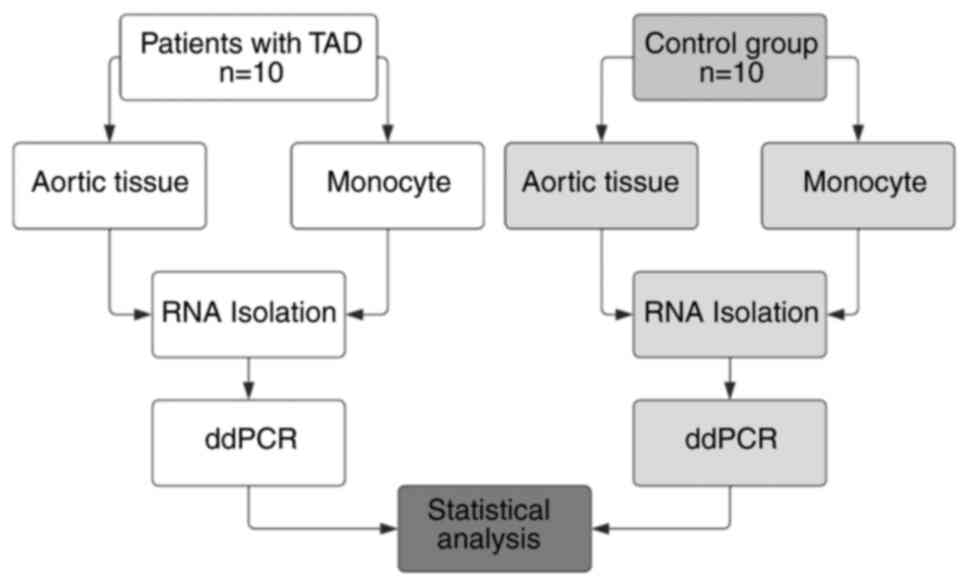
Introduction
Thoracic aortic dissection (TAD) is defined as the
separation of the aortic layers along the vessel wall following a
tear in the intima layer of the aorta (1). TAD is quite a life-threatening and
fatal condition. Based on estimations, the incidence of TAD is
between 3 to 5 cases per 100.000 individuals per year (2-4).
The primary characterization finding for TAD is the degraded aortic
media layer (1). Media is the
middle layer of the aorta which is composed of smooth muscle cells
(SMC), elastic and collagen fibers, proteoglycans,
glycosaminoglycans, and some other proteins. The elastic fibers and
supportive protein structures located in the media layer are the
components of the extracellular matrix (ECM) which has a key role
in maintaining the shape and strength of the aortic wall (1,5,6). In
the dissected aorta, an excess amount of protease such as matrix
metalloproteinases (MMPs) is also found, whose substrates are
mainly the ECM components. The functions of MMPs are variable, thus
they can either degrade or activate proteins in the ECM (7). Numerous studies have revealed that
most of the MMPs exhibit differentiated expression in cases with
TAD (8-10).
In particular, it has been reported that MMP2 and MMP9 can degrade
collagen types I, II and III that have been cleaved by MMP1 at the
beginning of the process (11). In
addition, it has been stated that both MMP2 and MMP9 can be
regulated by transforming growth factor β (TGF-β) signaling, which
is known to be involved in matrix synthesis and degradation
(12).
MMPs, which have an important role in the formation
of TAD (8-10),
have natural inhibitors in the cell (13). The tissue inhibitors of matrix
metalloproteinases (TIMPs) inhibit the MMP function by binding in
the active site of MMPs and prevent matrix degradation (14). Although the inhibitory capacity of
TIMP2 and TIMP3 varies, they act on all MMPs. TIMP2 has been
reported to be highly effective in inhibiting MMP2. Additionally,
TIMP3 has been found to inhibit MMP2 and MMP9 successfully
(15,16). In cases with TAD it was shown that
the expression levels of TIMPs can be both increased and decreased
(8,17,18).
Additionally, the MMP/TIMP gene expression ratio appears to direct
the cell function into a proteolytic stage, which is a key factor
in TAD (1,19).
On the site of the tear, an inflammation also occurs
in TAD. Macrophages are one of the main players of inflammation in
TAD. Since the circulating monocytes give rise to the macrophages,
they are also important components of the process (20,21).
It was observed that the percentage of total CD14+
monocytes was increased in patients with TAD, and there were
expression changes in the genes managing the formation of TAD
(22).
Most of the studies in literature are not focused on
the gene expression changes at the mRNA level in patients with TAD.
In such complex diseases such as TAD, mRNA levels of target genes
both in aortic vascular tissue and in the circulating counterparts,
may provide important knowledge in the understanding of the
pathogenesis of the disease. Since MMP2 and MMP9 are closely
related to the maintenance and degradation of the ECM and its
components (7-10,12),
they were predicted to make significant contributions to the
investigation of the pathogenesis of TAD and were selected among
other MMPs. In addition, the fact that TIMP2 and TIMP3 have an
effect on all MMPs and are particularly effective in the regulation
of MMP2 and MMP9 (15-18)
are the reasons for their inclusion in the present study. A
two-step study was conducted, in which gene expression analysis was
performed both in aortic tissue and circulating monocyte cells in
patients with TAD and controls. The mRNA levels of MMP2,
MMP9, TIMP2 and TIMP3 genes were investigated to
identify candidate factors driving the development of TAD.
Materials and methods
Study design and subjects
The present study included 20 aortic tissue samples
and 20 whole blood samples obtained from 10 patients with TAD (2
women and 8 men) and 10 individuals with coronary artery disease (2
women and 8 men) considered as a control group. All the
participants underwent a surgical intervention between August 2019
and February 2021. Patients with TAD who were enrolled in the
present study were diagnosed by clinicians with acute Stanford Type
A aortic dissection. Patients with aortic aneurysms or connective
tissue disorders such as Marfan syndrome were excluded. Individuals
who underwent coronary artery bypass grafting (CABG) were used as
the control. The ascending aorta tissue from the site of the
central anastomosis of the CABG was obtained (17,18,23).
