Introduction
Atherosclerosis (AS), a chronic inflammatory
disease, is featured by forming of atherosclerotic plaques in the
arterial wall (1,2), which may lead to various diseases
including coronary artery disease and stroke as well as
cerebrovascular disease (3-5),
producing high morbidity and mortality worldwide (6). Increasing evidence has indicated that
abnormal proliferation and migration of VSMCs play a crucial role
in the progression of AS (7,8).
Therefore, it is urgently necessary to explore the molecular
pathogenesis of VSMCs in AS and to find significant therapeutic
targets for the suppression of VSMCs development.
microRNAs (miRNAs/miRs) are small non-coding
single-stranded RNAs with ~22 nucleotides that show key regulatory
roles by post-transcriptionally targeting mRNAs of target genes
(9). Studies have reported that
miRNAs are tightly involved in the progression of VSMCs, for
example, Farina et al (10)
suggested that miR-128-3p was a novel regulator of VSMCs phenotypic
switch and vascular diseases. Wang et al (11) indicated that CTBP1-AS2 inhibited
proliferation and induces autophagy in ox-LDL-stimulated VSMCs
through regulating miR-195-5p/ATG14. You et al (12) found that overexpression of
miR-29a-3p suppressed proliferation, migration of VSMCs in
atherosclerosis via targeting TNFRSF1A. Jing et al (13) suggested that LINC00472 regulated
VSMCs migration and proliferation by regulating miR-149-3p.
Additionally, various studies reported that miR-374a played
important roles in cancer progression. For example, Hao et
al (14) found that LINC-PINT
suppressed tumor cell proliferation, migration by targeting
miR-374a-5p in ovarian cancer. Lu et al (15) identified that miR-374a promoted the
proliferation of osteosarcoma cell proliferation via targeting
Axin2. Ma et al (16)
indicated that miR-374a could promote pancreatic cancer cell
proliferation and epithelial to mesenchymal transition by targeting
SRCIN1, whereas the potential role of miR-374a in VSMCs
proliferation, migration remains to be elucidated.
The present study aimed to explore the effect of
miR-374a on the proliferation and migration of VSMCs, and the
underlying mechanism.
Materials and methods
Cell culture and transfection
The Human Brain Vascular Smooth Muscle Cells
(HBVSMCs) were bought from Sciencell Research Laboratories, Inc.
(cat. no. sc-1100) and the cells were maintained in Dulbecco's
Modified Eagle's Medium (DMEM) supplemented with 10% FBS (Gibco;
Thermo Fisher Scientific, Inc.), 1% penicillin and 1% streptomycin
(Gibco; Thermo Fisher Scientific, Inc.) with an atmosphere of 5%
CO2 at 37˚C.
The isolation and culture of primary mouse VSMCs
were performed according to previous protocol (17). Briefly, mVSMCs were isolated from
the aorta (from the aortic arch to the iliac bifurcation) of two
6-week-old, male, C57BL/6J mice (weight, 20 g; at 20˚C, with a
light/dark cycle of 10/14 h and humidity of 55%; Charles River
Laboratories International, Inc.) and plated in DMEM supplemented
with 20% FBS, 1% penicillin and 1% streptomycin at 37˚C in a
humidified atmosphere of 5% CO2.
The miR-374a mimics (50 nM), miR-374a inhibitors (50
nM) and respective controls (50 nM) were bought from Guangzhou
RiboBio Co., Ltd. The pcDNA (Vector) and pcDNA-RAR-related orphan
receptor A (RORA)/GATA2 overexpression (RORA-OE/GATA2-OE) plasmids
were purchased from Guangzhou RiboBio Co., Ltd. Plasmid cloning DNA
(Wild-type), miR-374a mimics or inhibitors as well as the
respective controls (empty/inhibitor-NC/mimic-NC) were first mixed
with Opti-MEM (Invitrogen; Thermo Fisher Scientific, Inc.),
incubated for 5 min at 37˚C, co-incubated with
Lipofectamine® 3000 (Invitrogen; Thermo Fisher
Scientific, Inc.) for 20 min and transfected into cells for 72 h at
37˚C according to the manufacturer's instructions. Follow-up
experiments were conducted 2 days later. PDGF-BB (MilliporeSigma)
was adopted as a concentration gradient assays to identify the
final intervention dose. The sequences of the miRNA mimic/inhibitor
and miR-NCs are shown in Table
I.
 | Table IPrimers used in the present
study. |
Table I
Primers used in the present
study.
| Primer | Sequences |
|---|
| miR-374a | Forward:
5'-CGCGCGTTATAATACAACCTGA-3' |
| | Reverse:
5'-AGTGCAGGGTCCGAGGTATT-3' |
| U6 | Forward:
5'-ATTGGAACGATACAGAGAAGATT-3' |
| | Reverse:
5'-GGAACGCTTCACGAATTTG-3' |
| GAPDH | Forward:
5'-TCGGAGTCAACGGATTTGGT-3' |
| | Reverse:
5'-TTCCCGTTCTCAGCCTTGAC-3' |
| RORA | Forward:
5'-ATGGAGTCAGCTCCGGCA-3' |
| | Reverse:
5'-TCGTTACTGAGATACCTCTGCTG-3' |
| TNPO1 | Forward:
5'-ACCGTGCAACAAAAACTGGA-3' |
| | Reverse:
5'-TGGGAAGTTCTGAAAGTGTGCT-3' |
| MIB1 | Forward:
5'-CCTCTGGGATAATGGTGCT-3' |
| | Reverse:
5'-GGACAATGTTCGCTTTAGTG-3' |
| CCND1 | Forward:
5'-GATCAAGTGTGACCCGGACT-3' |
| | Reverse:
5'-CTTGGGGTCCATGTTCTGCT-3' |
| YOD1 | Forward:
5'-AGACAACTCTTGCCTCTTTA-3' |
| | Reverse:
5'-TTTCCCAGTATTGCCTCAC-3' |
| PTBP1 | Forward:
5'-TTTTCCAAGCTCACCAGCCT-3' |
| | Reverse:
5'-TATACCAGGTGCACCGAAGG-3' |
| GATA2 | Forward:
5'-CCTGTAGTTCCTGCCCCTCT-3' |
| | Reverse:
5'-AGCTGCCGACTCCCAGA-3' |
| HESX1 | Forward:
5'-CCTGCAGCTCATCAGGGAAA-3' |
| | Reverse:
5'-CAGTTCTTGGTCTTCGGCCT-3' |
| LBX2 | Forward:
5'-AGCATCGCAGACATCCTAGC‐3' |
| | Reverse:
5'-CTGCCCGCCCTTCAGAG‐3' |
| NR4A1 | Forward:
5'-TACGAAACTTGGGGGAGTGC-3' |
| | Reverse:
5'-CTGCACCCTACCCGGC-3' |
| MEF2A | Forward:
5'-CGGAATCATAAAATCGCACCTGG-3' |
| | Reverse:
5'-GTTAACGTTGAGCTGGCTGC-3' |
| HSPA13 | Forward:
5'-AACCCGAGCAATGTCTGGAAA‐3' |
| | Reverse:
5'-CTAGAGGGCACGAAGCCATA‐3' |
| UBE2K | Forward:
5'-CTGAAGAGCGAGGAGACGAG‐3' |
| | Reverse:
5'-ACCGGACCTTAGGGGGATTA‐3' |
| TACC1 | Forward:
5'-AGTGGGCGAAATGGACGTG‐3' |
| | Reverse:
5'-CCTTCAGAATCCGAGCTGAAAC‐3' |
| circTADA2A | Forward:
5'-CCCTCTGTTTGCATCTACCC‐3' |
| (divergent) | Reverse:
5'‐GCTGGGATCAAGGACAGGAA-3' |
| circTADA2A | Forward:
5'‐GGCTTTGGAAATTGGCAGGA-3' |
| (convergent) | Reverse:
w5'-TGAAATGGAATGGCTGTGTCA‐3' |
| Mimics |
UUAUAAUACAACCUGAUAAGUGCU (Sense) |
| (miR-374a) |
CACUUAUCAGGUUGUAUUAUAAUU (Antisense) |
| NC mimics |
UUGUACUACACAAAAGUACUGCU (Sense) |
| |
CAGUACUUUUGUGUAGUACAAUU (Antisense) |
| Inhibitors |
CACUUAUCAGGUUGUAUUAUAA (miR-374a) |
| NC inhibitors |
CAGUACUUUUGUGUAGUACAA |
| sh-NC |
CCGGGCGAACGATCGAGTAAACGGACTCGAGTCCGTTTACTCGATCGTTCGCTTTTT
(sense) |
| |
AATTCAAAAAGCGAACGATCGAGTAAACGGACTCGAGTCCGTTTACTCGATCGT
TCGC (antisense) |
| sh-circTADA2A |
CCGGCCATTTCACTACTTCAGATTTTCTCGAGAAAATCTGAAGTAGTGAAATGGT
TTTTG (sense) |
| |
AATTCAAAAACCATTTCACTACTTCAGATTTTCTCGAGAAAATCTGAAGTAGTGA
AATGG (antisense) |
Reverse transcription-quantitative
(RT-qPCR)
Total RNA was extracted from VSMCs with
TRIzol® reagent (Thermo Fisher Scientific, Inc.) on the
basis of manufacturer's instructions (90% density of cells used for
RNA extraction). Next, the isolated RNA was reverse-transcribed by
the cDNA Synthesis Kit (Takara Bio, Inc.) in accordance to the
manufacturer's instructions. PCR amplification was carried out
based on the protocols of SYBR Premix Ex Taq™ II (Takara
Bio, Inc.). U6 and GAPDH were employed as housekeeping genes of
miRNAs or mRNAs, and the results were assessed with
2-ΔΔCq method (1). The
conditions for PCR cycling were as follows: Activation of TaqMan at
95˚C for 10 min, and then 40 cycles of denaturation at 95˚C for 10
sec, and annealing/extension at 60˚C for 60 sec. The experiments
were repeated three times. The primers for RT-qPCR are in Table I.
