Introduction
Diabetes is a metabolic disease characterized by
hyperglycemia due to defects in insulin production/function of
insulin. Chronic hyperglycemia can cause damage to the organs,
including the kidneys, eyes, nerves and heart. Multiple pathogenic
processes contribute to the onset of diabetes, including the
autoimmune destruction of pancreatic β-cells leading to insulin
deficiency, as well as various abnormalities causing insulin
resistance, while deficient insulin function can also lead to
abnormalities in metabolism. Insufficient secretion of insulin and
tissue responses to insulin often coexist, making the primary cause
of hyperglycemia unclear (1).
Reactive oxygen species (ROS) are molecules
containing chemically active oxygen that are produced within living
systems. They are natural by-products of oxygen metabolism in all
aerobic organisms (2,3). Elevated levels of ROS result in
oxidative stress, which serves an important role in damaging
cellular components, such as lipids, proteins and DNA (4). The antioxidant defense system plays a
vital role in protecting biological systems by mitigating the
detrimental effects of ROS. Numerous antioxidant enzymes, such as
superoxide dismutase (SOD), glutathione peroxidase, glutathione
reductase, catalase (CAT) and paraoxonase, actively contribute to
this protective mechanism (5).
Apart from the enzymatic antioxidants, the non-enzymatic
antioxidant defense system [including ascorbate, tocopherols,
retinol, carotenoids, reduced glutathione (GSH), melatonin,
polyphenols, ceruloplasmin and carnosine, among others] is equally
important in the regulation of ROS levels and preservation of
normal cellular function (6).
Elevated levels of oxygen free radicals are associated with lipid
peroxidation, non-enzymatic protein glycation and glucose
oxidation, all of which contribute to the development of diabetes
mellitus (DM) and its complications (2).
Cellular and animal model studies have revealed that
essential oils possess notable anti-inflammatory, antioxidant and
anticancer properties (7,8). Researchers are increasingly
interested in essential oils due to their natural phenolic content
and potential antioxidant and free radical scavenging activities.
Essential oils from basil, cinnamon, clove, nutmeg, oregano and
thyme have demonstrated significant radical-scavenging and
antioxidant effects in the DPPH radical assay at room temperature
(9). However, it is important to
note that the free radical scavenging ability of these essential
oils is not solely attributed to their phenolic components, as
monoterpene alcohols, ketones, aldehydes, hydrocarbons and ethers
also contribute to their antioxidant activity (10).
Linalool (LIN), an acyclic monoterpene alcohol, is a
naturally occurring compound found in aromatic plants and is widely
used as a fragrance ingredient in various products. Its versatile
application extends from decorative cosmetics, fine fragrances and
toiletries to non-cosmetic items, such as household cleaners and
detergents (11,12). LIN has been reported to possess
pharmacological properties, including sedative, analgesic,
anti-inflammatory, antioxidant, antimicrobial and antitumor effects
(13,14).
The interest in the antioxidant properties of
essential oils has increased recently (15,16).
There is a growing trend to substitute synthetic antioxidants with
natural compounds, leading to the emergence of their potential use
as natural additives (17).
Cinnamomum osmophloeum (Lauraceae) oil exhibited
2,2-diphenyl-1-picrylhydrazyl (DPPH) radical scavenging activity
(IC50, 29.7 µg/ml), which was associated with its major
component, LIN (73%) (18).
Despite the association between the antioxidant activity of
essential oils and the presence of major compounds, such as LIN, in
certain plants, van Zyl et al (19) showed that LIN alone has minimal
antioxidant activity (IC50 >648 µM) against the DPPH
radical.
LIN has been reported to reduce oxidative
stress-induced damage by inhibiting superoxide anion and hydroxyl
radical formation under in vitro conditions (20). In particular, studies have
indicated that its antioxidant effect is mediated by the prevention
of H2O2-induced decreases in cell viability,
the reduction of LDH release, excessive ROS production, apoptosis
and G2/M phase cell cycle arrest (21), as well as the inhibition of NF-κB
activation (20).
Streptozotocin (STZ), also known as streptozocin,
was identified in 1959 as a natural antibiotic originating from
Streptomyces achromogenes (22). The toxic effects of STZ on
pancreatic β-cells, known as its diabetogenic action, were first
reported in 1963 (23,24). The STZ molecule, with a molecular
formula of C8H15N3O7
and a molecular weight of ~265 g/mol, comprises two components: i)
A glucopyranosyl group, which aids its absorption by pancreatic
β-cells through glucose transporter 2; and ii) a nitrosourea group,
which is responsible for the destruction of pancreatic β-cells
(25). STZ has been observed to
induce diabetic conditions in animal studies due to its selective
destruction of insulin-producing β-cells within the pancreatic
islets (23). The detailed
description of this effect in preclinical rat laboratory models has
encouraged the use of STZ to induce diabetes in laboratory animals
for research purposes (26).
Numerous studies have reported the diverse
biological activities of LIN (27-31).
While most of these studies were conducted in vitro or in
animal models using different administration methods (i.e.
intraperitoneally and inhalation) there is a lack of consistent
clinical investigations specifically focused on LIN. Considering
that processes such as oxidative stress and inflammation are
involved in the Considering that processes such as oxidative stress
and inflammation are involved in the pathogenesis of diabetes
(32), the antioxidant properties
of LIN may support a potential activity of this phytochemical
against oxidative stress in diabetes. Therefore, the present study
aimed to investigate the antioxidant activity of LIN in an
STZ-induced diabetic rat model.
Materials and methods
Chemicals and reagents
LIN and STZ were supplied by Glentham Life Sciences
Ltd. The kits for the SOD (cat. no. 706002), CAT (cat. no. 707002),
GSH (cat. no. 703002) and malondialdehyde (MDA) (cat. no. 10009055)
assays were purchased from Cayman Chemical Company.