Whole blood samples were collected during surgery. The experimental
flowchart is provided in Fig. 1.
The present study was approved (approval no. 2019/525) by the
Istanbul Medical Faculty Clinical Research Ethics Committee and the
Institute of Graduate Studies in Sciences, Istanbul University
(Istanbul, Turkey) and written informed consent was obtained from
all the participants.
Monocyte enrichment and RNA
isolation
Venous whole blood (10 ml) was drawn from each
patient with TAD and the control group, and stored in EDTA tubes.
The human monocyte cells were collected from the whole blood
through negative selection by using RosetteSep Human Monocyte
Enrichment Cocktail (cat. no. 15668; StemCell Technologies, Inc.)
with the help of the Ficoll-Hypaque centrifugation method. To apply
this method, a whole blood-Rosette mixture prepared according to
the manufacturer's recommended protocol was added to a Falcon tube
containing 15 ml Ficoll-Hypaque. The addition was performed by
spreading the mixture slowly on the surface of the Ficoll to avoid
mixing of the blood-Rosette mixture. The tubes were centrifuged at
1,200 x g for 20 min at 4˚C with the brake off. The desired cells
were collected by pipette from the layer between the Ficoll and
serum layer. All samples were processed for the RNA isolation using
the PureLink RNA Mini Kit (cat. no. 12183018A; Thermo Fisher
Scientific, Inc.). The obtained total RNA for each sample was
reverse transcribed to cDNA with the iScript cDNA Synthesis Kit
(cat. no. 1708890; Bio-Rad Laboratories, Inc.).
Gene expression analysis
To analyze the mRNA expression of target genes, the
QX200 Droplet Digital PCR System (ddPCR) (Bio-Rad Laboratories,
Inc.) was used. The PCR was set up with EvaGreen Supermix (Bio-Rad
Laboratories, Inc.) in 20 µl total volume. The following primer
sequences for MMP2, MMP9, TIMP2, TIMP3 and
GAPDH genes were used: MMP2 gene forward,
5'-GCTACGATGGAGGCGCTAATG-3' and reverse,
5'-GGGCAGCCATAGAAGGTGTTC-3'; MMP9 gene forward,
5'-TTTGGTGTCGCGGAGCAC-3' and reverse, 5'-CGAGTTGGAACCACGACGC-3';
TIMP2 gene forward, 5'-CTGGACGTTGGAGGAAAGAAGG-3' and
reverse, 5'-CATCTGGTACCTGTGGTTCAGG-3'; TIMP3 gene forward,
5'-GCAACTCCGACATCGTGATCC-3' and reverse,
5'-TGGTGAAGCCTCGGTACATCTTC-3'; GAPDH gene forward,
5'-GCACCGTCAAGGCTGAGAAC-3' and reverse, 5'-TGGTGAAGACGCCAGTGGA-3'.
The reaction mixture of each sample was partitioned into droplets
with the QX200 droplet generator and transferred into a 96-well
plate. After sealing, the plate was placed into a T100 Thermal
Cycler (Bio-Rad Laboratories, Inc.) and the cycling protocol was
activated. PCR cycling conditions were as follows: 1 cycle of
preincubation at 94˚C for 180 sec, then 39 cycles of 3-step
amplification at 94˚C for 40 sec, 60˚C for 40 sec and 72˚C for 50
sec, and 1 cycle of final extension at 72˚C for 300 sec. Since the
EvaGreen is a fluorescent dye, the expression values of the target
genes were obtained using the QX200 reader (Bio-Rad Laboratories,
Inc.) which is the last component of the ddPCR system.
Statistical analysis
All statistical analyses were performed using
GraphPad Prism version 9.3.1 (Dotmatics). The numerical and
categorical data were presented as the mean, standard error of the
mean (SEM), and percentage (%). To reveal whether the data were
normally distributed or not, the Shapiro-Wilk test was used. For
descriptive statistical analysis of the study group, Mann-Whitney U
and Fisher's exact test were used for continuous and categorical
variables, respectively. In the ddPCR method (24,25),
the expression values were obtained as copy/ml for each sample
using the QuantaSoft™ software (version 1.7.4; Quantosoft, s.r.o.)
for the absolute quantification of the target genes. For each test
subject, copy/1ng RNA values were calculated by considering the
initial amount of RNA used for the individual reactions. In
addition to the target genes, a reference gene (GAPDH) was
also included in the study to perform the normalization. Since the
data was not normally distributed, the expression levels of the
target genes (MMP2, MMP9, TIMP2 and TIMP3) between
the patients with TAD and the control group were compared using the
two-tailed Mann-Whitney U test. The correlation of the expression
levels of target genes between aortic tissue and the monocyte cells
was performed with the Spearman's correlation test due to the not
normally distributed data type. Receiver operating characteristic
(ROC) curve analysis was performed and area under the curve (AUC)
values were calculated to investigate sensitivity and specificity.
The results also revealed the differentiation degree between the
patients with TAD and the control group samples and what the
diagnostic values of the targeted mRNAs were. For the
all-statistical analysis, P<0.05 was considered to indicate a
statistically significant difference.
Results
Descriptive characteristics
The descriptive characteristics of the patients with
TAD and the control group are revealed in Table I. There was no statistically
significant difference in age, height, weight, body mass index
(BMI), smoking status, sex and hypertension between the patients
with TAD and the respective control group.
 | Table IDescriptive characteristics of
patients with TAD and the control group. |
Table I
Descriptive characteristics of
patients with TAD and the control group.