Nucleic acid electrophoresis and
Sanger sequencing
PCR products were separated by electrophoresis on a
2% agarose gels, and gels were cut and sent to Shanghai Sangon
Bioengineering Co., Ltd., for sequencing following observation in a
UV imaging system.
RNase R and actinomycin D Assays
RNase and actinomycin D assays were used to
determine the RNA stability. For the RNase R assay, 2 µg RNA was
treated with 3 U/µg RNase R (BioVision, Inc.; Abcam) or
diethylpyrocarbonate-treated water (control) at 37˚C for 30 min.
Thereafter, RNA was extracted using an RNeasy MinElute Cleaning Kit
(Qiagen, Inc.) and subjected to RT-qPCR quantification as
aforementioned. For the actinomycin D assay, cells were treated
with 2 µg/ml actinomycin D (Shanghai Aladdin Biochemical Technology
Co., Ltd.) to halt RNA synthesis. The remaining RNA in the cells
was extracted and subjected to RT-qPCR quantification as
aforementioned.
Western blotting
Total protein of VSMCs was extracted using RIPA
lysis buffer (Beyotime Institute of Biotechnology) and the protein
concentrations were measured via a protein assay kit (Boster
Biological Technology) according to the manufacturer's protocol.
Proteins (20 µg) were separated on a 10% gel using SDS-PAGE and
then transferred to PVDF membrane (MilliporeSigma). After being
blocked using 5% skimmed milk in TBST (0.1% Tween) buffer at room
temperature for 1 h, the membrane was incubated with primary
antibodies (GAPDH; 1:5,000; cat. no. ab8245; Abcam; RORA; 1:1,000;
cat. no. ab256799; Abcam; GATA2; 1:2,000; cat. no. ab109241; Abcam)
overnight at 4˚C. After that, membranes were incubated using a
peroxidase-conjugated secondary antibody (cat. no. 58802; 1:1,000;
Cell Signaling Technology, Inc.) for 1 h at room temperature.
Finally, chemiluminescent detection was carried out with the ECL
system (MilliporeSigma) and analyzed using the ChemiDoc™
XRS Molecular Imager 3.0 system (Bio-Rad Laboratories, Inc.).
MTT assay
MTT assay was performed to assess cell proliferation
ability. The VSMCs were seeded in 96-well plates (2x103
cells/well). MTT solution (20 µl) was added to each well for 1, 2,
3, 4 and 5 days respectively. After incubation for 4 h at 37˚C, 100
µl of dimethyl sulfoxide was added into each well to dissolve the
precipitate. The absorbance was detected at 490 nm with a
microplate reader (Molecular Devices, LLC). The assay was conducted
in triplicate.
EdU cell proliferation assay
Cell proliferation was detected using the EdU assay
kit (Guangzhou RiboBio Co., Ltd.). Briefly, cells were seeded into
96-well plates at a density of 1x104 cells/well. The OS
cells were then treated with culture medium containing 50 µM EdU
reagent at 37˚C for 2 h, and fixed with 4% formaldehyde for 30 min
at room temperature. The nuclei were stained with Hoechst 33342 (10
µg/ml; cat. no. HY-15559; MedChemExpress). Finally, the results
were photographed using a fluorescence microscope (Nikon
Corporation), and the number of EdU-positive cells were quantified
and analyzed.
Transwell assay
The capacity of cell migration was evaluated by
Transwell assay. The cell suspension with serum-free media was
added to an upper chamber, whereas the full media containing 30%
FBS were seeded to the lower chamber. Following that, cells that
transferred to the lower chamber were fixed using 4%
paraformaldehyde for 20 min at room temperature and stained
applying 0.1% crystal violet for 15 min at room temperature and
counted using a light microscope (Olympus Corporation; x200
magnification). A total of five fields of view were randomly
selected for the images.
TUNEL staining assay
Apoptosis in VSMCs was detected using the One Step
TUNEL Apoptosis Assay Kit (Nanjing KeyGen Biotech Co., Ltd.)
according to the manufacturer's protocol. Briefly, cells were fixed
by 4% paraformaldehyde for 10 min at room temperature and
permeabilized by 0.25% Triton-X 100 for 30 min at room temperature.
Cells were then subjected to TUNEL staining reagents, and nuclei
were counterstained with DAPI for 30 min at room temperature.
Images of TUNEL and DAPI-positive cells were obtained using a
fluorescence microscope (BX63; Olympus Corporation).
Luciferase reporter assay
The specific binding linkage between miR-374a and
RORA 3'UTR, PMIR-REPORT luciferase vector (Guangzhou RiboBio Co.,
Ltd.) with wild-type-RORA-3'UTR (Wt-RORA-3'UTR) or mutant
(Mut)-RORA-3'UTR, miR-374a mimic or miR-374a inhibitors or miR-NC
(Guangzhou RiboBio Co., Ltd.) was transfected into VSMCs,
respectively, co-incubated with Lipofectamine® 3000
(Invitrogen; Thermo Fisher Scientific, Inc.) for 48 h at 37˚C. The
measurement of the luciferase activity was conducted with
Dual-Luciferase Reporter Assay (Promega Corporation) after 24 h.
All experiments were conducted in triplicate. Renilla
luciferase activity was used for normalization. The sequences of
the miRNA mimic/inhibitor and miR-NCs are shown in Table I.
Chromatin immunoprecipitation (ChIP)
assay
ChIP assay was performed in VSMCs cells (90% density
in a 15-cm Petri dish) with a ChIP assay kit (MilliporeSigma) based
on the manufacturer's instructions. The amount of lysate per
reaction was 100 µl, with 10 µl (1%) supernatant removed as the
input, as previously described (18). Immunoprecipitation was conducted
using the anti-GATA2 antibody (dilution, 1:100; cat. no. ab109241;
Abcam) or the control IgG at 4˚C overnight. The precipitates were
washed with low-salt wash buffer, high-salt wash buffer and LiCI
wash buffer, and rinsed with TE buffer twice. The primer for qPCR
is in Table I. The qPCR was
performed as aforementioned.
Statistical analysis
Analysis of results was conducted using GraphPad
Prism 5 (Dotmatics). All data was shown as mean ± SD. The
significance of differences between two groups was evaluated via
Student's unpaired t-test, and the comparison among multiple groups
was conducted via one-way ANOVA followed by Tukey's post hoc test.
P<0.05 was considered to indicate a statistically significant
difference.