Animals
In the present study, 40 male Wistar albino rats
(age, 8 weeks; weight, 250-300 g) were used. Care of the rats and
experimental procedures were performed at the Experimental Medicine
Application and Research Center of Karabük University. Rats were
housed in transparent plastic conventional cages (5 rats in each
cage) according to the experimental animal care conditions of the
research center (21±2˚C, 50±5% humidity), with a 12/12 h light/dark
cycle, with standard pellet rat chow and tap water given ad
libitum. Ethics approval for the study was obtained from the
Karabük University Animal Experiments Local Ethics Committee
(approval no. 2022/2/3).
Induction of diabetes
In the present study, rats were intraperitoneally
injected with a single dose of STZ (65 mg/kg) to induce
experimental diabetes (33). On
experimental day 1, all rats were fasted for 6-8 h before STZ
administration. Water was given normally. Immediately before
injection, STZ was dissolved in 50 mM cold (4˚C) sodium citrate
buffer (pH 4.5). Animals in the CONTROL and LIN groups were
injected with an equal volume of citrate buffer (pH 4.5). Rats were
returned to their cages and were provided with normal food and
water containing 5% glucose. On day 2 of the experiment, 5% glucose
in the water was replaced with normal water (33,34).
Fasting blood glucose levels were measured with a
glucometer device (Accu-Chek Active; Roche Diagnostics) in ~1-2 µl
of blood taken from the tail vein by pricking and bleeding the tail
48 h after STZ administration, and rats with glucose levels
exceeding 200 mg/dl were considered diabetic (35).
Administration of the experimental
drug
Animals in the CONTROL were included in the
experiment as healthy controls (n=10). The rats in the DM group
(untreated diabetic group) received no drugs (n=10). The CONTROL
and DM groups were given physiological saline solution by oral
gavage for 21 days. In the DM + LIN group, 48 h after STZ
injection, 100 mg/kg LIN (in physiological serum) (36) was administered via oral gavage once
a day, every day for 21 days (n=10). In the LIN group, 100 mg/kg
LIN was administered via oral gavage. Throughout the 21-day
experiment (37), the animals were
provided with ad libitum access to food and water. Body
weight and fasting blood glucose levels (on the 7th, 14th and 21st
days) were measured once a week before and after LIN treatment
initiation.
Collection of blood and tissue
samples
At the end of the 21-day experimental period,
anesthesia was induced in rats using a combination of ketamine (80
mg/kg) and xylazine (10 mg/kg) (38), and euthanasia was performed by
cardiac puncture and exsanguination. Blood samples from the heart
of the rats were placed in tubes without anticoagulant and were
left to coagulate at room temperature for 30 min, followed by
centrifugation at 2,000 x g for 15 min at 4˚C.
Following euthanasia, the liver tissues were
removed, washed with saline and stored at -80˚C until further
analysis.
Analysis of oxidant and antioxidant
parameters in blood and tissue
CAT and SOD activities and GSH and MDA levels in
serum and liver tissue samples were determined using the
aforementioned commercial kits in accordance with the protocols
specified by the manufacturer. All measurements were performed
using a microplate reader (Multiskan™ GO Microplate
Spectrophotometer; Thermo Fisher Scientific Inc.).
Statistical analysis
Data are presented as mean ± SD of multiple animals
used in one assay, and were analyzed using SPSS Statistics, version
17.0 (SPSS, Inc.). Normality was checked using the Shapiro-Wilk
test. One-way ANOVA followed by Tukey's post hoc test was used for
determining significant differences among the studied groups.
P<0.05 considered to indicate a statistically significant
difference.
Results
Blood glucose measurement
In all groups, blood glucose was measured in the
blood collected from the tail veins once a week before and after
LIN treatment initiation, four times in total during the
experiment. The blood glucose values of the rats in the LIN-treated
and untreated groups were assessed (Table I). There was no statistically
significant difference in blood glucose levels between the CONTROL
and LIN groups and between the DM + LIN and DM groups at all
measurement times. This indicated that LIN alone did not have an
impact on blood glucose levels in non-diabetic rats, nor did it
significantly alter glucose levels in diabetic rats compared with
untreated diabetic rats.
 | Table IBlood glucose levels of rats
(mg/dl). |
Table I
Blood glucose levels of rats
(mg/dl).
| Time point | CONTROL (n=10) | DM (n=10) | DM + LIN
(n=10) | LIN (n=10) |
|---|
| Before model
establishment | 113.40±15.67 |
413.20±46.94a |
465.20±95.20a,b |
98.60±7.71c |
| 1 week after model
establishment | 97.56±9.96 |
528.50±81.37a |
547.50±76.77a,b |
102.80±15.75c |
| 2 weeks after model
establishment | 104.89±5.88 |
564.70±40.72a |
572.10±56.26a,b |
106.10±11.50c |
| 3 weeks after model
establishment | 103.44±6.52 |
566.20±39.47a |
579.90±39.64a,b |
98.70±9.73c |
A statistically significant difference was found
between the blood glucose values of the CONTROL vs. DM, CONTROL vs.
DM + LIN, DM vs. LIN and DM + LIN vs. LIN groups at all time points
(P<0.001). This demonstrates that the blood glucose levels of
the diabetic groups (DM and DM + LIN) were consistently elevated
compared with the non-diabetic groups (CONTROL and LIN), confirming
the successful induction of diabetes in these groups.
Results of oxidative stress markers.