| Parameters | Patients with TAD
(n=10) | Control group
(n=10) | P-value |
|---|
| Mean age ± SEM,
years | 53.80±3.15 | 55.10±1.95 | 0.492 |
| Mean height ± SEM,
cm | 167.30±2.26 | 167.60±2.08 | 0.896 |
| Mean weight ± SEM,
kg | 84.20±3.60 | 80.80±2.24 | 0.245 |
| Mean BMI ± SEM,
kg/m2 | 30.29±1.67 | 28.99±1.42 | 0.393 |
| Smokers, % | 60 (n=6) | 80 (n=8) | 0.639 |
| Sex (female/male),
% | 20/80 (n=2/8) | 20/80 (n=2/8) | 0.999 |
| Hypertension,
% | 100 (n=10) | 80 (n=8) | 0.478 |
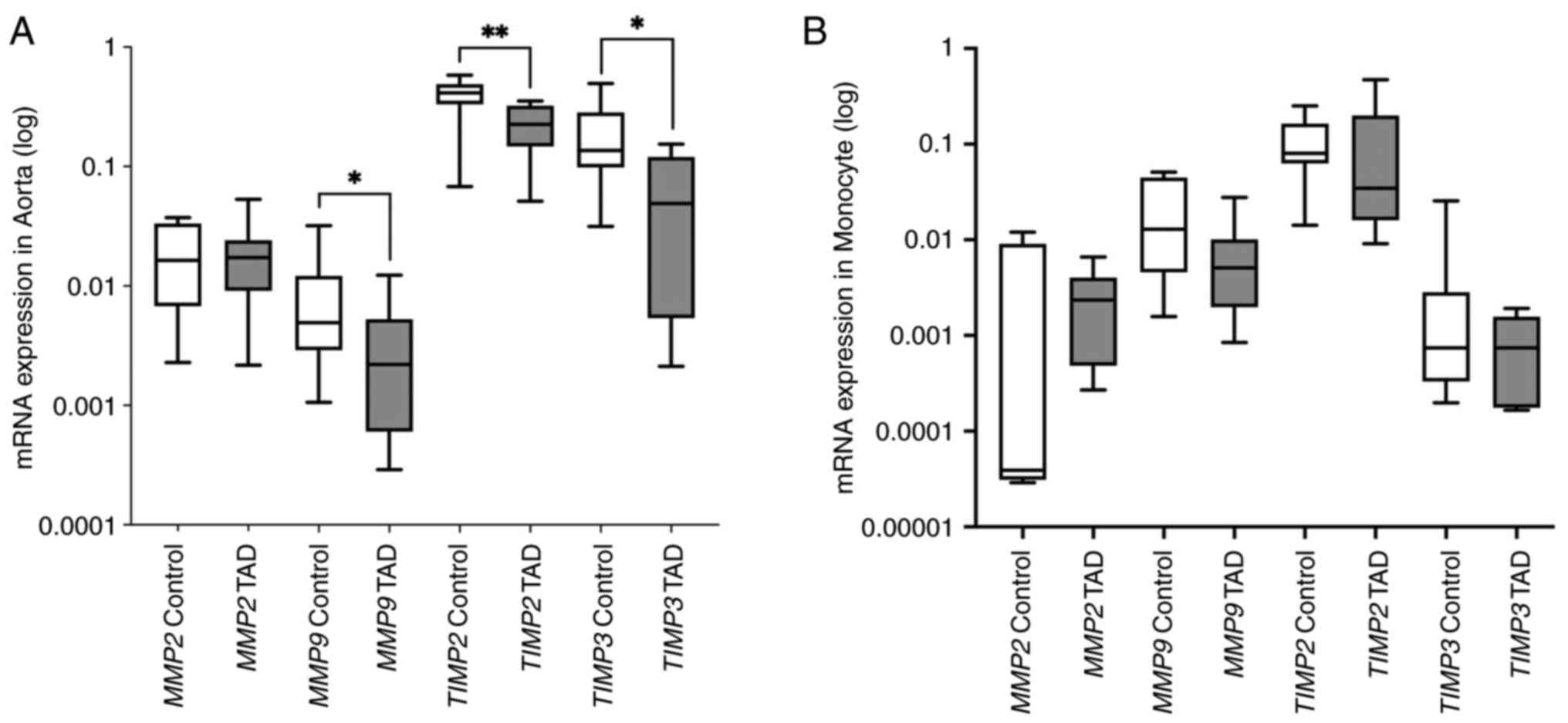
mRNA expression analysis
In the aortic wall tissue, the expression level of
MMP2 did not demonstrate a significant difference between
patients with TAD and the control group (P=0.85). Expression levels
of MMP9, TIMP2 and TIMP3 genes were revealed
to be significantly lower in patients with TAD compared with the
control group (P=0.043, P=0.009 and P=0.028, respectively). In the
monocyte cells, while the expression level of TIMP2
(P=0.248) increased, the expression levels of MMP2
(P=0.148), MMP9 (P=0.114) and TIMP3 (P=0.370)
slightly decreased in patients with TAD compared with the
respective control groups (Table
II). However, the observed changes in the expression level of
any target genes were not statistically significant in monocyte
cells (Fig. 2).
 | Table IImRNA expression levels of target
genes in aortic tissue and monocyte cells. |
Table II
mRNA expression levels of target
genes in aortic tissue and monocyte cells.
