Introduction
Epilepsy is a common neurological disorder caused by
abnormal brain discharge, characterized by recurrent limb twitching
and loss of consciousness. It affects ~1% of the global population.
Currently, antiseizure medication (ASM) is the primary treatment
for epilepsy (1). However ~30% of
cases remain medically intractable, resulting in a heavy economic
burden on patients and society (2). Therefore, it is necessary to develop
novel ASMs that effectively control seizures.
Platycladi cacumen (PC), a widely used
traditional Chinese medicine, is derived from dry twigs and leaves
of Platycladi orientalis (L.) Franco. It is traditionally
used to cool blood, stanch bleeding, dispel pathogenic winds,
remove dampness, eliminate phlegm and raise hair and blacken hair
(3). Recently, PC was shown to
exert anti-inflammatory, antioxidative and neuroprotective effects
(4). Aqueous extracts of PC
suppress lipopolysaccharide (LPS)-induced intestinal inflammation
by increasing colon length and inhibiting fecal occult blood,
severe diarrhea and enteritis (5).
PC carbonisata-derived nanoparticles inhibit ulcerative colitis
induced by 2,4,6,-trinitrobenzenesulfonic acid in rats by
decreasing tumor necrosis factor-α (TNF-α) and interleukin-6 and
upregulating interleukin-10(4).
Furthermore, denuded mice treated with water extract of PC for 4
weeks exhibit increased hair growth with increasing hair bulb size
and dermis and epidermal thickness (6). A similar effect was found with
volatile oil extracts of PC (7).
Furthermore, PC exerts renoprotective effects by targeting renal
organic anion transporters 1 and 3 to inhibit protein activity
(8). A total of 43 compounds have
been extracted and separated by 75% methanol from PC and grouped as
organic acids, flavonoids, phenylpropanoids, volatile oils and
tannins (9). Among them,
myricitrin, quercitrin and amentoflavone are the primary compounds
(10) that ameliorate liver
ischemia-reperfusion and (11)
kidney injury (11) and inhibit
platelet activation in arterial thrombosis (12) and human breast cancer (13).
The efficacy of PC against epilepsy has been
reported in ‘Effective Prescription for Epilepsy Treatment’
(14). However, its anti-epileptic
components and underlying mechanisms remain unclear. Therefore, in
the present study, the anti-epileptic compound PC was explored
using network pharmacology and in vitro experiments.
Materials and methods
Construction of an
‘Herbs-Components-Targets’ (H-C-T) network
The Traditional Chinese Medicine System Pharmacology
Database (TCMSP) was used to identify the active ingredients of PC
(15), of which, the components
whose toxicokinetic absorption, distribution, metabolism and
excretion (ADME) adhered to oral bioavailability (OB) ≥30% and
drug-likeness (DL) ≥0.18 were defined as the main compounds.
Druggable compounds that may cross the blood-brain barrier (BBB) as
predicted by SwissADME (swissadme.ch/index.ph) were further used to identify
targets on the SwissTargetPrediction (SWISS; new.swisstargetprediction.ch/) and similarity ensemble
approach (SEA; sea.bkslab.org/) websites by using
relative Canonical Simplified Molecular Input Line Entry System
(SMILES) numbers.
Genes associated with epilepsy were obtained from
GeneCards (version 4.9.0; genecards.org/). Overlapping genes between targets of
the druggable compounds and epilepsy-associated targets were
retrieved using VENN map (bioinformatics.psb.ugent.be/webtools/Venn/). The
protein-protein interaction (PPI) network was analyzed using the
protein-protein interaction networks functional enrichment analysis
online tool (STRING; string-db.org/) and core genes were obtained using the
CytoNCA of Cytoscape3.9.1 (cytoscape.org/) with the criteria of two-fold the
median value of degree centrality (DC), median values of
betweenness centrality (BC) and closeness centrality (16). The H-C-T network of PC was
constructed using Cytoscape3.9.1.
Gene functions and pathway
analysis
Gene Ontology (GO) biological process, cellular
component and molecular function and Kyoto Encyclopedia of Genes
and Genomes (KEGG) pathways were analyzed among the overlapping
genes using the web-based tool DAVID v6.8 (david.ncifcrf.gov/tools.jsp) (17). P<0.05 (Bonferroni-corrected) was
considered to indicate statistical significance.
Cell culture and proliferation
assay
The murine microglial cell line BV2 was purchased
from Procell Life Science & Technology Co., Ltd. and cultured
in high-glucose Dulbecco's modified Eagle's medium (DMEM; Gibco;
Thermo Fisher Scientific, Inc.) with 10% fetal bovine serum (Clark
Bioscience) and 1% streptomycin/penicillin (Biosharp Life Sciences)
in a humidified incubator with 5% CO2 at 37˚C.
Cell proliferation was assessed using Cell Counting
Kit-8 (CCK8; Dojindo Molecular Technologies, Inc.). Briefly, cells
(1x104/ml) were seeded and cultured in 96-well
microplates for 24 h. Cells were treated with isopimaric acid (IPA,
Sigma-Aldrich; Merck KGaA; 0.1, 1.0, 10.0, 100.0 and 1,000.0 µM)
for 24 h, followed by incubation with 10 µl CCK8 reagent for 1 h
all at 37˚C. Absorbance was measured by a microplate reader (BioTek
Instruments, Inc.; EPOCH2NS) at 450 nm. Survival rate was
calculated as follows (18):
Survival rate %=absorbance of IPA/absorbance of control x100%.
Wound healing assay
Confluent BV2 cells (90%) were scratched using a
pipette tip and washed three times with PBS to remove non-adherent
cells. The cells were incubated with LPS (1 µg/ml; Sigma-Aldrich;
Merck KGaA) in the presence or absence of 0.0, 0.1, 1.0, 10.0 or
100.0 µM IPA for 24 h ay 37˚C. Images of the central cell-free zone
before and after treatment were obtained by light field microscopy
(magnification, x200; Zeiss X-Cite; Carl Zeiss AG) (19). The migratory area was calculated as
follows: Migratory area (%)=[(area at 0 h - area at 24 h)/area at 0
h] x100%.
LDH assay
The medium of cells treated with LPS in the presence
or absence of IPA was collected to determine the released LDH
content using assay kits (no. A020-2-2; Nanjing Jiancheng
Bioengineering Institute) as previously described (20).
Flow cytometric analysis of cellular
reactive oxygen species (ROS)
Following treatment with LPS in the presence or
absence of IPA for 24 h, cells were incubated at 37˚C with 10 µM
2',7'-dichlorofluorescin diacetate (MedChemExpress) diluted in DMEM
for 30 min in the dark. Images were captured using a fluorescence
microscope (Zeiss X-Cite; Zeiss AG) and mean intensity was measured
using a flow cytometer (NovoCyte; Agilent Technologies, Inc.) in
the fluorescein isothiocyanate (FITC) channel.
Annexin Ⅴ-FITC/propidium iodide (PI)
analysis for apoptosis
BV2 cells were digested 37˚C for 5 min using
trypsin, followed by incubation with Annexin-FITC and PI for 5 min
at room temperature (Boster Biological Technology). Fluorescence
intensities were detected using a flow cytometer (NovoCyte) with
FITC and PI channels, as previously described (21). A total of four populations of cells
were distinguished: Viable (no staining), early apoptosis (Annexin
Ⅴ+PI-), late apoptotic cells (Annexin
Ⅴ+PI+), and necrotic (Annexin
Ⅴ-PI+) cells. Apoptosis was determined as
early + late apoptosis.
Determination of mitochondrial
membrane potential (MMP)
Cells were treated with IPA and LPS for 24 h,
followed by incubation with 500 µl JC-1 working solution in the
dark (Beyotime Institute of Biotechnology) for 20 min at 37˚C.
Images were obtained using a fluorescence microscope in the FITC
and PI channels (magnification, x200; Zeiss X-Cite; Carl Zeiss
AG).