Serum and liver CAT activity
Serum and liver tissue CAT activity levels were
evaluated as an indicator of antioxidant status between the groups
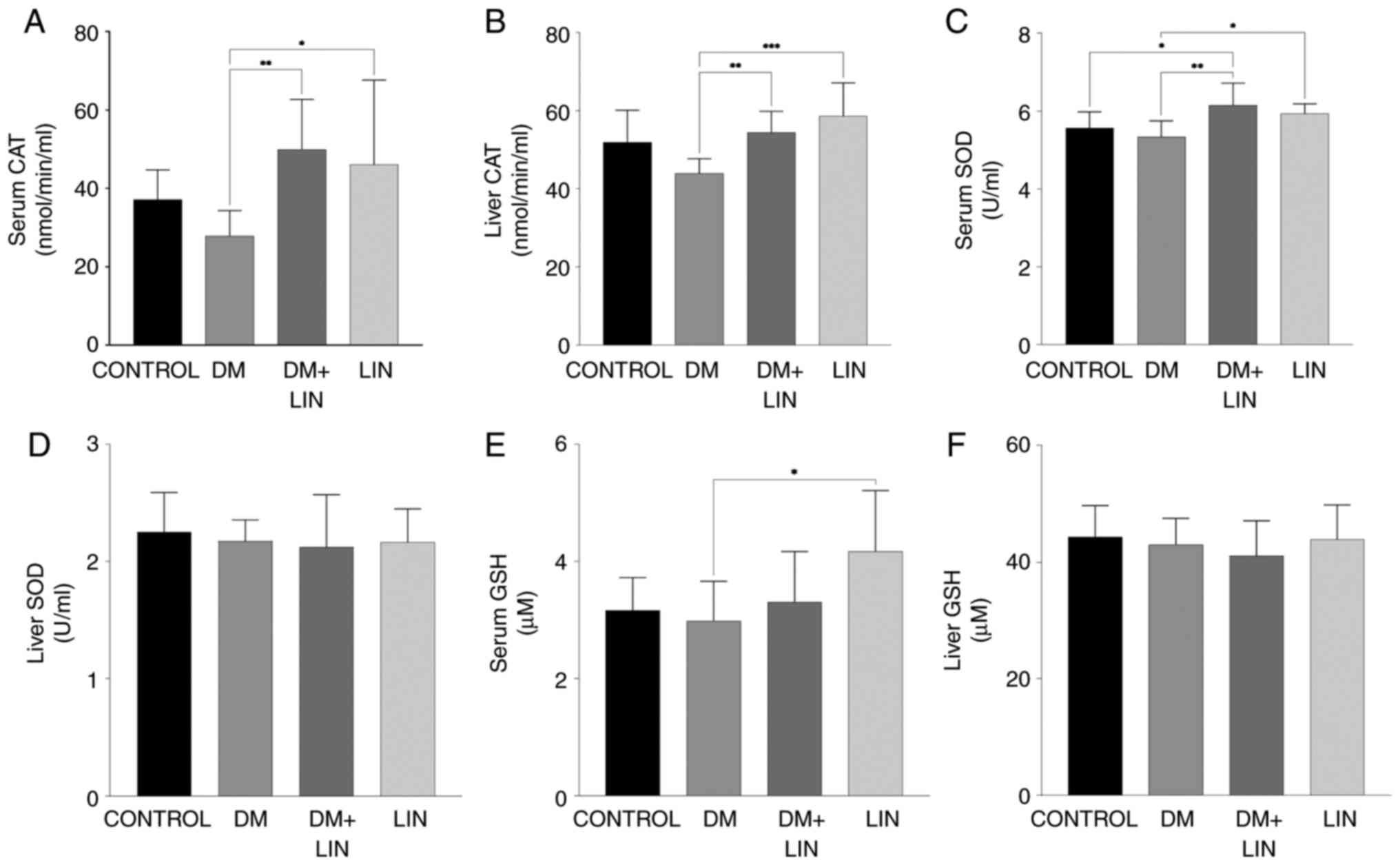
(Fig. 1A and B).
Serum and tissue CAT activity were significantly
higher (P<0.01) in LIN-treated diabetic rats (DM+LIN) compared
with untreated diabetic rats (DM). This indicated that LIN
treatment enhanced antioxidant enzyme activity in diabetic rats,
which suggests a potential protective effect against oxidative
stress induced by diabetes. This increase in CAT activity was also
observed in the LIN-treated group compared with the DM group,
further supporting the antioxidant role of LIN.
However, no significant difference was found in CAT
activity levels when DM, DM + LIN and LIN groups were compared with
the CONTROL group (P>0.05). This suggests that while LIN
treatment significantly improved CAT activity in diabetic rats, it
did not lead to a statistically significant increase in
non-diabetic rats compared with their respective controls.
Serum and liver tissue CAT activity values indicate
the potential of LIN in modulating antioxidant CAT activity in
diabetic conditions, although its effects were less pronounced in
non-diabetic conditions.
Serum and liver SOD activity. Serum SOD
values were assessed at the end of the study (Fig. 1C). There were no statistically
significant differences in serum SOD levels between the CONTROL vs.
DM, CONTROL vs. LIN or DM + LIN vs. LIN groups (P>0.05). This
indicates that LIN treatment alone did not significantly alter
serum SOD levels in non-diabetic rats, nor did it result in
significant differences when comparing LIN-treated diabetic rats
with LIN-treated non-diabetic rats.
However, there were statistically significant
differences between the CONTROL vs. DM + LIN (P<0.05), DM vs.
LIN (P<0.05) and DM vs. DM + LIN groups (P<0.01). These
results suggest that LIN treatment in diabetic rats (DM + LIN) led
to significant changes in serum SOD levels compared with untreated
diabetic rats (DM), indicating a potential beneficial effect of LIN
on antioxidant enzyme activity in diabetic conditions.
No statistically significant difference was found in
liver tissue SOD values among the groups (P>0.05; Fig. 1D). This suggests that while LIN may
affect serum SOD levels in diabetic rats, its impact on liver
tissue SOD activity was not significant under the conditions of the
present study. The serum and liver tissue SOD values were presented
These data provide a detailed overview of the antioxidant SOD
enzyme activity, indicating that LIN specifically enhances serum
SOD levels in diabetic conditions while having no significant
effect on liver tissue SOD activity.
Serum and liver GSH levels. No statistically
significant difference was found in serum GSH levels between the
CONTROL vs. DM, CONTROL vs. DM + LIN, CONTROL vs. LIN, DM vs. DM +
LIN and DM + LIN vs. LIN groups (P>0.05). A statistically
significant difference was found only between the DM and LIN groups
(P=0.013; Fig. 1E). This indicates
that LIN treatment did not significantly alter serum GSH levels in
most group comparisons, with the exception of a notable increase in
the LIN group compared with the DM group.