| A, Aortic
tissue |
|---|
| | MMP2 | MMP9 | TIMP2 | TIMP3 |
|---|
| Groups | Mean ± SEM | P-value | Mean ± SEM | P-value | Mean ± SEM | P-value | Mean ± SEM | P-value |
|---|
| TAD | 0.0186±0.0045 | | 0.0035±0.0013 | | 0.23±0.035 | | 0.058±0.018 | |
| Control | 0.0187±0.0041 | 0.853 | 0.0088±0.0029 | 0.043a | 0.39±0.049 | 0.009a | 0.180±0.048 | 0.028a |
| B, Monocyte
cells |
| Groups | Mean ± SEM | P-value | Mean ± SEM | P-value | Mean ± SEM | P-value | Mean ± SEM | P-value |
| TAD | 0.028±0.025 | | 0.0074±0.0027 | | 0.12±0.053 | |
0.00081±0.00024 | |
| Control | 0.0030±0.003 | 0.148 | 0.021±0.0067 | 0.114 | 0.11±0.022 | 0.248 | 0.0037±0.0027 | 0.370 |
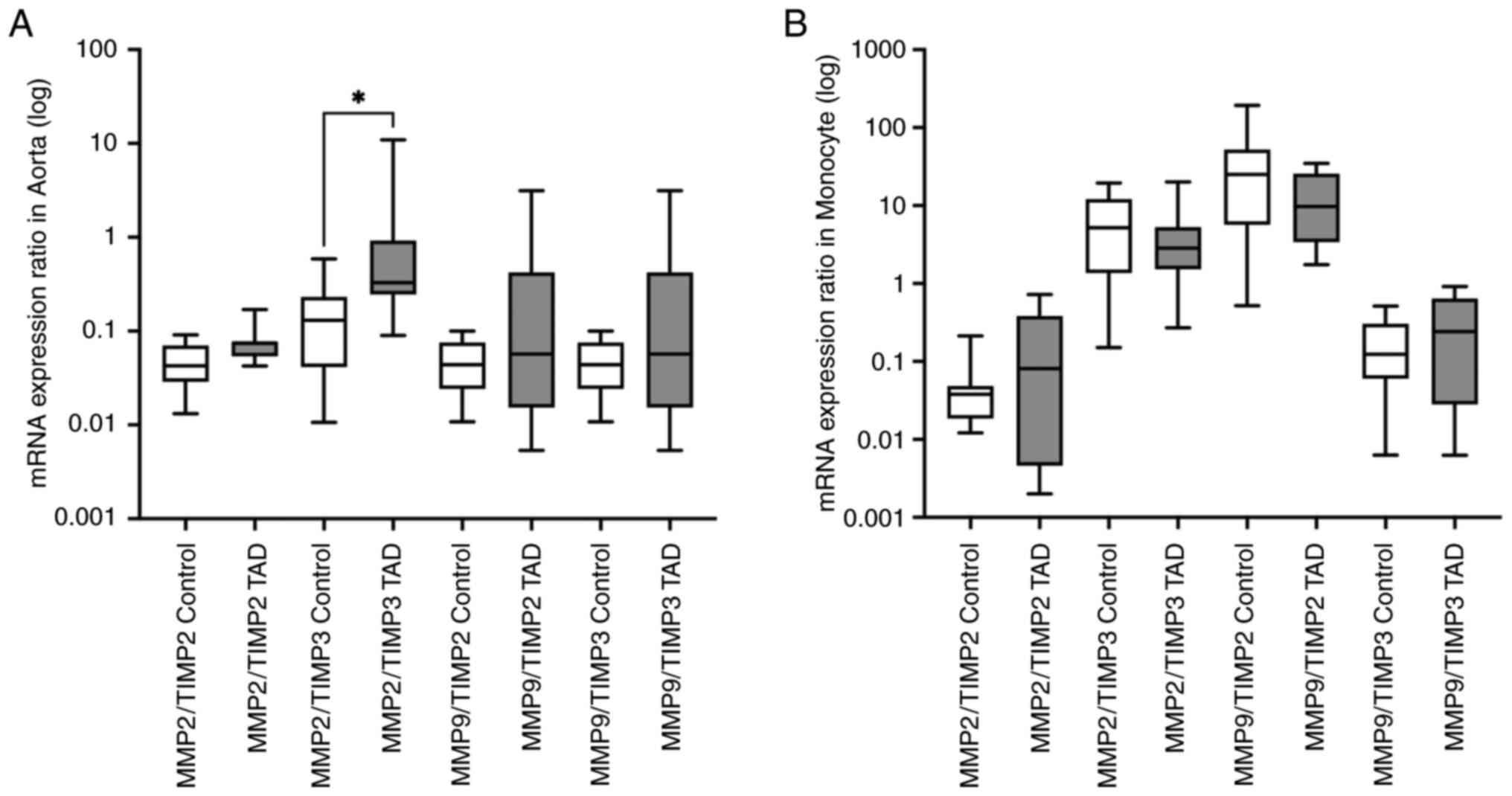
The gene expression level ratios of MMP2/TIMP2,
MMP2/TIMP3, MMP9/TIMP2 and MMP9/TIMP3 were also
calculated in both aorta samples and the circulated monocyte cells.
In the aorta samples, MMP2/TIMP3 (P=0.012) expression ratio
was higher in the patients with TAD compared with the control group
and this difference was statistically significant. There was no
significant difference in MMP2/TIMP2 (P=0.063),
MMP9/TIMP2 (P=0.248) and MMP9/TIMP3 (P=0.796) gene
expression ratios between the tested groups. In monocyte cells,
none of the MMP/TIMP ratios of the examined target genes exhibited
a significant difference between the patients with TAD and the
respective control group. The following values were found:
MMP2/TIMP2 (P=0.447), MMP2/TIMP3 (P=0.681),
MMP9/TIMP2 (P=0.796) and MMP9/TIMP3 (P=0.279)
(Fig. 3).
Findings of the ROC curve analyses of the target
genes, which demonstrated statistically significant changes at the
mRNA expression levels between the patients with TAD and the
control group, are as follows. MMP9 (P=0.041; AUC=0.77; 95%
CI, 0.490-0.971), TIMP2 (P=0.010; AUC=0.840; 95% CI,
0.633-1.00), TIMP3 (P=0.028; AUC=0.800; 95% CI,
0.600-0.999), MMP2/TIMP3 (P=0.013; AUC=0.830; 95% CI,
0.644-1.00). The data are presented in Fig. 4. The correlation analysis was
performed to reveal whether there was a relationship between the
obtained expression levels of the target genes in the aorta and the
monocyte cells. Additionally, the correlation analysis was also
performed to check the possible relationship between the expression
levels of target genes in the same tissue. No significant
correlation was identified in the expression levels of MMP2
(rs=-0.771, P=0.103), MMP9 (rs=0.267,
P=0.493), TIMP2 (rs=0.503, P=0.144) and
TIMP3 (rs=-0.524, P=0.197) genes between the
aorta and the monocyte cells. In addition, a significant positive
correlation between the expression of MMP2 and TIMP2
(rs=0.93, P<0.001) genes in aorta was observed. All
the r and P values are presented in Table III.
