Introduction
Breast-conserving surgery (BCS) is widely accepted
as an oncologically secure procedure and has become one of the
predominant breast cancer surgical techniques (1). The evolution of oncoplastic surgery
alongside BCS aims to reduce breast deformities post-surgery
(2). Nonetheless, significant
tissue removal during BCS can result in a noticeable depression,
which is particularly pronounced in patients with smaller breasts
(3). Recent research indicates
that utilizing acellular dermal matrices (ADM) for breast
reconstruction presents a promising solution to address these
challenges (4).
In breast reconstruction, ADMs are predominantly
utilized as a covering for implants (5). Their use has expanded recently,
particularly in implant-based reconstructions following
nipple-sparing and skin-sparing mastectomies (6,7). The
application methods for ADM in breast surgery are well-established,
with a solid safety profile that has been corroborated by the
literature (8,9). Yet, instances of ADM being employed
in BCS reconstructions remain infrequently documented.
The present study documented the impact of two
methods of manipulating ADM on the restoration of breast volume
during conserving surgeries. By varying the dimensions and
configuration of the ADM, superior cosmetic results were achieved
with a concomitant decrease in complication rates compared with
traditional breast-conserving surgery. Furthermore, the study
suggested the viability of a specific type of ADM material.
Utilizing ADM with a honeycomb-like, open three-dimensional
structure was presented as a viable alternative for addressing the
limitations associated with breast-conserving surgery.
Methods
In the present study, two patients with breast
cancer were treated at Tangshan People's Hospital (Tangshan,
China). The admission dates for Patient 1 was July 2023 and for
Patient 2 was August 2023. The patients underwent lumpectomies to
excise the cancerous tissue, ensuring clean surgical margins, and
then received reconstruction with ADM (National Medical Products
Administration reg. no. 20223130801) (Beijing Ruijian High-tech
Biotechnology Co., Ltd.). Neither of the individuals had
pre-existing conditions such as diabetes or any connective tissue
disorders. Both were released from the hospital 2 days
post-operation. The patients were monitored every 24 h for 72 h
after surgery for post-surgical complications, which included the
development of seromas, infections and pain levels. Cosmetic
outcomes were self-rated by the patients using a 4-point scale in a
survey taken immediately after the operation and again 3 months
post-radiotherapy (10,11). The present study also reviewed the
clinicopathological data. For follow-up assessments,
ultrasonography and breast magnetic resonance imaging (MRI) were
employed.
Surgical techniques. Prior to the surgical
procedure, a radiologist used an ultrasound to guide the placement
of a needle, pinpointing the primary tumor and any adjacent areas
of concern, which were then delineated on the surface of the
breast. The patients were positioned on their backs with their arms
outstretched. Local anesthetic was administered, followed by the
insertion of a needle to re-confirm the locations of any
questionable lesions through the use of intraoperative ultrasound,
ensuring accurate tumor localization. The surgical team then
proceeded with the standard lumpectomy, maintaining clear margins
of 1 to 2 cm. Additionally, for patient 1, sentinel lymph node
sampling was conducted; for patient 2, axillary lymph node
dissection was conducted.
During the operation, the present study conducted an
immediate frozen section analysis of tissue from the resection
cavity to verify the margins were free of cancer cells. Once clear
margins were established, the surgical team proceeded with securing
hemostasis and rinsing the wound. The ADM was then prepared for
implantation; it was first hydrated in saline solution, after which
multiple 5-mm incisions were created with a no. 11 scalpel blade.
Gentamicin sulfate (Thermo Fisher Scientific, Inc.) was prepared in
sterile 0.9% saline at a concentration of 3.2 µ/ml. The ADM, now
perforated, was immersed in the antibiotic solution for 5 to 10
min. Following this, the ADM was thoroughly rinsed with saline once
more before being placed into the surgical defect.