Results
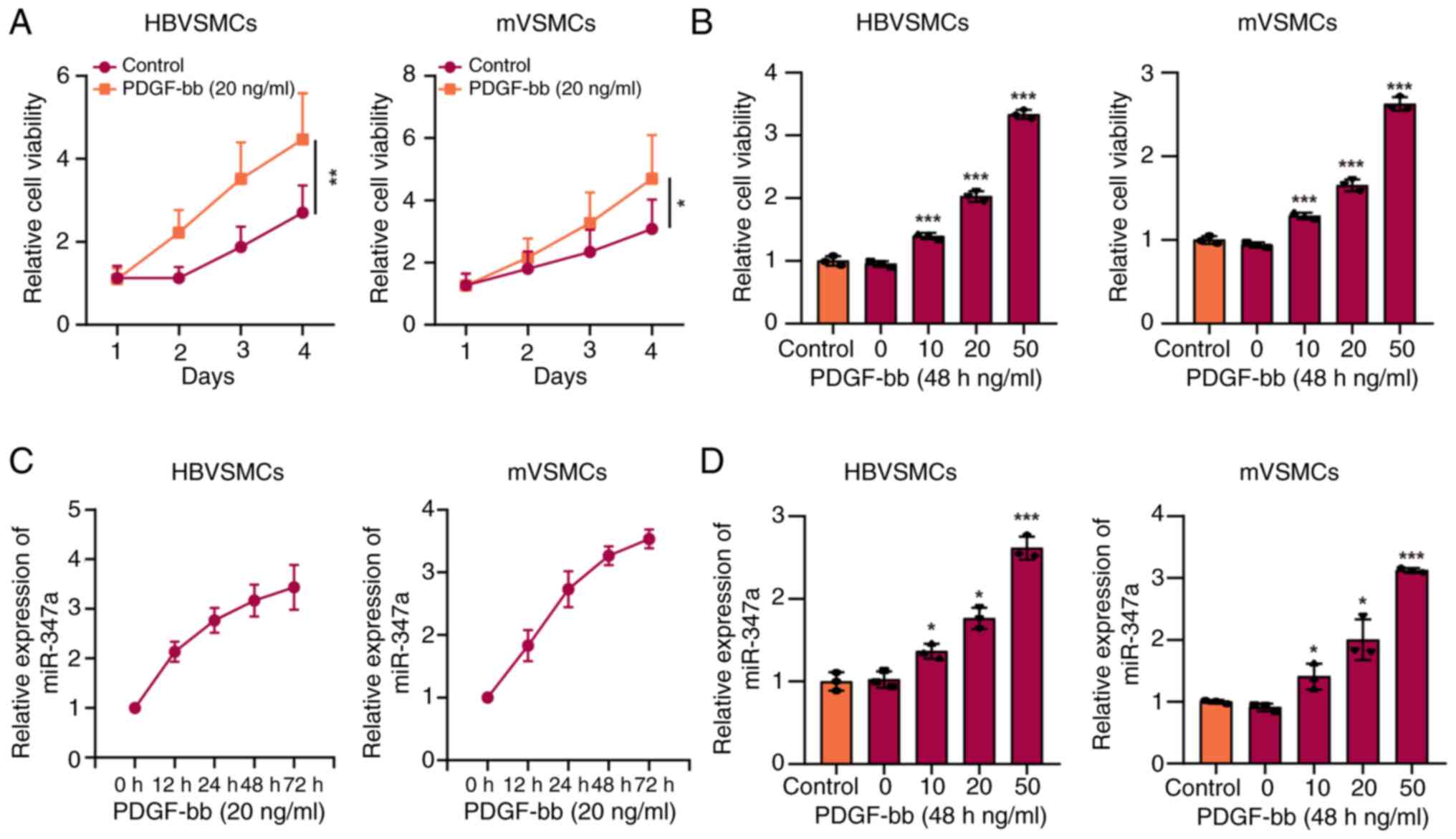
miR-374a is elevated in
PDGF-bb-induced VSMCs
MTT assay was applied to assess whether PDGF-bb
could promote the proliferation viability of VSMCs and RT-qPCR
assay was used to detect the expression level of miR-374a. The
result of VSMCs and mVSMCs viability exhibited a significant
increase following treatment using 20 ng/ml PDGF-bb in comparison
to that in control group and the cell viability was clearly
promoted over time (Fig. 1A). As
demonstrated in Fig. 1B, the
augment of VSMCs viability induced by various concentrations of
PDGF-bb also showed concentration-dependence. The expression of
miR-374a was significantly increased in VSMCs treated with 20 ng/ml
PDGF-bb in a time-dependence manner compared with control group
(Fig. 1C). Additionally, PDGF-bb
promoted the level of miR-374a in a concentration-dependent method
(Fig. 1D).
Upregulation of miR-374a promotes
proliferation, migration of VSMCs in vitro
To investigate the function of miR-374a in the
progression of VSMCs, HBVSMCs and mVSMCs were transfected with
miR-374a mimic or miR-374a inhibitor and the level of miR-374a was
overexpression following transfection using miR-374a mimic was
detected. Meanwhile, miR-374a was downregulated following
transfection with miR-374a inhibitor (Fig. 2A). As expected, upregulation of
miR-374a could clearly facilitate the proliferation, migration of
VSMCs compared with that in control groups, while, downregulation
of miR-374a had a reverse effect (Fig.
2B-D). Furthermore, the result showed that high miR-374a
expression reduced the number of TUNEL-positive cells in HBVSMCs
and mVSMCs, whereas, miR-374a inhibitor produced a reverse result
(Fig. 2E), indicating that
miR-374a demonstrated a promoting role in cell proliferation and
metastasis of VSMCs.
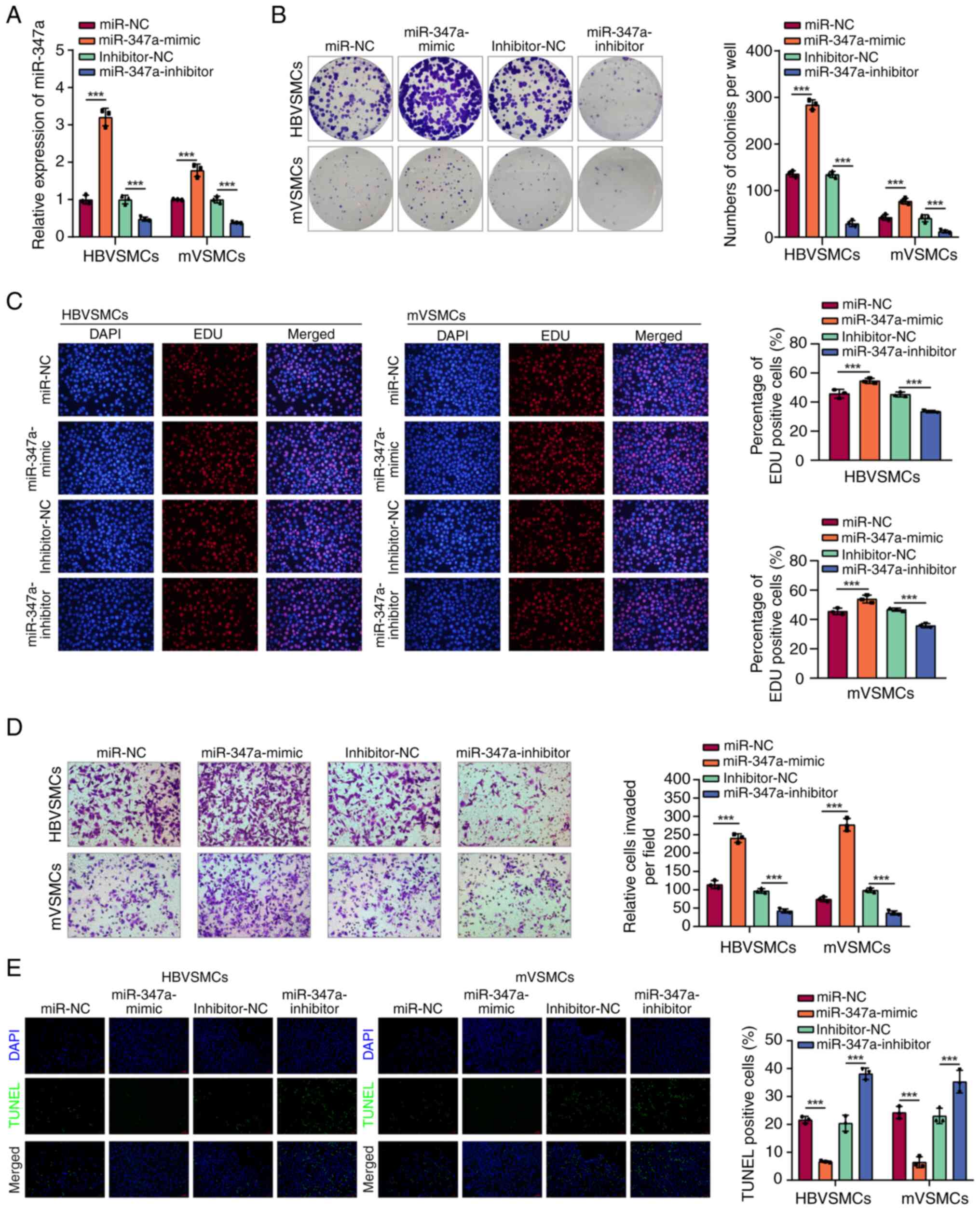 | Figure 2miR-374a overexpression promoted
proliferation, migration of VSMCs in vitro. (A) RT-qPCR
assay was adopted to analyze miR-374a expression in HBVSMCs and
mVSMCs after treatment with miR-374a mimics, inhibitor and
scramble. (B) The proliferation ability of HBVSMCs and mVSMCs were
evaluated via MTT assay in miR-374a mimics, inhibitor, scramble and
control group. (C) EdU incorporation assays. (D) The Transwell
assay was employed to detect migration of HBVSMCs and mVSMCs in
miR-374a mimics, inhibitor, scramble and control group. (E) The
TUNEL staining assay indicated the apoptotic rate of HBVSMCs and
mVSMCs. Magnification, x20. ***P<0.001. miR, micro
RNA; VSMCs, vascular smooth muscle cells; RT-qPCR, reverse
transcription-quantitative PCR; HB, human brain; m, mouse. |