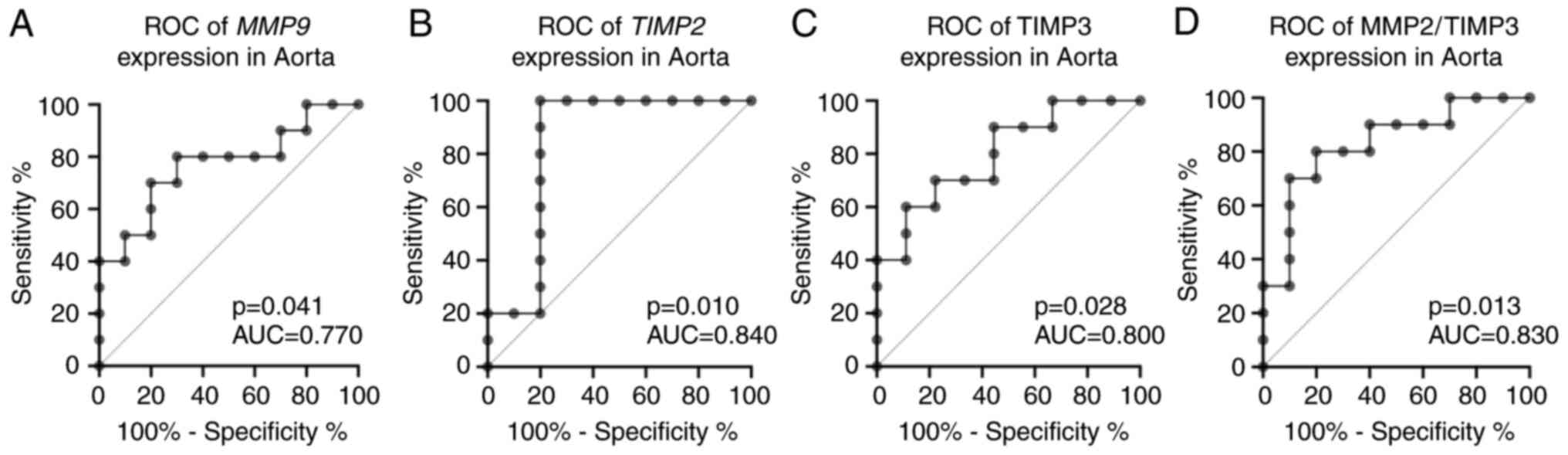 | Figure 4ROC curve analyses and AUC of
MMP9, TIMP2, TIMP3 and MMP2/TIMP3 gene expression
levels in patients with TAD and the control group in aorta. (A)
MMP9 (P=0.041; AUC=0.77; 95% CI, 0.490-0.971), (B)
TIMP2 (P=0.010; AUC=0.840; 95% CI, 0.633-1.00), (C)
TIMP3 (P=0.028; AUC=0.800; 95% CI, 0.600-0.999), (D)
MMP2/TIMP3 (P=0.013; AUC=0.830; 95% CI, 0.644-1.00). ROC,
receiver operating characteristic; AUC, area under curve; CI,
confidence interval; MMP, matrix metalloproteinases; TIMP, tissue
inhibitors of metalloproteinases; TAD, thoracic aortic
dissection. |
 | Table IIISpearman's correlation analysis of
the target genes. |
Table III
Spearman's correlation analysis of
the target genes.
| A, Gene expression
in aortic tissue |
|---|
| | MMP2 | TIMP2 | MMP9 | TIMP3 |
|---|
| Genes | rs | P-value | rs | P-value | rs | P-value | rs | P-value |
|---|
| MMP2 | 1.00 | - | 0.93 |
<0.001a | 0.02 | 0.973 | 0.30 | 0.407 |
| TIMP2 | 0.93 |
<0.001a | 1.00 | - | -0.03 | 0.946 | 0.33 | 0.349 |
| MMP9 | 0.02 | 0.973 | -0.03 | 0.946 | 1.00 | - | 0.04 | 0.918 |
| TIMP3 | 0.30 | 0.407 | 0.33 | 0.349 | 0.04 | 0.918 | 1.00 | - |
| B, Gene expression
in monocyte cells |
| | MMP2 | TIMP2 | MMP9 | TIMP3 |
| Genes | rs | P-value | rs | P-value | rs | P-value | rs | P-value |
| MMP2 | -0.77 | 0.103 | -0.60 | 0.242 | 0.14 | 0.803 | -0.54 | 0.297 |
| TIMP2 | 0.38 | 0.279 | 0.50 | 0.144 | 0.24 | 0.513 | 0.41 | 0.247 |
| MMP9 | -0.05 | 0.912 | 0.15 | 0.708 | 0.26 | 0.493 | -0.60 | 0.097 |
| TIMP3 | -0.21 | 0.619 | -0.07 | 0.882 | 0.17 | 0.703 | -0.52 | 0.197 |
Discussion
TAD is a lethal condition that occurs when there is
a tear in the intima and media layer of the aorta, and can result
in death if not treated immediately. Since the aorta is a
multilayered tissue, it is composed of numerous different types of
cells and molecules. In TAD, a tear in the intima layer extends
along the vessel, providing a new route for blood flow and
weakening the aortic tissue (20).
Although the details of the molecular background and pathogenesis
of TAD have not been completely elucidated, MMPs are one of the
most important players in TAD and are considered to be responsible
for ECM degradation (26,27). Numerous studies have revealed that
the expression levels of MMPs differ in cases of TAD (8,9,17,28).
The importance of MMPs for the pathogenesis of TAD makes it
reasonable to consider their tissue inhibitors as well (14). TIMPs regulate the proteolytic
ability and activities of MMPs (29,30).
