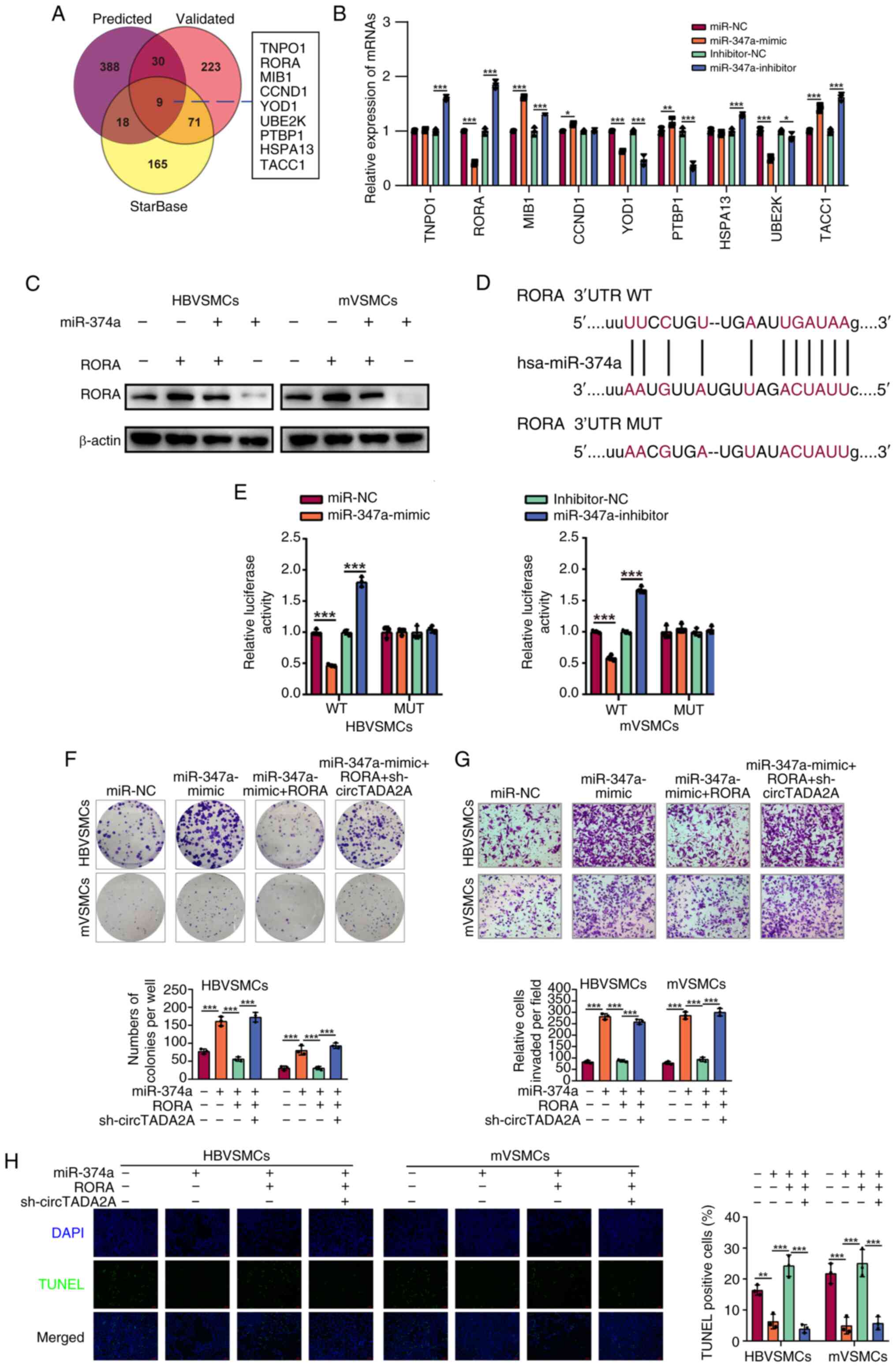
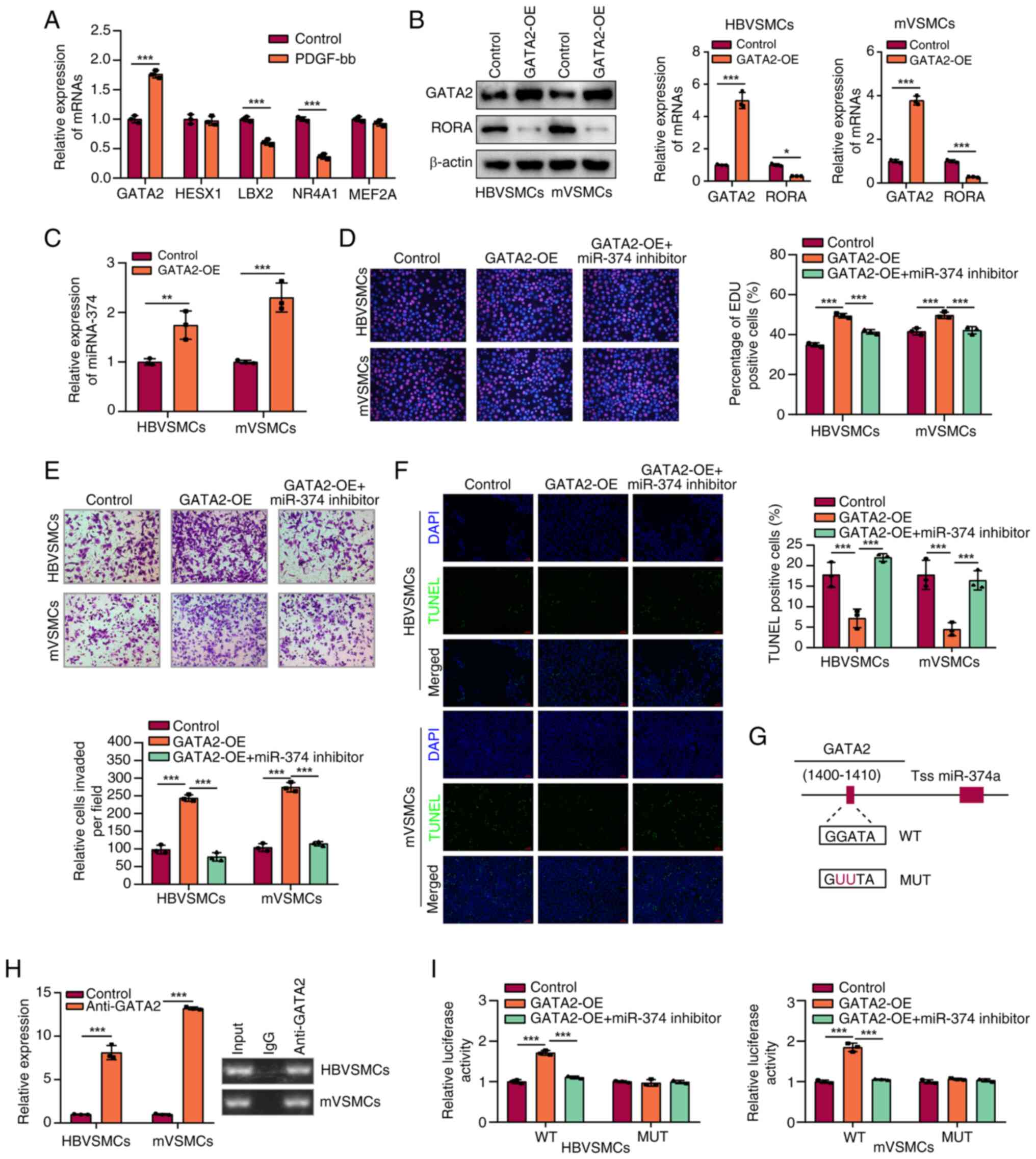
|
1
|
Robinson JG and Davidson MH: Can we cure
atherosclerosis? Rev Cardiovasc Med. 19 (S1):S20–S24.
2018.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Libby P: Inflammation in atherosclerosis.
