect on skeletal muscles of the ABS could not be excluded.
The present study focused on the neurodegenerative effects of the
ABS in the acute phase (7 days post-treatment). Previous studies
performed with mice have shown axonal regeneration, remyelination,
and increased neuromuscular junctions 4 weeks after peripheral
nerve injury (51,52). Long-term degeneration and
dysfunction were not investigated at 4 weeks. Thus, we recommend
repeating these tests after 4 weeks to clarify whether ABS-induced
sciatic nerve degeneration is permanent and whether regeneration
has started after degeneration. Persistent neurotoxic side effects
of the ABS, including nerve dysfunction, diminish patient quality
of life (23,46). Another limitation of the present
study is the need for deciphering peripheral nerve degeneration
molecular mechanisms. It cannot be estimated which results clinical
trials will produce based on sensory and motor function findings
obtained from animal models. The primary limitation of this study
is the lack of clinical proof of the ABS neurodegenerative
effect.
The results of the present study generally support
previous studies (23,32-36,46).
The present study demonstrated that ABS led to the degeneration of
the cytoskeletal structures of axons, fragmentation and
demyelination of the myelin sheaths surrounding the axons and a
decline in the number of Schwann cells, which were demonstrated by
IHC. Furthermore, ABS caused dysfunction (indicated by foot
placement deficits and/or gait impairment) in the sciatic nerves of
mice. This dysfunction was confirmed by immunofluorescence imaging
of neurodegeneration and demyelination markers. The present results
indicate that the ABS can induce sensory and motor function
disturbances. To prevent harm to in humans, ABS should be tested
for neurotoxic and neurodegenerative effects via further
experimental studies as well as clinical trials. Long-term outcomes
of ABS treatment should be identified. The effect on skeletal
muscle of ABS treatment should be assessed in preclinical and
clinical studies. Nerve function in clinical trials is the most
important outcome parameter for clinicians. Finally, the neurotoxic
molecule among ABS components should be revealed.
In conclusion, the present study demonstrated that
the topical application of ABS to the sciatic nerve led to notable
gait impairment in the LRWT, degeneration and deformation in the
axon and the myelin sheath.
Supplementary Material
Supplementary Data
Foot placement score of 5.81 was found
in the control group. Post-treatment, the control group showed
correct foot placement and smooth gait while walking.
Foot placement score of 5.88 was found
in the saline group. Post-treatment, the saline group showed
correct foot placement and primarily smooth gait while
walking.
Foot placement score of 4.08 was found
in the ABS group. Post-treatment, the ABS group showed a high rate
of errors in foot placement and gait impairment while walking.
Acknowledgements
The authors would like to thank Professor Sıddık
Keskin (Department of Biostatistics, School of Medicine, Van
Yüzüncü Yıl University, Van, Turkey) for assistance with the
statistical analysis and Dr Seda Keskin (Department of Histology
and Embryology, School of Medicine, Van Yüzüncü Yıl University,
Van, Turkey) for technical contributions to the experiments.
Funding
Funding: The present study was supported by the Van Yüzüncü Yıl
University Scientific Research Project Directorate (grant no.
2015-HIZ-TF317).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
RU conceived the study and wrote the manuscript. EO,
FT and AS performed experiments. AS analyzed data and wrote the
manuscript. FT and AS confirm the authenticity of all the raw data.
All authors have read and approved the final version of the final
manuscript.
Ethics approval and consent to
participate
All experiments involving animals and surgical
procedures were approved (approval no. 27552122-604.01.02-E.69647)
by the Ethical Committee of University of Yüzüncü Yıl Sciences
Committee in accordance with the European Community Council
Directive 86/609/ECC for the care and use of laboratory
animals.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Xu B, Jin HY, Wu K and Chen C, Li L, Zhang
Y, Gu WZ and Chen C: Primary and secondary postoperative hemorrhage
in pediatric tonsillectomy. World J Clin Cases. 9:1543–1553.