Case report
The characteristics of both patients are summarized
in Table I.
 | Table IClinicopathological profiles of
patients with breast cancer who were treated with lumpectomy
followed by reconstruction using acellular dermal matrix. |
Table I
Clinicopathological profiles of
patients with breast cancer who were treated with lumpectomy
followed by reconstruction using acellular dermal matrix.
| Parameter | Patient 1 | Patient 2 |
|---|
| Sex/age, years | F/59 | F/40 |
| Type of tumor | Invasive ductal
carcinoma | Invasive ductal
carcinoma |
| Multifocality | No | No |
| Removed breast
tissue, g | 13.4 | 21.0 |
| Pathological tumor
size, cm | 0.8 | 2.0 |
| Resected tissue
volume, mla | 14 | 22 |
| Hormone receptor
status | Positive | Positive |
| Her2 gene
expression | Negative | Positive |
| Resection margin | Negative | Negative |
| No. of
metastatic/total removed axillary lymph nodes | 0/4 | 11/20 |
| TNM stage | pT1N0M0 | pT1N3M0 |
| Adjuvant
chemotherapy | No | Yes |
| Adjuvant
radiotherapy | Yes | Yes |
| Adjuvant hormone
therapy | Yes | Yes |
| Postoperative
complication | No | No |
| Postoperative pain
score based on a visual analog scale | 3 | 4 |
| A satisfactory degree
of cosmesis after surgery | Excellent | Excellent |
| A satisfactory degree
