1. Introduction
Pulmonary thromboembolism (PE) represents a serious
condition and is a significant cause of mortality worldwide. This
has led to an increase in research studying predictive prognostic
factors to improve mortality rates. Several factors have been
identified including major trauma and surgery, hip or knee
prosthesis, fractures, prolonged immobilization, malignancy, the
use of oral contraceptives or hormonal substitution therapy and
pregnancy, all of which can lead to a worse prognosis (1,2).
Patients with cancer have an eight-fold increased
risk of PE compared with healthy individuals (3,4). The
incidence of embolic events differs according to age, location of
the malignancy, staging and histopathological features, as well as
various hospital-related factors such as length of hospitalization,
central venous catheters and administration of chemotherapy
(3,5-7).
For example, patients with pancreatic, lung, colorectal, prostate,
breast, brain and hematological cancers exhibit a higher risk of
PE. Similarly, patients with metastatic stomach, liver and lung
neoplasia are more likely to develop a PE (3,5-7).
The evolution of these thromboembolic events differs
by patient, therefore identifying prognostic parameters followed by
optimized treatment criteria can reduce morbidity and mortality.
Biomarkers such as natriuretic peptides (NTproBNP and BNP),
troponins (cTnT) and D-dimers have allowed for the stratification
of PE into various subtypes, which can aid in the development of
diagnostic and therapeutic regimens.
According to a study on a group of 100 patients with
PE that assessed mortality, measurement of NTproBNP and troponin T
biomarkers at the time of patient admission assisted with risk
stratification. In patients with cancer with troponin T serum
values >0.07 µg/l, the total mortality rate was 15% and the
mortality rate by acute PE was 8%. Similarly, NTproBNP serum values
>7,600 ng/l were predictive of mortality by any disease
including acute PE (8). Mortality
rates in patients with acute PE who had serum levels of NTproBNP
≥600 ng/l and T troponin serum values ≥0.07 µg/l were 33%. The
mortality rate of patients with an NTproBNP serum level <600
ng/l and cTnT serum level <0.07 µg/l was found to be only 3.7%
(8).
The aim of the present review was to provide a
reference article for clinicians by summarizing the findings of
data from multiple studies, including observational studies that
evaluated the accuracy of diagnosing PE in patients with cancer and
also to compare the effectiveness of different anticoagulant
therapeutic regimens in a category of patients with a complex
pathology, which often poses a significant challenge.
2. Clinical prognostic biomarkers and
paraclinical investigations
Typically, the primary cause of PE is deep venous
thrombosis (DVT), which is present in ~70% of patients diagnosed
with PE (9,10). The incidence of DVT may vary
according to the age and sex of patients, with it being more
prevalent in men and the elderly (11). Emboli originating from the lower
limb deep venous system are ten times more likely to migrate
compared with emboli in the upper body.
A significant number of conditions present Virchow's
triad (venous stasis, hypercoagulability state and injury of the
vessel wall) (12), which may
predispose an individual to PE and a patient with cancer typically
presents with the following features: They are bedridden, often
dehydrated, exhibit increased secretion of parathyroid hormone with
a procoagulant effect and venous access is required for curative or
palliative treatment (1,13). Predisposing factors to venous
thromboembolism and PE are classified according to the associated
risk level (1,2); high-risk predisposing factors include
orthopedic surgery, hospitalization for heart failure or rhythm
disturbances in the past 3 months, major trauma, recent myocardial
infarction, or a history of venous thromboembolism. Moderate risk
predisposing factors include minimally invasive orthopedic surgery,
blood transfusions, central venous catheters, pacemakers,
chemotherapy, congestive heart failure, respiratory failure,
neoplasia, oral contraceptive medication, stroke, post-partum
period, superficial venous thrombosis and thrombophilia. Low-risk
predisposing factors include being bedridden for >3 days,
diabetes mellitus, hypertension, prolonged trips by plane or
vehicles, being elderly, minimally invasive surgery, obesity,
pregnancy and chronic venous insufficiency.
An increasing number of cancer patients are
exhibiting moderate risk factors and this is exacerbated by the
cumulative effect of additional factors including dehydration,
paraneoplastic syndromes, major resection surgery, multiple venous
access points (port-a-cath and venous catheters) and chemotherapy.
The type of malignancy markedly influences the risk for DVT and
according to this, Khorana et al (14) developed a predictive model for DVT
risk based on five factors (Table
I): Site of cancer (2 points for very high-risk site, 1 point
for high-risk site), platelet count of 350x109/l or
more, hemoglobin levels <100 g/l (10 g/dl) and/or use of
erythropoiesis-stimulating agents, leukocyte count
>11x109/l and a body mass index of ≥35
kg/m2 (1 point each) (14).
 | Table IPredictive model for chemotherapy
associated venous thromboembolism (14). |
Table I
Predictive model for chemotherapy
associated venous thromboembolism (14).
| Item | | Risk score |
|---|
| Site of
cancer: | Very high
risk: | |
| | • Stomach | |
| | • Pancreas | 2 |
| | High risk: | |
| | • Lung | |
| | • Lymphoma | |
| | • Gynecologic | |
| | • Bladder, | |
| | • Testicular | 1 |
| Prechemotherapy
platelet count ≥350x109/l or more | 1 |
| Hemoglobin level
<10 g/dl or use of red cell growth factors | 1 |
| Prechemotherapy
leukocyte count >11x109/l | 1 |
| Body mass index ≥35
kg/m2 | 1 |
These parameters were combined into a simple risk
assessment model that allows providers to classify patients into
three discrete categories corresponding to the risk of
chemotherapy-associated venous thromboembolism (VTE; low-risk, 0;
intermediate-risk, 1-2; and high-risk, >3 of VTE-0.3, 2 and
6.7%) and this classification could be used to assist in deciding
the appropriate therapeutic regimen for thromboprophylaxis.
A literature review of articles published on Web of
Science over the past 6 years (between January 2018 and January
2024) revealed articles indicating various cellular mechanisms of
venous thrombotic events leading to PE. Chemotherapy was associated
with bone marrow suppression and consequently thrombocytopenia. In
cancers that pose an increased risk of DVT and consequently,
therapeutic and preventative challenges, thrombocytopenia was
associated with both the risk of thromboembolism and bleeding
during anticoagulant treatment (15).
There is a paradox; 20-25% of patients with solid
tumors of the pancreas (16),
stomach, genitourinary tract, or brain (glioblastoma) (17) have thrombocytopenia, induced by
platin-based chemotherapy (such as gemcitabine or temozolomide)
which implicitly leads to an increased risk of bleeding
(proportional to the severity of thrombocytopenia). Additionally,
these same tumors are associated with a high incidence of
developing PE. Thus, this category of patients requires safe and
personalized therapeutic strategies. The selection of an
appropriate type of anticoagulant treatment and the dose of this
medication must be balanced with the risk of recurrence of PE and
the risk of bleeding associated with thrombocytopenia due to
chemotherapeutic treatment (15).
Another mechanism implicated in chemotherapy-induced
thromboembolisms is the induction of arrhythmias, with an
elongation of the QTc interval, necessitating the need for
anticoagulant measures in these patients. Additionally, the use of
central venous catheters on the same arm as lymphadenectomy is
associated with an increased risk of thrombosis and it is
recommended that a peripheral venous system is used in such cases
(18). The facilitation of the
release of specific tumor markers can increase the risk of DVT, as
observed by Naito et al (19) in a study on colon cancer treated
with polaprezinc, leading to an increase in CA19.9 levels.
The cellular mechanism of thrombosis induction
involves the cytotoxic effect of chemotherapy through the release
of lipoproteins, intracellular or cell membrane-related elements,
which later serve as precipitating factors for subsequent
thrombotic phases (20). Another
implicated mechanism involves the activation of endothelial cells
through elevated levels of von Willebrand factor and soluble
P-selectin, secondary to the cytotoxic effect with the release of
thrombospondin type 13. This endothelial cell activation triggers
the cascade of intravascular thrombosis (21).
Chemotherapy can induce the establishment of
inflammatory conditions triggering the NF-κB signaling pathway,
leading to the production of proinflammatory cytokines. IL-6
enhances the procoagulant status by inducing tissue factor (TF)
expression. TF expression initiates the coagulation system,
characterized by increased D-dimer levels. A previous study
evaluated the changes in plasma IL-6 and D-dimer levels in patients
with cancer at a high risk of thrombosis undergoing chemotherapy
(22). In serous ovarian cancers,
Ward et al (23) identified
a pronounced procoagulant effect of cisplatin and paclitaxel via
the activation of the protein C pathway of coagulation.
Patients with cancer should be made familiar with
the risk of such complications to raise awareness of the need for
preventative medications and lifestyle adaptations, including
adequate hydration and adopting an active lifestyle. Programs
addressing this risk among patients exist (24).
Although DVT is responsible for ~75% of PE cases,
there are other rare causes such as intracardiac thrombosis caused
by arrhythmias, embolism with tumor fragments, septic embolism and
iatrogenic causes (such as inferior vena cava filters or broken
fragments of guiding devices) (1).
The highest risk of PE occurrence is within the
first 12 months of neoplasia diagnosis, subsequently decreasing
progressively after this period, approaching that of the general
population after ~10 years. The increased risk in the first 12
months is associated with treatments such as chemotherapy and major
surgical interventions (3,5,25).
The annual incidence of VTE in patients receiving chemotherapy is
estimated to be 11%, which can rise to 20% or higher depending on
the drug(s) administered. In addition to chemotherapy, several
other anti-neoplastic and supportive therapies are also associated
with an increased risk of VTE development (17,26,27).
Not every surgical treatment is associated with a high risk of PE.
Surgical interventions involving prolonged bed rest and extensive
resections associated with peripheral venous or pulmonary arterial
vascular sutures are recommended against. An observational study by
Sweetland et al (28),
demonstrated that the risk of thromboembolism in patients
undergoing orthopedic procedures (hip and knee replacement) is
higher in the first 6 weeks postoperatively, surpassing that of
cancer surgery. This study also suggests that the long-term risk of
PE in patients with cancer may be up to eight times higher than
other surgical treatments (28).
