Introduction
The transient receptor potential channel canonical 5
(TRPC5) is localized in the cell membrane and capable of conducting
mono- and divalent ions such as Na+, Ca2+, or
Mg2+ along their electrochemical gradient into the cell
(1). By doing so, TRPC5 can
depolarize excitable cells or activate various downstream signaling
pathways (1,2). The channel can be activated by
signaling pathways or other stimuli such as mechanical stress or
ligands (1,2). Therefore, TRP channels, to which
TRPC5 belongs, are often referred to as multimodal sensory proteins
(1). Next to the canonical (TRPC)
subfamily, the polycystin (TRPP), mucolipin (TRPML), vanilloid
(TRPV), melastatin (TRPM), and ankyrin (TRPA) have been described.
TRP channels share structural similarities and are typically
tetrameters. Each monomer consists of six transmembrane domains
with an amino- and carboxyl-terminal end that extends into the
cytoplasm. Among the different groups and members, these endings
exhibit major structural differences (1,2).
The most important mechanism of stimulation of TRPC5
is the activation through G proteins and phospholipases C (3,4).
While the function of TRPC5 as a store-operated channel is still
being discussed, TRPC5 seems to be a major regulator of cellular
Ca2+-homeostasis (3,5). In
smooth muscle cells, TRPC5's role as a regulator of cellular
Ca2+-homeostasis is critical for cell functionality
(6). TRPC5 has been reported in
different localizations that include the central nervous system,
the kidney, and the cardiovascular system (3,5). In
the lung, TRPC5 has been detected in smooth muscle cells, of
airways and of venous and arterial blood vessels, as well as in
neuroepithelial bodies of the intrapulmonary airway (7–11). In
their study, Peng et al (7)
isolated rat pulmonary venous smooth muscle cells and detected
TRPC5 messenger ribonucleic acid (mRNA) by performing real-time
polymerase chain reaction (RT-PCR). Similarly, Lu et al
(8) detected TRPC5 mRNA in rat
pulmonary arterial smooth muscle cells. White et al
(9) detected TRPC5 in human airway
smooth muscle cells through western blot analysis and the
corresponding mRNA through RT-PCR. In airway smooth muscle cells of
guinea pigs TRPC5 mRNA has also been detected using RT-PCR by Ong
et al (10). Finally,
Lembrechts et al (11)
detected the TRPC5-protein using immunohistochemical staining in
neuroepithelial bodies of mice in the intrapulmonary airway
epithelium. These are clusters of pulmonary neuroendocrine cells
(12). Additionally, there is also
evidence that TRPC5 is present in macrophages (13). Tao et al (14) provided evidence suggesting that
TRPC5 in macrophages may inhibit the polarization into a
proinflammatory M1 phenotype, by showing that macrophages of the M1
phenotype were increased in the aortic wall of TRPC5-knockout mice.
Another study, conducted by Pereira et al (15) also revealed that TRPC4/TRPC5
complexes in macrophages may mediate a protective role in sepsis.
Although TRPC5 appears to be important for the physiological
function of macrophages, to our knowledge, it has not been
described in lung-specific alveolar macrophages.
A recent study, conducted by Yang et al
(16) provided evidence that TRPC5
promotes proliferation and invasion of cancer cells through the
upregulation of the hypoxia-inducible factor-1α (HIF-1α) signaling
pathway in papillary thyroid carcinoma. Similar findings in colon
carcinoma cells were presented by Chen et al (17). Furthermore, overexpression of both
TRPC5 and dysregulation of cellular Ca2+-homeostasis are
associated with higher levels of p-glycoproteins (p-GP) in the
plasma membrane of cancer cells. P-GP are associated with the
outward transfer of chemotherapeutic drugs like paclitaxel or
adriamycin. Thus, TRPC5 may play an important role in the
development of chemoresistance in cancer cells (18). To our knowledge, the role of TRPC5
in lung cancer has not been subjected to extensive research
yet.
In summary, TRPC5 has already been detected in many
cell types as a critical player in physiological processes
(3). While some studies have
already detected TRPC5 in the lung, only a few cell types have been
examined. Most studies used single cell cultures, animal tissue, or
detected the levels of TRPC5 mRNA and not the protein directly.
Furthermore, many studies focused on the function and regulation of
TRPC5. While these aspects are important, knowledge of the
localization of TRPC5 in the human lung is critical to acquire a
better understanding of the channel. Therefore, the aim of the
present study was to detect the TRPC5-protein in the human lung
using immunohistochemistry and to determine its histological
distribution profile.