Nature. 420:868–874. 2002.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Choromańska B, Myśliwiec P, Choromańska K,
Dadan J and Chabowski A: The role of CD36 receptor in the
pathogenesis of atherosclerosis. Adv Clin Exp Med. 26:717–722.
2017.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Kuliczkowska-Płaksej J,
Bednarek-Tupikowska G, Płaksej R and Filus A: Scavenger receptor
CD36: Its expression, regulation, and role in the pathogenesis of
atherosclerosis. Part I. Postepy Hig Med Dosw (Online). 60:142–151.
2006.PubMed/NCBI
|
|
5
|
Millen AE, Nie J, Sahli MW, Mares JA,
Meyers KJ, Klein BEK, LaMonte MJ, Lutsey PL, Andrews CA and Klein
R: Vitamin D status and prevalent early age-related macular
degeneration in african americans and caucasians: The
atherosclerosis risk in communities (ARIC) study. J Nutr Health
Aging. 21:772–780. 2017.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Escárcega RO, Garcia-Carrasco M, Jara LJ
and Cervera R: Accelerated atherosclerosis in systemic lupus
erythematosus: Perspectives towards decreasing cardiovascular
morbidity and mortality. Lupus. 18:383–386. 2009.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Doran AC, Meller N and McNamara CA: Role
of smooth muscle cells in the initiation and early progression of
atherosclerosis. Arterioscler Thromb Vasc Biol. 28:812–819.
2008.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Wang P, Xu TY, Guan YF, Zhao Y, Li ZY, Lan
XH, Wang X, Yang PY, Kang ZM, Vanhoutte PM and Miao CY: Vascular
smooth muscle cell apoptosis is an early trigger for hypothyroid
atherosclerosis. Cardiovasc Res. 102:448–459. 2014.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Bartel DP: Metazoan MicroRNAs. Cell.
173:20–51. 2018.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Farina FM, Hall IF, Serio S, Zani S,
Climent M, Salvarani N, Carullo P, Civilini E, Condorelli G, Elia L
and Quintavalle M: miR-128-3p is a novel regulator of vascular
smooth muscle cell phenotypic switch and vascular diseases. Circ
Res. 126:e120–e135. 2020.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Wang Y, Zhang CX, Ge SL and Gong WH:
CTBP1-AS2 inhibits proliferation and induces autophagy in
ox-LDL-stimulated vascular smooth muscle cells by regulating
miR-195-5p/ATG14. Int J Mol Med. 46:839–848. 2020.PubMed/NCBI View Article : Google Scholar
|
|
12
|
You L, Chen H, Xu L and Li X:
Overexpression of miR-29a-3p suppresses proliferation, migration,
and invasion of vascular smooth muscle cells in atherosclerosis via
targeting TNFRSF1A. Biomed Res Int. 2020(9627974)2020.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Jing R, Pan W, Long T, Li Z and Li C:
LINC00472 regulates vascular smooth muscle cell migration and
proliferation via regulating miR-149-3p. Environ Sci Pollut Res
Int. 28:12960–12967. 2021.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Hao T, Huang S and Han F: LINC-PINT
suppresses tumour cell proliferation, migration and invasion
through targeting miR-374a-5p in ovarian cancer. Cell Biochem
Funct. 38:1089–1099. 2020.PubMed/NCBI View
Article : Google Scholar
|
|
15
|
Lu T, Zhang C, Chai MX, An YB and Jia JL:
MiR-374a promotes the proliferation of osteosarcoma cell
proliferation by targeting Axin2. Int J Clin Exp Pathol.
8:10776–10783. 2015.PubMed/NCBI
|
|
16
|
Ma L, Shao Z and Zhao Y: MicroRNA-374a
promotes pancreatic cancer cell proliferation and epithelial to
mesenchymal transition by targeting SRCIN1. Pathol Res Pract.
215(152382)2019.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Ruan C, Lu J, Wang H, Ge Z, Zhang C and Xu
M: miR-26b-5p regulates hypoxia-induced phenotypic switching of
vascular smooth muscle cells via the TGF-β/Smad4 signaling pathway.