Common symptoms and signs encountered in these patients include
dyspnea, syncope, chest pain and hemoptysis (1). Massive PE, acute cor pulmonale
(right ventricular dysfunction, acute heart failure, low cardiac
output syndrome, hypoxemia) occurs due to increased pressure in the
pulmonary artery above the mean pressure value (>40 mmHg),
leading to the rapid development of pulmonary hypertension
(1,13,29).
Patients with sub-massive PE are hemodynamically stable and
symptoms develop gradually. Pulmonary infarction occurs due to the
obstruction of a peripheral pulmonary segmentary or sub-segmentary
arterial branch (13).
Physical examination may reveal signs suggestive of
the diagnosis: Distended jugular veins, hypotension, cardiogenic
shock, tachycardia, paradoxical pulse, right ventricular gallop,
tricuspid regurgitation systolic murmur, pleural friction rub and
intensified vesicular sound, among others (1). Due to the nonspecific symptoms and
physical examination, the European Society of Cardiology recommends
the use of prediction scores (Wells and Geneva) for diagnosis,
based on predisposing factors for DVT and PE (1). The issue of gastrointestinal cancers
associated with cancer-related thrombosis, where anticoagulant
administration is imperative has been addressed in a previous study
where the role of low molecular weight heparin (LMWH) over oral
anticoagulants was advocated for to avoid uncontrollable bleeding
in the digestive system (30).
Once a PE has been established, paraclinical
evaluation of the patient is performed in the same manner as in
patients with cancer. Thus, laboratory analyses highlighting
specific biomarkers of myocardial injury (NT-proBNP or troponins)
can be altered due to right ventricular dysfunction resulting from
sudden increases in pulmonary artery pressure (1). The levels of all biomarkers
(NT-proBNP, D-dimer, myoglobin and troponins) are associated with
right ventricular dysfunction (31,32).
Elevated levels of NT-proBNP and troponins may be found in acute PE
and are associated with the risk of PE-associated mortality
(33-36).
Normal NT-proBNP levels are predictive of a good prognosis
(37-40).
Increased troponin and myoglobin levels signify myocardial
involvement, but there is no clear evidence that they are more
significant markers of severity than increased NTproBNP levels,
which is currently considered the strongest predictor of severity,
as shown by a study of Vuilleumier et al (32). This hypothesis is supported by the
fact that only NT-proBNP levels are associated with right
ventricular dysfunction on a chest CT scan (32), a factor considered a marker of poor
prognosis in PE (41). However,
NTproBNP levels are not a suitable decisive marker for thrombolysis
(41). NTproBNP and troponin I
levels are considered predictors of a poor prognosis in patients
with acute PE (42-44).
NT-proBNP
In response to left ventricular overload (45) and myocyte stretching (46), an inactive prohormone (proBNP) is
synthesized that is cleaved into the active hormone BNP and the
inactive N-terminal fragment (NTproBNP) (47,48).
BNP is released in response to ventricular strain and is predictive
of a negative outcome for patients with PE (49-51).
The NTproBNP fraction can also increase in several other disorders
including pre-existing left ventricular dysfunction, kidney failure
and chronic pulmonary disease, as well as in the elderly (52). Natriuretic peptides are useful
prognostic and diagnostic biomarkers in patients with congestive
heart failure and, unlike atrial natriuretic peptide, which is
primarily produced in the atrial tissue, BNP is primarily produced
by the ventricular myocytes and the main stimulus for its
production is myocyte stretch (37,48,53,54).
Increased serum levels of natriuretic peptides are found in
patients with right ventricular pressure overload due to causes
other than PE including primary pulmonary hypertension, chronic
thromboembolic pulmonary hypertension and chronic pulmonary disease
(55-58).
NTproBNP can be considered a marker of short-term mortality risk.
Additional studies are required to demonstrate whether NTproBNP
measurement may play a role in the decision-making for thrombolysis
and in identifying patients who could be treated on an outpatient
basis (43). In a 2008 study by
Klok et al (43), it was
found that the incidence of right ventricular dysfunction was 45%
in patients with elevated NTproBNP levels compared with 4.5% in
patients with normal NTproBNP levels (43). NTproBNP measurement in patients
with PE is recommended, considering it is a prognostic biomarker
(59,60). Data from a study (61) indicate that NTproBNP seems to be
the strongest predictor of mortality and hospitalization (for
complications such as PE and dyspnea, with or without chest pain) 3
months after the acute event. This biomarker has proven to be the
best predictor for identifying low-risk patients when NTproBNP
levels are 300 pg/ml (61).
A total of five meta-analyses have investigated the
prognostic value of natriuretic peptide measurement in PE,
concluding that elevated levels of natriuretic peptides are
associated with a poor short-term prognosis (42,43,62-64).
An NTproBNP value of 600 ng/l is the threshold for stratifying the
risk of death in hemodynamically stable patients with PE (65) and is associated with a poor
prognosis in patients with levels NTproBNP >600 ng/l, right
ventricular systolic dysfunction and a high PESI score (66-71).
Patients with PE and NTproBNP levels <600 ng/l, with absence of
right ventricular dysfunction and a PESI score of 0 have a good
prognosis (65). A study by
Lankeit et al (65),
consisting of 688 patients, concluded that patients with PE, that
were hemodynamically stable but had right ventricular systolic
dysfunction and NTproBNP levels >600 ng/l, may benefit from
early thrombolytic therapy, as supported by the randomized
pulmonary embolism thrombolysis study (65,72),
to prevent hemodynamic instability in patients with right
ventricular dysfunction and increased NTproBNP levels (65,73).
A high mortality rate in PE is associated with NTproBNP levels
>600 ng/l, while low NTproBNP levels <600 ng/l are associated
with a good prognosis (8).
Elevated NTproBNP levels can be used to identify patients at high
risk of complications or death but do not justify the initiation of
invasive treatments. Normal NTproBNP levels can help identify
patients who could be treated on an outpatient basis (43).
Troponins (cTnT and cTnI)
Troponin levels increase following myocardial
necrosis due to severe pressure overload or prolonged overload on
the right ventricle, leading to microscopic myocardial necrosis
(45,48). Troponins are sensitive and specific
biomarkers for myocardial cell injury, reflecting microscopic
myocardial necrosis. In PE, the increase in troponin levels
correlates well with the severity of right ventricular dysfunction
(33,34,36,74).
Serum troponin levels (cTnT >0.07 µg/l) have a sensitivity of
75% and specificity of 87% in identifying patients at high risk of
all-cause mortality. Serum troponin levels (cTnT <0.07 µg/l) are
associated with a good prognosis (8). Patients with elevated serum levels of
both biomarkers have a higher short-term mortality risk compared
with patients with NTproBNP levels >600 ng/l, but patients with
serum levels of cTnT <0.07 µg/l have an intermediate risk of
short-term mortality (8). The best
short-term prognosis is seen in patients with NTproBNP levels
<600 ng/l and cTnT levels <0.07 µg/l. Elevated serum levels
of troponins and natriuretic peptides can be used to predict
patients at high risk of in-hospital death. The primary role of
biomarkers is to differentiate between low-risk and
intermediate-risk patients (Table
II) (8,43,65,75).
Elevated levels of troponins and/or BNP in hemodynamically stable
patients with PE and right ventricular dysfunction but low risk of
bleeding should be considered for thrombolysis, whereas low levels
of troponins and natriuretic peptides can be used to identify
patients at low risk for complications (76).
 | Table IIMortality risk in patients with
modified biomarkers (8,43,65,75). |
Table II
Mortality risk in patients with
modified biomarkers (8,43,65,75).
| First author/s,
year | Studies | NTproBNP >600
ng/l | cTnT >0.07
g/l | Mortality of all
cause at 40 days | Mortality with PE
patients | Complications | (Refs.) |
|---|
| Kostrubiec et
al, 2005 | 100 patients | - | + | 15% | 8% | - | (8) |
| | | + | + | - | 33% | | |
| | | - | - | - | 3.7% | | |
| Lankeit et
al, 2014 | 688 patients | + | - | - | 4.2% | - | (65) |
| Klok et al,
2008 | meta-analysis of 13
studies | + | - | 10% | - | 23% | (43) |
| Vuilleumier et
al, 2008 | 146 patients | Hospitalization and
death have been evaluated for complications. Result achieved at 12%
with NTproBNP being the strongest predictor for command death. | (75) |
D-dimers
Studies suggest that the sensitivity and negative
predictive value of D-dimers is low in patients with neoplasms
(77). A negative D-dimer result
cannot safely exclude a diagnosis of PE in patients suspected of PE
with a cancer (78); however,
other studies have shown that the negative predictive value is
comparable to patients without neoplasms (78,79).
A study by Di Nisio et al (79) suggested measuring D-dimer levels in
patients with neoplasms to exclude PE, although these results
require confirmation in a larger study (79). The safety and accuracy of a
diagnosis based on D-dimer levels in patients with neoplasms have
not been established. Neoplasms and their treatment can both reduce
accuracy due to an increased likelihood of abnormal results than in
patients without neoplasms (80).
Reliably excluding PE in patients with neoplasms is of utmost
importance as PE is associated with a high mortality rate in these
patients and anticoagulant therapy significantly increases the risk
of major bleeding (81). D-dimers
are not specific for PE. Serum values may also be elevated in other
conditions such as myocardial infarction, pneumonia and cancer
without PE (80), especially in
the elderly, pregnancy, trauma and inflammatory states (82,83).
Normal D-dimer levels are more reliable for excluding rather than
confirming a diagnosis of PE (82-84).
The role of D-dimer measurement in diagnosing PE in patients with
clinical suspicion aims to avoid invasive and costly examinations
(Table III) (79).
 | Table IIIImportance of D-dimers measurement
(79). |
Table III
Importance of D-dimers measurement
(79).
| D-dimers |
|---|
| Patients with
cancer | Patients without
cancer |
|---|
| Sensitivity | Specificity | Negative predictive
value | Positive predictive
value | Sensitivity | Specificity | Negative predictive
value | Positive predictive
value |
|---|
| 100% | 21% | 100% | 31% | 93% | 53% | 97% | 31% |
Electrocardiogram
Electrocardiographic changes are nonspecific and may
suggest small, medium, or large arterial obstruction (Table IV) (85-87).
 | Table IVCommon electrocardiographic changes
in patients with pulmonary embolism (86,87). |
Table IV
Common electrocardiographic changes
in patients with pulmonary embolism (86,87).
|
Electrocardiographic changes suggestive in
PE with small or medium arterial obstruction |
Electrocardiographic changes suggestive in
PE with large arterial obstruction |
|---|
| • Sinus
tachycardia | • Right axis
deviation |
| | • Major or minor
right bundle branch block |
| | • Q, negative T
waves in lead III + negative T waves in V1-V4 |
| | • Negative T waves
in V1-V3 |
| | • Pulmonary P
wave |
| | • S1Q3T3 |
Pulse oximetry
Secondary to PE in the pulmonary arterial bed, there
is an imbalance between ventilation and perfusion. CO2
elimination is disrupted, clinically resulting in hypoxia (13).