The present study aimed to investigate the mRNA gene
expression levels of MMP2, MMP9, TIMP2 and TIMP3
genes in the aortic wall and circulating monocyte cells in patients
with TAD. The mRNA expression level of the MMP2 gene both in the
aortic wall and monocyte cells did not reveal a statistically
significant change in patients with TAD and control subjects
(P=0.85 and P=0.15, respectively). MMP2 can be expressed by
the SMC in the aorta. During formation of TAD, a considerable
amount of SMC might be lost due to apoptosis (31). MMP2 also has an important
role in tissue remodeling in patients with TAD, which can be
considered as an important part of the wound healing process. The
MMP2 level starts to increase after the acute phase in TAD
(32). It is clearly observed that
MMP2 levels can vary, and can either be increased or decreased in
the aortic tissues of patients with TAD, as revealed in previous
studies (8,17). These inconsistencies with regard to
the MMP2 levels may be due to the disease stage differences in
patients with TAD (31).
Additionally, the results of a meta-analysis study supported that
there was no difference between patients with TAD and control
groups for the circulating mRNA expression level of the MMP2 gene
(10). The complex molecular
background of TAD and the fact that MMPs often work cooperatively
with other MMPs and TIMPs to exert different functions (degradation
of ECM and remodeling of tissues) rather than working alone may
explain the undifferentiated gene expression level of MMP2
in cases of TAD.
In addition, it was further revealed in the present
study that the mRNA gene expression level of the MMP9 gene
in aortic tissue was significantly decreased (P=0.043) in patients
with TAD. Even though a decreased mRNA level for the MMP9
gene was also detected in monocyte cells in cases with TAD, the
result was not statistically significant (P=0.114). Moreover, the
results of the MMP9 ROC curve analysis in the aorta revealed
that MMP9 expression levels may be used to differentiate
patients with TAD and the control group (AUC=0.770; P=0.041). It
was revealed that the level of MMP9 did not present a statistically
significant difference in patients with TAD (17). Since MMP9 is an important player in
the wound-healing process in the intima layer of the aorta
(33), it can be inferred that its
measured amount might be affected by the rupture within the onset
of the dissection. By contrast, there are a number of studies that
conclude that the expression level of MMP9 was increased in
patients with TAD whether in aortic tissue or in plasma (8-10,17).
It is known that MMP9 is also stimulated in response to
mechanical injury (34). The
aortic region where the medial dissection begins may be the site of
ECM alteration caused by hemodynamic stress (35,36).
This may explain the changes in the expression level of MMP9
in the patients with TAD (9). The
decreased MMP9 mRNA expression level obtained in patients
with TAD in the present study expresses the level at a particular
time point, not the time-dependent variation. It can be surmised
that the aortic samples obtained during the surgical operation
might not represent the exact same disease stage. The aortic tissue
may still be under the influence of mechanical signaling or the
wound may not have started the healing process. This difference may
lead to different MMP9 levels in patients with TAD. It can
be concluded that further studies are required to confirm the
importance of the findings on MMP9 for improved
understanding of the pathogenesis of TAD.
TIMPs are found in aortic tissue concurrently with
MMPs. Any change in the expression level of a TIMP may change the
MMP/TIMP ratio, resulting in a change in MMP activity (14). In the present study, it was
revealed that there was a statistically significant decrease in the
mRNA expression levels of TIMP2 (P=0.009) and TIMP3
(P=0.028) in the aorta of cases with TAD. Furthermore, ROC curve
analyses of TIMP2 (AUC=0.840; P=0.010) and TIMP3
(AUC=0.800; P=0.028) in aortic tissue demonstrated that patients
with TAD and control groups can be differentiated by using
TIMP2 and TIMP3 mRNA expression levels in aorta. No
significant difference was observed in terms of TIMP2 and
TIMP3 gene expression in monocyte cells. In a previous
study, in which ELISA was used, it was revealed that the expression
level of TIMP2 was decreased in the aorta of cases with TAD. It is
stated that the decreased expression level of TIMP2 might represent
the pre-dissection situation rather than the dissection-related
function such as wound healing (17). In addition, it was revealed that
the mRNA level of TIMP3 was decreased in aortic tissue in
patients with TAD. It was shown that the decrease in TIMP3 level
may have occurred as a result of stimulation by TNF-α and TGF-β
signalling in vitro (37).
Considering the role of TGF-β on ECM component regulation,
fibrosis, and the regulation of MMP2 and MMP9 genes
(1), it can be inferred that the
decrease of TGF-β may directly affect the expression levels of
TIMP3, MMP2, and MMP9. The present study has provided
supporting results to the previous studies (1,17,37)
for TIMP2 and TIMP3 gene expression in patients with
TAD. Since TIMPs are the regulators of the MMPs, the decrease in
their gene expression levels might be associated with the elevated
MMP function or the elevated MMP/TIMP ratio, which may cause
increased degradation in aorta tissue in patients with TAD.
MMP/TIMP ratio is as important as the individual
expression changes of MMP and TIMP genes in explaining the
pathogenesis of TAD. It has been reported that due to impaired
MMP/TIMP balance, MMP functions in the cell are moving toward the
proteolytic state (1,19). Decreased TIMP2/MMP2 results were
also obtained in patients with TAD in a study by Manabe et
al (17), and they supported
the theory that an increased MMP/TIMP ratio has shifted the cell
into the proteolytic stage. In the results of the present study,
the increase of MMP2/TIMP2 (P=0.063), MMP2/TIMP3
(P=0.012) gene expression ratios were revealed in aortic tissue.
Based on these findings, it can be considered that increasing
MMP/TIMP ratios may cause a tendency to increase aortic lysis.
Moreover, ROC curve analysis of the MMP2/TIMP3 expression ratio
revealed that MMP2/TIMP3 (AUC=0.830; P=0.013) may differentiate
patients with TAD from the control group.
The aim of the present study was to also investigate
whether there was a relationship between aortic tissue and monocyte
cells in terms of the expression levels of target genes. As a
result of the correlation analysis, it was revealed that gene
expression levels of MMP2 and TIMP2 exhibited a
positive correlation in the aortic tissue (rs=0.93;
P<0.001). This result supports the close functional relationship
of MMP2 and TIMP2 genes with each other (14,15).