The liver tissue GSH values obtained in the present
study are shown in Fig. 1F. No
statistically significant difference was found in liver tissue GSH
levels between any of the groups (P>0.05). This suggests that
LIN treatment did not have a significant impact on liver GSH levels
under the conditions of the present study.
Serum and liver tissue GSH values provide a
comprehensive overview of GSH levels, highlighting that while LIN
increased serum GSH compared with the DM group, its overall impact
on liver tissue GSH levels was not significant. These findings
contribute to the better understanding of the diabetic conditions
under which LIN affects antioxidant defense.
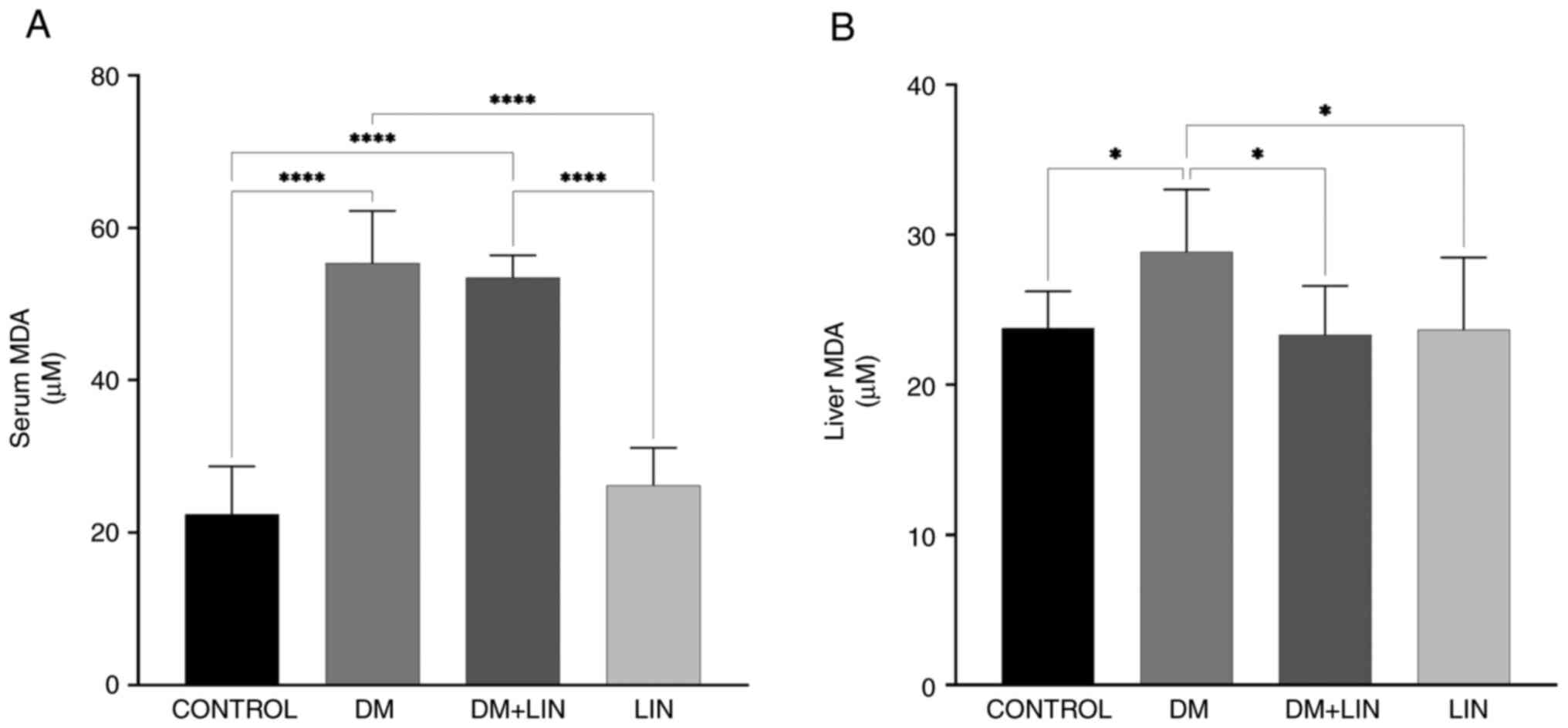
Serum and liver tissue MDA levels. A
statistically significant difference in serum MDA levels was found
between the CONTROL vs. DM, CONTROL vs. DM + LIN, DM vs. LIN and DM
+ LIN vs. LIN groups (P<0.0001). However, there was no
statistically significant difference between the CONTROL vs. LIN
and DM vs. DM + LIN groups (P>0.05) (Fig. 2). This suggests that LIN did not
significantly alter serum MDA levels in non-diabetic rats, nor did
it result in a significant difference in serum MDA levels between
treated and untreated diabetic groups.
Liver tissue MDA values were also assessed (Fig. 2). A statistically significant
difference was found between the CONTROL vs. DM (P=0.0328), DM vs.
DM + LIN (P=0.0174) and DM vs. LIN (P=0.0228) groups. There were no
statistically significant differences between the CONTROL vs. DM +
LIN, CONTROL vs. LIN and DM + LIN vs. LIN groups (P>0.05). LIN
significantly reduced liver MDA levels, specifically in diabetic
rats compared with untreated diabetic rats, but this effect was not
observed between non-diabetic groups or between certain
non-diabetic and treated diabetic groups.
Serum and liver tissue MDA values were assessed and
provide a detailed overview of the MDA oxidative stress marker,
highlighting the specific conditions under which LIN exerts its
antioxidant effects, particularly in reducing MDA levels in
diabetic rats.
Discussion
Diabetes is a chronic metabolic disorder
characterized by elevated blood sugar levels, resulting from either
insufficient insulin production or ineffective insulin utilization
by the body. It is a complex disease with various complications
(such as retinopathy, nephropathy, hypertension, coronary heart
disease and neuropathy) that affect multiple organ systems, in
particular the eyes, kidneys, heart and nerves (39). Oxidative stress is a condition that
occurs when there is an imbalance between the production of ROS and
the body's ability to neutralize them with antioxidants. ROS are
highly reactive molecules that contain oxygen and can cause damage
to cells and tissues if their levels become too high (40). Persistently high blood glucose
levels, known as hyperglycemia, are a hallmark of diabetes.