Echocardiography
In patients with PE, echocardiography provides data
on right ventricular dysfunction (85) (right ventricular dilation-right
ventricular telediastolic diameter >30 mm, right
ventricular/left ventricular ratio >1; paradoxical movement of
the interventricular septum; presence of a McConnell sign);
presence or absence of pulmonary hypertension and presence or
absence of clots in the right cavities (13). In acute PE, evidence of systolic
pressure in the pulmonary artery >60 mmHg is rarely found; such
a value is more indicative of chronic pulmonary hypertension,
especially when accompanied by other echocardiographic signs such
as right ventricular hypertrophy (13). Increased pressure in the pulmonary
artery leads to right ventricular dysfunction and right heart
failure, results in the release of cardiac biomarkers such as BNP,
NT-proBNP and troponins (13,88).
In addition to right ventricular dysfunction and right heart
failure, which are the primary causes of death in PE, left
ventricular function may also be affected. Left ventricular filling
is affected by septal bulging into the left ventricle due to
increased volume and pressure in the right ventricle. Diastolic
function can also altered. Reduced cardiac output leads to arterial
hypotension and shock (13,89).
Due to the decrease in left ventricular blood flow, coronary artery
blood flow decreases, leading to ischemia and even right
ventricular myocardial infarction or mortality in individuals with
massive PE, where compensatory mechanisms are overwhelmed and
cannot balance oxygen consumption at the myocardial level (13). Echocardiography in acute PE can be
used to assist in risk stratification and, using this, patients can
be divided into three groups of patients from a prognostic point of
view (76): i) No right
ventricular dysfunction, in-hospital mortality rate of <4%; ii)
sub-massive PE in hemodynamically stable patients with right
ventricular dysfunction, in-hospital mortality rate of between
5-10%; iii) severe right ventricular dysfunction and cardiogenic
shock, in-hospital mortality up to 30%.
Right ventricular dysfunction diagnosed by
echocardiography is a frequent clinical finding in patients with PE
and is considered a poor prognostic factor (42,45,49-51,90)
and an independent predictor of early mortality in patients with PE
(89). Identifying hemodynamically
stable patients with right ventricular dysfunction is crucial for
initiating therapies such as thrombolysis or embolectomy (91,92),
to prevent early hemodynamic deterioration. Echocardiography plays
an important role in identifying hemodynamically right ventricle
dysfunction (50,93,94)
but of significant importance is also computed tomography (CT)
angiography, which can be used to predict the risk of complications
or death by measuring the ratio between the right ventricle and the
left ventricle (41,95-100).
However, data from studies evaluating right ventricular dysfunction
by CT angiography are limited (101,102).
Chest X-ray
The role of chest X-rays is to exclude other acute
causes of respiratory failure in patients with suspected PE such as
pneumothorax, acute pneumonia, pulmonary edema, tumors, or pleurisy
(103).
CT angiography
CT angiography is the investigation of choice in
patients clinically suspected of PE. Multi-slice CT angiography
allows visualization of the arterial tree up to the segmental and
subsegmental levels, enabling the diagnosis of even subsegmental
pulmonary arterial micro-emboli with a sensitivity of up to 96%
(13).
Lung ventilation-perfusion
scintigraphy
This is a less commonly used diagnostic method for
PE compared with CT angiography. In patients with PE, the perfusion
scintigram is abnormal, but the ventilation scintigram is normal
(13).
Venous Doppler ultrasound
Diagnosing DVT in patients with suspected PE allows
for the administration of anticoagulant treatment without the need
for additional investigations (1).
The combination of a negative D-dimer result and negative Doppler
ultrasound for DVT safely excludes the diagnosis of PE and can lead
to the safe discontinuation of anticoagulant treatment in patients
with malignancies (78).
Pulmonary angiography
This is the gold standard for diagnosing PE and as
it allows for direct visualization of thrombi up to 1-2 mm in size
in the pulmonary arteries or their subsegmental branches (1).
Nuclear magnetic resonance (NMR)
With reduced availability, NMR is reserved for
patients with inferior vena cava thrombosis, iliac vein thrombosis,
during pregnancy and for patients with contraindications to
contrast agent administration (13).
3. The anticoagulant treatment dilemma
Clinically, patients with suspected PE can be
classified as high, intermediate, or low risk for early in-hospital
or 30-day mortality. Independent predictive factors for mortality
include neoplastic conditions, present in 10-20% of patients
diagnosed with PE. In ~10% of patients, PE represents the early
clinical status before a diagnosis of a malignancy in the 10 years
following the acute event; however, most malignancies are diagnosed
within 12-24 months following the acute event. Therefore, the
European guidelines recommend screening for neoplastic conditions
in patients with an apparently unprovoked PE (1). In the absence of hemodynamic
instability, signs of right ventricular dysfunction and positive
biomarkers, the PESI score can be used to categorize patients with
PE as intermediate or low risk. The presence of PE in patients with
neoplasms is associated with an increased long-term mortality risk
and is considered the second leading cause of mortality (25,85,104). The hemodynamic status of patients
with clinically suspected PE plays an important role in the
diagnostic and therapeutic strategy. The diagnostic and therapeutic
strategy depends on patient risk; for high-risk patients, CT
angiography should be performed. If the imaging investigation
confirms the presence of a PE, primary reperfusion therapy should
be initiated (13). Acute phase
treatment of PE aims to alleviate symptoms, limit the progression
of thrombosis and prevent death. Long-term treatment aims to
prevent recurrent thromboembolic events and chronic thromboembolic
pulmonary hypertension (1).
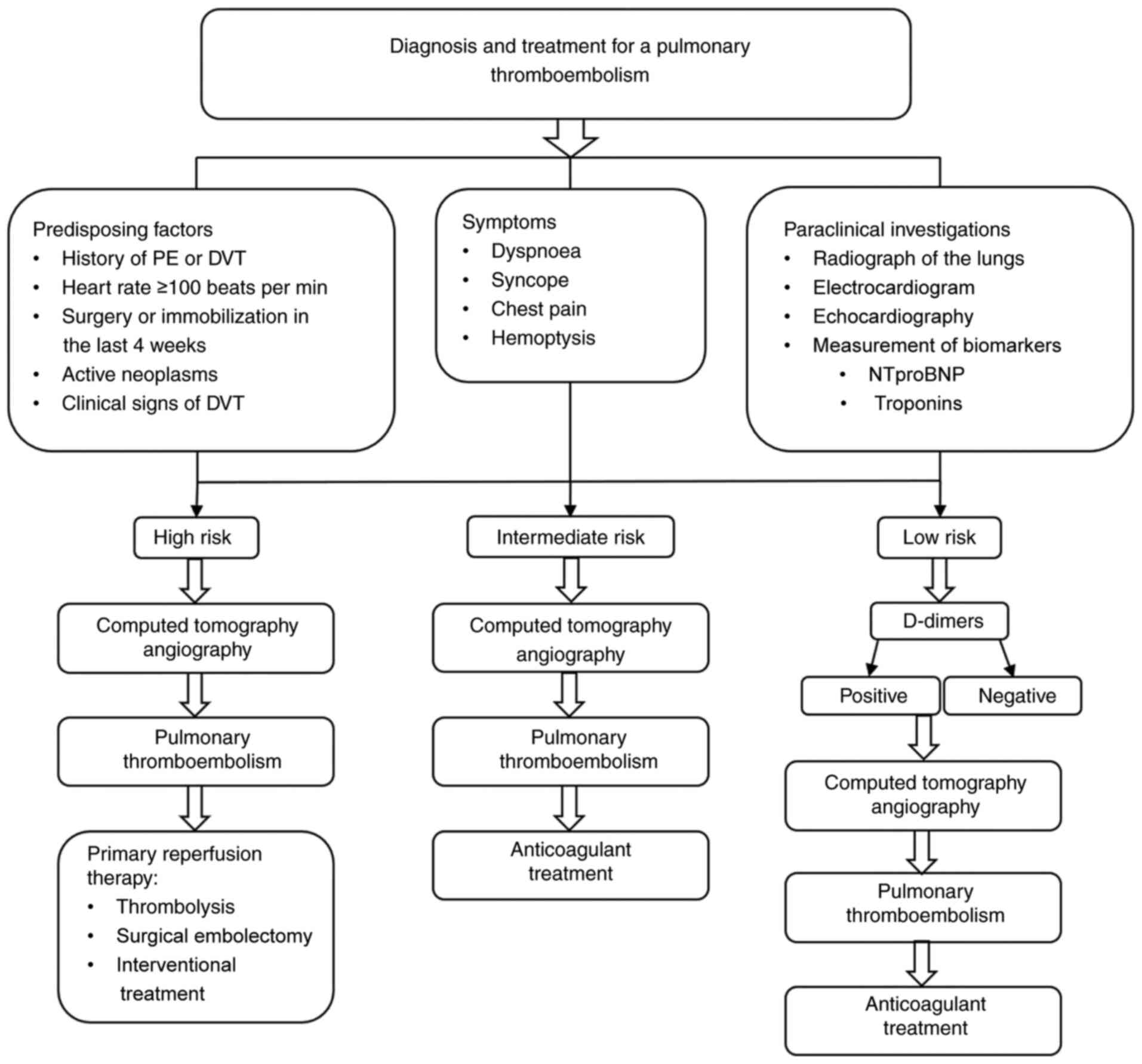
Patients at high risk should undergo reperfusion therapy:
Thrombolysis, surgical embolectomy, or interventional treatment;
along with respiratory and hemodynamic support. Intermediate and
low-risk patients should receive anticoagulant treatment (Fig. 1).