miR-374a is a direct target of
circTADA2A in VSMCs cells
Previous studies have reported that miR-374a can
sponge hsa_circ_0043278(19) and
it was hypothesized whether miR-374a also adsorbed hsa_circ_0043278
in VSMCs. A schematic diagram illustrated the formation of
hsa_circ_0043278 derived from the TADA2A gene exon 5 and 6
(Fig. 3A). The circular properties
of hsa_circ_0043278 (named circTADA2A) was identified with
divergent primers and convergent primers, respectively (Fig. 3B). Moreover, their head-to-tail
splicing structure was confirmed by Sanger sequencing (Fig. 3C). Under RNase R treatment, the
linear form of TADA2A was significantly reduced compared with mock,
while circTADA2A was significantly resistant to RNase R in HBVSMCs
and mVSMCs (Fig. 3D and E). The expression of circTADA2A was
significantly reduced in HBVSMCs and mVSMCs treated with various
concentrations of PDGF-bb, which also showed
concentration-dependence manner compared with control group
(Fig. 3F and G). The RT-qPCR results showed the
miR-374a mimics significantly reduced the expression of circTADA2A,
and miR-374a inhibitor increased the expression of circTADA2A
(Fig. 3H). Thus, these data
suggested that miR-374a might be the target of circTADA2A, and the
potential binding site between circTADA2A and miR-374a is shown in
Fig. 3I. Accordingly, the
luciferase reporter assays demonstrated that the relative
luciferase activity of circTADA2A wild type (WT) and miR-374a mimic
group was significantly lower than that of other groups (Fig. 3J and K).
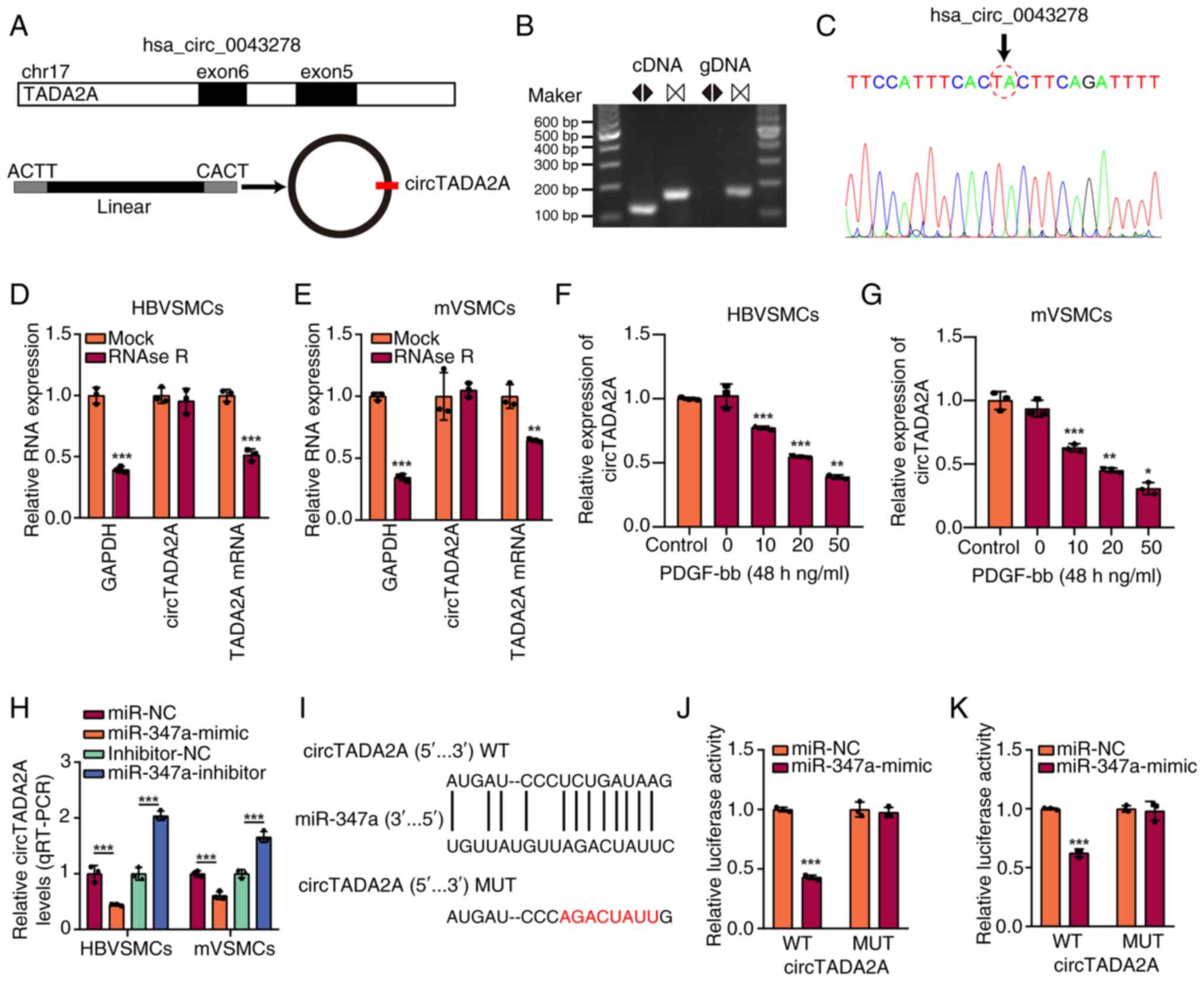 | Figure 3miR-374a sponged circTADA2A in VSMCs
cells. (A) Schematic diagram illustrated the formation of
hsa_circ_0043278 originated from TADA2A pre-mRNA. (B) RT-qPCR
products using divergent primers indicating circularization of
hsa_circ_0043278. cDNA represents complementary DNA. gDNA
represents genomic DNA. (C) Sanger sequencing illustrated the joint
site of circTADA2A. RT-qPCR showed the expression of circTADA2A and
TADA2A mRNA in (D) HBVSMCs and (E) mVSMCs administered with RNase R
or Mock control. PDGF-bb caused a dose-dependent upregulation in
circTADA2A expression levels in (F) HBVSMCs and (G) mVSMCs after
treatment for 48 h, as indicated via RT-qPCR assay. (H) RT-qPCR
analysis of circTADA2A expression levels with miR-374a
overexpression and miR-374a knockdown in HBVSMCs and mVSMCs. (I)
The binding sites between circTADA2A and miR-374a, and the mutant
sequence of circTADA2A based on binding region. (J and K) miR-374a
mimics suppressed the luciferase activity of circTADA2A wild-type
vector in HBVSMCs and mVSMCs. Magnification, x20.