2021.PubMed/NCBI View Article : Google Scholar
|
|
2
|
von Ahnen T, Schardey J, von Ahnen M,
Busch P, Schardey E, Ezzy MA, Schopf S and Wirth U: Neck
circumference measurement for surveillance and early detection of
hemorrhage after thyroidectomy: A diagnostic accuracy study. JAMA
Otolaryngol Head Neck Surg. 148:646–653. 2022.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Hiroshi I, Natsuko SY, Yutaka I, Masayori
S, Hiroyuki N and Hirohisa I: Frequency of hemorrhage after tooth
extraction in patients treated with a direct oral anticoagulant: A
multicenter cross-sectional study. PLoS One.
17(e0266011)2022.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Wahl MJ, Pinto A, Kilham J and Lalla RV:
Dental surgery in anticoagulated patients-stop the interruption.
Oral Surg Oral Med Oral Pathol Oral Radiol. 119:136–157.
2015.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Amer MZ, Mourad SI, Salem AS and
Abdelfadil E: Correlation between International Normalized Ratio
values and sufficiency of two different local hemostatic measures
in anticoagulated patients. Eur J Dent. 8:475–480. 2014.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Keceli HG, Aylikci BU, Koseoglu S and
Dolgun A: Evaluation of palatal donor site haemostasis and wound
healing after free gingival graft surgery. J Clin Periodontol.
42:582–589. 2015.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Cakarer S, Eyupoglu E, Gunes CO, Kuseoglu
BG, Berberoglu HK and Keskin C: Evaluation of the hemostatic
effects of Ankaferd blood stopper during dental extractions in
patients on antithrombotic therapy. Clin Appl Thromb Hemost.
19:96–99. 2013.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Baker RI and O'Donnell JS: How I treat
bleeding disorder of unknown cause. Blood. 138:1795–1804.
2021.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Kar M, Cetinkaya EA and Konsuk-Unlu H:
Comparison of the ankaferd blood stopper tampon and the merocel
nasal tampon after septoplasty surgery. Aesthetic Plast Surg.
47:294–300. 2023.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Huri E, Akgul T, Ayyildiz A, Bagcioglu M
and Germiyanoglu C: First clinical experience of Ankaferd
BloodStopper as a hemostatic agent in partial nephrectomy.
Kaohsiung J Med Sci. 26:493–495. 2010.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Garber A and Jang S: Novel therapeutic
strategies in the management of non-variceal upper gastrointestinal
bleeding. Clin Endosc. 49:421–424. 2016.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Iynen I, Bozkus F, San I and Alatas N: The
hemostatic efficacy of Ankaferd Blood Stopper in adenoidectomy. Int
J Pediatr Otorhinolaryngol. 75:1292–1295. 2011.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Koyuncu N: The effectiveness of ankaferd
blood stopper in the management of traumatic bleeding. Adv Ther.
36:1143–1149. 2019.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Gilman R and Ganesh Kumar N: Invited
discussion on: Comparison of the ankaferd blood stopper tampon and
the merocel nasal tampon after septoplasty surgery. Aesthetic Plast
Surg. 47:301–303. 2023.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Ozseker B, Shorbagi A, Efe C, Haznedaroglu
IC and Bayraktar Y: Controlling of upper gastrointestinal bleeding
associated with severe immune thrombocytopenia via topical
adjunctive application of Ankaferd blood stopper. Blood Coagul
Fibrinolysis. 23(464)2012.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Dincol ME, Ozbas H, Yilmaz B, Ersev H,
Gokyay S and Olgac V: Effect of the plant-based hemostatic agent
Ankaferd Blood Stopper(R) on the biocompatibility of mineral
trioxide aggregate. BMC Oral Health. 16(111)2016.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Jang Y, Kim H, Roh BD and Kim E: Biologic
response of local hemostatic agents used in endodontic
microsurgery. Restor Dent Endod. 39:79–88. 2014.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Shin JE and Cho Y: Epigenetic regulation
of axon regeneration after neural injury. Mol Cells. 40:10–16.
2017.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Sullivan R, Dailey T, Duncan K, Abel N and
Borlongan CV: Peripheral nerve injury: Stem cell therapy and
peripheral nerve transfer. Int J Mol Sci. 17(2101)2016.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Nave KA and Werner HB: Myelination of the
nervous system: Mechanisms and functions. Annu Rev Cell Dev Biol.