For the acute phase treatment patient should be
treated as follows: i) Respiratory support, administered to
patients with hypoxia including oxygen administration via nasal
cannula; ii) hemodynamic support, reserved for hemodynamically
unstable patients with hypotension, low cardiac output, or
cardiogenic shock; or iii) thrombolytic treatment. reserved for
high-risk patients with a Class I indication and Level B evidence
and for intermediate-risk or hemodynamically unstable patients with
a Class IIa indication and Level B evidence, according to the
European Society of Cardiology Guidelines for the Diagnosis and
Management of PE (1).
Thrombolytic treatment removes thromboembolic
obstruction and, in the short term, it improves right ventricular
function and the hemodynamic status. A long-term benefit of
thrombolytic treatment is the reduced incidence of chronic
thromboembolic pulmonary hypertension (1). Thrombolytic therapy includes
recombinant tissue plasminogen activators such as streptokinase and
urokinase. Absolute contraindications for thrombolytic therapy are
hemorrhagic stroke, ischemic stroke of an unknown etiology in the
past 6 months, ischemic stroke in the last 6 months, central
nervous system impairment or a cerebral tumor, major trauma,
surgery, or cranial trauma in the preceding 3 weeks,
gastrointestinal bleeding in the preceding 30 days and a high risk
of bleeding. Relative contraindications for thrombolytic therapy
include transient ischemic attack in the preceding 6 months, oral
anticoagulant treatment, pregnancy, postpartum-first week, puncture
in an uncompressible location, trauma resuscitation, uncontrolled
hypertension (BP >180 mmHg), advanced liver disease, infectious
endocarditis, or an active peptic ulcer (1).
The primary goal of anticoagulant treatment is to
prevent the mortality of a patient or the recurrence of PE
(105,106). Anticoagulant treatments can be
classified as follows: Parenteral administration agents, which
include unfractionated heparin (indicated for intermediate-risk
patients with signs of hemodynamic instability) (1,103);
LMWH (indicated for intermediate or low-risk patients, with a lower
risk of bleeding and heparin-induced thrombocytopenia) (13); fondaparinux (low bleeding risk,
indicated for intermediate and low-risk patients for 5-10 days);
oral anticoagulants; vitamin K antagonists (similar to LMWH
regarding mortality and bleeding risk but inferior in recurrent
thromboembolic events) (107,108); and novel oral anticoagulants
(NOACs; which do not need to be monitored). Certain NOACs
(apixaban, rivaroxaban) can be administered immediately after
diagnosis without the need for initiation of parenteral
anticoagulant.
Surgical pulmonary embolectomy is indicated for
patients at high risk of PE where thrombolysis is contraindicated
or has failed. It is contraindicated in patients with recurrent
pulmonary emboli or severe pulmonary hypertension with suspected
chronic thromboembolic pulmonary hypertension (1).
Inferior Cava Vein filters are indicated for
patients with recurrent PE secondary to DVT and under effective
anticoagulant treatment and for patients with an absolute
contraindication to anticoagulant treatment (1,109).
Long-term anticoagulant treatment aims to reduce the
number of recurrent thromboembolic events (1). The duration of anticoagulant
treatment for patients with ‘provoked’ or ‘unprovoked’ PE differs,
extending from 3 months in provoked cases to an undetermined period
in the case of the second unprovoked embolic episode. This
extension requires careful risk/benefit assessment when considering
prolonged anticoagulant treatment (1). For patients with recurrent PE and
chronic thromboembolic pulmonary hypertension, long-term
anticoagulant treatment is recommended indefinitely. For patients
with PE and associated neoplastic conditions, initial treatment
with LMWH for the first 3-6 months is preferred, given the observed
superiority in preventing recurrent thromboembolic events compared
with vitamin K antagonists, without being inferior in terms of
bleeding risk and mortality. Continuing anticoagulant treatment
beyond 6 months after the acute event is important considering that
the risk of recurrence is three times higher in patients with
cancer compared with the general population (110,111). Anticoagulant treatment after the
acute event should be continued until the neoplastic disease is
successfully treated. The decision to continue or discontinue
anticoagulant treatment must be made together with the patient,
taking into consideration the risk of recurrence, bleeding and the
preferences of the patient (1,112).
Recurrent thromboembolic events are associated with a significantly
higher long-term risk of mortality. Studies (113,114) have suggested that higher doses of
LMWH are required for patients with cancer after the second
thromboembolic event to lower the mortality rate in these patients
(114,115).
NOACs, such as apixaban, rivaroxaban, edoxaban and
dabigatran, have been directly compared in a study (115) and the results show a similar
efficiency to LMWH regarding the risk of recurrence and the
mortality rate. Regarding the safety profile of apixaban and
edoxaban, they presented the lowest risk of bleeding (Table V) (114,115).
 | Table VAnticoagulant treatment and class of
indication in pulmonary embolism associated with cancer (114). |
Table V
Anticoagulant treatment and class of
indication in pulmonary embolism associated with cancer (114).
| Anticoagulants | Class of
Indication |
|---|
| Low molecular
weight heparin | 2B |
| Vitamin K
antagonist | 2B |
| Novel oral
anticoagulants (apixaban, rivaroxaban, edoxaban and
dabigatran) | 2C |
The use of LMWH is preferable for the long-term
treatment of PE associated with cancer; it is unknown whether
vitamin K antagonists are superior to NOACs in this category of
patients because (Table VI)
(114): i) There are no direct
comparisons between the different types of NOACs; ii) NOACs have
not been directly compared with vitamin K antagonists in a broad
spectrum of patients with PE and cancer; and iii) indirect
comparisons have not convincingly shown different outcomes between
different NOACs.
 | Table VITherapeutic strategies in recurrent
PE associated with cancer (114) |
Table VI
Therapeutic strategies in recurrent
PE associated with cancer (114)
| Recurrent PE under
treatment with: | Consideration for a
therapeutic scheme: |
|---|
| Vitamin K
antagonists (within therapeutic range) | Switch from vitamin
K antagonists to full-dose LMWH |
| LMWH | Increase LMWH dose
by 25% |
| | or |
| | introduction of an
inferior vena cava filter if the anticoagulant dose cannot be
increased (considered as a last resort) |
| Dabigatran | Switch from
Dabigatran to full-dose LMWH, at least temporarily (2C) |
| Rivaroxaban | Switch from
Rivaroxaban to full-dose LMWH, at least temporarily (2C) |
| Apixaban | Switch from
Apixaban to full-dose LMWH, at least temporarily (2C) |
| Edoxaban | Switch from
Edoxaban to full-dose LMWH, at least temporarily (2C) |
The Hokusai-VTE study (116) concluded that treating patients
with PE and NT-proBNP levels >500 ng/l with edoxaban reduced
recurrence of PE compared with patients treated with warfarin.
These results suggest that treatment with novel anticoagulants in
patients with PE and elevated NT-proBNP is superior to warfarin
treatment (65). Assessing the
risk of thromboembolic events in hospitalized patients can be
achieved using the Padua score, which classifies a patient with a
cumulative score of 4 points as at high risk for thromboembolic
events (103). Prophylactic
treatment of thromboembolic events can be achieved by administering
fixed-dose anticoagulants or mechanical methods (103). Routine prophylaxis is recommended
in patients with cancer after surgery and for hospitalized
patients, but it is not recommended for patients treated on an
outpatient basis except if they have multiple myeloma (27,117).
Levine et al (117), in a randomized phase II study,
compared unfractionated heparin, LMWH and apixaban as the primary
treatment for the prevention of venous thromboembolism in patients
with a metastatic neoplasm (lung cancer, colon cancer, breast
cancer, pancreatic cancer, stomach cancer, bladder cancer, ovarian
cancer, prostate cancer, multiple myeloma, lymphomas and cancers of
an unspecified primary site) undergoing chemotherapy in the first 6
weeks from chemotherapy initiation with a duration of at least 90
days of chemotherapy. It was concluded that apixaban was
well-tolerated in the study population and supported further phase
III studies of apixaban for the prevention of venous
thromboembolism in patients with cancer receiving chemotherapy
(good safety profile, 93.5% risk of bleeding and the risk of major
bleeding rate in the apixaban 5 mg group was 2.2%) (117).
Cohen et al (118) evaluated the effectiveness of
rivaroxaban (10 mg/day for 35-39 days) with enoxaparin (40 mg/day
for 10-14 days) for the primary prevention of PE. It was concluded
that the efficacy of standard-duration rivaroxaban was similar to
enoxaparin, while the extended-duration rivaroxaban was superior to
enoxaparin, but it was also associated with a higher bleeding risk
(118) (Table VII).
 | Table VIIPrimary prophylaxis of pulmonary
embolism in oncology patients (117,118). |
Table VII
Primary prophylaxis of pulmonary
embolism in oncology patients (117,118).
| Anticoagulant | Dose | Duration | Safety profile |
|---|
| Enoxaparin | 40 mg/day | 10-14 days | Good |
| Rivaroxaban | 10 mg/day | 10-14 days | Similar to
enoxaparin |
| Rivaroxaban | 10 mg/day | 35-39 days | Superior efficacy
to enoxaparin but higher bleeding risk (requires additional
studies) |
| Apixaban | 5 mg/day | First 6 weeks from
chemotherapy initiation | Good safety profile
93.5% |
| | | | Major bleeding risk
2.2% (requires additional studies) |
Anticoagulant treatment in these patients is
associated with numerous bleeding-associated complications and the
recurrence rate of thromboembolic events should be considered,
given it is >3 times higher than in the general population
(119). Patients with cancer who
develop PE have a reduced life expectancy and the risk of mortality
after PE is four times higher compared with patients without cancer
(120,121). This can be explained by a more
aggressive evolution of the neoplastic process associated with PE
(121,122). However, the risk of recurrent PE
and death under anticoagulant treatment is reduced from 26 to 2.9%
over 36 months (123).
4. Conclusions
PE is considered the second leading cause of
mortality in patients with neoplastic conditions. Increased
NTproBNP levels are associated with acute right ventricular
dysfunction. Right ventricular dysfunction diagnosed by
echocardiography is a frequent clinical finding in patients with PE
and is considered a poor prognostic factor and an independent
predictor of early mortality.
In hemodynamically stable patients with PE with
elevated troponin and/or BNP levels and evidence of right
ventricular dysfunction in the absence of a risk of bleeding,
thrombolytic therapy should be considered (76). It is strongly contraindicated in
cases of apparent macroscopic bleeding, such as hemoptysis, gross
hematuria, or melena and is relatively contraindicated in occult
bleeding.