*P<0.05, **P<0.01 and
***P<0.001. miR, micro RNA; circ, circular RNA;
VSMCs, vascular smooth muscle cells; RT-qPCR, reverse
transcription-quantitative PCR; HB, human brain; m, mouse; PDGF-bb,
platelet-derived growth factor-BB; WT, wild type; MUT, mutant. |
It was subsequently investigated whether circTADA2A
plays an inhibitory role in the progression of VSMCs by sponging
miR-374a. First, the circTADA2A overexpression vectors and
sh-circTADA2A were transfected into HBVSMCs and mVSMCs to
upregulate or downregulate circTADA2A expression. The efficiency of
transfection was confirmed by RT-qPCR (Fig. 4A). The HBVSMCs and mVSMCs were
co-transfected with miR-374a mimics and circTADA2A overexpression
vectors. As expected, circTADA2A overexpression significantly
blocked the promoting effect of miR-374a mimics on cell
proliferation and migration ability in HBVSMCs and mVSMCs (Fig. 4B-D). Taken together, these data
indicated that circTADA2A acts as a miRNA sponge for miR-374a in
VSMCs.
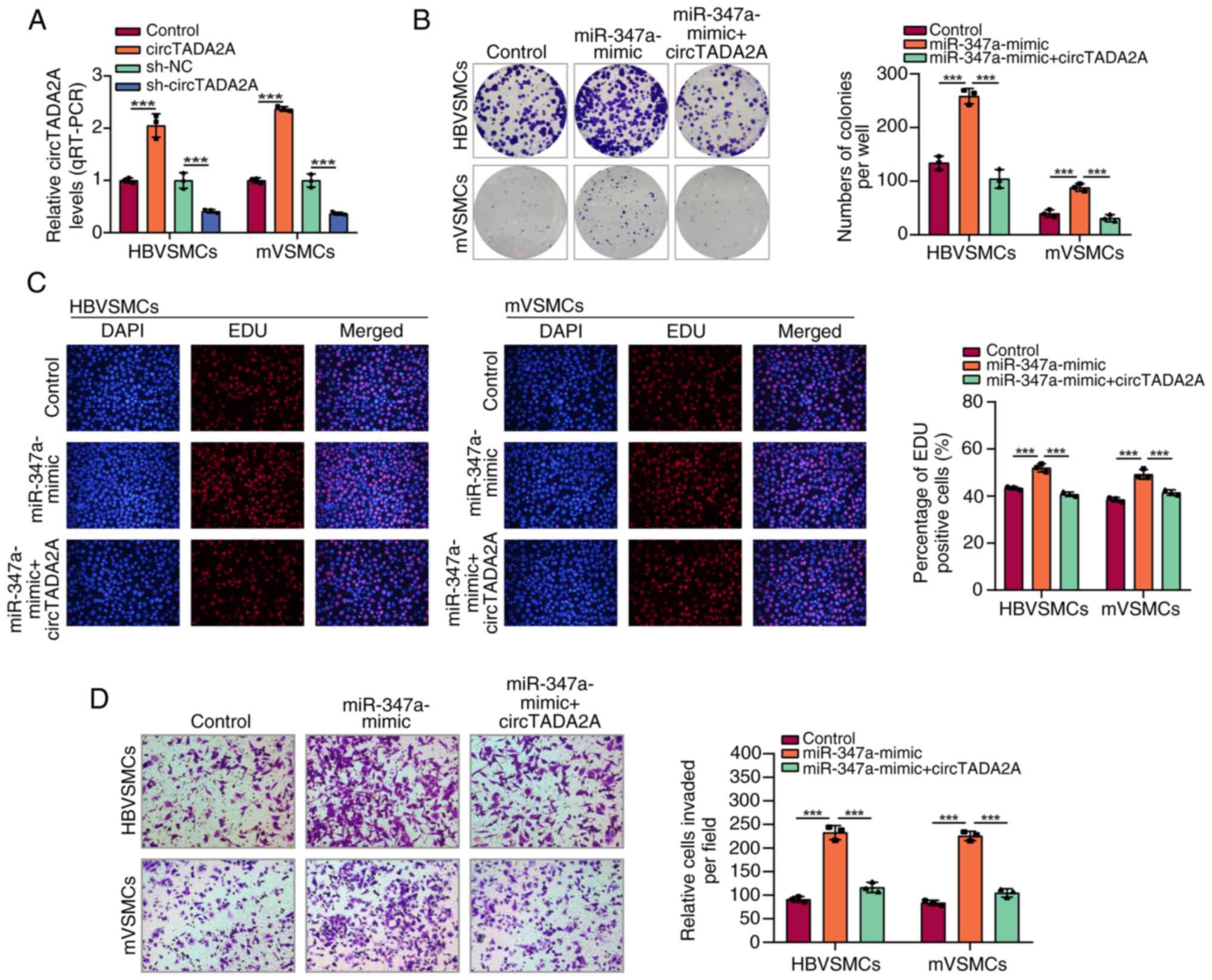 | Figure 4CircTADA2A reverses the promoting
effect of miR-374a on VSMCs. (A) CircTADA2A expression in HBVSMCs
and mVSMCs with circTADA2A overexpression vectors or sh-circTADA2A.
HBVSMCs and mVSMCs were transfected with miR-control, miR-374a
mimics, or miR-374a mimics + circTADA2A. Then the ability of cell
proliferation was, respectively, assessed by (B) colony formation
and (C) EdU incorporation assays. (D) HBVSMCs and mVSMCs
transfected with miR-control, miR-374a mimics, or miR-374a mimics +
circTADA2A. Then the TUNEL staining assay indicated the apoptotic
rate of HBVSMCs and mVSMCs. Magnification, x20.
***P<0.001. circ, circular RNA; miR, micro RNA;
VSMCs, vascular smooth muscle cells; m, mouse; HB, human brain; sh,
short hairpin. |
RORA is specifically regulated by
miR-374a in VSMCs
Next, the present study explored how miR-374a
exerted its roles in VSMCs. A total of nine common target genes
were revealed on the basis of overlapping analysis of DEGs among
miRWalk predicted, miRWalk validated and StarBase sets according to
the miRWalk website (Fig. 5A). It
was found that the expression level of RORA in HBVSMCs was
significantly reduced in comparison to that in control groups
(Fig. 5B). Furthermore, the result
of western blotting in Fig. 5C
showed a similar result. Afterwards, StarBase database was used to
predict the potential binding site of miR-374a to RORA (Fig. 5D). Luciferase reporter assay was
adopted to further verify the association between miR-374a and
RORA. Luciferase activity results indicated that the luciferase
activity of miR-374a mimic and RORA wildtype co-transfected VSMCs
was apparently reduced, whereas no obvious change was found in the
luciferase intensity in mutant RORA (Fig. 5E). The results suggested that RORA
expression was negatively regulated by miR-374a. Subsequently, it
was discovered that miR-374a upregulation clearly augmented the
proliferation and metastasis of VSMCs, which could be apparently
reversed by RORA increase. In addition, it was also found that
circTADA2A knockdown reversed the effects of RORA overexpression on
VSMCs proliferation and metastasis (Fig. 5F and G). Additionally, it was observed that
high miR-374a expression could reduce the number of TUNEL-positive
cells in VSMCs, and the effect was significantly rescued by RORA
overexpression, while downregulated circTADA2A expression
antagonized the effect of the RORA overexpression (Fig. 5H). These results indicated that
circTADA2A regulated the proliferation and metastasis of VSMCs via
miR-374a/RORA axis.