Materials and methods
Specimens
All samples used in this study originated from
voluntary body donors (n1=6) from the body donation
program of Saarland University's Institute for Anatomy and Cell
Biology. All samples were collected in April 2023. All sexes, ages,
body mass indices (BMI), and causes of death were susceptible to
inclusion. No specific exclusion criteria were applied. While four
of the body donors were male, two were female. All donors died of
natural causes. One donor died of pneumonia. Due to limited data,
we could not evaluate the bacteria that caused the infection or
whether the pneumonia was community- or hospital-acquired. The body
donor's characteristics are displayed in Table I. Following their decease, the body
donors were preserved using nitrite pickling salt and ethanol (NEP)
according to the protocol used in a previous study conducted by
Janczyk et al (19). There
is evidence that NEP preservation causes less protein denaturation
and retains antigenicity better than formaldehyde-based
preservation (20,21). After NEP fixation, a median
thoracotomy was performed to access the donor's lung. From each
donor, we collected 4 samples from different regions of the lung,
including the left and the right apex, the basal region of the
right lung, and the central region near the hilus of the right
lung. Out of these 24 samples, 10 (n2=10) were selected
for immunostaining; For tissue comparisons between donors, samples
taken from the left apex of each doner's lung were selected as they
provided the best overall tissue quality of each extraction site
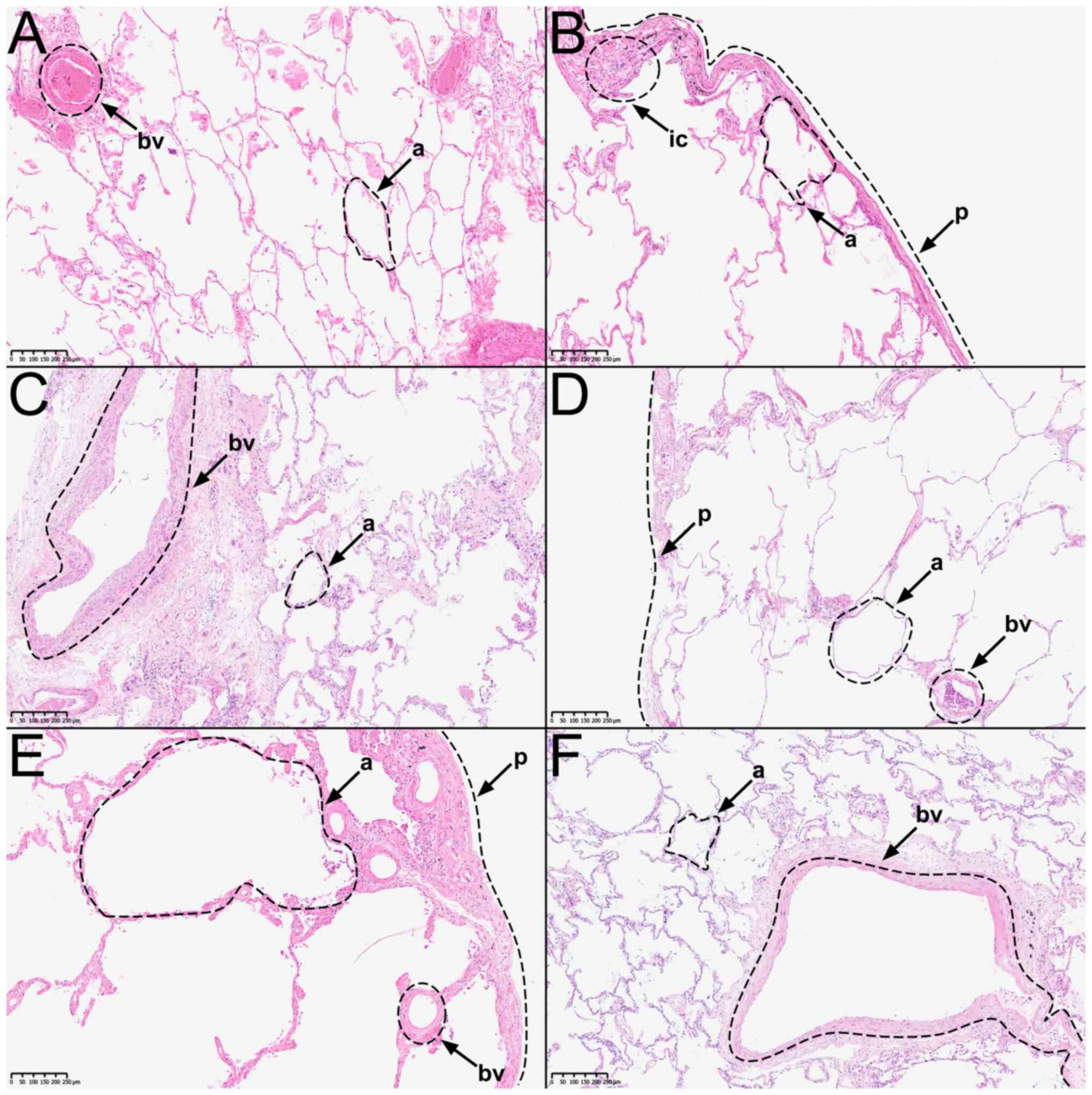
(Fig. 1A-F). Similarly, all
samples taken from donor 5 were selected to evaluate differences
between extraction sites. To further evaluate the difference
between peripheral and central extraction sites, an additional
sample from the hilus-near extraction site of donor 4 was
selected.