Reverse transcription-quantitative
(RT-q)PCR
Total RNA from BV2 cells was extracted using TRIzol
(Invitrogen; Thermo Fisher Scientific, Inc.) and
reverse-transcribed to cDNA using MonScript Reverse Transcriptase
(cat. no. MR05101; Monad Biotech Co., Ltd.), followed by SYBR Green
PCR (cat. no. MQ00401; Monad Biotech Co., Ltd.), according to the
manufacturer's protocol as previously described (21). Relative expression levels of target
genes were calculated based on the 2-∆∆Cq method using
actin as a reference housekeeping gene (22). The primer sequences are listed in
Table I.
 | Table ISequence and length of primers. |
Table I
Sequence and length of primers.
| Gene | Forward, 5'→3' | Reverse, 5'→3' | Length, bp |
|---|
| Actin |
CCACAGCTGAGAGGGAAATC |
AAGGAAGGCTGGAAAAGAGC | 193 |
| SOD-1 |
CCATCAGTATGGGGACAATACA |
GGTCTCCAACATGCCTCTCT | 109 |
| SOD-2 |
GACCCATTGCAAGGAACAA |
GTAGTAAGCGTGCTCCCACAC | 69 |
| IL-1β |
TGCCACCTTTTGACAGTGATG |
GGAGCCTGTAGTGCAGTTGT | 351 |
| TNF-α |
GTAGCCCACGTCGTAGCAA |
GTGAGGAGCACGTAGTCGG | 191 |
| Arg-1 |
GAACACGGCAGTGGCTTTAAC |
TGCTTAGCTCTGTCTGCTTTGC | 155 |
Western blotting
Total protein was extracted from BV2 cells using a
cell lysis buffer (cat. no. P0013, Beyotime Institute of
Biotechnology) with a phosphatase inhibitor, while concentrations
of proteins were determined by bicinchoninic acid method (Wuhan
Boster Biological Technology, Ltd.). Protein lysates (50 µg) were
resolved by 10% sodium dodecyl sulfate-polyacrylamide gel
electrophoresis and transferred onto a polyvinyl difluoride
membrane (MilliporeSigma) via electroblotting. Each blot was
blocked by QuickBlock (cat. no. P0256; Beyotime Institute of
Biotechnology) for 15 min at room temperature and incubated with
primary antibodies overnight at 4˚C (Table II). Membranes were incubated with
horseradish peroxidase-conjugated secondary antibodies (Proteintech
Group, Inc.; cat. no. 20000858; 1: 2,000; cat. no. 20000757;
1:5,000) for 1 h at 37˚C. The blots were visualized by the ChemiDoc
XRS imaging system (Bio-Rad Laboratories, Inc.) with BeyoECL kit
(cat. no. 081723240119; Beyotime Institute of Biotechnology) and
quantified using ImageJ Software (v1.52a; National Institutes of
Health).
 | Table IIAntibody information. |
Table II
Antibody information.
| Antibody | Supplier | Cat. no. | Dilution |
|---|
| Rabbit
anti-TNF-1α | Cell Signaling
Technology, Inc. | 8184 | 1:1,000 |
| Rabbit
anti-IL-1β | Cell Signaling
Technology, Inc. | 12703S | 1:1,000 |
| Mouse anti-AKT | Proteintech Group,
Inc. | 60203-2 | 1:5,000 |
| Rabbit
anti-p-AKT | Proteintech Group,
Inc. | 80455-1-RR | 1:5,000 |
| Rabbit
anti-mTOR | Abcam | ab2732 | 1:5,000 |
| Mouse
anti-p-mTOR | Proteintech Group,
Inc. | 67778-1 | 1:2,000 |
| Mouse
anti-PI3Kα | Proteintech Group,
Inc. | 67071-1-lg | 1:1,000 |
| Mouse
anti-PI3Kβ | Proteintech Group,
Inc. | 67644-1-lg | 1:5,000 |
| Rabbit
anti-GAPDH | Proteintech Group,
Inc. | 10494-1-AP | 1:6,000 |
Molecular docking
The crystallographic structure of AKT was obtained
from the Protein Data Bank (PDB code: 4GV1) (23) and docking by using Schrödinger
(version 2015) (24). Briefly, the
Protein Preparation Wizard and Receptor Grid Generation modules
were used to prepare the proteins. Ionization-generated possible
states of the LigPrep module were set at a target pH of 7.0±2.0 to
prepare IPA to dock flexibly into the ligand site using a Ligand
Docking module in standard precision mode, as previously described
(25).
Statistical analysis
Data are presented as the mean ± SD. Normally
distributed data were analyzed using the Shapiro-Wilk test and
one-way ANOVA followed by Dunn's post hoc test for multiple groups
using GraphPad Prism (version 9.0.0; Dotmatics) (26). Non-normally distributed data were
analyzed using Kruskal-Wallis test. P<0.05 was considered to
indicate a statistically significant difference.
Results
Targets prediction of PC and
visualization of H-C-T network
A total of seven primary compounds were obtained
from the TCMSP, of which hinokinin, IPA and
deoxypicropodophyllotoxin (DPT) exhibited the potential to cross
the BBB (Table III; Fig. 1A). Based on SMILES numbers of these
three components, 255 potential targets of PC were identified by
target fishing from the SWISS and SEA databases, of which 150 were
associated with epilepsy (Fig.
1B). These composite targets were input into STRING to
construct a PPI network with connected targets (combined score
>0.7), including 259 nodes and 308 edges (Fig. 1C). A total of 13 targets, including
TNF, tumor protein (TP53), estrogen receptor 1 (ESR1),
prostaglandin-endoperoxide synthase 2 (PTGS2), microtubule affinity
regulating kinase 3 (MARK3), peroxisome proliferative activated
receptor gamma (PPARG), caspase 3 (CASP3), B-cell lymphoma-2
(BCL2), glycogen synthase kinase 3 beta (GSK3B), mammalian target
of rapamycin (mTOR), protein tyrosine phosphatase non-receptor
type11 (PTPN11), sirtuin1 (SIRT1) and murine double minute 2 (MDM2)
exceeded the values (two-fold of DC, median of BC and closeness
centrality; Fig. 1D). Among these,
BCL2, GSK3B, and mTOR are potential anti-epileptic targets for
hinokinin; CASP3, mTOR, and SIRT1 for DPT; and TNF, TP53, ESR1,
PTGS2, MAPK3, PPARG, PTPN11, and MDM2 for IPA. In particular, TNF
showed the highest subgraph centrality value.