The Hokusai-VTE study (116) concluded that treating patients
with PE and NT-proBNP levels >500 ng/l with edoxaban was
associated with a reduction in recurrent PE compared with patients
treated with warfarin. These results suggest that treatment with
new anticoagulants in patients with PE and elevated NT-proBNP
levels is superior to warfarin treatment (65).
A negative D-dimer result safely excludes the
diagnosis of PE in patients with cancer. The combination of D-dimer
measurements with other imaging techniques such as CT or venous
ultrasound can improve the diagnosis but requires further
investigation (79).
Vitamin K antagonists are not considered superior
to NOACs (dabigatran, rivaroxaban, apixaban and edoxaban) in the
treatment of PE associated with cancer. NOACs have a similar
efficacy compared with vitamin K antagonists regarding the risk of
recurrence of thromboembolic events and mortality in patients with
PE associated with cancer. Edoxaban and apixaban have an improved
safety profile, with the lowest risk of bleeding risk in this
category of patients (124).
LMWH is preferred in the long-term treatment of PE
associated with cancer. In recurrent PE associated with cancer,
switching from vitamin K antagonists or NOACs to a full dose of
LMWH, at least temporarily (2C level of evidence according to the
European Society of Cardiology) (1), or increasing the dose of LMWH by 25%
in patients with recurrent PE currently being treated with an LMWH
is recommended. Anticoagulant treatment in patients with PE and
cancer may be associated with numerous hemorrhagic
complications.
The primary role of biomarkers is to differentiate
patients at low risk from patients at intermediate mortality risk.
NT-proBNP levels in patients with PE are considered a prognostic
biomarker. Serum levels of cTnT <0.07 µg/l is associated with a
favorable prognosis. Patients who have elevated serum levels of
both biomarkers are associated with a higher risk of short-term
mortality compared with patients who have NTproBNP values >600
ng/l but serum levels of cTnT <0.07 µg/l, which instead have an
intermediate short-term mortality risk. Patients with NTproBNP
levels <600 ng/l and cTnT levels <0.07 µg/l have the best
short-term prognosis.
Pulmonary angiography is the gold standard in the
diagnosis of PE. Prophylactic treatment for PE is routinely
recommended for patients with cancer following surgery and for
hospitalized patients. The recurrence rate of thromboembolic events
in patients with PE associated with cancer is >3 times higher
than that in the general population. Patients with cancer who
develop a PE have a reduced life expectancy and the risk of
mortality after PE is 4 times higher compared with the general
population.
The results of the present review could be applied
in helping differentiating patients at intermediate risk from
patients at low risk of mortality. That could be performed by
dosing biomarkers that are considered short-term prognostic markers
and by imaging studies (echocardiography and CT angiography to
identify right ventricular dysfunction) and thus to prevent early
hemodynamic deterioration that correlates with increased mortality
by initiating thrombolytic/anticoagulant therapy as soon as
possible. On the other hand, the present review wants to shed light
on the fact that in neoplastic patients, although they have an
increased risk of thromboembolic events and the risk of bleeding
associated with anticoagulant treatment is not negligible, the
prevention of thromboembolic events in the first 12 months of
neoplasia diagnosis may markedly reduce the risk of mortality in
these patients.
The present review tried to increase the interest
in new research studies, originals or meta-analyses, performed on
cohorts of cancer patients, studies that have to compare the
effectiveness in preventing and treating thromboembolic events and
safety profiles of different NOACs, of NOACs compared with LMWH and
antivitamins K. These research studies will have to address the
mortality, the recurrence of thromboembolic events as well as the
risk of bleeding in order to identify the classes of anticoagulants
with the lowest risk of bleeding and the maximum prophylactic
effect on thromboembolic events. Currently, there are no direct
studies to compare different NOACs in terms of effectiveness and
safety profile in cancer patients.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
Not applicable.
Authors' contributions
DMN conceived the topic and wrote this scientific
work. CAP and RU reviewed and edited the manuscript. RMR and AGR
revised the content of this article. All authors read and approved
the final manuscript. Data authentication is not applicable.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Konstantinides SV, Torbicki A, Agnelli G,
Danchin N, Fitzmaurice D, Galiè N, Gibbs JSR, Huisman MV, Humbert
M, Kucher N, et al: 2014 ESC guidelines on the diagnosis and
management of acute pulmonary embolism. The task force for the
diagnosis and management of acute pulmonary embolism of the
european society of cardiology (ESC). Endorsed by the European
respiratory society (ERS). Eur Heart J. 35:3033–3080. 2014.
|
|
2
|
Go AS, Mozaffarian D, Roger VL, Benjamin
EJ, Berry JD, Blaha MJ, Dai S, Ford ES, Fox CS, Franco S, et al:
Heart disease and stroke statistics-2014 update: A report from the
American heart association. Circulation. 129:e28–e292.
2014.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Timp JF, Braekkan SK, Versteeg HH and
Cannegieter SC: Epidemiology of cancer-associated venous
thrombosis. Blood. 122:1712–1723. 2013.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Cronin-Fenton DP, Søndergaard F, Pedersen
LA, Fryzek JP, Cetin K, Acquavella J, Baron JA and Sørensen HT:
Hospitalisation for venous thromboembolism in cancer patients and
the general population: A population-based cohort study in Denmark,
1997-2006. Br J Cancer. 103:947–953. 2010.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Madison CJ, Melson RA, Conlin MJ, Gundle
KR, Thompson RF and Calverley DC: Thromboembolic risk in patients
with lung cancer receiving systemic therapy. Br J Haematol.
194:179–190. 2021.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Patel JN, Jiang C, Hertz DL, Mulkey FA,
Owzar K, Halabi S, Ratain MJ, Friedman PN, Small EJ, Carducci MA,
et al: Bevacizumab and the risk of arterial and venous
thromboembolism in patients with metastatic, castration-resistant
prostate cancer treated on cancer and leukemia group B (CALGB)
90401 (alliance). Cancer. 121:1025–1031. 2015.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Heit JA, Silverstein MD, Mohr DN,
Petterson TM, O'Fallon WM and Melton LJ III: Predictors of survival
after deep vein thrombosis and pulmonary embolism: A
population-based, cohort study. Arch Intern Med. 159:445–453.
1999.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Kostrubiec M, Pruszczyk P, Bochowicz A,
Pacho R, Szulc M, Kaczynska A, Styczynski G, Kuch-Wocial A,
Abramczyk P, Bartoszewicz Z, et al: Biomarker-based risk assessment
model in acute pulmonary embolism. Eur Heart J. 26:2166–2172.
2005.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Dalen JE: Pulmonary embolism: What have we
learned since Virchow? Natural history, pathophysiology, and
diagnosis. Chest. 122:1440–1456. 2002.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Keron C: Natural history of venous
thromboembolism. Circulation. 107 (23 Suppl 1):S122–S130.
2003.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Cushman M, Tsai AW, White RH, Heckbert SR,
Rosamond WD, Enright P and Folsom AR: Deep vein thrombosis and
pulmonary embolism in two cohorts: The longitudinal investigation
of thromboembolism etiology. Am J Med. 117:19–25. 2004.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Drăgan A and Drăgan AŞ: Novel insights in
venous thromboembolism risk assessment methods in ambulatory cancer
patients: From the guidelines to clinical practice. Cancers
(Basel). 16(458)2024.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Goldhaber SZ: Pulmonary embolism. In:
Braunwald's Heart Disease: A Textbook of Cardiovascular Medicine.
9th edition. Philadelphia, pp1679-1695, 2012.
|
|
14
|
Khorana AA, Kuderer NM, Culakova E, Lyman
GH and Francis CW: Development and validation of a predictive model
for chemotherapy-associated thrombosis. Blood. 111:4902–4907.
2008.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Moik F, Makatsariya A and Cihan Ay:
Challenging anticoagulation cases: Cancer-associated venous
thromboembolism and chemotherapy-induced thrombocytopenia-A
case-based review of clinical management. Thromb Res. 199:38–42.
2021.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Willems RAL, Michiels N, Lanting VR,
Bouwense S, van den Broek BLJ, Graus M, Klok FA, Koerkamp BG, de
Laat B, Roest M, et al: Venous thromboembolism and primary
thromboprophylaxis in perioperative pancreatic cancer care. Cancers
(Basel). 15(3546)2023.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Kapteijn MY, Zwaan S, Ter Linden E,
Laghmani EH, van den Akker RFP, Rondon AMR, van der Zanden SY,
Neefjes J, Versteeg HH and Buijs JT: Temozolomide and lomustine
induce tissue factor expression and procoagulant activity in
glioblastoma cells in vitro. Cancers (Basel).
15(2347)2023.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Roberts R, Borley A, Hanna A, Dolan G,
Ganesh S and Williams EM: Identifying risk factors for
anthracycline chemotherapy-induced phlebitis in women with breast
cancer: An observational study. Clin Oncol (R Coll Radiol).
33:230–240. 2021.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Naito M, Torii R, Hashimoto Y, Kawamoto Y,
Hayashi K, Shinoda H, Honjo Y and Hiroyoshi M: Increase in
carbohydrate antigen 19-9 levels without tumor progression after
polaprezinc administration that induced deep vein thrombosis in a
colon cancer patient. Chemotherapy. 64:163–166. 2019.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Muhsin-Sharafaldine MR and McLellan AD:
Apoptotic vesicles: Deathly players in cancer-associated
coagulation. Immunol Cell Biol. 96:723–732. 2018.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Setiawan B, Permatadewi CO, de Samakto B,
Bugis A, Naibaho RM, Pangarsa EA, Santosa D and Suharti C: Von
Willebrand factor: Antigen and ADAMTS-13 level, but not soluble
P-selectin, are risk factors for the first asymptomatic deep vein
thrombosis in cancer patients undergoing chemotherapy. Thromb J.