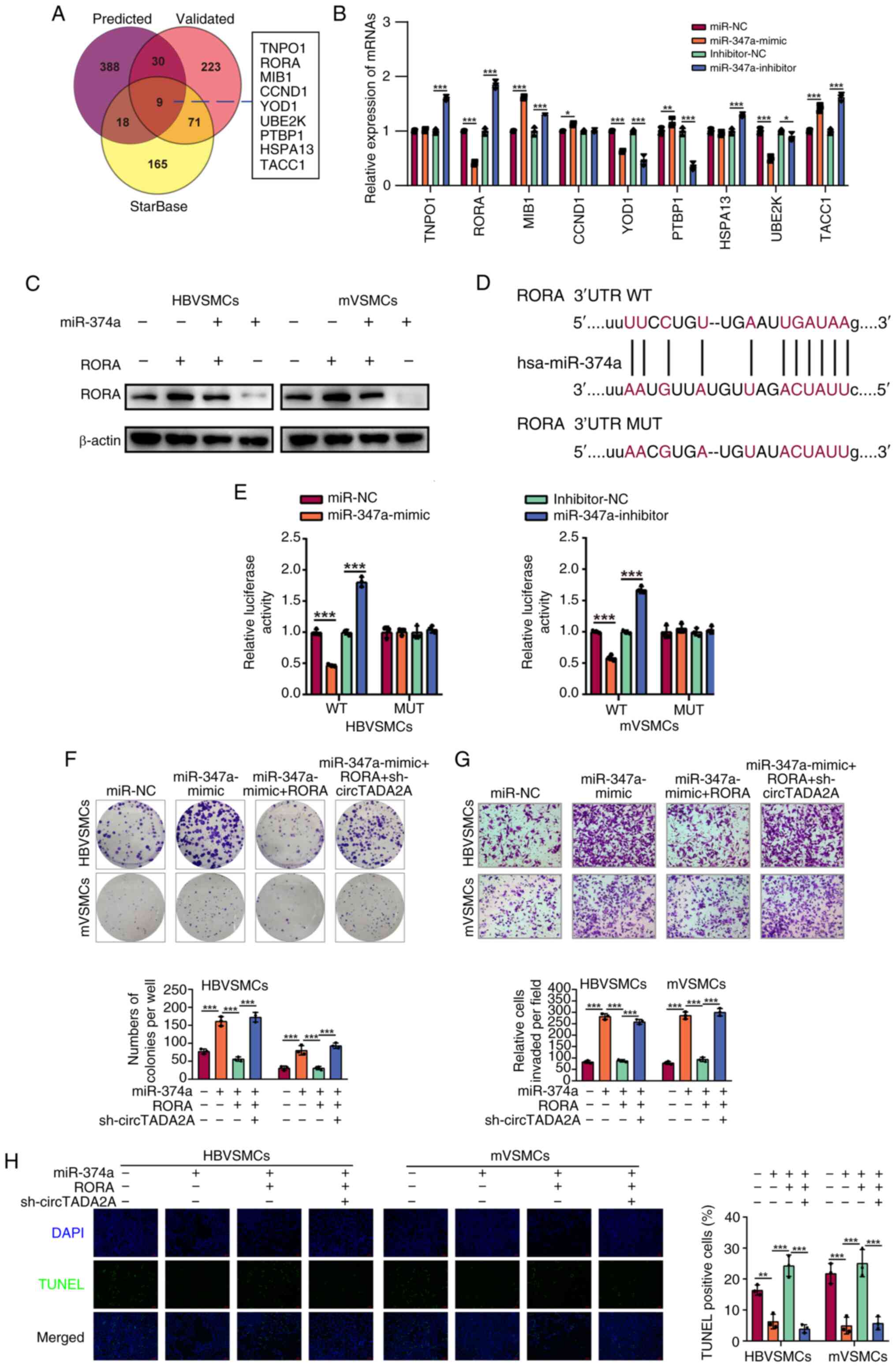 | Figure 5RORA is a downstream target of
miR-374a and negatively regulated by miR-374a in VSMCs. (A) Venn
diagram was conducted for miRWalk predicted, miRWalk validated and
StarBase sets. (B) RT-qPCR analysis was used to test the determined
nine intersected targets of miR-374a in HBVSMCs. (C) Western
blotting was used to measure the protein level of RORA in VSMCs
following treatment using miR-374a mimics, inhibitor, scramble or
control, respectively. (D) The putative binding site and the mutant
sites of miR-374a on the 3'-UTR of RORA mRNA were marked in purple.
(E) VSMCs co-transfection using RORA-WT or RORA-MUT and miR-374a
mimics or miR-NC mimics, at 48 h after transfection, luciferase
activities were measured. Each experiment was conducted in
triplicate. (F) HBVSMCs and mVSMCs transfected with miR-control,
miR-374a mimics, miR-374a mimics + RORA or miR-374a mimics + RORA +
sh-circTADA2A. The ability of cell proliferation was assessed by
colony formation assays. (G) HBVSMCs and mVSMCs transfected with
miR-control, miR-374a mimics, miR-374a mimics + RORA or miR-374a
mimics + RORA + sh-circTADA2A. The ability of cell migration was
assessed by Transwell migration assay. (H) HBVSMCs and mVSMCs
transfected with miR-control, miR-374a mimics, miR-374a mimics +
RORA or miR-374a mimics + RORA + sh-circTADA2A. The TUNEL staining
assay indicated the apoptotic rate of HBVSMCs and mVSMCs.
Magnification, x20. *P<0.05, **P<0.01
and ***P<0.001. RORA, RAR-related orphan receptor A;
miR, micro RNA; VSMCs, vascular smooth muscle cells; RT-qPCR,
reverse transcription-quantitative PCR; WT, wild type; MUT, mutant;
m, mouse; HB, human brain; sh, short hairpin; circ, circular
RNA. |
GATA2 transcriptionally promotes
miR-374a expression
To clarify the upstream regulatory molecule of
miR-374a, JASPAR software was used to predict transcription factors
(TFs) that modulated miR-374a promoters. RT-qPCR assay was
performed to measure the expression level of the top five potential
TFs that regulated miR-374a. The results of RT-qPCR indicated that
the expression level of GATA2 was much higher in HBVSMCs and mVSMCs
than in controls (Fig. 6A). In
addition, RORA expression was clearly decreased with GATA2
overexpression in HBVSMCs and mVSMCs (Fig. 6B). As illustrated in Fig. 6C, the result indicated that GATA2
could facilitate miR-374a expression. It was also observed that
high GATA2 expression could augment the proliferation, migration of
VSMCs, which could be significantly rescued by miR-374a
downregulation (P<0.05; Fig.
6D-F).