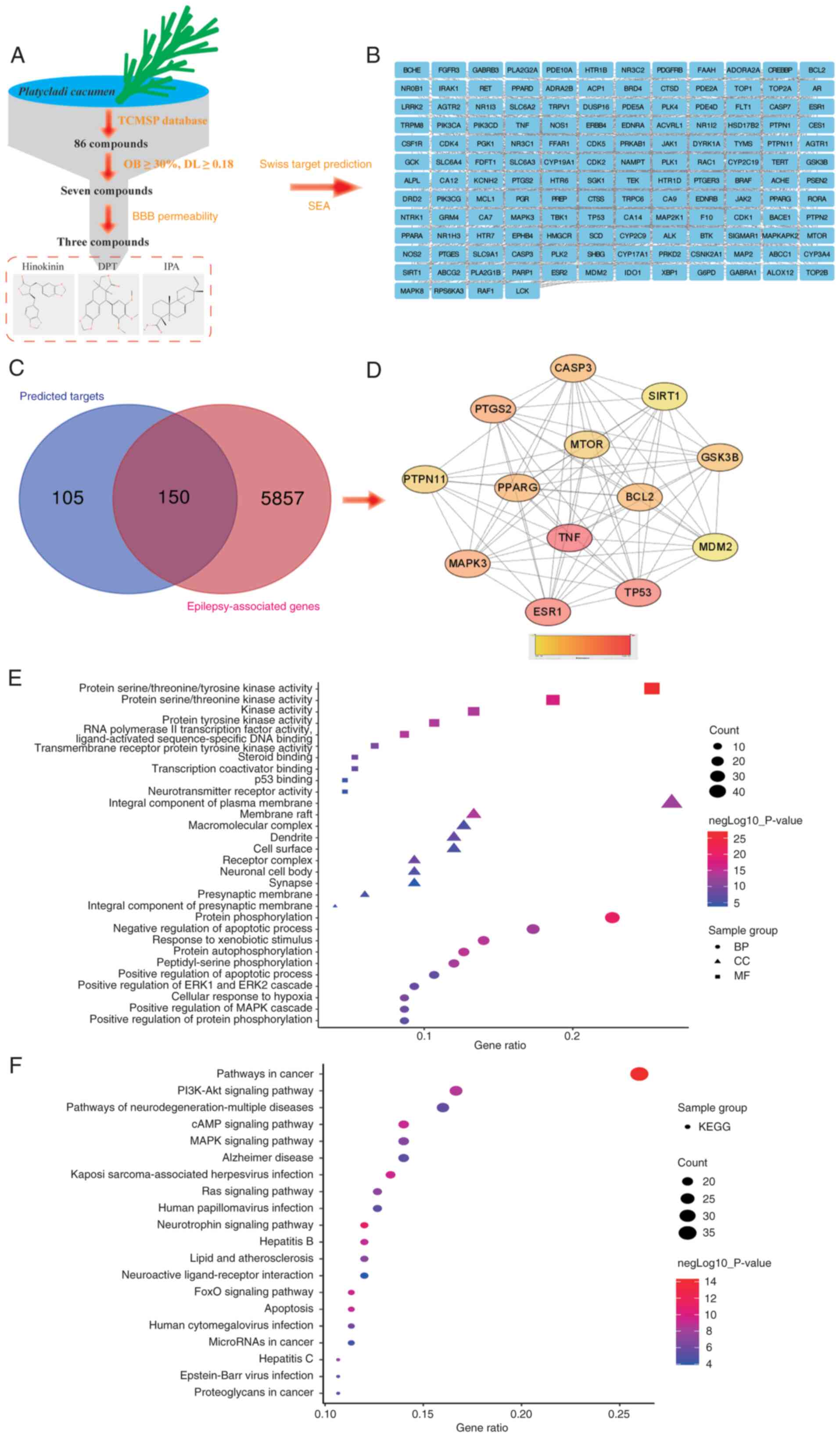 | Figure 1Network pharmacological study on the
anti-epileptic effect of Platycadi cacumen. (A) Analysis of
druggable compounds. A total of 86 compounds were obtained from
TCMSP database, of which seven fulfilled the criteria OB ≥30% and
DL ≥0.18. Hinokinin, DPT and IPA were predicted to cross BBB. (B)
Predicted targets of hinokinin, DPT and IPA from SwissTarget and
SEA database. (C) Venn diagram of overlapping target genes of
compounds and epilepsy-associated genes. (D) Hub gene analysis by
CytoNCA of 150 overlapping genes. (E) GO functional enrichment from
150 overlapping genes. (F) Pathways analysis of 50 overlapping
genes. TCMSP, Traditional Chinese Medicine System Pharmacology
Database; OB, oral bioavailability; DL, drug-likeness; DL,
deoxypicropodophyllotoxin; IPA, isopimaric acid; BBB, blood-brain
barrier; SEA, similarity ensemble approach; BP, biological
processes; CC, cellular component; MF, molecular function; KEGG,
Kyoto Encyclopedia of Genes and Genomes; GO, Gene Ontology. |
 | Table IIICharacteristics of seven active
constituents derived from PC. |
Table III
Characteristics of seven active
constituents derived from PC.
| ID no. | Molecule | MW (g/mol) | DL | OB, % | BBB-permeable |
|---|
| MOL000098 | Quercetin | 302.25 | 0.28 | 46.43 | No |
| MOL000358 | β-sitosterol | 414.79 | 0.75 | 36.91 | No |
| MOL000422 | Kaempferol | 286.25 | 0.24 | 41.88 | No |
| MOL002005 | Hinokinin | 354.38 | 0.64 | 56.5 | Yes |
| MOL002032 | DNOP | 390.62 | 0.40 | 40.59 | No |
| MOL002034 |
(5aR,8aS,9R)-9-(3,4,5-trimethoxyphenyl)-5a,6,8a,9-tetrahydro-5H-isobenzofurano[5,6-f][1,3]benzodioxol-8-one
(Deoxypicropodophyllotoxin) | 398.44 | 0.83 | 52.70 | Yes |
| MOL002039 | Isopimaric
acid | 302.45 | 0.28 | 36.20 | Yes |
GO and KEGG enrichment analysis
GO and pathway enrichment analyses were performed
for overlapping targets (150 genes) that were significantly
enriched ‘protein phosphorylation’, ‘negative regulation of
apoptotic process’, ‘response to xenobiotic stimulus’, ‘protein
autophosphorylation’ and ‘peptidyl-serine phosphorylation’ in the
biological processes. In terms of cellular component, the
overlapping targets were enriched in the ‘membrane raft’,
‘macromolecular complex’, ‘dendrite’, ‘cell surface’, and ‘receptor
complex’. With respect to molecular function, the core targets were
enriched in ‘protein serine/threonine/tyrosine kinase activity’,
‘protein serine/threonine kinase activity’, ‘kinase activity’,
‘protein tyrosine kinase activity’, and ‘RNA polymerase II
transcription factor activity, ligand-activated sequence-specific
DNA binding’ (Fig. 1E). A total of
150 genes were enriched in 140 pathways, of which ‘pathways in
cancer’, ‘PI3K-Akt signaling pathway’, ‘pathways of
neurodegeneration-multiple diseases’, ‘cAMP signaling pathway’ and
‘MAPK signaling pathway’ were the most enriched (Fig. 1F).
IPA inhibits elevation of ROS
production and migration of murine microglia cells induced by
glutamate and LPS
IPA serves a role in pathological processes,
including antibacterial activity (27) and anti-NLR family, pyrin domain
containing protein 3 (NLRP3) inflammasome (28) and anti-Alzheimer's disease effects
(29,30). IPA activates large-conductance
Ca2+-activated K+ channels (31,32)
by targeting gamma-aminobutuyric acid (GABA) receptors to induce
chloride ion currents (33).
Therefore, it was hypothesized that IPA may be a might be a target
compound to treat epilepsy. Hence, glutamate- and LPS-induced
excitotoxicity and neuroinflammation in murine microglia cells BV2
were examined to determine the anti-epileptic effects of IPA, as
previously described (34).