Elevated glucose levels can lead to increased production of ROS
through processes such as glucose autoxidation and the formation of
advanced glycation end products. These reactions generate ROS as
byproducts, which can promote oxidative stress (41,42).
To counteract the effects of oxidative stress in diabetes,
herbal-based therapeutic strategies to enhance the antioxidant
defense and reduce ROS production are being investigated (43). Numerous reports have provided
various results on the antidiabetic effects of plant extracts
containing LIN (44-47).
However, studies on the antioxidant effects of LIN in diabetic rats
are scarce.
In the present study, a significant increase in the
blood glucose concentration of STZ-induced diabetic rats compared
with the CONTROL group was observed. In STZ-treated groups, blood
glucose concentrations should typically exceed 200 mg/dl in
non-fasting animals, while blood glucose levels should be >150
mg/dl in fasting diabetic animals (33). The statistically significant
difference between the STZ-treated and CONTROL groups was one of
the most important points in this study, as it confirmed the
successful establishment of the diabetic model. Typically, it has
been reported that within 3 weeks following STZ injection, >50%
of animals develop severe hyperglycemia, with blood glucose
concentrations ranging from 300-600 mg/dl. Therefore, a 21-day
period was considered for the LIN treatment (48).
However, during the 21-day study period, no
significant decrease in the blood glucose levels was observed in
the DM + LIN group. Despite LIN administration, the blood glucose
concentrations remained elevated and were not statistically
different from those in the DM group. This lack of a significant
difference suggests that LIN did not exert a notable hypoglycemic
effect under the conditions of the present study, indicating the
necessity of further investigation into its potential therapeutic
benefits for diabetes management. It was concluded that LIN alone,
at a dose of 100 mg/kg, did not play an effective role in the
control of diabetic hyperglycemia. Lee et al (49) reported that indigenous cinnamon
leaf essential oil containing ~40% LIN significantly decreased
fasting blood glucose and fructosamine levels at all doses tested
(12.5, 25 and 50 mg/kg) and increased plasma and pancreatic insulin
levels under fasting conditions in the STZ-induced diabetic rat
model. The study attributed the effect of LIN in the leaf essential
oil from indigenous cinnamon to its hypoglycemic and
pancreas-protective effects. LIN, being the major component of
cinnamon leaf essential oil, was suggested to contribute to the
hypoglycemic effect observed in diabetic rats. Additionally, LIN
was reported to reverse the increase in hepatic pro-inflammatory
cytokine levels in diabetic rats (50). These findings suggest that LIN may
exert its effects through anti-inflammatory and antioxidant
mechanisms, contributing to its potential therapeutic benefits in
diabetes management (49). Deepa
and Venkatraman (50) reported an
improvement in blood glucose levels through inhibition of
gluconeogenesis in a study in which 25 mg/kg LIN was administered
for 45 days in diabetic rats. Although the results of the
aforementioned studies are different from those obtained in the
present study, it is hypothesized that these differences may be
attributed to the particular diabetes model used, the dose and
duration of LIN administration, the enantiomer differences of LIN
(51) or the type of experimental
animal used.
In the liver tissue of DM group, a decrease in CAT
activity was exhibited. Similarly, a notable reduction in serum CAT
activity was observed. Both liver and serum CAT activities were
significantly increased in DM + LIN and LIN groups compared with
those in the DM group Altınok-Yipel et al (36) reported that LIN increased CAT
activity in rats with liver damage induced by CCl4.
Consistent with this study, the present results demonstrated that
LIN can restore impaired CAT activity under stress. The restoration
of serum CAT levels suggests that LIN exhibits a systemic effect
and can ameliorate oxidative imbalances throughout the body. The
results of the present study highlight the efficacy of LIN in
combating systemic oxidative stress, as reflected in the serum and
its potential regulatory effects on various systems in the
organism. This may indicate that the antioxidant capacity of LIN is
beneficial for the general oxidative status of the organism beyond
the cellular level.
It was found that SOD levels in the liver tissue did
not show statistically significant differences among the groups.
The level of SOD activity in the LIN group was similar to that in
the CONTROL group, suggesting that LIN had no effect on SOD
activity under normal physiological conditions. Despite the
increase in serum SOD levels in the DM + LIN group compared with
those in the DM group, the effects of LIN on SOD activity in the
liver were not as pronounced as those in the serum. This suggests
that the antioxidant effects of LIN may differ between target
tissues. The results of the current study are in accordance with
those of previous studies (52-54),
which indicated that there were no substantial fluctuations in the
tissue activity of SOD in individuals with DM. However, other
studies have reported both elevated (55) and decreased (56) levels of SOD in patients with
DM.
Antioxidants help protect cells by neutralizing free
radicals caused by oxidative stress (57). Reversely, oxidative stress, by
increasing the formation of free radicals in cells, depletes
antioxidant molecules, such as GSH (58). Both in the serum and liver, no
significant effect of LIN treatment on GSH levels was observed in
diabetic rats. LIN is a compound with antioxidant properties, and
its antioxidant activity may protect GSH in cells from the harmful
effects of oxidative stress. However, it has also been indicated
that LIN improves GSH levels in brain tissue depending on the time
of administration. For example, it was reported that LIN was more
effective when it was administered to rats 3 days before or at the
same time as the acrylamide-induced neurotoxicity model
establishment, while no effect was observed when LIN was
administered 3 days after the model was established (59). Even if this model and the diabetes
model of the presented study are not the same, LIN was administered
48 h after the onset of diabetes in the present study. Therefore,
its efficacy may have varied depending on the time of
administration.