Accordingly, the positive correlation of the mRNA levels of MMPs
and TIMPs, which may alter the MMP/TIMP ratio, might support the
importance of the MMP/TIMP ratio in cases with TAD. Further
analysis may provide further insight with regard to the MMP-TIMP
relationship in patients with TAD.
Additionally, a limitation of the present study is
the absence of protein level analysis. It is clear that performing
protein level analysis would contribute greatly to the validation
of the gene expression data. However, protein analysis could not be
included in the present study due to the limited amount of vascular
material used as control tissue. Considering the risks of dividing
the small amount of tissue into two parts, the obtained material
was only used for RNA isolation in sufficient quantity.
In conclusion, the present study revealed that
MMP9, TIMP2 and TIMP3 genes have differentiated gene
expression in aortic vascular tissue of patients with TAD.
Considering the role of MMPs in matrix maintenance, altered gene
expression might be expected in TAD. However, the altered
expression of MMP inhibitors (TIMPs) in cases with TAD are
noteworthy. It can be acknowledged that TIMPs as well as MMPs have
an important role in understanding the pathogenesis of TAD.
Additionally, it was considered that examining MMPs along with
TIMPs rather than alone is more informative in terms of
understanding the disease pathogenesis. Considering the
statistically significant differences in MMP9, TIMP2 and
TIMP3 gene expression, as well as MMP2/TIMP3 ratio,
it can be inferred that future studies in both aortic tissue and
circulation at the protein level by increasing the sample size may
be an important step for the early diagnosis of TAD. Furthermore,
considering the large number of members of the MMP and TIMP gene
families, it is likely that they affect each other in various
combinations. In accordance with this point of view, the possible
cross-interference of all MMPs and TIMPs are planned to be analyzed
in future studies.
Acknowledgements
Not applicable.
Funding
Funding: The funding of the present study was provided by the
Scientific Research Projects Coordination Unit of Istanbul
University (grant no. 34118).
Availability of data and materials
The data generated in the present study are not
publicly available due to sensitivity reasons but may be requested
from the corresponding author.
Authors' contributions
TK, TG and AA designed the study. AA provided the
patient and control samples through the surgical process. TK
performed the isolations and ddPCR applications. TK and TG
performed the statistical analysis and prepared the manuscript. AA
revised the final version of the manuscript. TK and TG confirm the
authenticity of all the raw data. All authors read and approved the
final version of the manuscript.
Ethics approval and consent to
participate
The present study was approved (approval no.
2019/525) by the Istanbul Medical Faculty Clinical Research Ethics
Committee and the Institute of Graduate Studies in Sciences,
Istanbul University (Istanbul, Turkey). Written informed consent
was obtained from all the participants. All the procedures and
applications conducted in the present study adhere to the tenets of
The Declaration of Helsinki.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Wu D, Shen YH, Russell L, Coselli JS and
LeMaire SA: Molecular mechanisms of thoracic aortic dissection. J
Surg Res. 184:907–924. 2013.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Clouse WD, Hallett JW Jr, Schaff HV,
Spittell PC, Rowland CM, Ilstrup DM and Melton LJ: Acute aortic
dissection: Population-based incidence compared with degenerative
aortic aneurysm rupture. Mayo Clin Proc. 79:176–180.
2004.PubMed/NCBI View
Article : Google Scholar
|
|
3
|
Mészáros I, Mórocz J, Szlávi J, Schmidt J,
Tornóci L, Nagy L and Szép L: Epidemiology and clinicopathology of
aortic dissection. Chest. 117:1271–1278. 2000.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Golledge J and Eagle KA: Acute aortic
dissection. Lancet. 372:55–66. 2008.PubMed/NCBI View Article : Google Scholar
|
|
5
|
He R, Guo DC, Estrera AL, Safi HJ, Huynh
TT, Yin Z, Cao SN, Lin J, Kurian T, Buja LM, et al:
Characterization of the inflammatory and apoptotic cells in the
aortas of patients with ascending thoracic aortic aneurysms and
dissections. J Thorac Cardiovasc Surg. 131:671–678. 2006.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Ishii T and Asuwa N: Collagen and elastin
degradation by matrix metalloproteinases and tissue inhibitors of
matrix metalloproteinase in aortic dissection. Hum Pathol.
31:640–646. 2000.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Ra HJ and Parks WC: Control of matrix
metalloproteinase catalytic activity. Matrix Biol. 26:587–596.
2007.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Koullias GJ, Ravichandran P, Korkolis DP,
Rimm DL and Elefteriades JA: Increased tissue microarray matrix
metalloproteinase expression favors proteolysis in thoracic aortic
aneurysms and dissections. Ann Thorac Surg. 78:2106–2110;
discussion 2110-2101. 2004.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Sangiorgi G, Trimarchi S, Mauriello A,
Righini P, Bossone E, Suzuki T, Rampoldi V and Eagle KA: Plasma
levels of metalloproteinases-9 and -2 in the acute and subacute
phases of type A and type B aortic dissection. J Cardiovasc Med
(Hagerstown). 7:307–315. 2006.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Takagi H, Hari Y, Nakashima K, Kuno T and
Ando T: Matrix metalloproteinases and acute aortic dissection: Et
Tu, Brute? Interact Cardiovasc Thorac Surg. 30:465–476.
2020.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Wang X, LeMaire SA, Chen L, Shen YH, Gan
Y, Bartsch H, Carter SA, Utama B, Ou H, Coselli JS and Wang XL:
Increased collagen deposition and elevated expression of connective
tissue growth factor in human thoracic aortic dissection.
Circulation. 114 (1 Suppl):I200–I205. 2006.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Chung AW, Yang HH, Radomski MW and van
Breemen C: Long-term doxycycline is more effective than atenolol to
prevent thoracic aortic aneurysm in marfan syndrome through the
inhibition of matrix metalloproteinase-2 and -9. Circ Res.