of cosmesis after radiotherapy | Excellent | Excellent |
Patient 1
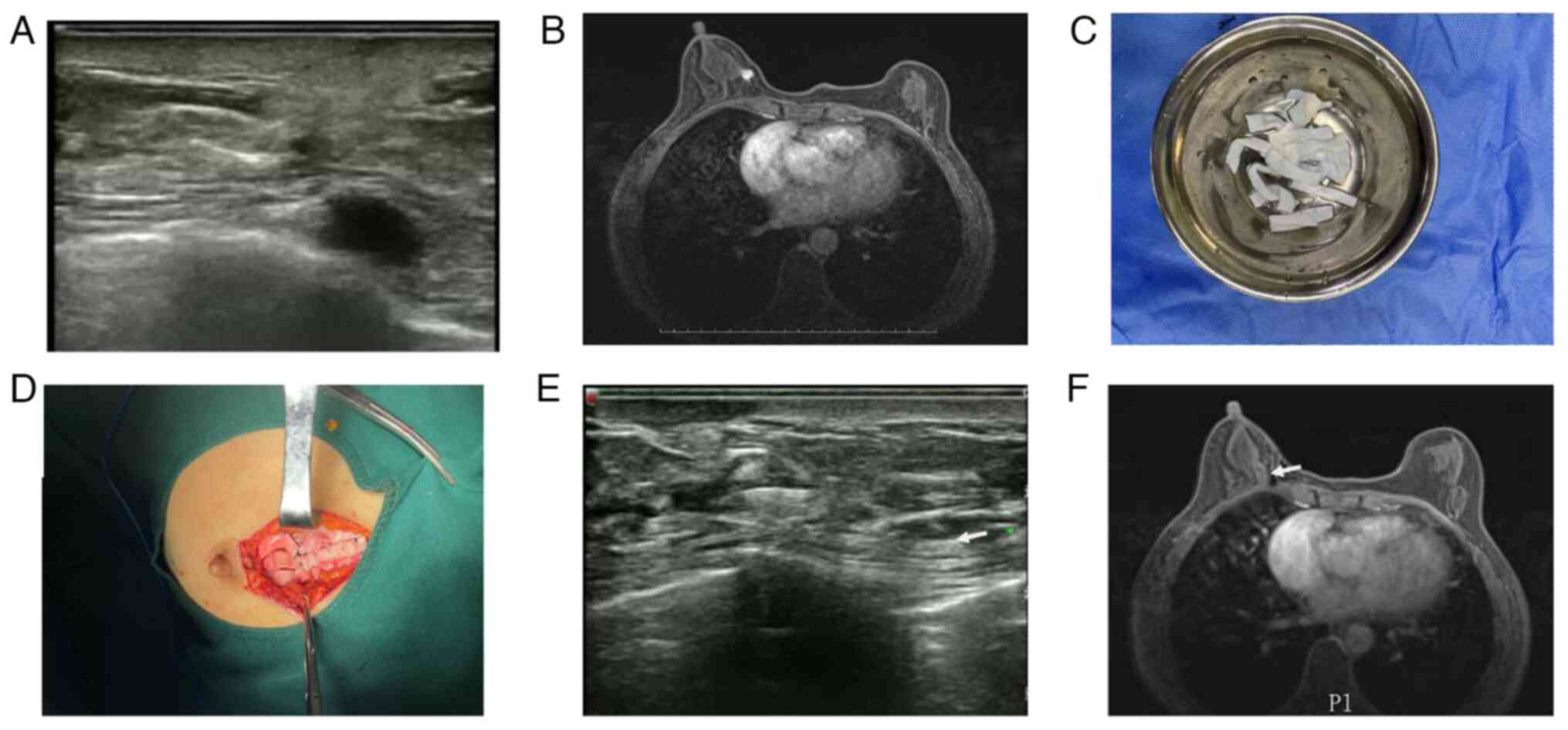
A female patient aged 59 received a diagnosis of
invasive ductal carcinoma in her right breast, confirmed via core
needle biopsy. She had a BMI of 20.12 kg/m2.
Ultrasonographic examination of the breast revealed an atypical
mass with ductal enlargement, measuring roughly 0.81 cm in its
largest dimension (Fig. 1A).
Subsequent breast MRI depicted an enhancing lesion consistent with
ductal architecture (Fig. 1B).
The patient was treated with a segmental mastectomy
and a sentinel node biopsy. The excised breast tissue weighed 13.4