18(33)2020.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Setiawan B, Manurung AKW, Zulizar AA,
Budianto W, Sukarnowati TW, Pangarsa EA, Santosa D, Setiabudy RD
and Suharti C: Changes in plasma levels of IL-6 and D-dimer in
high-risk thrombosis cancer patients undergoing chemotherapy. Bali
Med J. 11:520–527. 2022.
|
|
23
|
Ward MP, Saadeh FA, O'Toole SA, O'Leary
JJ, Gleeson N and Norris LA: Procoagulant activity in high grade
serous ovarian cancer patients following neoadjuvant
chemotherapy-the role of the activated protein C pathway. Thromb
Res. 200:91–98. 2021.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Baddeley E, Pease NJ, Nelson A, Sulma J,
Crabtree A and Noble SIR: PO-04 The impact of a patient information
video on patient awareness and understanding of chemotherapy
induced cancer associated thrombosis. Thromb Res. 200(S19)2021.
|
|
25
|
Horsted F, West J and Grainge MJ: Risk of
venous thromboembolism in patients with cancer: A systematic review
and meta-analysis. PLoS Med. 9(e1001275)2012.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Haddad TC and Greeno EW:
Chemotherapy-induced thrombosis. Thromb Res. 118:555–568.
2006.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Alikhan R, Gomez K, Maraveyas A, Noble S,
Young A and Thomas M: British Society for Haematology:
Cancer-associated venous thrombosis in adults (second edition): A
British society for haematology guideline. Br J Haematol: Apr 25,
2024 (Epub ahead of print).
|
|
28
|
Sweetland S, Green J, Liu B, de González
AB, Canonico M, Reeves G and Beral V: Million Women Study
collaborators. Duration and magnitude of the postoperative risk of
venous thromboembolism in middle aged women: Prospective cohort
study. BMJ. 339(b4583)2009.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Heit JA, Cohen AT and Anderson FA:
Estimated annual number of incident and recurrent, non-fatal and
fatal venous thromboembolism (VTE) events in the US. Blood.
106(910)2005.
|
|
30
|
Jain A, Amira M, Manoharan S, Mahmood S
and Yip D: Role of direct oral anticoagulants in gastrointestinal
cancer associated thrombosis ‘practical issues in clinical
practice’-narrative review. Ann Palliat Med. 10:10053–10061.
2021.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Kucher N and Goldhaber SZ: Cardiac
biomarkers for risc stratification of patients with acute pulmonary
embolism. Circulation. 108:2191–2194. 2003.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Vuilleumier N, Righini M, Perrier A,
Rosset A, Turck N, Sanchez JC, Bounameaux H, Le Gal G, Mensi N and
Hochstrasser D: Correlation between cardiac biomarkers and right
ventricular enlargement on chest CT in non massive pulmonary
embolism. Thromb Res. 121:617–624. 2008.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Giannitsis E, Müller-Bardorff M, Kurowski
V, Weidtmann B, Wiegand U, Kampmann M and Katus HA: Independent
prognostic value of cardiac troponin T in patients with confirmed
pulmonary embolism. Circulation. 102:211–217. 2000.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Kontantinides S, Geibel A, Olschewski M,
Kasper W, Hruska N, Jäckle S and Binder L: Importance of cardiac
troponins I and T in risk stratification of patients with acute
pulmonary embolism. Circulation. 106:1263–1268. 2002.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Mehta NJ, Jani K and Khan IA: Clinical
usefulness and prognostic value of elevated cardiac troponin I
levels in acute pulmonary embolism. Am Heart J. 145:821–825.
2003.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Meyer T, Binder L, Hruska N, Luthe H and
Buchwald AB: Cardiac troponin I elevation in acute pulmonary
embolism is associated with right ventricular dysfunction. J Am
Coll Cardiol. 36:1632–1636. 2000.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Kucher N, Printzen G and Goldhaber SZ:
Prognostic role of brain natriuretic peptide in acute pulmonary
embolism. Circulation. 107:2545–2547. 2003.PubMed/NCBI View Article : Google Scholar
|
|
38
|
ten Wolde M, Tulevski II, Mulder JW, Söhne
M, Boomsma F, Mulder BJ and Büller HR: Brain natriuretic peptide as
a predictor of adverse outcome in patients with pulmonary embolism.
Circulation. 107:2082–2084. 2003.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Sohne M, ten Wolde M and Büller HR:
Biomarkers in pulmonary embolism. Curr Opin Cardiol. 19:558–562.
2004.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Pruszczyk P, Kostrubiec M, Bochowicz A,
Styczyński G, Szulc M, Kurzyna M, Fijalkowska A, Kuch-Wocial A,
Chlewicka I and Torbicki A: N-terminal pro-brain natriuretic
peptide in patients with acute pulmonary embolism. Eur Respir J.
22:649–653. 2003.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Quiroz R, Kucher N, Schoepf UJ,
Kipfmueller F, Solomon SD, Costello P and Goldhaber SZ: Right
ventricular enlargement on chest computed tomography: Prognostic
role in acute pulmonary embolism. Circulation. 109:2401–2404.
2004.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Sanchez O, Trinquart L, Colombet I,
Durieux P, Huisman MV, Chatellier G and Meyer G: Prognostic value
of right ventricular dysfunction in patients with haemodynamically
stable pulmonary embolism: A systematic review. Eur Heart J.
29:1569–1577. 2008.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Klok FA, Mos ICM and Huisman MV:
Brain-type natriuretic peptide levels in the prediction of adverse
outcome in patients with pulmonary embolism: A systematic review
and meta-analysis. Am J Respir Crit Care Med. 178:425–430.
2008.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Horlander KT and Leeper KV: Troponin
levels as a guide to treatment of pulmonary embolism. Curr Opin
Pulm Med. 9:374–377. 2003.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Henzler T, Roeger S, Meyer M, Schoepf UJ,
Nance JW Jr, Haghi D, Kaminski WE, Neumaier M, Schoenberg SO and
Fink C: Pulmonary embolism: CT signs and cardiac biomarkers for
predicting right ventricular dysfunction. Eur Respir J. 39:919–926.
2012.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Hama N, Itoh H, Shirakami G, Nakagawa O,
Suga S, Ogawa Y, Masuda I, Nakanishi K, Yoshimasa T, Hashimoto Y,
et al: Rapid ventricular induction of brain natriuretic peptide
gene expression in experimental acute myocardial infarction.
Circulation. 92:1558–1564. 1995.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Hall C: Essential biochemistry and
physiology of (NT-pro)BNP. Eur J Heart Fail. 6:257–260.
2004.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Joolharzadeh P, Rodriguez M, Zaghlol R,
Pedersen LN, Jimenez J, Bergom C and Mitchell JD: Recent advances
in serum biomarkers for risk stratification and patient management
in cardio-oncology. Curr Cardiol Rep. 25:133–146. 2023.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Frémont B, Pacouret G, Jacobi D, Puglisi
R, Charbonnier B and de Labriolle A: Prognostic value of
echocardiographic right/left ventricular end-diastolic diameter
ratio in patients with acute pulmonary embolism: Results from a
monocenter registry of 1,416 patients. Chest. 133:358–362.
2008.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Grifoni S, Olivotto I, Cecchini P,
Pieralli F, Camaiti A, Santoro G, Conti A, Agnelli G and Berni G:
Short-term clinical outcome of patients with acute pulmonary
embolism, normal blood pressure, and echocardiographic right
ventricular dysfunction. Circulation. 101:2817–2822.
2000.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Goldhaber SZ: Pulmonary embolism. N Engl J
Med. 339:93–104. 1998.PubMed/NCBI View Article : Google Scholar
|
|
52
|
de Lemos JA, McGuire DK and Drazner MH:
B-type natriuretic peptide in cardiovascular disease. Lancet.
362:316–322. 2003.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Tulevski II, Hirsch A, Sanson BJ, Romkes
H, van der Wall EE, van Veldhuisen DJ, Büller HR and Mulder BJ:
Increased brain natriuretic peptide as a marker for right
ventricular dysfunction in acute pulmonary embolism. Thromb
Haemost. 86:1193–1196. 2001.PubMed/NCBI
|
|
54
|
Kucher N, Printzen G, Doernhoefer T,
Windecker S, Meier B and Hess OM: Low pro-brain natriuretic peptide
levels predict benign clinical outcome in acute pulmonary embolism.
Circulation. 107:1576–1578. 2003.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Nagaya N, Nishikimi T, Okano Y, Uematsu M,
Satoh T, Kyotani S, Kuribayashi S, Hamada S, Kakishita M, Nakanishi
N, et al: Plasma brain natriuretic peptide levels increase in
proportion to the extent of right ventricular dysfunction in
pulmonary hypertension. J Am Coll Cardiol. 31:202–208.
1998.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Nagaya N, Nishikimi T, Uematsu M, Satoh T,
Kyotani S, Sakamaki F, Kakishita M, Fukushima K, Okano Y, Nakanishi
N, et al: Plasma brain natriuretic peptide as a prognostic
indicator in patients with primary pulmonary hypertension.
Circulation. 102:865–870. 2000.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Bando M, Ishii Y, Sugiyama Y and Kitamura
S: Elevated plasma brain natriuretic peptide levels in chronic
respiratory failure with cor pulmonale. Respir Med. 93:507–514.
1999.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Tulevski II, Groenink M, van Der Wall EE,
van Veldhuisen DJ, Boomsma F, Stoker J, Hirsch A, Lemkes JS and
Mulder BJ: Increased brain and atrial natriuretic peptides in
patients with chronic right ventricular pressure overload:
Correlation between plasma neurohormones and right ventricular
dysfunction. Heart. 86:27–30. 2001.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Thygesen K, Mair J, Mueller C, Huber K,
Weber M, Plebani M, Hasin Y, Biasucci LM, Giannitsis E, Lindahl B,
et al: Recommendations for the use of natriuretic peptides in acute
cardiac care: A position statement from the study group on
biomarkers in cardiology of the ESC working group on acute cardiac
care. Eur Heart J. 33:2001–2006. 2012.PubMed/NCBI View Article : Google Scholar
|
|
60
|
Binder L, Pieske B, Olchewski M, Geibel A,
Klostermann B, Reiner C and Konstantinides S: N-terminal pro-brain
natriuretic peptide or troponin testing followed by
echocardiography for risk stratification of acute pulmonary
embolism. Circulation. 112:1573–1579. 2005.PubMed/NCBI View Article : Google Scholar
|
|
61
|
Wodzig KW, Pelsers MM, van der Vusse GJ,
Roos W and Glatz JF: One-step enzyme-linked immunosorbent assay
(ELISA) for plasma fatty acid-binding protein. Ann Clin Biochem.