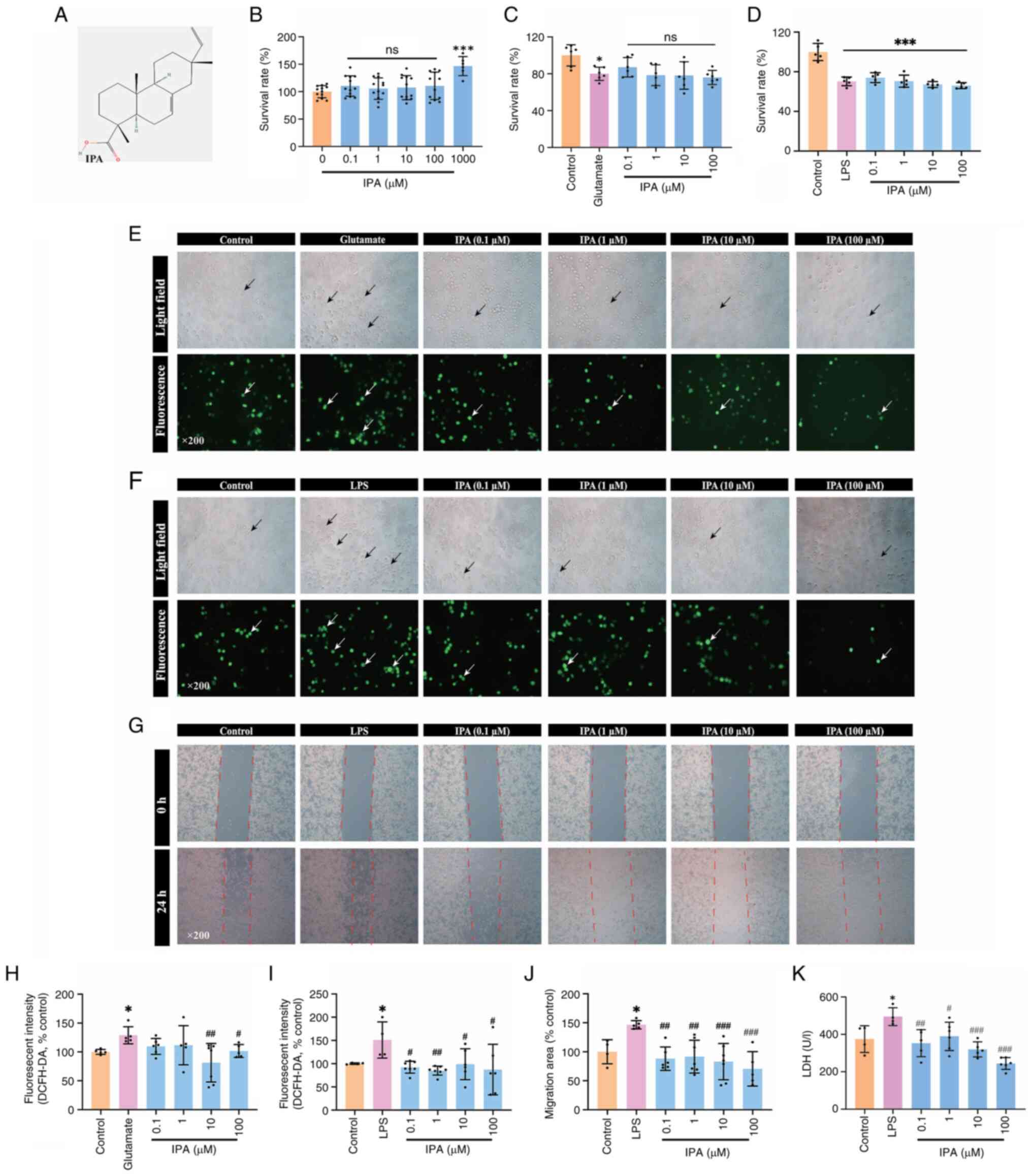
IPA (0.1-1,000.0 µM) was used to verify its effect
on the survival of BV2 cells. Concentrations of IPA from 0.1 to
100.0 µM did not affect the survival rate of BV2 cells and were
applied in subsequent experiments (Fig. 2A and B). BV2 cells treated with glutamate (5
mM) for 12 h and LPS (1 µg/ml) for 24 h notably suppressed the
survival rate. However, co-administration of IPA did not improve
the survival of BV2 cells (Fig. 2C
and D) but significantly
suppressed the production of ROS induced by glutamate (Fig. 2E and F) and LPS (Fig. 2G and H).
IPA significantly inhibited the wound closure of BV2
induced by LPS (0.1, 1.0, 10.0 and 100 µM IPA corresponded to
88.10±20.17, 91.55±28.29, 83.03±11.79 and 70.55±29.71%,
respectively, compared with 146.60±7.19% migration area in the LPS
group; Fig. 2I and J) and decreased LDH release (0.1, 1.0,
10.0 and 100.0 µM IPA doses corresponded to 352.93±72.37,
389.63±75.72, 319.76±40.31, and 244.11±31.44, respectively,
compared with 495.12±47.63 U/l in the LPS group; Fig. 2K).
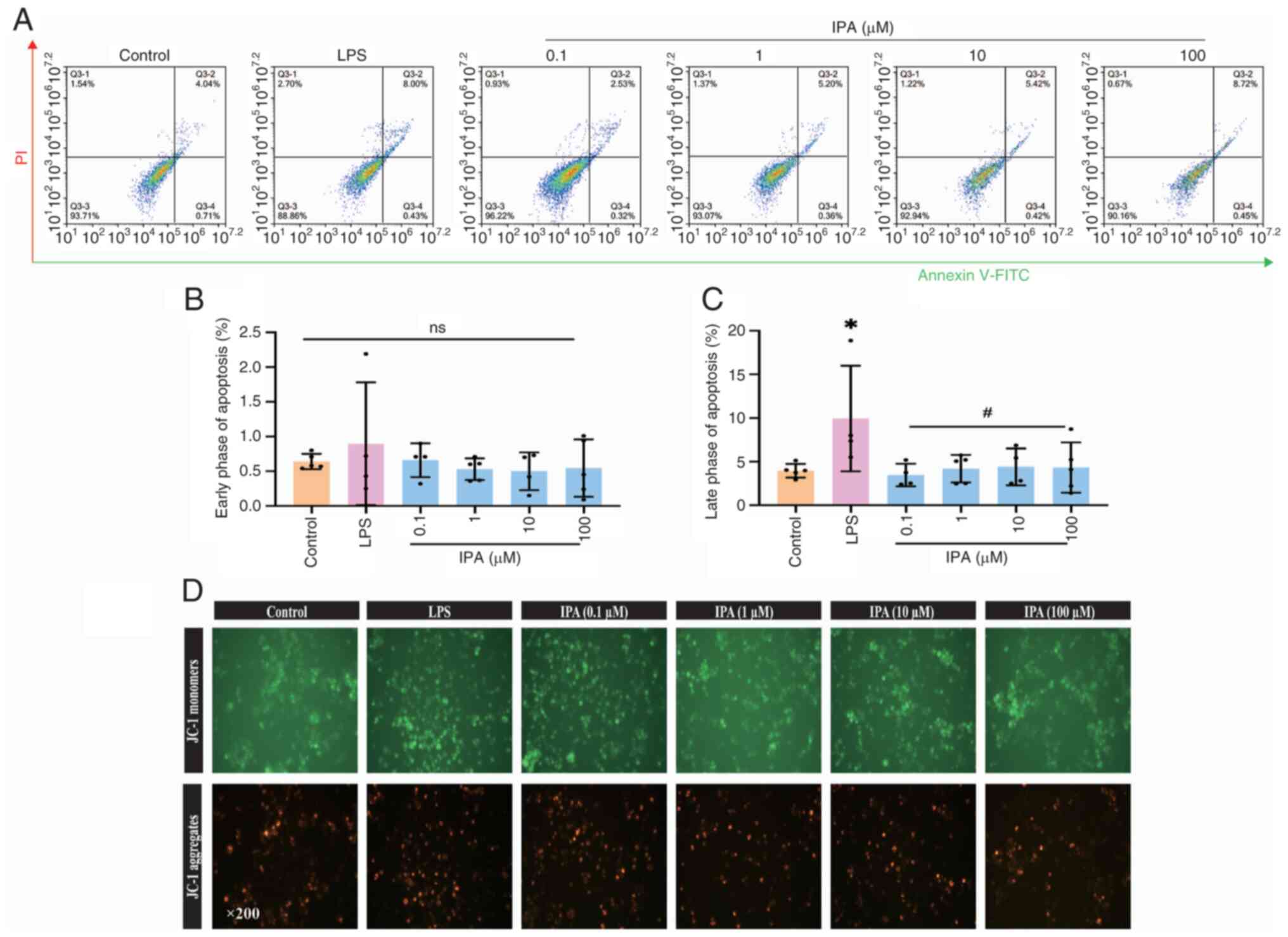
IPA suppresses LPS-induced apoptosis
in BV2 cells
Treatment with IPA in the range of 0.1-100.0 µM
prevented the LPS-induced late phase of apoptosis (LPS, 9.94±6.05%;
0.1 µM, 3.47±1.30%; 1 µM, 4.19±1.58%; 10 µM, 4.40±2.10%; 100 µM,
4.33±2.88%; Fig. 3A-C). It was
confirmed by MMP that BV2 cells treated with IPA showed decreased
JC-1 monomers compared with the LPS group (Fig. 3D).
IPA suppresses mRNA expression of
anti-oxidative genes including superoxide dismutase (SOD)-1 and
SOD-2, inflammatory genes (IL-1β and TNF-α) and M2-polarization
genes (Arg-1) in LPS-treated BV2 cells
To explore the mechanism of action of IPA, mRNA
expression of SOD-1 and SOD-2, which indicate ROS overload
(35), was assessed. IPA at
concentrations of 1, 10, and 100 µM significantly increased mRNA
expression of SOD-1 and SOD-2 (Fig.