MDA is a by-product of lipid peroxidation,
indicative of oxidative damage to cell membrane lipids (60). In the present study, a significant
increase in serum MDA levels were observed in the diabetic group
(DM) compared with the CONTROL group, as well as in the LIN-treated
diabetic group (DM + LIN) compared with the CONTROL group. These
findings confirm that diabetes induction substantially elevated
serum MDA levels, resulting in increased oxidative stress. The
results showed that the antioxidant effects of LIN were
significantly effective in reducing MDA levels, especially in the
liver of diabetic rats. The notable reduction in liver MDA levels
in LIN-treated diabetic rats compared with untreated diabetic rats
demonstrates the potential therapeutic benefits of LIN in
mitigating oxidative stress associated with diabetes. However, the
absence of significant differences in serum MDA levels between the
treated and untreated diabetic groups, as well as between the
non-diabetic groups, indicates that the effects of LIN may be more
pronounced in the liver tissue than in the serum. This differential
impact suggests that the antioxidant properties of LIN may be
tissue specific. Further research is required to elucidate the
precise mechanisms through which LIN modulates oxidative stress and
to explore its potential applications in other tissues and
diseases.
The present study assessed the antioxidant
properties of LIN in DM model. In STZ-induced diabetic rats, LIN
treatment alone failed to control hyperglycemia. However, a
significant restoration of CAT activity was observed in both the
liver and serum in diabetic rats treated with LIN. Nonetheless,
there was no significant change in liver SOD activity.
Additionally, LIN was observed to have a reducing effect on MDA
levels, an important indicator of diabetes-related oxidative
stress. It was also found that LIN did not affect GSH levels in
either the serum or liver in diabetic rats. In summary, the present
results suggest that LIN has the potential to reduce
diabetes-associated oxidative stress. Overall, the current study
may indicate that LIN has a regulatory effect on the overall
oxidative status of the organism by reducing liver MDA levels, an
important indicator of diabetes-related oxidative stress, while
increasing the activity of antioxidant enzymes, such as liver and
serum CAT activity, and serum SOD activity in diabetic rats. This
suggests that LIN may help in mitigating oxidative damage
associated with diabetes.
One of the limitations of the present study is the
absence of histopathologic tissue evaluation. Although blood
glucose levels and other biochemical parameters were thoroughly
assessed, a detailed examination of pancreatic or other relevant
tissue samples was not performed. Histopathologic analysis could
have provided valuable insights into the cellular and structural
changes associated with STZ-induced diabetes and the potential
protective effects of LIN. This limitation restricts the
understanding of the underlying mechanisms at the tissue level and
the broader implications of the present findings. Future studies
should incorporate comprehensive histopathologic evaluations to
complement biochemical data and offer a more holistic understanding
of the therapeutic potential of LIN in diabetic models.
These findings suggest that LIN has potential
antioxidant effects in the context of diabetes. Further research is
needed to understand the mechanisms underlying these effects and
determine the optimal dosage of LIN for diabetes management.
Acknowledgements
Not applicable.
Funding
Funding: This present study was funded by Karabük University
Scientific Research Projects Unit (grant no. KBÜBAP-22-YL-076).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
SB and MK conducted the experiments and edited the
manuscript. SB designed the study, and collected and processed the
data. MK conducted the statistical analysis, and reviewed and
revised the article. SB and MK confirm the authenticity of all the
raw data. Both authors read and approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by Karabük University
Animal Experiments Local Ethics Committee (approval no.
2022/2/3).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
American Diabetes Association. Diagnosis
and classification of diabetes mellitus. Diabetes Care. 37 (Suppl
1):S81–S90. 2014.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Sies H (ed): Oxidative Stress: Eustress
and Distress. Academic Press, Cambridge, MA, 2019.
|
|
3
|
Darenskaya MA, Kolesnikova LI and
Kolesnikov SI: Oxidative stress: Pathogenetic role in diabetes
mellitus and its complications and therapeutic approaches to
correction. Bull Exp Biol Med. 171:179–189. 2021.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Sifuentes-Franco S, Pacheco-Moisés FP,
Rodríguez-Carrizalez AD and Miranda-Díaz AG: The role of oxidative
stress, mitochondrial function, and autophagy in diabetic
polyneuropathy. J Diabetes Res. 2017(1673081)2017.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Ozougwu JC: The role of reactive oxygen
species and antioxidants in oxidative stress. Int J Res. 3:1–8.
2016.
|
|
6
|
Mirończuk-Chodakowska I, Witkowska AM and
Zujko ME: Endogenous non-enzymatic antioxidants in the human body.
Adv Med Sci. 63:68–78. 2018.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Miguel MG: Antioxidant and
anti-inflammatory activities of essential oils: A short review.
Molecules. 15:9252–9287. 2010.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Blowman K, Magalhães M, Lemos MFL, Cabral
C and Pires IM: Anticancer properties of essential oils and other
natural products. Evid Based Complement Alternat Med.
2018(3149362)2018.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Tomaino A, Cimino F, Zimbalatti V, Venuti
V, Sulfaro V, De Pasquale A and Saija A: Influence of heating on
antioxidant activity and the chemical composition of some spice
essential oils. Food Chem. 89:549–554. 2005.
|
|
10
|
Edris AE: Pharmaceutical and therapeutic
potentials of essential oils and their individual volatile
constituents: A review. Phytother Res. 21:308–323. 2007.PubMed/NCBI View
Article : Google Scholar
|
|
11
|
Lapczynski A, Letizia C and Api A:
Addendum to fragrance material review on linalool. Food Chem
Toxicol. 46 (Suppl 11):S190–S192. 2008.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Kamatou GPP and Viljoen AM: Linalool-A
review of a biologically active compound of commercial importance.
Nat Prod Commun. 3(1187)2008.
|
|
13
|
Aprotosoaie AC, Hăncianu M, Costache II
and Miron A: Linalool: A review on a key odorant molecule with
valuable biological properties. Flavour Fragr J. 29:193–219.