 | Table IBody donors' characteristics. |
Table I
Body donors' characteristics.
| Body donor | Sex | Age, years | Date of death,
month/year | Time between death
and preservation, days | Cause of death | Further clinical
conditions |
|---|
| 1 | M | 83 | 03/2023 | 1 | Metastatic prostate
carcinoma | Arterial
hypertension, pulmonary embolism (S/P) |
| 2 | M | 92 | 03/2023 | 2 | Pneumonia | Renal failure,
aortic valve replacement (S/P) |
| 3 | F | 80 | 03/2023 | 4 | Upper
gastrointestinal bleeding | Cardiac arrhythmia,
coronary artery disease |
| 4 | M | 81 | 03/2023 | 4 | Multi-organ
failure | Metastatic
urothelial carcinoma |
| 5 | F | 79 | 03/2023 | 4 | Heart failure | Absolute
tachyarrhythmia |
| 6 | M | 72 | 03/2023 | 3 | Multi-organ
failure | Pulmonary
insufficiency, diabetes mellitus type II |
For positive control, we gained tissue samples of
the heart, as TRPC5 has previously been detected in that tissue
(22). Afterwards, the samples
were processed using 4% formaldehyde and embedded in paraffin as
described before (23). A tissue
thickness of 6 μm was obtained using a microtome. Slices were then
placed onto microscope slides.
Immunohistochemistry
Hematoxylin & eosin (H&E) staining was
performed as previously described by Diebolt et al (23). Fig.
1 shows representative images of H&E-stained samples from
each body donor. For immunostaining, heat-induced epitope retrieval
(HIER) was performed to ensure complexing of the antibody with the
antigen (24). The samples were
placed in 1% citrate-buffer solution and incubated at 95˚C for up
to 60 min. After HIER, the samples were washed in
phosphate-buffered saline (PBS) and normal goat serum (NGS), used
as blocking solution, was added (cat. no. 01-6201; Invitrogen AG;
Thermo Fisher Scientific, Inc.). To ensure proper binding of the
knockout-validated primary anti-TRPC5 antibody (cat. no. ACC-020;
Alomone Labs) solution (1:50 in NGS), samples were incubated for 12
h at room temperature. The negative control group was incubated
with rabbit serum instead (Institute for Medical Biochemistry and
Molecular Biology, Saarland University). The samples were then
washed again with PBS and incubated in a 3%
H2O2 solution for 10 min to deactivate the
endogenous peroxidases. Goat anti-rabbit antibody, in conjunction
with horseradish peroxidase (1:500 in NGS), was added (cat. no.
A10547 Invitrogen AG; Thermo Fisher Scientific, Inc.). The samples
were incubated for 45 min at room temperature and washed with PBS.
In order to visualize the antibody binding sites, we incubated the
samples with diaminobenzidine (DAB) for 10 min (cat. no. SK-4103;
Vector Laboratories, Inc.). The samples were counterstained with
hematoxylin before cover slipping.
Analysis
The anti-TRPC5-stained samples (n2=10)
were analyzed under a light microscope equipped with a digital
camera (MikroCam SP 5.1; Bresser, GmbH). The staining signals were
then classified into three groups, based on their intensity, and
ranging from no staining signal to medium and strong staining
signals. Samples with no staining signal will only display the
blue-colored counterstain. Samples with a light to dark orange
color are considered to have a medium staining signal, the blue
counterstain may still be visible in these samples. Strongly
stained samples present a light to dark brown color; In these
samples, the blue counterstain is almost no more visible.
Subsequently, H&E- and immunohistochemically-stained samples
were digitalized by using the Nano Zoomer S210 (Hamamatsu, Japan)
to obtain representative high-resolution images (Figs. 1 and 2).