4A and B). LPS induced
significant increases of the mRNA expression of inflammatory genes
including IL-1β (Fig. 4C)
and TNF-α (Fig. 4D), while
100 µM of IPA significantly decreased the mRNA expression of
IL-1β and TNF-α. Furthermore, it was demonstrated
that LPS suppressed the mRNA expression of Arg-1, a specific
surface phenotype marker of M2(36). IPA at concentrations of 1, 10, and
100 µM significantly increased the mRNA expression of Arg-1,
indicating that IPA induced the polarization of BV2 to M2 (Fig. 4E).
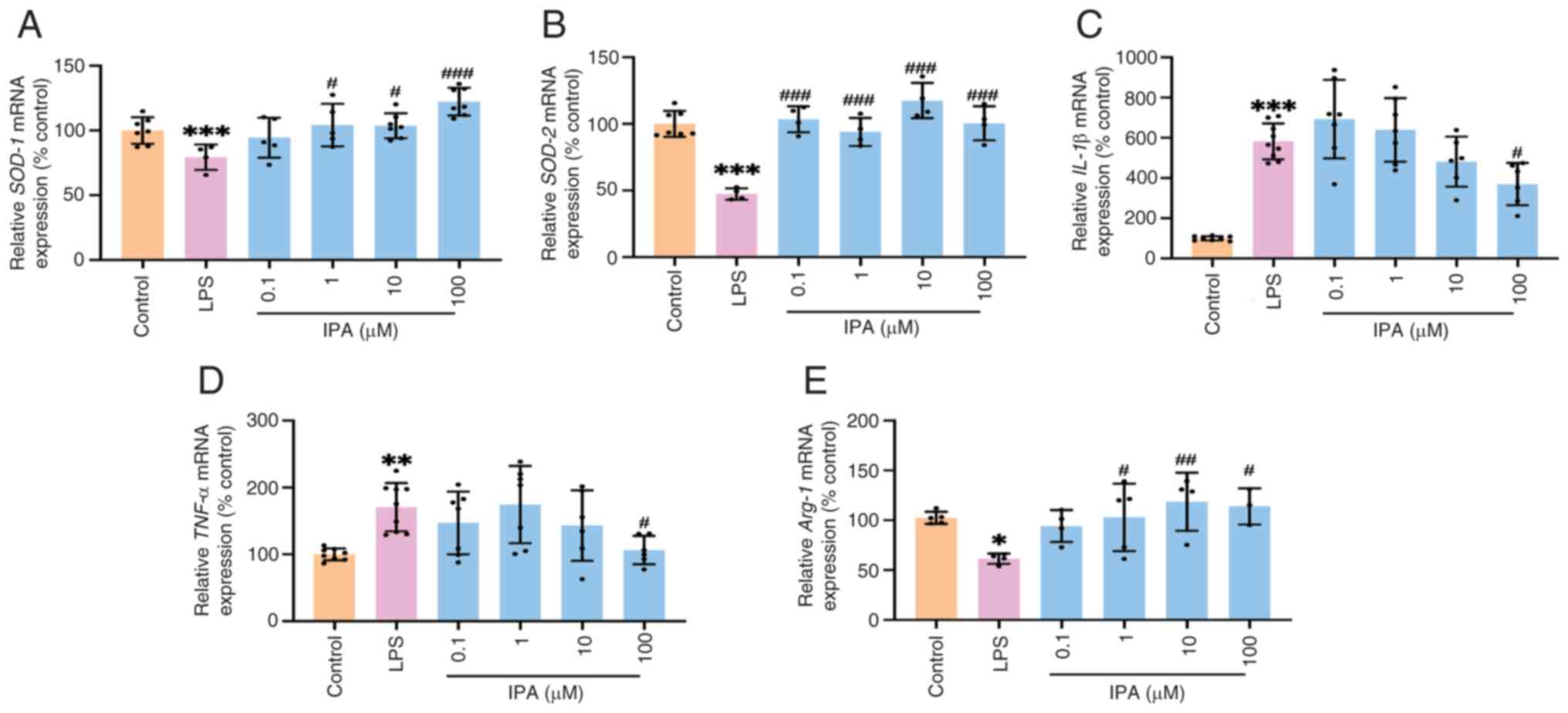 | Figure 4mRNA expression of genes associated
with anti-oxidation, inflammation and polarization. mRNA expression
of (A) SOD-1(B) SOD-2 (C) IL-1β, (D) TNF-α and (E) Arg-1. n=3-9,
*P<0.05, **P<0.01,
***P<0.001 vs. control; #P<0.05,
##P<0.01 ###P<0.001 vs. LPS. SOD,
superoxide dismutase; Arg, arginase; IPA, isopimaric acid; LPS,
lipopolysaccharide. |
IPA suppresses protein expression of
IL-1β and TNF-α by inhibiting the phosphorylation of hyperactive
mTOR and AKT
Protein expression of hub (TNF-α and mTOR) and
PI3K/AKT signaling pathway genes (AKT and p-AKT; Fig. 5A) were assessed. IPA inhibited
protein expression of IL-1β (Fig.
5B) and TNF-α (Fig. 5C)
compared with the LPS group. IPA also suppressed phosphorylation of
mTOR (Fig. 5D) and AKT (Fig. 5E) but not of phosphatidylinositol
3-kinase (PI3K)α and β (Fig. 5F
and G). Furthermore, molecular
docking results confirmed that two oxygen atoms of carboxyl in IPA
docked on the key Phe161 and Gly162 residue of AKT, which may
interrupt the phosphorylation of AKT (37) (Fig.
5H).
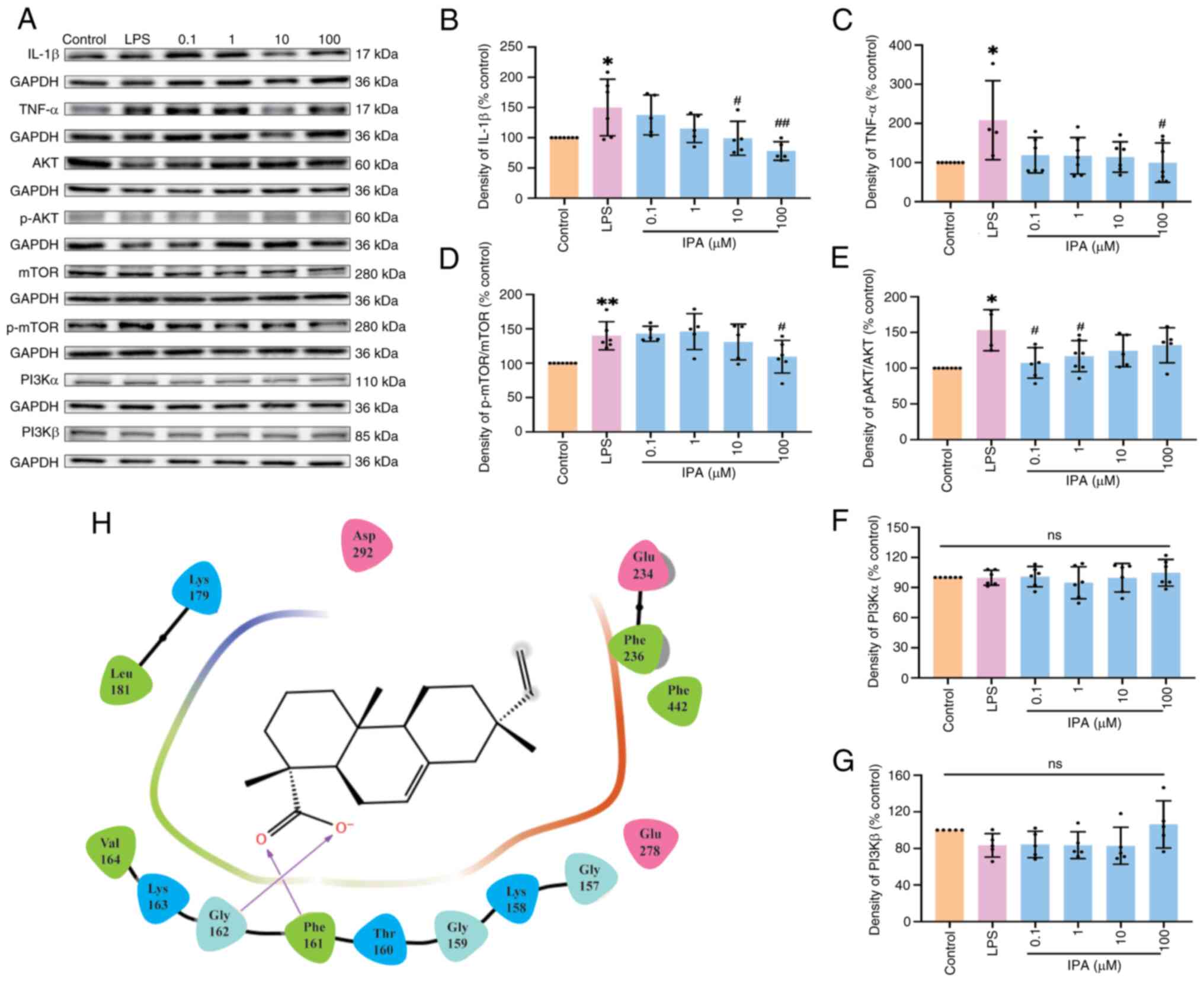 | Figure 5Protein expression of potential
targets. (A) Representative blots for IL-1β, TNF-α, AKT, p-AKT,
mTOR, p-mTOR, PI3Kα and PI3Kβ. Protein expression analysis of (B)
IL-1β (C) TNF-α, (D) ratio of mTOR and p-mTOR, (E) ratio ofAKT and
p-AKT, (F) PI3Kα and (G) PI3Kβ. (H) Binding model of IPA with
active pocket of AKT. n=4-7, *P<0.05,
**P<0.01 vs. control; #P<0.05,
##P<0.01 vs. LPS. IPA, isopimaric acid; LPS,
lipopolysaccharide; PI3K, phosphatidylinositol 3-kinase; p-,
phosphorylation. |
Discussion
A total of ~40 antiepileptic drugs have been used to
treat epileptic patients in clinical settings (38). However, most control the occurrence
of acute seizures and do not exert a true antiepileptogenic effect.