102:e73–e85. 2008.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Chow AK, Cena J and Schulz R: Acute
actions and novel targets of matrix metalloproteinases in the heart
and vasculature. Br J Pharmacol. 152:189–205. 2007.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Wang X and Khalil RA: Matrix
metalloproteinases, vascular remodeling, and vascular disease. Adv
Pharmacol. 81:241–330. 2018.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Bourboulia D and Stetler-Stevenson WG:
Matrix metalloproteinases (mmps) and tissue inhibitors of
metalloproteinases (timps): Positive and negative regulators in
tumor cell adhesion. Semin Cancer Biol. 20:161–168. 2010.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Arpino V, Brock M and Gill SE: The role of
timps in regulation of extracellular matrix proteolysis. Matrix
Biol. 44-46:247–254. 2015.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Manabe T, Imoto K, Uchida K, Doi C and
Takanashi Y: Decreased tissue inhibitor of
metalloproteinase-2/matrix metalloproteinase ratio in the acute
phase of aortic dissection. Surg Today. 34:220–225. 2004.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Lesauskaite V, Tanganelli P, Sassi C, Neri
E, Diciolla F, Ivanoviene L, Epistolato MC, Lalinga AV,
Alessandrini C and Spina D: Smooth muscle cells of the media in the
dilatative pathology of ascending thoracic aorta: Morphology,
immunoreactivity for osteopontin, matrix metalloproteinases, and
their inhibitors. Hum Pathol. 32:1003–1011. 2001.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Chow BS, Chew EG, Zhao C, Bathgate RA,
Hewitson TD and Samuel CS: Relaxin signals through a
rxfp1-perk-nnos-no-cgmp-dependent pathway to up-regulate matrix
metalloproteinases: The additional involvement of inos. PLoS One.
7(e42714)2012.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Shen YH and LeMaire SA: Molecular
pathogenesis of genetic and sporadic aortic aneurysms and
dissections. Curr Probl Surg. 54:95–155. 2017.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Samadzadeh KM, Chun KC, Nguyen AT, Baker
PM, Bains S and Lee ES: Monocyte activity is linked with abdominal
aortic aneurysm diameter. J Surg Res. 190:328–334. 2014.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Lu L, Tong Y, Wang W, Hou Y, Dou H and Liu
Z: Characterization and significance of monocytes in acute stanford
type B aortic dissection. J Immunol Res.
2020(9670360)2020.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Zhang L, Yu C, Chang Q, Luo X, Qiu J and
Liu S: Comparison of gene expression profiles in aortic dissection
and normal human aortic tissues. Biomed Rep. 5:421–427.
2016.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Hindson BJ, Ness KD, Masquelier DA,
Belgrader P, Heredia NJ, Makarewicz AJ, Bright IJ, Lucero MY,
Hiddessen AL, Legler TC, et al: High-throughput droplet digital PCR
system for absolute quantitation of DNA copy number. Anal Chem.
83:8604–8610. 2011.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Hindson CM, Chevillet JR, Briggs HA,
Gallichotte EN, Ruf IK, Hindson BJ, Vessella RL and Tewari M:
Absolute quantification by droplet digital PCR versus analog
real-time PCR. Nat Methods. 10:1003–1005. 2013.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Iyer RP, Patterson NL, Fields GB and
Lindsey ML: The history of matrix metalloproteinases: Milestones,
myths, and misperceptions. Am J Physiol Heart Circ Physiol.
303:H919–H930. 2012.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Coady MA, Rizzo JA, Goldstein LJ and
Elefteriades JA: Natural history, pathogenesis, and etiology of
thoracic aortic aneurysms and dissections. Cardiol Clin.
17:615–635, vii. 1999.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Weis-Müller BT, Modlich O, Drobinskaya I,
Unay D, Huber R, Bojar H, Schipke JD, Feindt P, Gams E, Müller W,
et al: Gene expression in acute stanford type A dissection: A
comparative microarray study. J Transl Med. 4(29)2006.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Djuric T and Zivkovic M: Overview of MMP
biology and gene associations in human diseases. In: The Role of
Matrix Metalloproteinase in Human Body Pathologies. IntechOpen,
London, 2017.
|
|
30
|
Brew K and Nagase H: The tissue inhibitors
of metalloproteinases (timps): An ancient family with structural
and functional diversity. Biochim Biophys Acta. 1803:55–71.
2010.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Zhang X, Wu D, Choi JC, Minard CG, Hou X,
Coselli JS, Shen YH and LeMaire SA: Matrix metalloproteinase levels
in chronic thoracic aortic dissection. J Surg Res. 189:348–358.
2014.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Akiyama M, Ohtani H, Sato E, Nagura H and
Tabayashi K: Up-regulation of matrix metalloproteinase-2 and
membrane-type 1-matrix metalloproteinase were coupled with that of
type I procollagen in granulation tissue response after the onset
of aortic dissection. Virchows Arch. 448:811–821. 2006.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Schneiderman J, Bordin GM, Adar R,
Smolinsky A, Seiffert D, Engelberg I, Dilley RB, Thinnes T and
Loskutoff DJ: Patterns of expression of fibrinolytic genes and
matrix metalloproteinase-9 in dissecting aortic aneurysms. Am J
Pathol. 152:703–710. 1998.PubMed/NCBI
|
|
34
|
James TW, Wagner R, White LA, Zwolak RM
and Brinckerhoff CE: Induction of collagenase and stromelysin gene
expression by mechanical injury in a vascular smooth muscle-derived
cell line. J Cell Physiol. 157:426–437. 1993.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Schlatmann TJ and Becker AE: Pathogenesis
of dissecting aneurysm of aorta. Comparative histopathologic study
of significance of medial changes. Am J Cardiol. 39:21–26.
1977.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Roberts WC: Aortic dissection: Anatomy,
consequences, and causes. Am Heart J. 101:195–214. 1981.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Kimura N, Futamura K, Arakawa M, Okada N,
Emrich F, Okamura H, Sato T, Shudo Y, Koyano TK, Yamaguchi A, et
al: Gene expression profiling of acute type A aortic dissection
combined with in vitro assessment. Eur J Cardiothorac Surg.
52:810–817. 2017.PubMed/NCBI View Article : Google Scholar
|