Mol Med Rep. 15:4185–4190. 2017.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Chen H, Xu L, Shan ZL, Chen S and Hu H:
GPX8 is transcriptionally regulated by FOXC1 and promotes the
growth of gastric cancer cells through activating the Wnt signaling
pathway. Cancer Cell Int. 20(596)2020.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Li Z, Yao H, Wang S, Li G and Gu X:
CircTADA2A suppresses the progression of colorectal cancer via
miR-374a-3p/KLF14 axis. J Exp Clin Cancer Res.
39(160)2020.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Li FP, Lin DQ and Gao LY: LncRNA TUG1
promotes proliferation of vascular smooth muscle cell and
atherosclerosis through regulating miRNA-21/PTEN axis. Eur Rev Med
Pharmacol Sci. 22:7439–7447. 2018.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Sun D, Xiang G, Wang J, Li Y, Mei S, Ding
H and Yan J: miRNA 146b-5p protects against atherosclerosis by
inhibiting vascular smooth muscle cell proliferation and migration.
Epigenomics. 12:2189–2204. 2020.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Afzal TA, Luong LA, Chen D, Zhang C, Yang
F, Chen Q, An W, Wilkes E, Yashiro K, Cutillas PR, et al: NCK
associated protein 1 modulated by miRNA-214 determines vascular
smooth muscle cell migration, proliferation, and neointima
hyperplasia. J Am Heart Assoc. 5(e004629)2016.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Son D, Kim Y, Lim S, Kang HG, Kim DH, Park
JW, Cheong W, Kong HK, Han W, Park WY, et al: miR-374a-5p promotes
tumor progression by targeting ARRB1 in triple negative breast
cancer. Cancer Lett. 454:224–233. 2019.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Li W, Meng Z, Zou T, Wang G, Su Y, Yao S
and Sun X: MiR-374a activates Wnt/β-catenin signaling to promote
osteosarcoma cell migration by targeting WIF-1. Pathol Oncol Res.
26:533–539. 2020.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Xu X, Wang W, Su N, Zhu X, Yao J, Gao W,
Hu Z and Sun Y: miR-374a promotes cell proliferation, migration and
invasion by targeting SRCIN1 in gastric cancer. FEBS Lett.
589:407–413. 2015.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Huang H, Liu X, Chen D, Lu Y, Li J, Du F,
Zhang C and Lu L: Melatonin prevents endothelial dysfunction in SLE
by activating the nuclear receptor retinoic acid-related orphan
receptor-α. Int Immunopharmacol. 83(106365)2020.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Erbilgin A, Seldin MM, Wu X, Mehrabian M,
Zhou Z, Qi H, Dabirian KS, Sevag Packard RR, Hsieh W, Bensinger SJ,
et al: Transcription factor Zhx2 deficiency reduces atherosclerosis
and promotes macrophage apoptosis in mice. Arterioscler Thromb Vasc
Biol. 38:2016–2027. 2018.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Qin W, Yang H, Liu G, Bai R, Bian Y, Yang
Z and Xiao C: Activating transcription factor 3 is a potential
target and a new biomarker for the prognosis of atherosclerosis.
Hum Cell. 34:49–59. 2021.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Nakagawa Y, Wang Y, Han SI, Okuda K, Oishi
A, Yagishita Y, Kumagai K, Ohno H, Osaki Y, Mizunoe Y, et al:
Enterohepatic transcription factor CREB3L3 protects atherosclerosis
via SREBP competitive inhibition. Cell Mol Gastroenterol Hepatol.
11:949–971. 2021.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Owens GK, Kumar MS and Wamhoff BR:
Molecular regulation of vascular smooth muscle cell differentiation
in development and disease. Physiol Rev. 84:767–801.
2004.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Oh S, Son M, Park CH, Jang JT, Son KH and
Byun K: Pyrogallol-phloroglucinol-6,6-bieckolon attenuates vascular
smooth muscle cell proliferation and phenotype switching in
hyperlipidemia through modulation of chemokine receptor 5. Mar
Drugs. 18(393)2020.PubMed/NCBI View Article : Google Scholar
|