Currently, ~30% of patients with epilepsy experience uncontrollable
seizures (39). Therefore,
exploring novel antiepileptic drugs that impede epileptogenesis is
vital for treating refractory epilepsy. Chinese herbal medicines
including Gastrodia elata, Uncaria rhynchophylla, Acrori
tatarinowii, Paeonia lactiflora, Bupleurum Chinese and PC have
been used to treat seizures and epilepsy for thousands of years
(40-42).
The present study demonstrated that three compounds from PC,
hinokinin, DPT and IPA showed druggability in crossing the BBB. In
total, 150 predicted targets were associated with epilepsy,
suggesting that hinokinin, DPT and IPA are potential components of
PC against epilepsy.
Microglia are the primary glial cells in the central
nervous system and act as immune protectors to maintain stability
of the nerve cell microenvironment. Upon abnormal stimulation,
microglia are transformed, exhibiting cellular structures varying
from ramified to amoeboid, and enhancing migration to the injured
region (43), where they induce
release of inflammatory cytokines (IL-1β and TNF-α), and cause
inflammation (44). Microglial
activation and inflammation have been observed in the brain of
patients with refractory epilepsy (45,46),
which increases neuronal excitability and contributes to
epileptogenesis (47). In the
present study, IPA, a diterpenoid compound separated from PC
(48), alleviated glutamate- and
LPS-induced oxidative stress and inflammation in BV2 cells,
confirming previous studies where IPA not only inhibited production
of inflammation protein NF-κB in HBEC3-KT (Homo sapiens lung
and bronchial epithelial cells), MRC-5 (Homo sapiens lung
fibroblasts), and THP-1 cells (Homo sapiens peripheral blood
monocyte) (49), but also
suppressed the proliferation and metastasis of breast cancer cells
including (MDA-MB-231 and MCF-7) via mitochondrial oxidative
phosphorylation signaling pathways (50). In particular, IPA significantly
increased mRNA expression of anti-oxidative kinases (SOD-1 and
SOD-2) and decreased gene expression of inflammatory factors (IL-1β
and TNF-α), suggesting an anti-inflammation role in microglia.
An increasing number of studies have confirmed that
hyperactive mTOR is involved in inflammation and apoptosis of
microglia during epileptogenesis and is a potential target for
epileptic treatment (51,52). Somatic mTOR variants, including
p.C1483Y and p.C1483R, have been identified in patients with
refractory epilepsy and focal cortical malformation (53), while, kainic acid- and LPS-induced
seizures significantly activate mTOR in rats (54,55).
The activation of mTOR in microglia enhances inflammatory responses
(56). Furthermore, the PI3K/Akt
pathway is key for mTOR-involved cell survival and migration
(57,58). The present study demonstrated that
IPA significantly inhibited phosphorylation of mTOR, confirming a
previous study showing that inhibiting the PI3K/Akt/mTOR pathway
prevents microglial apoptosis (59). As previous studies have
demonstrated that protein expression of PI3Kα and β is
significantly increased in acute and chronic epilepsy, independent
of phosphorylation levels (60,61),
the present study only analyzed the protein expression of PI3Kα and
β. However, the present study did not find any changes to PI3Kα and
β. Further analysis by molecular docking suggested that IPA may
directly combine with AKT at the Phe161 and Gly162 residues and
suppress activation of AKT, in line with a previous study that
demonstrated residue Phe161 serves a vital role on AKT (37). Overall, the present study suggested
that IPA inhibited LPS-induced neuroinflammation via the Akt/mTOR
pathway. However, lack of data on the selectivity and specificity
of IPA for AKT and mTOR in anti-epileptic activity is a limitation
of the present study. Hence, their direct association should be
investigated in the future.
In summary, IPA may be a potential anti-epileptic
compound in PC that acts by suppressing neuroinflammation,
apoptosis and polarization via the Akt/mTOR pathway. These findings
indicate that IPA may be a novel anti-epileptic drug.
Acknowledgements
The authors would like to thank Dr Junyu Xu (Hainan
Medical University, Haikou, China) for help with molecular
docking.
Funding
Funding: The present study was supported by the Hainan
Provincial Key Research and Development Program (grant nos.
ZDYF2021SHFZ092 and ZDYF2022SHFZ109); Hainan Provincial Natural
Science Foundation of China (grant no. 820RC630); Epilepsy Research
Science Innovation Group of Hainan Medical University (grant no.
2022); Hainan Province Clinical Medical Center (grant no. 2021);
Excellent Talent Team of Hainan Province (grant no. QRCBT202121)
and National Natural Science Foundation of China (grant nos.
81960249, 82260270 and 82360838).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
YaW conceived and designed the study, analyzed data,
wrote the manuscript and constructed figures. YuW, CL and DL
designed the experiments. YuW performed experiments and constructed
figures. YaW, YC, and QL confirm the authenticity of all the raw
data. YC and QL designed the experiment, wrote the manuscript, and
supervised the study. All authors have read and approved the final
manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Asadi-Pooya AA, Brigo F, Lattanzi S and
Blumcke I: Adult epilepsy. Lancet. 402:412–424. 2023.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Pitkanen A, Ekolle Ndode-Ekane X,
Lapinlampi N and Puhakka N: Epilepsy biomarkers-Toward etiology and
pathology specificity. Neurobiol Dis. 123:42–58. 2019.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Chinese Pharmacopoeia Commission:
Pharmacopoeia of the People's Republic of China. Vol 1. China
Medical Science and Technology Press, Beijing, pp292-293, 2015.
|
|
4
|
Zhang ML, Liu YH and Qu HH: Protective
effect of nanoparticles from Platycladi cacumen carbonisata
on 2,4,6-Trinitrobenzene Sulfonic Acid (TNBS)-Induced Colitis in
Rats. J Biomed Nanotechnol. 18:422–434. 2022.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Zhang HX, Li YY, Liu ZJ and Wang JF:
Quercetin effectively improves LPS-induced intestinal inflammation,
pyroptosis, and disruption of the barrier function through the
TLR4/NF-κB/NLRP3 signaling pathway in vivo and in vitro. Food Nutr
Res. 66(8948)2022.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Fu H, Li W, Weng Z, Huang Z, Liu J, Mao Q
and Ding B: Water extract of cacumen platycladi promotes
hair growth through the Akt/GSK3beta/beta-catenin signaling
pathway. Front Pharmacol. 14(1038039)2023.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Zhang Y, Chen S, Qu F, Su G and Zhao Y: In
vivo and in vitro evaluation of hair growth potential of Cacumen
Platycladi, and GC-MS analysis of the active constituents of
volatile oil. J Ethnopharmacol. 238(111835)2019.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Huo X, Meng Q, Wang C, Wu J, Zhu Y, Sun P,
Ma X, Sun H and Liu K: Targeting renal OATs to develop renal
protective agent from traditional Chinese medicines: Protective
effect of Apigenin against Imipenem-induced nephrotoxicity.