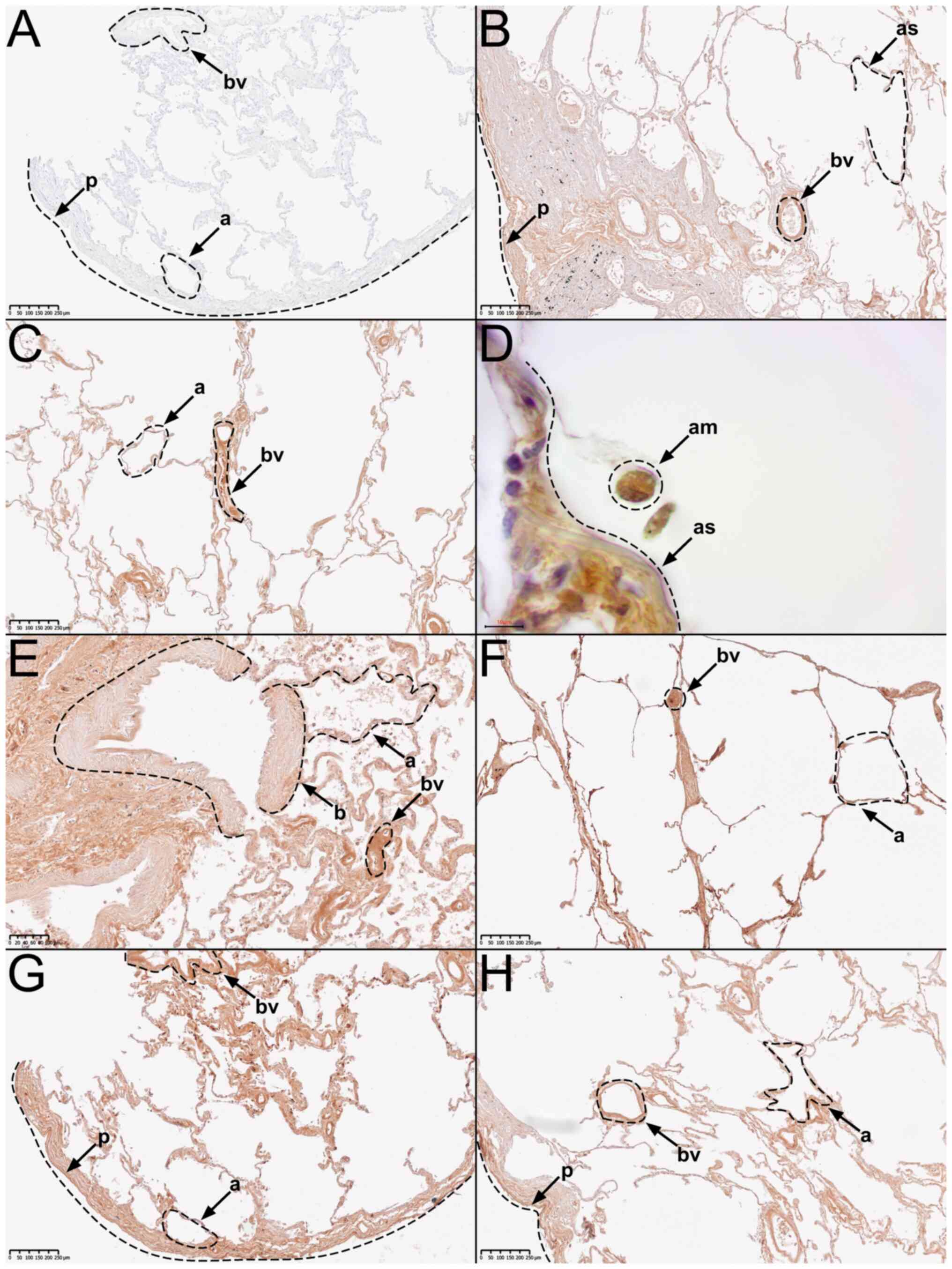 | Figure 2Representative immunohistochemical
staining using a primary knockout-validated anti-TRPC5 antibody.
(A) Negative control staining. (B) Tissue from body donor 1, tissue
from donor 2 in (C) low and (D) high magnification, (E) tissue from
donor 3, (F) tissue from donor 4, (G) tissue from donor 5, and (H)
tissue from donor 6. AM, alveolar macrophage; A, alveolus; AS,
alveolar septum; B, bronchiole; BV, blood vessel; P, pleura. |
Results
To confirm that the samples' histological structures
were intact, hematoxylin-eosin staining (H&E) was performed.
The overall histologic structure of all samples was intact.
Alveoli, blood vessels, and bronchioles were recognizable.
Proteolysis was limited and artifacts such as tissue detachment
were minimal. Donors 1, 2, 4, and 5 presented an emphysematous
enlargement of the alveoli (Fig.
1A, B, D, and E). In addition, eosinophilic exudate was
detectable in the alveoli of donor 1 (Fig. 1A). Samples taken from donor 2 show
slight signs of infiltrating immune cells in the subpleural
connective tissue. Bacterial infiltration is not visible (Fig. 1B). Donors 3, 5, and 6 showed signs
of fibrotic remodeling of the interalveolar septa and subpleural
tissue (Fig. 1C, E, and F). Furthermore, the incorporation of
anthracotic pigments into the connective tissue of the lung was
identifiable in all donors. There were no signs of malignant cell
infiltration in any sample. Sample quality was ultimately deemed
acceptable, as the general histologic lung structure of donors 1-6
was intact and artifacts due to tissue processing and embedding
were minimal.
In the overview of anti-TRPC5-stained samples
(n2=10), two presented an overall strong and eight a
medium staining signal, while none presented a negative staining
signal.
Further analysis, revealed that all major
histological structures of the human lung showed a medium to strong
immunostaining signal for TRPC5. This includes pleura, subpleural
connective tissue, pulmonary arteries and veins, bronchioles,
alveolar septa, type 1 and 2 pneumocytes, and alveolar macrophages
(Fig. 2).
All samples showed significant differences compared
to the negative control (Fig. 2A).
It can therefore be assumed that the TRPC5-protein is ubiquitously
distributed in human lung tissue. Fig.
2 displays representative images of important histological
features.