30:503–533. 2014.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Ramdharry G: Peripheral nerve disease.
Handb Clin Neurol. 159:403–415. 2018.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Hacioglu SK, Dogu MH, Sari I and Keskin A:
Successful treatment of refractory gastrointestinal bleeding by
systemic (Oral) ankaferd blood stopper in a patient with glanzmann
thrombasthenia. Balkan Med J. 32:218–220. 2015.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Adak BM, Lacin N, Simsek F, Uysal E, Soylu
FE and Ozkan I: Evaluation of the effects of different hemostatic
agent applications on mental nerve. Eur Arch Otorhinolaryngol.
279:5355–5362. 2022.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Percie du Sert N, Hurst V, Ahluwalia A,
Alam S, Avey MT, Baker M, Browne WJ, Clark A, Cuthill IC, Dirnagl
U, et al: The ARRIVE guidelines 2.0: Updated guidelines for
reporting animal research. BMJ Open Sci. 4(e100115)2020.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Arslan S, Haznedaroglu IC, Öz B and Goker
H: Endobronchial application of Ankaferd blood stopper to control
profuse lung bleeding leading to hypoxemia and hemodynamic
instability. Resp Med CME. 2:144–146. 2009.
|
|
26
|
Metz GA and Whishaw IQ: Cortical and
subcortical lesions impair skilled walking in the ladder rung
walking test: A new task to evaluate fore- and hindlimb stepping,
placing, and co-ordination. J Neurosci Methods. 115:169–179.
2002.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Farr TD, Liu L, Colwell KL, Whishaw IQ and
Metz GA: Bilateral alteration in stepping pattern after unilateral
motor cortex injury: A new test strategy for analysis of skilled
limb movements in neurological mouse models. J Neurosci Methods.
153:104–113. 2006.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Namgung U, Choi BH, Park S, Lee JU, Seo
HS, Suh BC and Kim KT: Activation of cyclin-dependent kinase 5 is
involved in axonal regeneration. Mol Cell Neurosci. 25:422–432.
2004.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Makker PG, Duffy SS, Lees JG, Perera CJ,
Tonkin RS, Butovsky O, Park SB, Goldstein D and Moalem-Taylor G:
Characterisation of immune and neuroinflammatory changes associated
with chemotherapy-induced peripheral neuropathy. PLoS One.
12(e0170814)2017.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Wang Y, Li WY, Sun P, Jin ZS, Liu GB, Deng
LX and Guan LX: Sciatic nerve regeneration in KLF7-transfected
acellular nerve allografts. Neurol Res. 38:242–254. 2016.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Hao GM, Liu YG, Wu Y, Xing W, Guo SZ, Wang
Y, Wang ZL, Li C, Lv TT, Wang HL, et al: The protective effect of
the active components of ERPC on diabetic peripheral neuropathy in
rats. J Ethnopharmacol. 202:162–171. 2017.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Ustun R and Oguz EK: Degenerative effect
of Ankaferd Blood Stopper (R) on mice peripheral sensory neurons in
vitro. Folia Neuropathol. 56:67–74. 2018.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Emes Y, Aybar B, Vural P, Issever H, Yalcn
S, Atalay B, Dincol E and Bilir A: Effects of hemostatic agents on
fibroblast cells. Implant Dent. 23:641–647. 2014.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Evren C, Ugur MB, Yildirim B, Bektas S,
Yigit VB and Cinar F: Unpredicted effects of Ankaferd(R) on
cartilage tissue. Int J Clin Exp Med. 8:922–927. 2015.
|
|
35
|
Kaya I, Gulabi D, Yilmaz M, Bas A, Cecen
GS and Sener N: Intraarticular Ankaferd blood stopper application
increases cartilagedegeneration: An experimental study. Turk J Med
Sci. 46:236–240. 2016.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Zhang SX, Huang F, Gates M, Shen X and
Holmberg EG: Early application of tail nerve electrical
stimulation-induced walking training promotes locomotor recovery in
rats with spinal cord injury. Spinal Cord. 54:942–946.