34:263–268. 1997.PubMed/NCBI View Article : Google Scholar
|
|
62
|
Cavallazzi R, Nair A, Vasu T and Marik PE:
Natriuretic peptides in acute pulmonary embolism: A systematic
review. Intensive Care Med. 34:2247–2256. 2008.PubMed/NCBI View Article : Google Scholar
|
|
63
|
Coutance G, Cauderlier E, Ehtisham J and
Hamon M and Hamon M: The prognostic value of markers of right
ventricular dysfunction in pulmonary embolism: A meta-analysis.
Crit Care. 15(R103)2011.PubMed/NCBI View Article : Google Scholar
|
|
64
|
Lega JC, Lacasse Y, Lakhal L and
Provencher S: Natriuretic peptides and troponins in pulmonary
embolism: A meta-analysis. Thorax. 64:869–875. 2009.PubMed/NCBI View Article : Google Scholar
|
|
65
|
Lankeit M, Jiménez D, Kostrubiec M, Dellas
C, Kuhnert K, Hasenfuß G, Pruszczyk P and Konstantinides S:
Validation of N-terminal pro-brain natriuretic peptide cut-off
values for risk stratification of pulmonary embolism. Eur Respir J.
43:1669–1677. 2014.PubMed/NCBI View Article : Google Scholar
|
|
66
|
Agterof MJ, Schutgens RE, Moumli N,
Eijkemans MJ, van der Griend R, Tromp EA and Biesma DH: A
prognostic model for short term adverse events in normotensive
patients with pulmonary embolism. Am J Hematol. 86:646–649.
2011.PubMed/NCBI View Article : Google Scholar
|
|
67
|
Klok FA, Van Der Bijl N, Eikenboom HC, Van
Rooden CJ, De Roos A, Kroft LJ and Huisman MV: Comparison of CT
assessed right ventricular size and cardiac biomarkers for
predicting short-term clinical outcome in normotensive patients
suspected for having acute pulmonary embolism. J Thromb Haemost.
8:853–856. 2010.PubMed/NCBI View Article : Google Scholar
|
|
68
|
Kostrubiec M, Pruszczyk P, Bochowicz A,
Pacho R, Szulc M, Kaczynska A, Styczynski G, Kuch-Wocial A,
Abramczyk P, Bartoszewicz Z, et al: Biomarker-based risk assessment
model in acute pulmonary embolism. Eur Heart J. 26:2266–2272.
2005.PubMed/NCBI View Article : Google Scholar
|
|
69
|
Lankeit M, Friesen D, Aschoff J, Dellas C,
Hasenfuss G, Katus H, Konstantinides S and Giannitsis E: Highly
sensitive troponin T assay in normotensive patients with acute
pulmonary embolism. Eur Heart J. 31:1836–1844. 2010.PubMed/NCBI View Article : Google Scholar
|
|
70
|
Dellas C, Puls M, Lankeit M, Schäfer K,
Cuny M, Berner M, Hasenfuss G and Konstantinides S: Elevated
heart-type fatty acid-binding protein levels on admission predict
an adverse outcome in normotensive patients with acute pulmonary
embolism. J Am Coll Cardiol. 55:2250–2257. 2010.PubMed/NCBI View Article : Google Scholar
|
|
71
|
Ozsu S, Karaman K, Mentese A, Ozsu A,
Karahan SC, Durmus I, Oztuna F, Kosucu P, Bulbul Y and Ozlu T:
Combined risk stratification with computerized tomography
/echocardiography and biomarkers in patients with normotensive
pulmonary embolism. Thromb Res. 126:486–492. 2010.PubMed/NCBI View Article : Google Scholar
|
|
72
|
Steering Committee: Single-bolus
tenecteplase plus heparin compared with heparin alone for
normotensive patients with acute pulmonary embolism who have
evidence of right ventricular dysfunction and myocardial injury:
Rationale and design of the pulmonary embolism thrombolysis
(PEITHO) trial. Am Heart J. 163:33–38.e1. 2012.PubMed/NCBI View Article : Google Scholar
|
|
73
|
Meyer G, Vicaut E, Danays T, Agnelli G,
Becattini C, Beyer-Westendorf J, Bluhmki E, Bouvaist H, Brenner B,
Couturaud F, et al: Fibrinolysis for patients with acute
intermediate-risk pulmonary embolism. N Engl J Med. 370:1402–1411.
2014.PubMed/NCBI View Article : Google Scholar
|
|
74
|
Pruszczyk P, Bochowicz A, Torbicki A,
Szulc M, Kurzyna M, Fijałkowska A and Kuch-Wocial A: Cardiac
troponin T monitoring identifies high-risk group of normotensive
patients with acute pulmonary embolism. Chest. 123:1947–1952.
2003.PubMed/NCBI View Article : Google Scholar
|
|
75
|
Vuilleumier N, Le Gal G, Verschuren F,
Perriers A, Bounameaux H, Turck N, Sanchez JC, Mensi N, Perneger T,
Hochstrasser D and Righini M: Cardiac biomarkers for risk
stratification in non-massive pulmonary embolism: A multicenter
prospective study. J Thromb Haemost. 7:391–398. 2008.PubMed/NCBI View Article : Google Scholar
|
|
76
|
Kucher N and Goldhaber SZ: Cardiac
biomarkers for risk stratification of patients with acute pulmonary
embolism. Circulation. 108:2191–2194. 2003.PubMed/NCBI View Article : Google Scholar
|
|
77
|
Lee AYY, Julian JA, Levine MN, Weit JI,
Kearon C, Wells PS and Ginsberg JS: Clinical utility of a rapid
whole-blood D-dimer assay in patients with cancer who present with
suspected acute deep venous thrombosis. Am Intern Med. 131:417–423.
1999.PubMed/NCBI View Article : Google Scholar
|
|
78
|
ten Wolde M, Kraaijenhagen RA, Prins MH
and Buller HR: The clinical usefulness of D-dimer testing in cancer
patients with suspected deep venous thrombosis. Arch Intern Med.
162:1880–1884. 2002.PubMed/NCBI View Article : Google Scholar
|
|
79
|
Di Nisio M, Sohne M, Kamphuisen PW and
Büller HR: D-dimer test in cancer patients with suspected acute
pulmonary embolism. J Thromb Haemost. 3:1239–1242. 2005.PubMed/NCBI View Article : Google Scholar
|
|
80
|
Kelly J, Rudd A, Lewis RR and Hunt BJ:
Plasma D-dimers in the diagnosis of venous thromboembolism. Arch
Intern Med. 162:747–756. 2002.PubMed/NCBI View Article : Google Scholar
|
|
81
|
Levine MN, Raskob G, Beyth RJ, Kearon C
and Schulman S: Hemorrhagic complication of anticoagulant
treatment. The seventh ACCP conference on antithrombotic and
thrombolytic therapy. Chest. 126 (3 Suppl):287S–310S.
2004.PubMed/NCBI View Article : Google Scholar
|
|
82
|
Stein PD, Hull RD, Patel KC, Olson RE,
Ghali WA, Brant R, Biel RK, Bharadia V and Kalra NK: D-dimer for
the exclusion of acute venous thrombosis and pulmonary embolism: A
systematic review. Am Intern Med. 140:589–602. 2004.PubMed/NCBI View Article : Google Scholar
|
|
83
|
Brown MD, Rowe BH, Reeves MJ, Bermingham
JM and Goldhaber SZ: The accuracy of the enzyme-linked
immunosorbent assay D-dimer test in the diagnosis of pulmonary
embolism: A meta-analysis. Ann Emerg Med. 40:133–144.
2002.PubMed/NCBI View Article : Google Scholar
|
|
84
|
Wikan VE, Tøndel BG, Morelli VM, Brodin
EE, Brækkan SK and Hansen JB: Diagnostic blood biomarkers for acute
pulmonary embolism: A systematic review. Diagnostics (Basel).
13(2301)2023.PubMed/NCBI View Article : Google Scholar
|
|
85
|
Silva BV, Calé R, Menezes MN, Jorge C,
Pinto FJ and Caldeira D: How to predict prognosis in patients with
acute pulmonary embolism? Recent advances. Kardiol Pol. 81:684–691.
2023.PubMed/NCBI View Article : Google Scholar
|
|
86
|
Stein PD, Terrin ML, Hales CA, Palevsky
HI, Saltzman HA, Thompson BT and Weg JG: Clinical, laboratory,
roentgenographic, and electrocardiographic findings in patients
with acute pulmonary embolism and no pre-existing cardiac or
pulmonary disease. Chest. 100:598–603. 1991.PubMed/NCBI View Article : Google Scholar
|
|
87
|
Chou T and Knilans TK: Electrocardiography
in clinical practice: Adult and pediatric; 4th edition.
Philadelphia: Saunders, pp167-170, 1996.
|
|
88
|
Konstantinides S: Pulmonary Embolism:
Impact of right ventricular dysfunction. Curr Opin Cardiol.
20:496–501. 2005.PubMed/NCBI View Article : Google Scholar
|
|
89
|
Goldhaber SZ, Visani L and De Rosa M:
Acute pulmonary embolism: clinical outcomes in the international
cooperative pulmonary embolism registry (ICOPER). Lancet.
353:1386–1389. 1999.PubMed/NCBI View Article : Google Scholar
|
|
90
|
Becattini C, Vedovati MC and Agnelli G:
Prognostic value of troponins in acute pulmonary embolism: A
meta-analysis. Circulation. 116:427–433. 2007.PubMed/NCBI View Article : Google Scholar
|
|
91
|
Kreit JW: The impact of right ventricular
dysfunction on the prognosis and therapy of normotensive patients
with pulmonary embolism. Chest. 125:1539–1545. 2004.PubMed/NCBI View Article : Google Scholar
|
|
92
|
Leacche M, Unic D, Goldhaber SZ, Rawn JD,
Aranki SF, Couper GS, Mihaljevic T, Rizzo RJ, Cohn LH, Aklog L and
Byrne JG: Modern surgical treatment of massive pulmonary embolism:
Results in 47 consecutive patients after rapid diagnosis and
aggressive surgical approach. J Thorac Cardiovasc Surg.
129:1018–1023. 2005.PubMed/NCBI View Article : Google Scholar
|
|
93
|
Kasper W, Konstantinides S, Geibel A,
Tiede N, Krause T and Just H: Prognostic significance of right
ventricular afterload stress detected by echocardiography in
patients with clinically suspected pulmonary embolism. Heart.