We also compared the differences in anti-TRPC5
staining signal between the male and the female donors, while
excluding the male donor, who died of pneumonia (donor 2). Two of
the three remaining male donors presented a medium staining signal
(Fig. 2B and H), while one showed a strong staining
signal (Fig. 2F). The tissue from
both female donors presented a medium staining signal (Fig. 2E and G).
The comparison between the tissue from the male
donor (donor 2) who died of pneumonia, and the other five donors
without pneumonia revealed the following: The tissue from donor 2
presented a strong staining signal (Fig. 2C). In contrast, only one of the
five donors without pneumonia presented a strong staining signal
(Fig. 2F), while the other four
presented a medium staining signal (Fig. 2B, E, G, and
H).
Discussion
The anti-TRPC5 antibody we employed has already been
used previously for immunohistochemistry (25,26).
Additionally, the antibody has been validated on TRPC5-knockout
mice in a previous study (27). We
therefore assume that the antibodies were sufficiently specific to
be used in our experiments. In each staining run, we included
negative controls of the lung and positive controls of the heart.
To ensure that DAB was converted by the horseradish peroxidase of
the antibody and not by the endogenous peroxidases of the examined
tissue, we deactivated the endogenous peroxidases by treating the
tissue with 3% H2O2 solution, as
aforementioned.
Whilst TRPC5 has already been detected in a few cell
types of the lung, most studies have used cell cultures, animal
cells or simply investigated the expression of TRPC5 mRNA. To our
best knowledge, our study is the first to report TRPC5 in human
lung tissue using immunohistochemistry. However, our study design
has certain methodological limitations; The average age of the body
donors we used was 81.3 years. Each donor died of natural causes.
One of the donors died of pneumonia, caused by a bacterial
infection. For staining, we used ten samples obtained from six body
donors, four of which were male and two female. On one side the
sample collective was rather small, since body donor availability
is often restricted. Therefore, further validating research is
needed to enable a generalized statement about the distribution and
expression of the TRPC5-protein in the human lung. On the other
side, the donors were of advanced age and because of that, the
tissue might be prone to pathophysiological changes. The quality of
the samples was assessed beforehand, using H&E staining, and
deemed acceptable. However, some more subtle pathophysiological
changes cannot be detected by this method. Due to the advanced age
of the body donors, the distribution of the TRPC5-protein in lungs
of younger specimens remains elusive. For instance, expression
levels of various proteins are known to change throughout lifetime
(28). Therefore, the distribution
and expression intensity of TRPC5 may differ in younger
individuals. Ultimately, the sole use of elderly body donors
presents a methodological limitation of our study. In order to
evaluate possible differences, further research should be conducted
based on lung samples of younger specimens. However, this may prove
complicated because of the rarity of young body donors, as most
body donors die at an advanced age. Samples from younger, living
donors could also be obtained from patients who have undergone
biopsy or surgery for diagnostic or therapeutic purposes.
In our study we did not apply any exclusion criteria
to the body donors. This poses a limitation to our study, as lung
diseases could present confounding variables.
As mentioned above, we also compared the staining
signals from lung tissues between male and female specimens. While
one of the sections from the male donors presented a strong
staining signal, and none of the female sections did, we cannot
make a statement about the differences between male and female lung
tissue in terms of TRPC5 immunostaining levels. Our sample size is
too small and unbalanced in terms of sex distribution. For the
comparison between the tissue of the male donor (donor 2) who died
of pneumonia and the other five donors without pneumonia similar
circumstances applied. While the tissue from donor 2 shows a strong
staining signal, the sample of donor 4 also presents a strong
staining signal. The sample size is too small to draw conclusions
about differences in TRPC5 immunostaining levels. In addition, an
infiltration of inflammatory cells in the lung tissue of donor 2
could cause changes to the staining signal due to the
superimposition of positively and negatively stained cells.
Therefore, staining signals could be misinterpreted. However, we
found only slight signs of infiltration (Fig. 1B), and in most segments of the
immunostained samples, no pneumonic infiltration is visible
(Fig. 2C).
As mentioned above, we also stained all samples
obtained from donor 5 to evaluate differences between extraction
sites. Yet, we were unable to detect any differences. Similarly, we
compared the sample from the central hilus-near extraction site of
donors 4 and 5 with the peripheral lung samples. Again, no
differences were detected. However, these findings are only
preliminary. Due to the small sample size, we cannot make any
statement about the difference in anti-TRPC5 staining signals
between extraction sites or the difference between central and
peripheral regions of the lung.
The fact that we only used immunostaining to detect
the TRPC5-protein in the human lung is a restriction of our study.
Further assays should be performed to solidify the evidence that
TRPC5 is widely distributed in the human lung. This would also
enable supportive investigation of possible differences in
TRPC5-expression between donors with different characteristics,
such as sex and age or between healthy and diseased tissue.