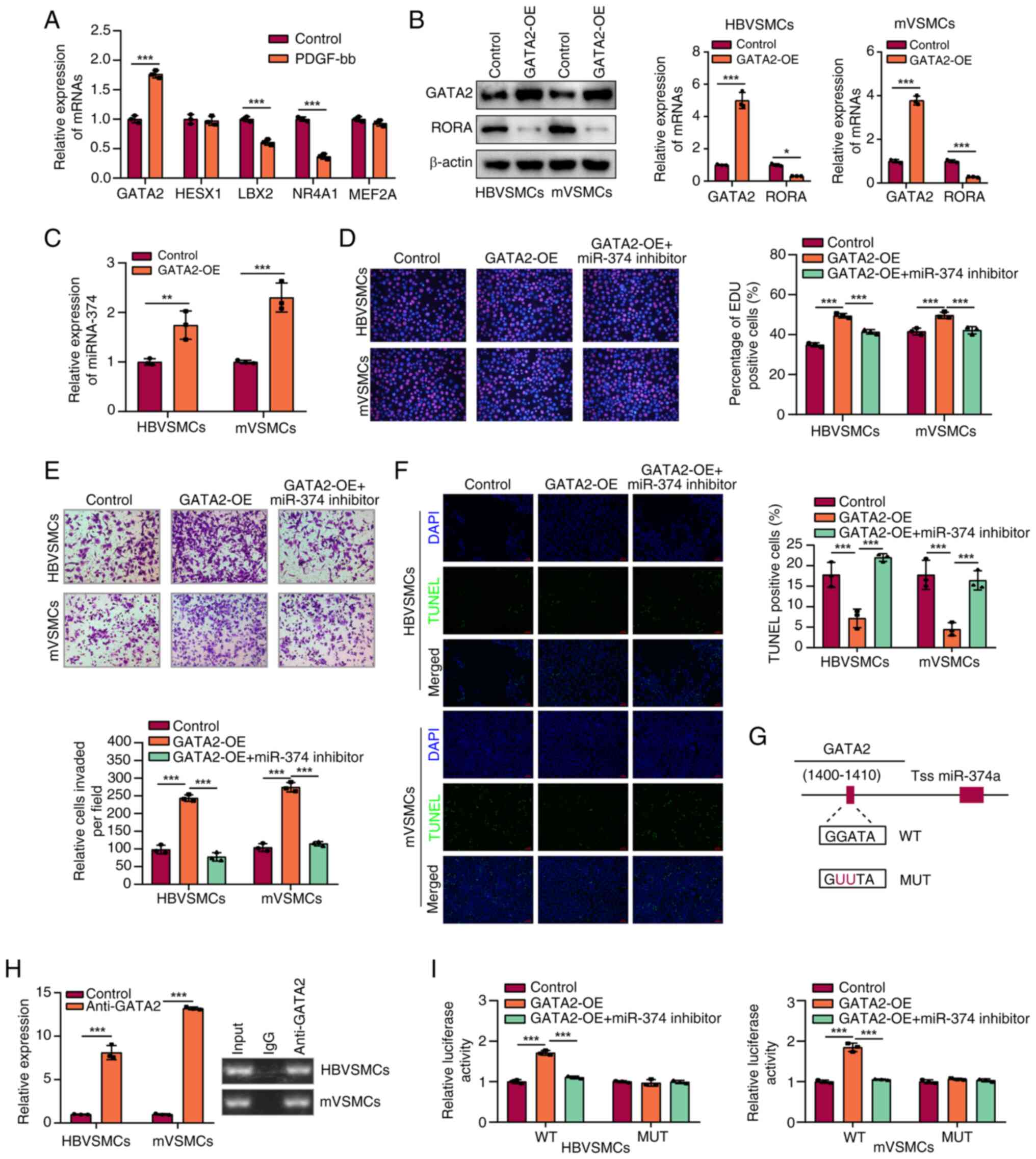 | Figure 6GATA2 transcriptionally promoted
miR-374a expression. (A) Analysis of the top five TFs expression
mRNA expression levels in HBVSMCs by RT-qPCR. (B) The protein level
of RORA and GATA2 were assessed by western blotting in VSMCs
transfected with GATA2-OE vector. (C) The role of high GATA2
expression in miR-374a level in VSMCs was assessed through RT-qPCR
assay. (D) The role of GATA2 and miR-374a in proliferation of VSMCs
by MTT analysis. (E) Influence of GATA2 and miR-374a on the
migration of VSMCs was analyzed by Transwell assay. (F) The TUNEL
staining assay indicated the apoptotic rate of HBVSMCs and mVSMCs.
(G) A schematic for the proximal region of the miR-374a promoter on
the basis of ChIP assay. (H) The binding relationship between GATA2
and the miR-374a promoter in VSMCs was explored by ChIP assay. (I)
The luciferase activity in the WT miR-374a promoter was tested in
VSMCs with GATA2 overexpression which was rescued with transfection
with miR-374a inhibitor; no alteration was observed in luciferase
activity with mutation of GATA2 binding site at -1,400 to -1,410 bp
in the pre-miR-374a promoter region upstream.
*P<0.05, **P<0.01 and
***P<0.001. GATA2, GATA binding protein 2; miR, micro
RNA; TF, transcription factor; VSMCs, vascular smooth muscle cells;
RT-qPCR, reverse transcription-quantitative PCR; RORA, RAR-related
orphan receptor A; OE, overexpression; ChIP, Chromatin
Immunoprecipitation. |
ChIP assay was conducted to further explore the
direct association of GATA2 with the promoter of miR-374a. Binding
sites of GATA2 inside the assumed miR-374a promoter region were
predicted according to the JASPAR database. ChIP assays further
confirmed that GATA2 binds to the ARE site in promoter miR-374a. As
illustrated in Fig. 6G, miR-374a
promoter activity was upregulated with the elevation of GATA2
binding to the miR-374a promoter region. Additionally, ChIP assay
confirmed the direct binding of GATA2 to the miR-374a promoter
(Fig. 6H and I). These results indicated that GATA2
regulated miR-374a expression via binding to its promoter in
HBVSMCs and mVSMCs.
Discussion
A number of studies have shown that miRNAs played
significant roles in facilitating or suppressing the proliferation
and motion of VSMCs. For instance, Li et al (20) suggested that lncRNA TUG1 promoted
proliferation of VSMC and atherosclerosis by regulating
miRNA-21/PTEN axis. Sun et al (21) indicated that miRNA 146b-5p
protected against atherosclerosis by inhibiting VSMC proliferation
and migration. Afzal et al (22) demonstrated that NCK associated
protein 1 modulated by miRNA-214 determined VSMC migration,
proliferation and neointima hyperplasia. Evidence indicates that
miR-374a was tightly related to the development of various types of
carcinomas. For instance, Son et al (23) reported that miR-374a-5p promoted
tumor progression via targeting ARRB1 in triple negative breast
cancer. Li et al (24)
found that miR-374a activated Wnt/β-catenin signaling to promote
osteosarcoma cell migration by targeting WIF-1. Xu et al
(25) indicated that miR-374a
promoted cell proliferation, migration through targeting SRCIN1 in
gastric cancer. However, the effect of miR-374a and its molecular
mechanism in AS remains to be elucidated.
The present study confirmed that miR-374a was indeed
upregulated in PDGF-bb-induced VSMCs. Also, overexpression of
miR-374a via transfection could promote the proliferation and
migration of HBVSMCs and mVSMCs, whereas miR-374a inhibitors showed
the opposite effect, which demonstrated that high-expression of
miR-374a could promote AS development. Moreover, circTADA2A was
found to sponge miR-374a and RORA could serve as a direct target of
miR-374a on the basis of miRWalk and dual-luciferase assay.
Finally, mechanism studies showed that circTADA2A may suppress
proliferation and migration of HBVSMCs and mVSMCs via sponging
miR-374a, and then upregulating RORA expression. Huang et al
(26) reported that melatonin and
its nuclear receptor retinoid-related orphan receptor α (RORα) had
some protective effects on the progression of atherosclerosis,
which was consistent with the present research.
Previous studies reported that TFs were closely
associated with AS. For example, Erbilgin et al (27) found that TF ZHX2 deficiency could
decrease AS and facilitate macrophage apoptosis in mice. Qin et
al (28) discovered that
activating TF 3 was a potential target and a new biomarker for the
prognosis of AS. Nakagawa et al (29) reported that enterohepatic TF
CREB3L3 protected AS by SREBP competitive inhibition. The present
study used bioinformatics method to predict TFs regulating
promoters of miR-374a and found that GATA2 may serve as a direct
upstream regulatory molecule of miR-374a, which was further
confirmed based on ChIP assay and luciferase reporter assay.
VSMCs serve as highly specialized contractile cells
with a crucial function in maintaining normal vascular structure
and blood pressure (30).
AS could induce vascular wall remodeling process,
which results in VSMC dedifferentiation or phenotype switching from
contractile phenotype to synthetic phenotype (31). The alteration of the markers for
contractile phenotype and synthetic phenotype in VSMCs needs to be
analyzed in the future.
Apart from inspiring outcomes, limitations still
existed in the present study. First, although the role of the
GATA2/circTADA2A-miR-374a-RORA axis in the proliferation of VSMCs
was clear, the pathway through which this axis functions remains to
be elucidated. Second, the present study lacked the support of
clinical samples and animal experiments and needs to be further
verified.
In spite of the aforementioned limitations, there
are still numerous valuable implications in the present study. The
findings showed a novel function of miR-374a in promotion of
PDGF-bb-induced VSMC proliferation and migration by inhibition of
RORA. Moreover, circTADA2A bound to miR-374a and decreased the
expression of miR-374a, which resulted in inhibition of cell growth
and migration. In addition, the present study verified that GATA2
was a direct upstream regulatory molecule of miR-374a. Thus, it was
suggested that GATA2/circTADA2A-miR-374a-RORA axis might be
therapeutic targets in VSMCs-associated disease. Further
large-scale, well-designed studies should be conducted to confirm
these findings before the application of
GATA2/circTADA2A-miR-374a-RORA axis for targeted therapy of AS.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Author contributions
WT, YD and MW were the administrators of the present
study. WT, MF, MW and YD performed data curation. WT, MF, YD and QZ
implemented formal analysis. YW, DG and QZ conducted the
investigation. DG and JL were in charge of methodology. WT, MF and
YD confirm the authenticity of all the raw data. WT, MF and YD
wrote the original daft. YD and MW wrote, reviewed and edited the
manuscript. All authors read and approved the final version of the
manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Robinson JG and Davidson MH: Can we cure
atherosclerosis? Rev Cardiovasc Med. 19 (S1):S20–S24.