2016.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Crescenzi A and Baloch Z:
Immunohistochemistry in the pathologic diagnosis and management of
thyroid neoplasms. Front Endocrinol (Lausanne).
14(1198099)2023.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Harms PW, Frankel TL, Moutafi M, Rao A,
Rimm DL, Taube JM, Thomas D, Chan MP and Pantanowitz L: Multiplex
Immunohistochemistry and Immunofluorescence: A practical update for
pathologists. Mod Pathol. 36(100197)2023.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Dudas B, Lane M, Mupparaju N, Kim HM and
Merchenthaler I: A forgotten principle in immunocytochemistry:
Optimal dilution. J Histochem Cytochem. 70:759–765. 2022.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Kim SW, Roh J and Park CS:
Immunohistochemistry for pathologists: Protocols, pitfalls, and
tips. J Pathol Transl Med. 50:411–418. 2016.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Rosso G, Liashkovich I, Young P and Shahin
V: Nano-scale biophysical and structural investigations on intact
and neuropathic nerve fibers by simultaneous combination of atomic
force and confocal microscopy. Front Mol Neurosci.
10(277)2017.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Ma M, Ferguson TA, Schoch KM, Li J, Qian
Y, Shofer FS, Saatman KE and Neumar RW: Calpains mediate axonal
cytoskeleton disintegration during Wallerian degeneration.
Neurobiol Dis. 56:34–46. 2013.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Tricaud N and Park HT: Wallerian
demyelination: Chronicle of a cellular cataclysm. Cell Mol Life
Sci. 74:4049–4057. 2017.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Zhou Z, Liu Y, Nie X, Cao J, Zhu X, Yao L,
Zhang W, Yu J, Wu G, Liu Y and Yang H: Involvement of upregulated
SYF2 in Schwann cell differentiation and migration after sciatic
nerve crush. Cell Mol Neurobiol. 34:1023–1036. 2014.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Starobova H and Vetter I: Pathophysiology
of chemotherapy-induced peripheral neuropathy. Front Mol Neurosci.
10(174)2017.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Ustun R, Oguz EK, Delilbasi C, Seker A,
Taspinar F, Oncu MR and Oguz AR: Neuromuscular degenerative effects
of Ankaferd Blood Stopper((R)) in mouse sciatic nerve model.
Somatosens Mot Res. 34:248–257. 2017.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Turkoz D, Demirel C, Sataloglu H and
Cokluk C: Analysing the blood-stemming effect of Ankaferd Blood
Stopper in medulla spinalis surgery. Turk J Med Sci. 50:1131–1135.
2020.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Stamm B, Moschopulos M, Hungerbuehler H,
Guarner J, Genrich GL and Zaki SR: Neuroinvasion by Mycoplasma
pneumoniae in acute disseminated encephalomyelitis. Emerg Infect
Dis. 14:641–643. 2008.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Bitirgen G, Akpinar Z, Malik RA and
Ozkagnici A: Use of corneal confocal microscopy to detect corneal
nerve loss and increased dendritic cells in patients with multiple
sclerosis. JAMA Ophthalmol. 135:777–782. 2017.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Pampu AA, Yildirim M, Tuzuner T, Baygin O,
Abidin I, Dayisoylu EH and Senel FC: Comparison of the effects of
new folkloric hemostatic agent on peripheral nerve function: An
electrophysiologic study in rats. Oral Surg Oral Med Oral Pathol
Oral Radiol. 115:e1–e6. 2013.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Okuwa Y, Toriumi T, Nakayama H, Ito T,
Otake K, Kurita K, Nakashima M and Honda M: Transplantation effects
of dental pulp-derived cells on peripheral nerve regeneration in
crushed sciatic nerve injury. J Oral Sci. 60:526–535.
2018.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Li L, Yokoyama H, Kaburagi H, Hirai T,
Tsuji K, Enomoto M, Wakabayashi Y and Okawa A: Remnant
neuromuscular junctions in denervated muscles contribute to
functional recovery in delayed peripheral nerve repair. Neural
Regen Res. 15:731–738. 2020.PubMed/NCBI View Article : Google Scholar
|