77:346–349. 1997.PubMed/NCBI View Article : Google Scholar
|
|
94
|
Ribeiro A, Lindmarker P, Juhlin-Dannfelt
A, Johnsson H and Jorfeldt L: Echocardiography Doppler in pulmonary
embolism: Right ventricular dysfunction as a predictor of mortality
rate. Am Heart J. 134:479–487. 1997.PubMed/NCBI View Article : Google Scholar
|
|
95
|
Araoz PA, Gotway MB, Harrington JR,
Harmsen WS and Mandrekar JN: Pulmonary embolism: Prognostic CT
findings. Radiology. 242:889–897. 2007.PubMed/NCBI View Article : Google Scholar
|
|
96
|
Ghaye B, Ghuysen A, Willems V, Lambermont
B, Gerard P, D'Orio V, Gevenois PA and Dondelinger RF: Severe
pulmonary embolism:Pulmonary artery clot load scores and
cardiovascular parameters as predictors of mortality. Radiology.
239:884–891. 2006.PubMed/NCBI View Article : Google Scholar
|
|
97
|
Ghuysen A, Ghaye B, Willems V, Lambermont
B, Gerard P, Dondelinger RF and D'Orio V: Computed tomographic
pulmonary angiography and prognostic significance in patients with
acute pulmonary embolism. Thorax. 60:956–961. 2005.PubMed/NCBI View Article : Google Scholar
|
|
98
|
Mansencal N, Joseph T, Vieillard-Baron A,
Langlois S, El Hajjam M, Qanadli SD, Lacombe P, Jardin F and
Dubourg O: Diagnosis of right ventricular dysfunction in acute
pulmonary embolism using helical computed tomography. Am J Cardiol.
95:1260–1263. 2005.PubMed/NCBI View Article : Google Scholar
|
|
99
|
Schoepf UJ, Kucher N, Kipfmueller F,
Quiroz R, Costello P and Goldhaber SZ: Right ventricular
enlargement on chest computed tomography: A predictor of early
death in acute pulmonary embolism. Circulation. 110:3276–3280.
2004.PubMed/NCBI View Article : Google Scholar
|
|
100
|
van der Meer RW, Pattynama PM, van Strijen
MJ, van den Berg-Huijsmans AA, Hartmann IJ, Putter H, de Roos A and
Huisman MV: Right ventricular dysfunction and pulmonary obstruction
index at helical CT: Prediction of clinical outcome during 3-month
follow-up in patients with acute pulmonary embolism. Radiology.
235:798–803. 2005.PubMed/NCBI View Article : Google Scholar
|
|
101
|
Contractor S, Maldjian PD, Sharma VK and
Gor DM: Role of helical CT in detecting right ventricular
dysfunction secondary to acute pulmonary embolism. J Comput Assist
Tomogr. 26:587–591. 2002.PubMed/NCBI View Article : Google Scholar
|
|
102
|
Lim KE, Chan CY, Chu PH, Hsu YY and Hsu
WC: Right ventricular dysfunction secondary to acute massive
pulmonary embolism detected by helical computed tomography
pulmonary angiography. Clin Imaging. 29:16–21. 2005.PubMed/NCBI View Article : Google Scholar
|
|
103
|
Mann DL, Zipes DP, Libby P and Bonow RO
(eds): Braunwaldʼs heart disease. A textbook of cardiovascular
medicine, single volume, 10th edition. Elsevier Saunders, 2015.
|
|
104
|
Oride T, Sawada K, Shimizu A, Kinose Y,
Takiuchi T, Kodama M, Hashimoto K, Kobayashi E, Nakatani E and
Kimura T: Clinical trial assessing the safety of edoxaban with
concomitant chemotherapy in patients with gynecological
cancer-associated thrombosis (EGCAT study). Thromb J.
21(57)2023.PubMed/NCBI View Article : Google Scholar
|
|
105
|
Konstantinides S and Goldhaber SZ:
Pulmonary embolism: Risk assessment and management. Eur Heart J.
33:3014–3022. 2012.PubMed/NCBI View Article : Google Scholar
|
|
106
|
Guyatt GH, Akl EA, Crowther M, Gutterman
DD and Schuünemann HJ: American College of Chest Physicians
Antithrombotic Therapy and Prevention of Thrombosis Panel.
Executive summary: Antithrombotic therapy and prevention of
thrombosis, 9th ed: American college of chest physicians
evidence-based clinical practice guidelines. Chest. 141 (2
Suppl):7S–47S. 2012.PubMed/NCBI View Article : Google Scholar
|
|
107
|
Limbrey R and Howard L: Developments in
the management and treatment of pulmonary embolism. Eur Respir Rev.
24:484–497. 2015.PubMed/NCBI View Article : Google Scholar
|
|
108
|
Lewis JE and Pilcher DV: The management of
pulmonary embolism. Anaesth Intensive Care Med. 18:126–132.
2017.
|
|
109
|
Muriel A, Jiménez D, Aujesky D, Bertoletti
L, Decousus H, Laporte S, Mismetti P, Muñoz FJ, Yusen R and Monreal
M: RIETE Investigators. Survival effects of inferior vena cava
filter in patients with acute symptomatic venous thromboembolism
and a significant bleeding risk. J Am Coll Cardiol. 63:1675–1683.
2014.PubMed/NCBI View Article : Google Scholar
|
|
110
|
Zamorano JL, Lancellotti P, Rodriguez
Muñoz D, Aboyans V, Asteggiano R, Galderisi M, Habib G, Lenihan DJ,
Lip GYH, Lyon AR, et al: 2016 ESC position paper on cancer
treatments and cardiovascular toxicity developed under the auspices
of the ESC committee for practice guidelines: The task force for
cancer treatments and cardiovascular toxicity of the European
society of cardiology (ESC). Eur Heart J. 37:2768–2801.
2016.PubMed/NCBI View Article : Google Scholar
|
|
111
|
Louzada ML, Carrier M, Lazo-Langner A, Dao
V, Kovacs MJ, Ramsay TO, Rodger MA, Zhang J, Lee AYY, Meyer G and
Wells PS: Development of a clinical prediction rule for risk
stratification of recurrent venous thromboembolism in patients with
cancer-associated venous thromboembolism. Circulation. 126:448–454.
2012.PubMed/NCBI View Article : Google Scholar
|
|
112
|
Akl EA, Vasireddi SR, Gunukula S, Barba M,
Sperati F, Terrenato I, Muti P and Schünemann H: Anticoagulation
for the initial treatment of venous thromboembolism in patients
with cancer. Cochrane Database Syst Rev: CD006649, 2011.
|
|
113
|
Carrier M, Le Gal G, Cho R, Tierney S,
Rodger M and Lee AY: Dose escalation of low molecular weight
heparin to manage recurrent venous thromboembolic events despite
systemic anticoagulation in cancer patients. J Thromb Haemost.
7:760–765. 2009.PubMed/NCBI View Article : Google Scholar
|
|
114
|
Kearon C, Akl EA, Ornelas J, Blaivas A,
Jimenez D, Bounameaux H, Huisman M, King CS, Morris TA, Sood N, et
al: Antithrombotic therapy for VTE disease: CHEST guideline and
expert panel report. Chest. 149:315–352. 2016.PubMed/NCBI View Article : Google Scholar
|
|
115
|
Mantha S and Ansell J: Indirect comparison
of dabigatran, rivaroxaban, apixaban and edoxaban for the treatment
of acute venous thromboembolism. J Thromb Thrombolysis. 39:155–156.
2015.PubMed/NCBI View Article : Google Scholar
|
|
116
|
Hokusai-VTE Investigators. Büller HR,
Décousus H, Grosso MA, Mercuri M, Middeldorp S, Prins MH, Raskob
GE, Schellong SM, Schwocho L, et al: Edoxaban versus warfarin for
the treatment of symptomatic venous thromboembolism. N Engl J Med.
369:1406–1415. 2013.PubMed/NCBI View Article : Google Scholar
|
|
117
|
Levine MN, Gu C, Liebman HA, Escalante CP,
Solymoss S, Deitchman D, Ramirez L and Julian J: A randomized phase
II trial of apixaban for the prevention of thromboembolism in
patients with metastatic cancer. J Thromb Haemost. 10:807–814.
2012.PubMed/NCBI View Article : Google Scholar
|
|
118
|
Cohen AT, Spiro TE, Büller HR, Haskell L,
Hu D, Hull R, Mebazaa A, Merli G, Schellong S, Spyropoulos AC, et
al: Rivaroxaban for thromboprophylaxis in acutely ill medical
patients. N Engl J Med. 368:513–523. 2013.PubMed/NCBI View Article : Google Scholar
|
|
119
|
Gussoni G, Frasson S, La Regina M, Di
Micco P and Monreal M: RIETE Investigators. Three-month mortality
rate and clinical predictors in patients with venous
thromboembolism and cancer. Findings from the RIETE registry.
Thromb Res. 131:24–30. 2013.PubMed/NCBI View Article : Google Scholar
|
|
120
|
Lee AYY: Epidemiology and management of
venous thromboembolism in patients with cancer. Thromb Res.
110:167–172. 2003.PubMed/NCBI View Article : Google Scholar
|
|
121
|
Kakkar AK: An expanding role for
antithrombotic therapy in cancer patients. Cancer Treat Rev. 29
(Suppl 2):S23–S26. 2003.PubMed/NCBI View Article : Google Scholar
|
|
122
|
Sørensen HT, Mellemkjaer L, Olsen JH and
Baron JA: Prognosis of cancers associated with venous
thromboembolism. N Engl J Med. 343:1846–1850. 2000.PubMed/NCBI View Article : Google Scholar
|
|
123
|
Buller HR, Agnelli G, Hull RD, Hyers TM,
Prins MH and Raskob GE: Antithrombotic therapy for venous
thromboembolic disease: The Seventh ACCP conference on
antithrombotic and thrombolytic therapy. Chest. 126 (3
Suppl):401S–428S. 2004.PubMed/NCBI View Article : Google Scholar
|
|
124
|
Masini M, Toma M, Spallarossa P, Porto I
and Ameri P: Direct oral anticoagulants for cancer-associated
venous thromboembolism. Curr Oncol Rep. 25:979–987. 2023.PubMed/NCBI View Article : Google Scholar
|