2018.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Libby P: Inflammation in atherosclerosis.
Nature. 420:868–874. 2002.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Choromańska B, Myśliwiec P, Choromańska K,
Dadan J and Chabowski A: The role of CD36 receptor in the
pathogenesis of atherosclerosis. Adv Clin Exp Med. 26:717–722.
2017.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Kuliczkowska-Płaksej J,
Bednarek-Tupikowska G, Płaksej R and Filus A: Scavenger receptor
CD36: Its expression, regulation, and role in the pathogenesis of
atherosclerosis. Part I. Postepy Hig Med Dosw (Online). 60:142–151.
2006.PubMed/NCBI
|
|
5
|
Millen AE, Nie J, Sahli MW, Mares JA,
Meyers KJ, Klein BEK, LaMonte MJ, Lutsey PL, Andrews CA and Klein
R: Vitamin D status and prevalent early age-related macular
degeneration in african americans and caucasians: The
atherosclerosis risk in communities (ARIC) study. J Nutr Health
Aging. 21:772–780. 2017.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Escárcega RO, Garcia-Carrasco M, Jara LJ
and Cervera R: Accelerated atherosclerosis in systemic lupus
erythematosus: Perspectives towards decreasing cardiovascular
morbidity and mortality. Lupus. 18:383–386. 2009.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Doran AC, Meller N and McNamara CA: Role
of smooth muscle cells in the initiation and early progression of
atherosclerosis. Arterioscler Thromb Vasc Biol. 28:812–819.
2008.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Wang P, Xu TY, Guan YF, Zhao Y, Li ZY, Lan
XH, Wang X, Yang PY, Kang ZM, Vanhoutte PM and Miao CY: Vascular
smooth muscle cell apoptosis is an early trigger for hypothyroid
atherosclerosis. Cardiovasc Res. 102:448–459. 2014.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Bartel DP: Metazoan MicroRNAs. Cell.
173:20–51. 2018.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Farina FM, Hall IF, Serio S, Zani S,
Climent M, Salvarani N, Carullo P, Civilini E, Condorelli G, Elia L
and Quintavalle M: miR-128-3p is a novel regulator of vascular
smooth muscle cell phenotypic switch and vascular diseases. Circ
Res. 126:e120–e135. 2020.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Wang Y, Zhang CX, Ge SL and Gong WH:
CTBP1-AS2 inhibits proliferation and induces autophagy in
ox-LDL-stimulated vascular smooth muscle cells by regulating
miR-195-5p/ATG14. Int J Mol Med. 46:839–848. 2020.PubMed/NCBI View Article : Google Scholar
|
|
12
|
You L, Chen H, Xu L and Li X:
Overexpression of miR-29a-3p suppresses proliferation, migration,
and invasion of vascular smooth muscle cells in atherosclerosis via
targeting TNFRSF1A. Biomed Res Int. 2020(9627974)2020.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Jing R, Pan W, Long T, Li Z and Li C:
LINC00472 regulates vascular smooth muscle cell migration and
proliferation via regulating miR-149-3p. Environ Sci Pollut Res
Int. 28:12960–12967. 2021.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Hao T, Huang S and Han F: LINC-PINT
suppresses tumour cell proliferation, migration and invasion
through targeting miR-374a-5p in ovarian cancer. Cell Biochem
Funct. 38:1089–1099. 2020.PubMed/NCBI View
Article : Google Scholar
|
|
15
|
Lu T, Zhang C, Chai MX, An YB and Jia JL:
MiR-374a promotes the proliferation of osteosarcoma cell
proliferation by targeting Axin2. Int J Clin Exp Pathol.
8:10776–10783. 2015.PubMed/NCBI
|
|
16
|
Ma L, Shao Z and Zhao Y: MicroRNA-374a
promotes pancreatic cancer cell proliferation and epithelial to
mesenchymal transition by targeting SRCIN1. Pathol Res Pract.
215(152382)2019.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Ruan C, Lu J, Wang H, Ge Z, Zhang C and Xu
M: miR-26b-5p regulates hypoxia-induced phenotypic switching of
vascular smooth muscle cells via the TGF-β/Smad4 signaling pathway.
Mol Med Rep. 15:4185–4190. 2017.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Chen H, Xu L, Shan ZL, Chen S and Hu H:
GPX8 is transcriptionally regulated by FOXC1 and promotes the
growth of gastric cancer cells through activating the Wnt signaling
pathway. Cancer Cell Int. 20(596)2020.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Li Z, Yao H, Wang S, Li G and Gu X:
CircTADA2A suppresses the progression of colorectal cancer via
miR-374a-3p/KLF14 axis. J Exp Clin Cancer Res.
39(160)2020.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Li FP, Lin DQ and Gao LY: LncRNA TUG1
promotes proliferation of vascular smooth muscle cell and
atherosclerosis through regulating miRNA-21/PTEN axis. Eur Rev Med
Pharmacol Sci. 22:7439–7447. 2018.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Sun D, Xiang G, Wang J, Li Y, Mei S, Ding
H and Yan J: miRNA 146b-5p protects against atherosclerosis by
inhibiting vascular smooth muscle cell proliferation and migration.
Epigenomics. 12:2189–2204. 2020.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Afzal TA, Luong LA, Chen D, Zhang C, Yang
F, Chen Q, An W, Wilkes E, Yashiro K, Cutillas PR, et al: NCK
associated protein 1 modulated by miRNA-214 determines vascular
smooth muscle cell migration, proliferation, and neointima
hyperplasia. J Am Heart Assoc. 5(e004629)2016.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Son D, Kim Y, Lim S, Kang HG, Kim DH, Park
JW, Cheong W, Kong HK, Han W, Park WY, et al: miR-374a-5p promotes
tumor progression by targeting ARRB1 in triple negative breast
cancer. Cancer Lett. 454:224–233. 2019.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Li W, Meng Z, Zou T, Wang G, Su Y, Yao S
and Sun X: MiR-374a activates Wnt/β-catenin signaling to promote
osteosarcoma cell migration by targeting WIF-1. Pathol Oncol Res.
26:533–539. 2020.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Xu X, Wang W, Su N, Zhu X, Yao J, Gao W,
Hu Z and Sun Y: miR-374a promotes cell proliferation, migration and
invasion by targeting SRCIN1 in gastric cancer. FEBS Lett.
589:407–413. 2015.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Huang H, Liu X, Chen D, Lu Y, Li J, Du F,
Zhang C and Lu L: Melatonin prevents endothelial dysfunction in SLE
by activating the nuclear receptor retinoic acid-related orphan
receptor-α. Int Immunopharmacol. 83(106365)2020.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Erbilgin A, Seldin MM, Wu X, Mehrabian M,
Zhou Z, Qi H, Dabirian KS, Sevag Packard RR, Hsieh W, Bensinger SJ,
et al: Transcription factor Zhx2 deficiency reduces atherosclerosis
and promotes macrophage apoptosis in mice. Arterioscler Thromb Vasc
Biol. 38:2016–2027. 2018.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Qin W, Yang H, Liu G, Bai R, Bian Y, Yang
Z and Xiao C: Activating transcription factor 3 is a potential
target and a new biomarker for the prognosis of atherosclerosis.
Hum Cell. 34:49–59. 2021.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Nakagawa Y, Wang Y, Han SI, Okuda K, Oishi
A, Yagishita Y, Kumagai K, Ohno H, Osaki Y, Mizunoe Y, et al:
Enterohepatic transcription factor CREB3L3 protects atherosclerosis
via SREBP competitive inhibition. Cell Mol Gastroenterol Hepatol.
11:949–971. 2021.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Owens GK, Kumar MS and Wamhoff BR:
Molecular regulation of vascular smooth muscle cell differentiation
in development and disease. Physiol Rev. 84:767–801.
2004.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Oh S, Son M, Park CH, Jang JT, Son KH and
Byun K: Pyrogallol-phloroglucinol-6,6-bieckolon attenuates vascular
smooth muscle cell proliferation and phenotype switching in
hyperlipidemia through modulation of chemokine receptor 5. Mar
Drugs. 18(393)2020.PubMed/NCBI View Article : Google Scholar
|