Another factor that needs to be discussed are the
possible postmortem changes in protein levels and histological
structures. In the present study, a major factor that contributes
to unwanted postmortem changes is the time between the onset of
death and the beginning of the preservation process. The start of
preservation can be delayed by various factors like long transport
time, delayed determination of death, or by official administrative
matters. In our case, there were 1-4 days between the onset of
death and the beginning of the preservation process. In a previous
study, Cocariu et al (29)
examined the correlation of refrigeration time and autolytic
histological changes in lung tissue. They concluded that the
deterioration of histological structures correlates with the time
spent in the refrigeration unit (29). Protein levels could also be lowered
due to autolysis. However, this process can be slowed down by lower
temperatures (30). It is
therefore essential that body donors are processed quickly after
death. If this is not possible, the donor should be stored in a
refrigeration unit until preservation.
To our knowledge, we were the first to detect the
TRPC5-protein in human alveolar macrophages, by using
immunohistochemistry. As mentioned in the introduction, Tao et
al (14) showed that TRPC5
inhibits macrophage polarization into a proinflammatory phenotype
and Pereira et al (15)
suggested that TRPC5 in macrophages may play a protective role in
sepsis. Thus, TRPC5 appears to be important for macrophage
function. Alveolar macrophages are major players in the innate
immune response and are critical for the proper maintenance of lung
homeostasis (14,31). The topic of TRPC5 in alveolar
macrophages may therefore be a promising area of research to better
understand inflammatory lung diseases such as chronic obstructive
pulmonary disease (COPD) (32).
To further solidify the evidence that TRPC5 is
expressed in human alveolar macrophages, other experiments, such as
RT-PCR to detect TRPC5 mRNA or Western blot to directly detect the
TRPC5-protein could be performed.
Considering previous studies on the physiological
function of TRPC5, a dysregulation of the channel may contribute to
the pathogenesis of several lung diseases. These studies showed
that disturbed cellular Ca2+-homeostasis in airway
smooth muscle may be due to dysregulation of TRPC5 (7,10,33,34).
In smooth muscle cells, dysregulation of cellular
Ca2+-homeostasis is associated with bronchial asthma,
pulmonary hypertension, and COPD (6,7,35).
Moreover, the study by Chen et al (36) pointed out that impaired
TRPC5-expression may contribute to the proliferation of pulmonary
artery smooth muscle cells. Therefore, TRPC5 may become a target
for pharmacological intervention in such conditions (6,37).
In this regard, TRPC5 has already been identified as
a target for multiple pharmacological agents such as clemizole
hydrochloride, GFB-887 or AC1903 (37-39).
It is likely that other compounds will follow. Some of them may
even be considered as candidates for the treatment of diseases that
involve TRPC5-dysregulation (37).
Interestingly, the TRPC5-inhibitor GFB-887 has already entered
clinical trials, in patients with focal segmental
glomerulosclerosis, treatment-resistant minimal change disease, and
diabetic nephropathy (39).
As mentioned in the introduction, dysregulation of
TRPC5 may be involved in the promotion of chemoresistance,
proliferation, and invasion of cancer cells. It may be interesting
to further investigate the role of TRPC5 in lung cancer. In that
regard, TRPC5 could also present a drug target in cancer cells
(16-18).
In conclusion, it can be stated that TRPC5 is likely
to play an important role in pulmonary cell function, particularly
in cellular cation homeostasis. Dysregulation of cellular cation
homeostasis is associated with various lung diseases such as
bronchial asthma and pulmonary hypertension. Previous studies have
already implicated dysregulation of TRPC5 as part of the
pathogenesis of such lung diseases (6,7,10,33-35).
Our study suggests that TRPC5 is widely distributed throughout the
human lung. Pharmacological agents targeting TRPC5 have already
been discovered. Therefore, TRPC5 may present a new target for the
treatment of various lung diseases (37-39).
Since the importance of TRPC5 in such pharmacological interventions
is highly speculative at present, it is clear that the properties
of TRPC5 and its involvement in lung physiology and pathophysiology
should be further investigated.
Acknowledgements
The authors would like to thank Dr Dirk Schaudien
(Frauenhofer Institute for Toxicology and Experimental Medicine,
Hannover, Germany) for digitalizing the samples, as well as Ms.
Irina Scheck, Ms. Katja Schäfer, Mr. Alexander Grissmer and Ms.
Kerstin Simon (Institute of Anatomy and Cell Biology, Saarland
University, Homburg, Germany) for the technical support.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
TT and FU planned and conducted the study. FU
performed the experiments. FU, FF, TT, CMD and CNE evaluated and
interpreted the data. FU and TT confirm the authenticity of all the
raw data. FU wrote the first draft of the manuscript. TT, CMD and
CNE reviewed and edited the manuscript. All authors read and
approved the final version of the manuscript.
Ethics approval and consent to
participate
All body donors gave written consent during their
lifetime for their bodies and tissues to be used for scientific
research. In addition, the conducted study was approved by the
ethical commission of the Saarland Medical Association (Ärztekammer
des Saarlandes) under the approval number 163/20. The
responsibility of the above mentioned committee for the conducted
study was approved by the Dean of the Saarland University and this
document was deposited at the publisher (Spandidos
Publications).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Ramsey IS, Delling M and Clapham DE: An
introduction to TRP channels. Annu Rev Physiol. 68:619–647.