2014.
|
|
14
|
de Cássia da Silveira e Sá R, Andrade LN
and de Sousa DP: A review on anti-inflammatory activity of
monoterpenes. Molecules. 18:1227–1254. 2013.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Tit DM and Bungau SG: Antioxidant activity
of essential oils. Antioxidants (Basel). 12(383)2023.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Chen X, Shang S, Yan F, Jiang H, Zhao G,
Tian S, Chen R, Chen D and Dang Y: Antioxidant activities of
essential oils and their major components in scavenging free
radicals, inhibiting lipid oxidation and reducing cellular
oxidative stress. Molecules. 28(4559)2023.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Sökmen M, Serkedjieva J, Daferera D,
Gulluce M, Polissiou M, Tepe B, Akpulat HA, Sahin F and Sokmen A:
In vitro antioxidant, antimicrobial, and antiviral activities of
the essential oil and various extracts from herbal parts and callus
cultures of Origanum acutidens. J Agric Food Chem. 52:3309–3312.
2004.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Lin KH, Yeh SY, Lin MY, Shih MC, Yang KTU
and Hwang SY: Major chemotypes and antioxidative activity of the
leaf essential oils of Cinnamomum osmophloeum Kaneh. from a
clonal orchard. Food Chem. 105:133–139. 2007.
|
|
19
|
van Zyl RL, Seatlholo ST, van Vuuren SF
and Viljoen AM: The Biological activities of 20 nature identical
essential oil constituents. Journal of Essential Oil Research. 18
(Suppl 1):S129–S133. 2006.
|
|
20
|
Gunaseelan S, Balupillai A, Govindasamy K,
Ramasamy K, Muthusamy G, Shanmugam M, Thangaiyan R, Robert BM,
Prasad Nagarajan R, Ponniresan VK and Rathinaraj P: Linalool
prevents oxidative stress activated protein kinases in single
UVB-exposed human skin cells. PLoS One. 12(e0176699)2017.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Migheli R, Lostia G, Galleri G, Rocchitta
G, Serra PA, Bassareo V, Acquas E and Peana AT: Neuroprotective
effect of (R)-(-)-linalool on oxidative stress in PC12 cells.
Phytomed Plus. 1(100073)2021.
|
|
22
|
Vavra JJ, Deboer C, Dietz A, Hanka LJ and
Sokolski WT: Streptozotocin, a new antibacterial antibiotic.
Antibiot Annu. 7:230–235. 1959.PubMed/NCBI
|
|
23
|
Rakieten N, Rakieten ML and Nadkarni MV:
Studies on the diabetogenic action of streptozotocin (NSC-37917).
Cancer Chemother Rep. 29:91–98. 1963.PubMed/NCBI
|
|
24
|
Ghasemi A and Jeddi S: Streptozotocin as a
tool for induction of rat models of diabetes: A practical guide.
EXCLI J. 22:274–294. 2023.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Elsner M, Guldbakke B, Tiedge M, Munday R
and Lenzen S: Relative importance of transport and alkylation for
pancreatic beta-cell toxicity of streptozotocin. Diabetologia.
43:1528–1533. 2000.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Gunnarsson R, Berne C and Hellerström C:
Cytotoxic effects of streptozotocin and N-nitrosomethylurea on the
pancreatic B cells with special regard to the role of
nicotinamide-adenine dinucleotide. Biochem J. 140:487–494.
1974.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Huo M, Cui X, Xue J, Chi G, Gao R, Deng X,
Guan S, Wei J, Soromou LW, Feng H and Wang D: Anti-inflammatory
effects of linalool in RAW 264.7 macrophages and
lipopolysaccharide-induced lung injury model. J Surg Res.
180:e47–e54. 2013.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Sun XB, Wang SM, Li T and Yang Y:
Anticancer activity of linalool terpenoid: apoptosis induction and
cell cycle arrest in prostate cancer cells. Trop J Pharm Res.
14:619–625. 2015.
|
|
29
|
Cho SY, Jun HJ, Lee JH, Jia Y, Kim KH and
Lee SJ: Linalool reduces the expression of
3-hydroxy-3-methylglutaryl CoA reductase via sterol regulatory
element binding protein-2- and ubiquitin-dependent mechanisms. FEBS
Lett. 585:3289–3296. 2011.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Herman A, Tambor K and Herman A: Linalool
affects the antimicrobial efficacy of essential oils. Curr
Microbiol. 72:165–172. 2016.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Katsuyama S, Kuwahata H, Yagi T, Kishikawa
Y, Komatsu T, Sakurada T and Nakamura H: Intraplantar injection of
linalool reduces paclitaxel-induced acute pain in mice. Biomed Res.
33:175–181. 2012.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Asmat U, Abad K and Ismail K: Diabetes
mellitus and oxidative stress-a concise review. Saudi Pharm J.
24:547–553. 2016.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Furman BL: Streptozotocin-induced diabetic
models in mice and rats. Curr Protoc. 1(e78)2021.PubMed/NCBI View
Article : Google Scholar
|
|
34
|
Fararh KM, Shimizu Y, Shiina T, Nikami H,
Ghanem MM and Takewaki T: Thymoquinone reduces hepatic glucose
production in diabetic hamsters. Res Vet Sci. 79:219–223.
2005.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Faisal Lutfi M, Abdel-Moneim AH,
Alsharidah AS, Mobark MA, Abdellatif AAH, Saleem IY, Al Rugaie O,
Mohany KM and Alsharidah M: Thymoquinone lowers blood glucose and
reduces oxidative stress in a rat model of diabetes. Molecules.
26(2348)2021.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Altınok-Yipel F, Tekeli İO, Özsoy ŞY,
Güvenç M, Kaya A and Yipel M: Hepatoprotective activity of linalool
in rats against liver injury induced by carbon tetrachloride. Int J
Vitam Nutr Res. 90:302–308. 2020.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Deeds MC, Anderson JM, Armstrong AS,
Gastineau DA, Hiddinga HJ, Jahangir A, Eberhardt NL and Kudva YC:
Single dose streptozotocin-induced diabetes: Considerations for
study design in islet transplantation models. Lab Anim. 45:131–140.