Phytother Res. 34:2998–3010. 2020.PubMed/NCBI View
Article : Google Scholar
|
|
9
|
Zhuang B, Bi ZM, Wang ZY, Duan L, Lai CJ
and Liu EH: Chemical profiling and quantitation of bioactive
compounds in Platycladi Cacumen by UPLC-Q-TOF-MS/MS and
UPLC-DAD. J Pharm Biomed Anal. 154:207–215. 2018.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Ding M, Li J, Zou S, Tang G, Gao X and
Chang YX: Simultaneous extraction and determination of compounds
with different polarities from Platycladi cacumen by AQ
C(18)-Based vortex-homogenized matrix solid-phase dispersion with
ionic liquid. Front Pharmacol. 9(1532)2019.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Shen Y, Shen X, Cheng Y and Liu Y:
Myricitrin pretreatment ameliorates mouse liver ischemia
reperfusion injury. Int Immunopharmacol. 89 (Pt
A)(107005)2020.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Oh TW, Do HJ, Jeon JH and Kim K:
Quercitrin inhibits platelet activation in arterial thrombosis.
Phytomedicine. 80(153363)2021.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Qiu S, Zhou Y, Kim JT, Bao C, Lee HJ and
Chen J: Amentoflavone inhibits tumor necrosis factor-alpha-induced
migration and invasion through AKT/mTOR/S6k1/hedgehog signaling in
human breast cancer. Food Funct. 12:10196–10209. 2021.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Zhang T (ed): Effective Prescription for
Treating Epilepsy. 1st edition. People's Military Medical Press,
Beijing, 1996 (In Chinese).
|
|
15
|
Wang Y, Li C, Xiong Z, Chen N, Wang X, Xu
J, Wang Y, Liu L, Wu H, Huang C, et al: Up-and-coming
anti-epileptic effect of aloesone in Aloe vera: Evidenced by
integrating network pharmacological analysis, in vitro, and in vivo
models. Front Pharmacol. 13(962223)2022.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Tang Y, Li M, Wang J, Pan Y and Wu FX:
CytoNCA: A cytoscape plugin for centrality analysis and evaluation
of protein interaction networks. Biosystems. 127:67–72.
2015.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Sherman BT, Hao M, Qiu J, Jiao X, Baseler
MW, Lane HC, Imamichi T and Chang W: DAVID: a web server for
functional enrichment analysis and functional annotation of gene
lists (2021 update). Nucleic Acids Res. 50(W1):W216–W221.
2022.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Wang J, Sun Y, Zhang X, Cai H, Zhang C, Qu
H, Liu L, Zhang M, Fu J, Zhang J, et al: Oxidative stress activates
NORAD expression by H3K27ac and promotes oxaliplatin resistance in
gastric cancer by enhancing autophagy flux via targeting the
miR-433-3p. Cell Death Dis. 12(90)2021.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Wang Y, Li Y, Liu D, Zheng D, Li X, Li C,
Huang C, Wang Y, Wang X, Li Q and Xu J: A potential
anti-glioblastoma compound LH20 induces apoptosis and arrest of
human glioblastoma cells via CDK4/6 inhibition. Molecules.
28(5047)2023.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Wang Y, Li Y, Wang G, Lu J and Li Z:
Overexpression of Homer1b/c induces valproic acid resistance in
epilepsy. CNS Neurosci Ther. 29:331–343. 2023.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Wang Y, Xiong Z, Li C, Liu D, Li X, Xu J,
Chen N, Wang X, Li Q and Li Y: Multiple beneficial effects of
aloesone from aloe vera on LPS-Induced RAW264.7 cells, including
the inhibition of oxidative stress, inflammation, M1 polarization,
and apoptosis. Molecules. 28(1617)2023.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) Method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Addie M, Ballard P, Buttar D, Crafter C,
Currie G, Davies BR, Debreczeni J, Dry H, Dudley P, Greenwood R, et
al: Discovery of
4-amino-N-[(1S)-1-(4-chlorophenyl)-3-hydroxypropyl]-1-(7H-pyrrolo[2,3-d]pyrimidin-4-yl)piperidine-4-carboxamide
(AZD5363), an orally bioavailable, potent inhibitor of Akt kinases.
J Med Chem. 56:2059–2073. 2013.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Yang Y, Yao K, Repasky MP, Leswing K, Abel
R, Shoichet BK and Jerome SV: Efficient Exploration of Chemical
Space with Docking and Deep Learning. J Chem Theory Comput.
17:7106–7119. 2021.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Xu J, Li H, Wang X, Huang J, Li S, Liu C,
Dong R, Zhu G, Duan C, Jiang F, et al: Discovery of coumarin
derivatives as potent and selective cyclin-dependent kinase 9
(CDK9) inhibitors with high antitumour activity. Eur J Med Chem.
200(112424)2020.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Ying J, Huang Y, Ye X, Zhang Y, Yao Q,
Wang J, Yang X, Yu C, Guo Y, Zhang X, et al: Comprehensive study of
clinicopathological and immune cell infiltration and lactate
dehydrogenase expression in patients with thymic epithelial
tumours. Int Immunopharmacol. 126(111205)2024.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Smith E, Williamson E, Zloh M and Gibbons
S: Isopimaric acid from Pinus nigra shows activity against
multidrug-resistant and EMRSA strains of Staphylococcus aureus.
Phytother Res. 19:538–542. 2005.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Wu XW, Wang Q, Li Q, Cui YM, Pu YK, Shi
QQ, Bi DW, Zhang JJ, Zhang RH, Li XL, et al: Rubellawus A-D, four
new diterpenoids isolated from callicarpa rubella and Their
Anti-NLRP3 inflammasome effects. Chem Biodivers.
17(e2000798)2020.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Yamamoto K, Ueta Y, Wang L, Yamamoto R,
Inoue N, Inokuchi K, Aiba A, Yonekura H and Kato N: Suppression of
a neocortical potassium channel activity by intracellular
amyloid-beta and its rescue with Homer1a. J Neurosci.
31:11100–11109. 2011.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Wang L, Kang H, Li Y, Shui Y, Yamamoto R,
Sugai T and Kato N: Cognitive recovery by chronic activation of the
large-conductance calcium-activated potassium channel in a mouse
model of Alzheimer's disease. Neuropharmacology. 92:8–15.
2015.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Salari S, Silvera Ejneby M, Brask J and
Elinder F: Isopimaric acid-a multi-targeting ion channel modulator
reducing excitability and arrhythmicity in a spontaneously beating
mouse atrial cell line. Acta Physiol (Oxf).
222(e12895)2018.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Imaizumi Y, Sakamoto K, Yamada A, Hotta A,
Ohya S, Muraki K, Uchiyama M and Ohwada T: Molecular basis of
pimarane compounds as novel activators of large-conductance
Ca(2+)-activated K(+) channel alpha-subunit. Mol Pharmacol.
62:836–846. 2002.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Zaugg J, Khom S, Eigenmann D, Baburin I,
Hamburger M and Hering S: Identification and characterization of
GABA(A) receptor modulatory diterpenes from Biota orientalis
that decrease locomotor activity in mice. J Nat Prod. 74:1764–1772.