2006.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Venkatachalam K and Montell C: TRP
channels. Annu Rev Biochem. 76:387–417. 2007.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Zholos AV: TRPC5. Handb Exp Pharmacol.
222:129–156. 2014.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Won J, Kim J, Jeong H, Kim J, Feng S,
Jeong B, Kwak M, Ko J, Im W, So I and Lee HH: Molecular
architecture of the Gαi-bound TRPC5 ion channel. Nat Commun.
14(2550)2023.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Du SL, Jia ZQ, Zhong JC and Wang LF: TRPC5
in cardiovascular diseases. Rev Cardiovasc Med. 22:127–135.
2021.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Dietrich A, Chubanov V, Kalwa H, Rost BR
and Gudermann T: Cation channels of the transient receptor
potential superfamily: Their role in physiological and
pathophysiological processes of smooth muscle cells. Pharmacol
Ther. 112:744–760. 2006.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Peng G, Lu W, Li X, Chen Y, Zhong N, Ran P
and Wang J: Expression of store-operated Ca2+ entry and transient
receptor potential canonical and vanilloid-related proteins in rat
distal pulmonary venous smooth muscle. Am J Physiol Lung Cell Mol
Physiol. 299:L621–L630. 2010.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Lu W, Wang J, Shimoda LA and Sylvester JT:
Differences in STIM1 and TRPC expression in proximal and distal
pulmonary arterial smooth muscle are associated with differences in
Ca2+ responses to hypoxia. Am J Physiol Lung Cell Mol Physiol.
295:L104–L113. 2008.PubMed/NCBI View Article : Google Scholar
|
|
9
|
White TA, Xue A, Chini EN, Thompson M,
Sieck GC and Wylam ME: Role of transient receptor potential C3 in
TNF-alpha-enhanced calcium influx in human airway myocytes. Am J
Respir Cell Mol Biol. 35:243–251. 2006.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Ong HL, Brereton HM, Harland ML and
Barritt GJ: Evidence for the expression of transient receptor
potential proteins in guinea pig airway smooth muscle cells.
Respirology. 8:23–32. 2003.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Lembrechts R, Brouns I, Schnorbusch K,
Pintelon I, Timmermans JP and Adriaensen D: Neuroepithelial bodies
as mechanotransducers in the intrapulmonary airway epithelium:
involvement of TRPC5. Am J Respir Cell Mol Biol. 47:315–323.
2012.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Domnik NJ and Cutz E: Pulmonary
neuroepithelial bodies as airway sensors: Putative role in the
generation of dyspnea. Curr Opin Pharmacol. 11:211–217.
2011.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Wu J, Li Z, Deng Y, Lu X, Luo C, Mu X,
Zhang T, Liu Q, Tang S, Li J, et al: Function of TRP channels in
monocytes/macrophages. Front Immunol. 14(1187890)2023.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Tao L, Guo G, Qi Y, Xiong Y, Ma X, Wu N,
Dong C and Yang C: Inhibition of canonical transient receptor
potential 5 channels polarizes macrophages to an M1 phenotype.
Pharmacology. 105:202–208. 2020.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Pereira DMS, Mendes SJF, Alawi K, Thakore
P, Aubdool A, Sousa NCF, da Silva JFR, Castro JA Jr, P Pereira IC,
Silva LCN, et al: Transient receptor potential canonical Channels 4
and 5 mediate Escherichia coli-Derived thioredoxin effects
in lipopolysaccharide-injected mice. Oxid Med Cell Longev.
2018(4904696)2018.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Yang J, Cheng Y, Nie Y, Tian B, Huang J,
Gong R, Li Z, Zhu J and Gong Y: TRPC5 expression promotes the
proliferation and invasion of papillary thyroid carcinoma through
the HIF-1α/Twist pathway. Transl Oncol. 39(101809)2024.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Chen Z, Zhu Y, Dong Y, Zhang P, Han X, Jin
J and Ma X: Overexpression of TrpC5 promotes tumor metastasis via
the HIF-1α-Twist signaling pathway in colon cancer. Clin Sci
(Lond). 131:2439–2450. 2017.PubMed/NCBI View Article : Google Scholar
|
|
18
|
He DX and Ma X: Transient receptor
potential channel C5 in cancer chemoresistance. Acta Pharmacol Sin.
37:19–24. 2016.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Janczyk P, Weigner J, Luebke-Becker A,
Kaessmeyer S and Plendl J: Nitrite pickling salt as an alternative
to formaldehyde for embalming in veterinary anatomy-A study based
on histo- and microbiological analyses. Ann Anat. 193:71–75.
2011.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Werner M, Chott A, Fabiano A and Battifora
H: Effect of formalin tissue fixation and processing on
immunohistochemistry. Am J Surg Pathol. 24:1016–1019.