2011.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Bhatia A, Saikia PP, Dkhar B and Pyngrope
H: Anesthesia protocol for ear surgery in Wistar rats (animal
research). Animal Model Exp Med. 5:183–188. 2022.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Banday MZ, Sameer AS and Nissar S:
Pathophysiology of diabetes: An overview. Avicenna J Med.
10:174–188. 2020.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Pizzino G, Irrera N, Cucinotta M, Pallio
G, Mannino F, Arcoraci V, Squadrito F, Altavilla D and Bitto A:
Oxidative stress: Harms and benefits for human health. Oxid Med
Cell Longev. 2017(8416763)2017.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Caturano A, D'Angelo M, Mormone A, Russo
V, Mollica MP, Salvatore T, Galiero R, Rinaldi L, Vetrano E,
Marfella R, et al: Oxidative stress in type 2 diabetes: impacts
from pathogenesis to lifestyle modifications. Curr Issues Mol Biol.
45:6651–6666. 2023.PubMed/NCBI View Article : Google Scholar
|
|
42
|
González P, Lozano P, Ros G and Solano F:
Hyperglycemia and oxidative stress: An integral, updated and
critical overview of their metabolic interconnections. Int J Mol
Sci. 24(9352)2023.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Khalid M, Petroianu G and Adem A: Advanced
glycation end products and diabetes mellitus: Mechanisms and
perspectives. Biomolecules. 12(542)2022.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Vats V, Yadav SP and Grover JK: Ethanolic
extract of Ocimum sanctum leaves partially attenuates
streptozotocin-induced alterations in glycogen content and
carbohydrate metabolism in rats. J Ethnopharmacol. 90:155–160.
2004.PubMed/NCBI View Article : Google Scholar
|
|
45
|
More TA, Kulkarni BR, Nalawade ML and
Arvindekar AU: antidiabetic activity of linalool and limonene in
streptozotocin-induced diabetic rat: A combinatorial therapy
approach. Int J Pharm Pharm Sci. 6:159–163. 2014.
|
|
46
|
Garba HA, Mohammed A, Ibrahim MA and
Shuaibu MN: Effect of lemongrass (Cymbopogon citratus Stapf) tea in
a type 2 diabetes rat model. Clin Phytosci. 6(19)2020.
|
|
47
|
Tran N, Pham B and Le L: Bioactive
compounds in anti-diabetic plants: From herbal medicine to modern
drug discovery. Biology (Basel). 9(252)2020.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Camargo SB, Simões LO, Medeiros CFA, de
Melo Jesus A, Fregoneze JB, Evangelista A, Villarreal CF, Araújo
AAS, Quintans-Júnior LJ and Silva DF: Antihypertensive potential of
linalool and linalool complexed with β-cyclodextrin: Effects of
subchronic treatment on blood pressure and vascular reactivity.
Biochem Pharmacol. 151:38–46. 2018.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Lee SC, Xu WX, Lin LY, Yang JJ and Liu CT:
Chemical composition and hypoglycemic and pancreas-protective
effect of leaf essential oil from indigenous cinnamon
(Cinnamomum osmophloeum Kanehira). J Agric Food Chem.
61:4905–4913. 2013.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Deepa B and Venkatraman Anuradha C:
Effects of linalool on inflammation, matrix accumulation and
podocyte loss in kidney of streptozotocin-induced diabetic rats.
Toxicol Mech Methods. 23:223–234. 2013.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Peana AT, D'Aquila PS, Panin F, Serra G,
Pippia P and Moretti MD: Anti-inflammatory activity of linalool and
linalyl acetate constituents of essential oils. Phytomedicine.
9:721–726. 2002.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Peuchant E, Delmas-Beauvieux MC,
Couchouron A, Dubourg L, Thomas MJ, Perromat A, Clerc M and Gin H:
Short-term insulin therapy and normoglycemia. Effects on
erythrocyte lipid peroxidation in NIDDM patients. Diabetes Care.
20:202–207. 1997.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Kesavulu M, Rao BK, Giri R, Vijaya J,
Subramanyam G and Apparao C: Lipid peroxidation and antioxidant
enzyme status in type 2 diabetics with coronary heart disease.
Diabetes Res Clin Pract. 53:33–39. 2001.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Sözmen EY, Sözmen B, Delen Y and Onat T:
Catalase/superoxide dismutase (SOD) and catalase/paraoxonase (PON)
ratios may implicate poor glycemic control. Arch Med Res.
32:283–287. 2001.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Kimura F, Hasegawa G, Obayashi H, Adachi
T, Hara H, Ohta M, Fukui M, Kitagawa Y, Park H, Nakamura N, et al:
Serum extracellular superoxide dismutase in patients with type 2
diabetes: Relationship to the development of micro- and
macrovascular complications. Diabetes Care. 26:1246–1250.
2003.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Ciechanowski K, Kedzierska K, Gołembiewska
E, Safranow K, Bober J, Domański L, Rózański J and Myślak M:
Impaired synthesis is not the reason for decreased activity of
extracellular superoxide dismutase in patients with diabetes. Arch
Med Res. 36:148–153. 2005.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Lobo V, Patil A, Phatak A and Chandra N:
Free radicals, antioxidants and functional foods: Impact on human
health. Pharmacogn Rev. 4:118–126. 2010.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Bhattacharyya A, Chattopadhyay R, Mitra S
and Crowe SE: Oxidative stress: An essential factor in the
pathogenesis of gastrointestinal mucosal diseases. Physiol Rev.
94:329–354. 2014.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Mehri S, Meshki MA and Hosseinzadeh H:
Linalool as a neuroprotective agent against acrylamide-induced
neurotoxicity in Wistar rats. Drug Chem Toxicol. 38:162–166.
2015.PubMed/NCBI View Article : Google Scholar
|
|
60
|
Gaweł S, Wardas M, Niedworok E and Wardas
P: Malondialdehyde (MDA) as a lipid peroxidation marker. Wiad Lek.
57:453–455. 2004.PubMed/NCBI(In Polish).
|