2011.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Rong S, Wan D, Fan Y, Liu S, Sun K, Huo J,
Zhang P, Li X, Xie X, Wang F and Sun T: Amentoflavone affects
epileptogenesis and exerts neuroprotective effects by inhibiting
NLRP3 inflammasome. Front Pharmacol. 10(856)2019.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Chang YC, Fong Y, Tsai EM, Chang YG, Chou
HL, Wu CY, Teng YN, Liu TC, Yuan SS and Chiu CC: Exogenous
C(8)-Ceramide Induces Apoptosis by Overproduction of ROS and the
switch of superoxide dismutases SOD1 to SOD2 in human lung cancer
cells. Int J Mol Sci. 19(3010)2018.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Xu M, Yang Y, Peng J, Zhang Y, Wu B, He B,
Jia Y and Yan T: Effects of Alpinae Oxyphyllae Fructus on
microglial polarization in a LPS-induced BV2 cells model of
neuroinflammation via TREM2. J Ethnopharmacol. 302(Pt
A)(115914)2023.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Kashyap MP, Singh AK, Kumar V, Yadav DK,
Khan F, Jahan S, Khanna VK, Yadav S and Pant AB: Pkb/Akt1 mediates
Wnt/GSK3β/β-catenin signaling-induced apoptosis in human cord blood
stem cells exposed to organophosphate pesticide monocrotophos. Stem
Cells Dev. 22:224–238. 2013.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Ravizza T, Scheper M, Di Sapia R, Gorter
J, Aronica E and Vezzani A: mTOR and neuroinflammation in epilepsy:
Implications for disease progression and treatment. Nat Rev
Neurosci. 25:334–350. 2024.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Janson MT and Bainbridge JL: Continuing
burden of refractory epilepsy. Ann Pharmacother. 55:406–408.
2021.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Lin CH and Hsieh CL: Chinese herbal
medicine for treating epilepsy. Front Neurosci.
15(682821)2021.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Lu H, Luo M, Chen R, Luo Y, Xi A, Wang K
and Xu Z: Efficacy and safety of traditional Chinese medicine for
the treatment of epilepsy: A updated meta-analysis of randomized
controlled trials. Epilepsy Res. 189(107075)2023.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Wu J, Cao M, Peng Y, Dong B, Jiang Y, Hu
C, Zhu P, Xing W, Yu L, Xu R and Chen Z: Research progress on the
treatment of epilepsy with traditional Chinese medicine.
Phytomedicine. 120(155022)2023.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Block ML, Zecca L and Hong JS:
Microglia-mediated neurotoxicity: Uncovering the molecular
mechanisms. Nat Rev Neurosci. 8:57–69. 2007.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Hernandez VG, Lechtenberg KJ, Peterson TC,
Zhu L, Lucas TA, Bradshaw KP, Owah JO, Dorsey AI, Gentles AJ and
Buckwalter MS: Translatome analysis reveals microglia and
astrocytes to be distinct regulators of inflammation in the
hyperacute and acute phases after stroke. Glia. 71:1960–1984.
2023.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Butler T, Li Y, Tsui W, Friedman D, Maoz
A, Wang X, Harvey P, Tanzi E, Morim S, Kang Y, et al: Transient and
chronic seizure-induced inflammation in human focal epilepsy.
Epilepsia. 57:e191–194. 2016.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Kumar P, Lim A, Hazirah SN, Chua CJH, Ngoh
A, Poh SL, Yeo TH, Lim J, Ling S, Sutamam NB, et al: Single-cell
transcriptomics and surface epitope detection in human brain
epileptic lesions identifies pro-inflammatory signaling. Nat
Neurosci. 25:956–966. 2022.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Luo C, Koyama R and Ikegaya Y: Microglia
engulf viable newborn cells in the epileptic dentate gyrus. Glia.
64:1508–1517. 2016.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Cui X, Cheng L, Liu Q and Jia Y:
Isolation, identification and HPLC analysis of active component
isopimaric acid in leaves of Platycladus orientalis.
Lishizhen Med Mater Med Res. 15:78–79. 2004.(In Chinese).
|
|
49
|
Michavila Puente-Villegas S, Apaza Ticona
L, Rumbero Sanchez A and Acebes JL: Diterpenes of Pinus pinaster
aiton with anti-inflammatory, analgesic, and antibacterial
activities. J Ethnopharmacol. 318(Pt B)(117021)2024.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Li J, Liu X, Chen L, Zhu X, Yu Z, Dong L,
Zhao X, Zou H, Wei Q, Feng Y, et al: Isopimaric acid, an ion
channel regulator, regulates calcium and oxidative phosphorylation
pathways to inhibit breast cancer proliferation and metastasis.
Toxicol Appl Pharmacol. 462(116415)2023.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Hodges SL and Lugo JN: Therapeutic role of
targeting mTOR signaling and neuroinflammation in epilepsy.
Epilepsy Res. 161(106282)2020.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Hu Y, Mai W, Chen L, Cao K, Zhang B, Zhang
Z, Liu Y, Lou H, Duan S and Gao Z: mTOR-mediated metabolic
reprogramming shapes distinct microglia functions in response to
lipopolysaccharide and ATP. Glia. 68:1031–1045. 2020.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Lai D, Gade M, Yang E, Koh HY, Lu J,
Walley NM, Buckley AF, Sands TT, Akman CI and Mikati MA: , et
al: Somatic variants in diverse genes leads to a spectrum of
focal cortical malformations. Brain. 145:2704–2720. 2022.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Huang XY, Hu QP, Shi HY, Zheng YY, Hu RR
and Guo Q: Everolimus inhibits PI3K/Akt/mTOR and NF-kB/IL-6
signaling and protects seizure-induced brain injury in rats. J Chem
Neuroanat. 114(101960)2021.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Russo E, Andreozzi F, Iuliano R, Dattilo
V, Procopio T, Fiume G, Mimmi S, Perrotti N, Citraro R, Sesti G, et
al: Early molecular and behavioral response to lipopolysaccharide
in the WAG/Rij rat model of absence epilepsy and depressive-like
behavior, involves interplay between AMPK, AKT/mTOR pathways and
neuroinflammatory cytokine release. Brain Behav Immun. 42:157–168.
2014.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Zhao XF, Liao Y, Alam MM, Mathur R,
Feustel P, Mazurkiewicz JE, Adamo MA, Zhu XC and Huang Y:
Microglial mTOR is neuronal protective and antiepileptogenic in the
pilocarpine model of temporal lobe epilepsy. J Neurosci.
40:7593–7608. 2020.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Zhu F, Kai J, Chen L, Wu M, Dong J, Wang Q
and Zeng LH: Akt inhibitor perifosine prevents epileptogenesis in a
rat model of temporal lobe epilepsy. Neurosci Bull. 34:283–290.
2018.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Bai Q, Wang X, Yan H, Wen L, Zhou Z, Ye Y,
Jing Y, Niu Y, Wang L, Zhang Z, et al: Microglia-Derived Spp1
promotes pathological retinal neovascularization via activating
endothelial Kit/Akt/mTOR signaling. J Pers Med.
13(146)2023.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Du M, Sun Z, Lu Y, Li YZ, Xu HR and Zeng
CQ: Osthole inhibits proliferation and induces apoptosis in BV-2
microglia cells in kainic acid-induced epilepsy via modulating
PI3K/AKt/mTOR signalling way. Pharm Biol. 57:238–244.
2019.PubMed/NCBI View Article : Google Scholar
|
|
60
|
Xiao Z, Peng J, Gan N, Arafat A and Yin F:
Interleukin-1β plays a pivotal role via the PI3K/Akt/mTOR signaling
pathway in the chronicity of mesial temporal lobe epilepsy.
Neuroimmunomodulation. 23:332–344. 2016.PubMed/NCBI View Article : Google Scholar
|
|
61
|
Wang P, Nan S, Zhang Y and Fan J: Effects
of GABA(B) receptor positive allosteric modulator BHF177 and IRS-1
on apoptosis of hippocampal neurons in rats with refractory
epilepsy via the PI3K/Akt pathway. Cell Biol Int. 46:1775–1786.
2022.PubMed/NCBI View Article : Google Scholar
|