2000.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Ramos-Vara JA: Technical aspects of
immunohistochemistry. Vet Pathol. 42:405–426. 2005.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Bush EW, Hood DB, Papst PJ, Chapo JA,
Minobe W, Bristow MR, Olson EN and McKinsey TA: Canonical transient
receptor potential channels promote cardiomyocyte hypertrophy
through activation of calcineurin signaling. J Biol Chem.
281:33487–33496. 2006.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Diebolt CM, Schaudien D, Junker K,
Krasteva-Christ G, Tschernig T and Englisch CN: New insights in the
renal distribution profile of TRPC3-Of mice and men. Ann Anat.
252(152192)2024.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Okoh GR, Kazeem HM, Kia GSN and Ponfa ZN:
Heat induced epitope retrieval for rabies virus detection by direct
fluorescent antibody test in formalin-fixed dog brain tissues. Open
Vet J. 8:313–317. 2018.PubMed/NCBI View Article : Google Scholar
|
|
25
|
De March Z, Giampà C, Patassini S,
Bernardi G and Fusco FR: Cellular localization of TRPC5 in the
substantia nigra of rat. Neurosci Lett. 402:35–39. 2006.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Englisch CN, Steinhäuser J, Wemmert S,
Jung M, Gawlitza J, Wenzel G, Schick B and Tschernig T:
Immunohistochemistry reveals TRPC Channels in the human hearing
organ-A novel CT-guided approach to the cochlea. Int J Mol Sci.
24(9290)2023.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Zhu Y, Gao M, Zhou T, Xie M, Mao A, Feng
L, Yao X, Wong WT and Ma X: The TRPC5 channel regulates
angiogenesis and promotes recovery from ischemic injury in mice. J
Biol Chem. 294:28–37. 2019.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Bordet G, Lodhi N, Kossenkov A and Tulin
A: Age-Related changes of gene expression profiles in drosophila.
Genes (Basel). 12(1982)2012.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Cocariu EA, Mageriu V, Stăniceanu F,
Bastian A, Socoliuc C and Zurac S: Correlations between the
autolytic changes and postmortem interval in refrigerated cadavers.
Rom J Intern Med. 54:105–112. 2016.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Poloz YO and O'Day DH: Determining time of
death: Temperature-dependent postmortem changes in calcineurin A,
MARCKS, CaMKII, and protein phosphatase 2A in mouse. Int J Legal
Med. 123:305–314. 2009.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Aegerter H, Lambrecht BN and Jakubzick CV:
Biology of lung macrophages in health and disease. Immunity.
55:1564–1580. 2022.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Malainou C, Abdin SM, Lachmann N, Matt U
and Herold S: Alveolar macrophages in tissue homeostasis,
inflammation, and infection: Evolving concepts of therapeutic
targeting. J Clin Invest. 133(e170501)2023.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Liu BB, Peng YB, Zhang WJ, Zhao XX, Chen
LP, Liu MS, Wang GG, Liu YJ, Shen J, Zhao P, et al: NS8593 inhibits
Ca2+ permeant channels reversing mouse airway smooth
muscle contraction. Life Sci. 238(116953)2019.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Chen YY, Yu MF, Zhao XX, Shen J, Peng YB,
Zhao P, Xue L, Chen W, Ma LQ, Qin G, et al: Paracetamol inhibits
Ca2+ permeant ion channels and Ca2+
sensitization resulting in relaxation of precontracted airway
smooth muscle. J Pharmacol Sci. 142:60–68. 2020.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Koopmans T, Anaparti V, Castro-Piedras I,
Yarova P, Irechukwu N, Nelson C, Perez-Zoghbi J, Tan X, Ward JP and
Wright DB: Ca2+ handling and sensitivity in airway smooth muscle:
Emerging concepts for mechanistic understanding and therapeutic
targeting. Pulm Pharmacol Ther. 29:108–120. 2014.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Chen J, Zhang M, Liu Y, Zhao S, Wang Y,
Wang M, Niu W, Jin F and Li Z: Histone lactylation driven by
mROS-mediated glycolytic shift promotes hypoxic pulmonary
hypertension. J Mol Cell Biol. 14(mjac073)2023.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Sharma S and Hopkins CR: Review of
transient receptor potential canonical (TRPC5) channel modulators
and diseases. J Med Chem. 62:7589–7602. 2019.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Richter JM, Schaefer M and Hill K:
Clemizole hydrochloride is a novel and potent inhibitor of
transient receptor potential channel TRPC5. Mol Pharmacol.
86:514–521. 2014.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Walsh L, Reilly JF, Cornwall C, Gaich GA,
Gipson DS, Heerspink HJL, Johnson L, Trachtman H, Tuttle KR, Farag
YMK, et al: Safety and efficacy of GFB-887, a TRPC5 channel
inhibitor, in patients with focal segmental glomerulosclerosis,
treatment-resistant minimal change disease, or diabetic
nephropathy: TRACTION-2 trial design. Kidney Int Rep. 6:2575–2584.
2021.PubMed/NCBI View Article : Google Scholar
|