g. Prepared ADM was fashioned into strips measuring 1 cm by 5 cm
(Fig. 1C). Once a frozen section
confirmed the absence of residual cancer in the margins of the
excised tissue, the breast defect was filled with the prepared ADM
strips. To secure the ADM in place and prevent displacement, each
corner of the ADM strip was sutured using 3-0 silk sutures within
the defect area (Fig. 1D). This
process may take an extra 10 min compared with traditional
breast-conserving surgery. Closure of the surgical site involved a
two-tiered approach, with the deeper glandular tissue and the
superficial skin sutured separately (12). Drainage of excess fluid was managed
by a surgically placed closed drain. The incision was meticulously
sutured in two layers with resorbable 3-0 and 4-0 monofilament
sutures (Johnson & Johnson). Post-surgery, an elastic bandage
was applied, ensuring it was not too tight to prevent compression
of the reconstructed breast. Pain assessment using a visual analog
scale (13) indicated an average
postoperative pain level at three different times during the
patient's hospitalization. The patient was released from the
hospital on the third postoperative day. The final pathology report
described a 0.8 cm invasive ductal carcinoma with a hormone
receptor-positive profile. At 3 months post-radiotherapy, the
integrity of the ADM was confirmed via ultrasound and breast MRI
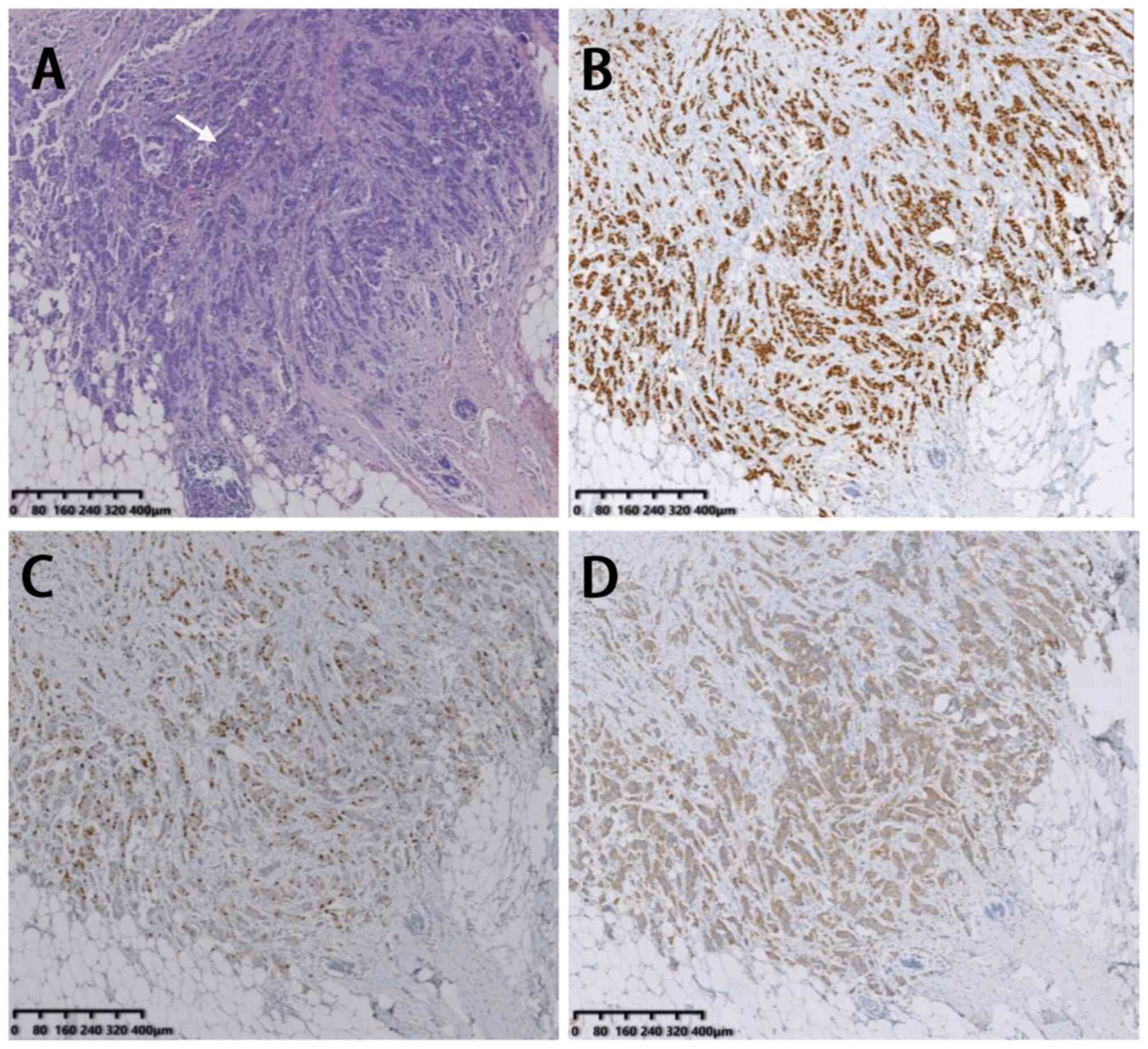
imaging (Fig. 1E-F). Tumor HE
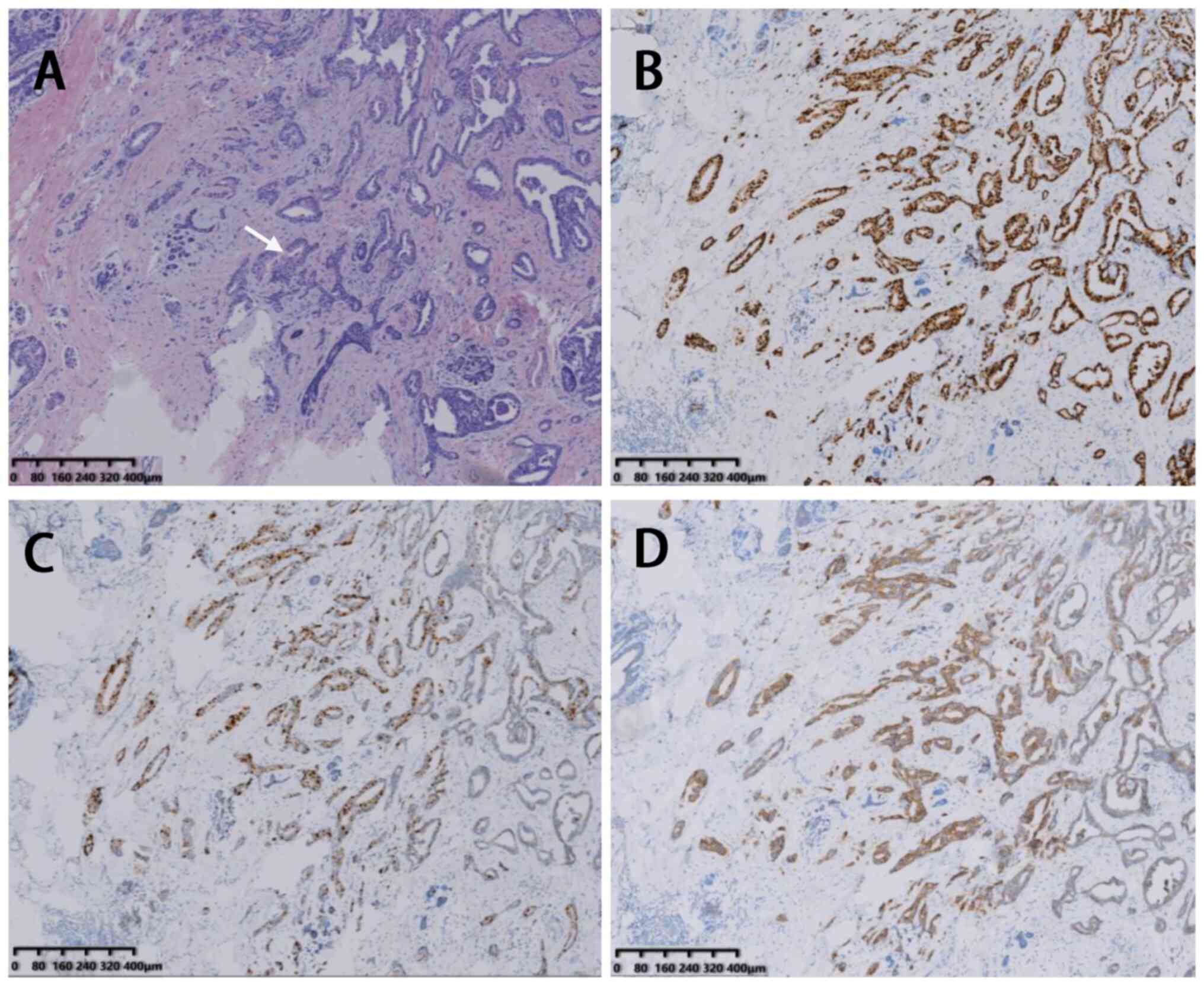
staining and immunohistochemical staining are shown in Fig. 2 (Department of Pathology, Tangshan
People's Hospital, Tangshan City, Hebei Province, China). The
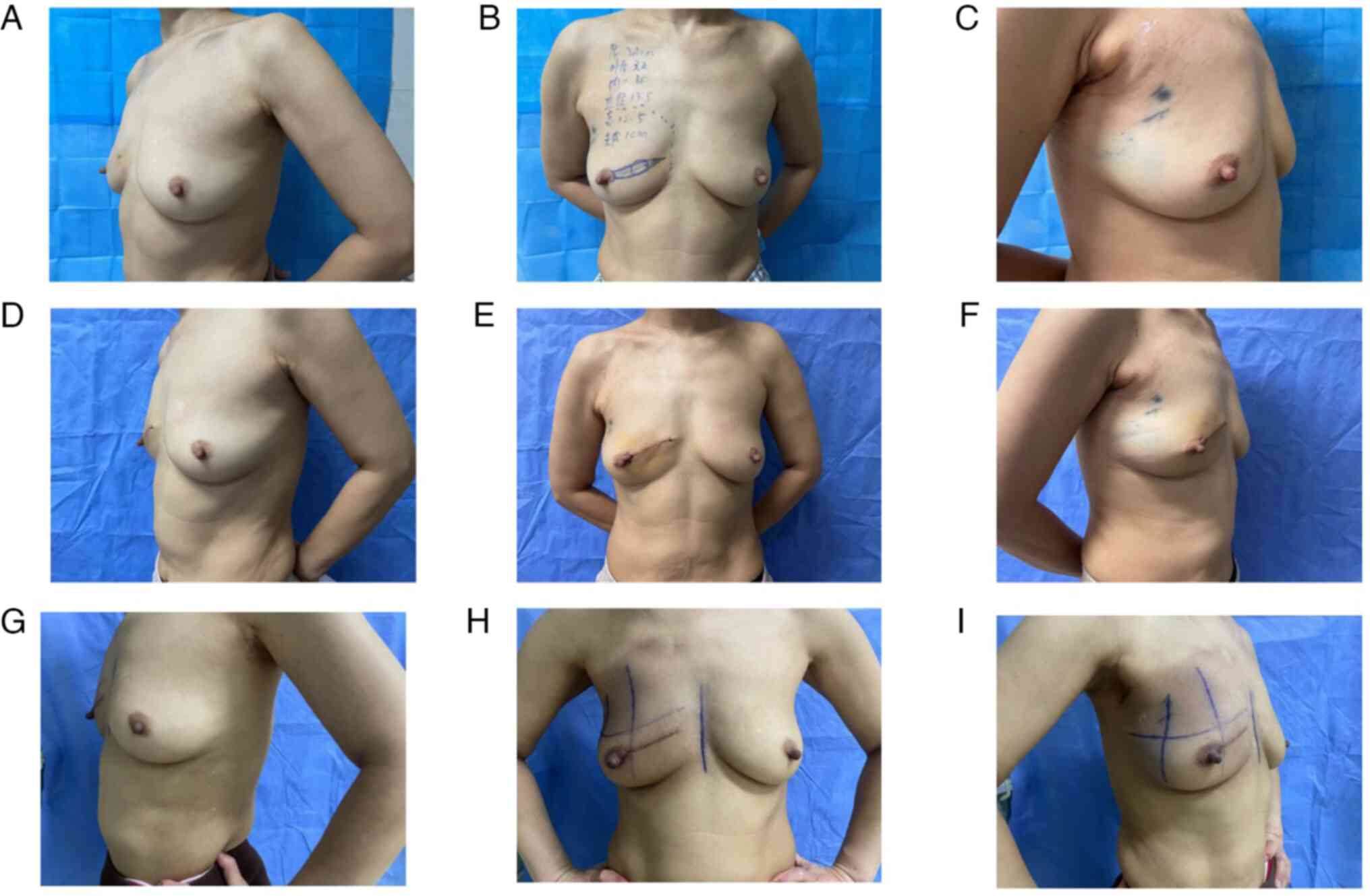
patient assessed the cosmetic outcome as excellent, referencing a
4-point scale (14,15), both 3 days after surgery and 3
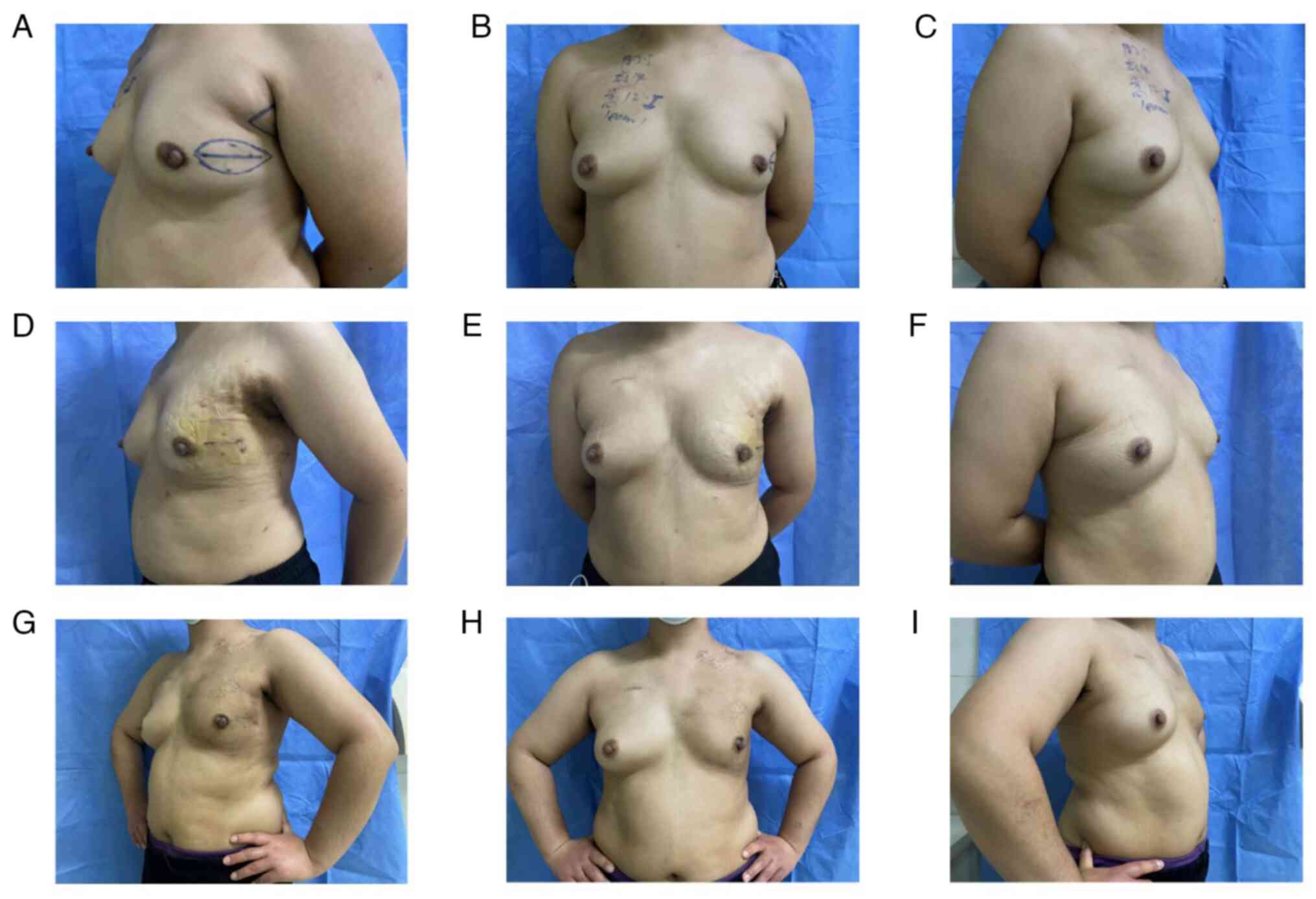
months post-radiotherapy (Fig.
3A-I).
Patient 2
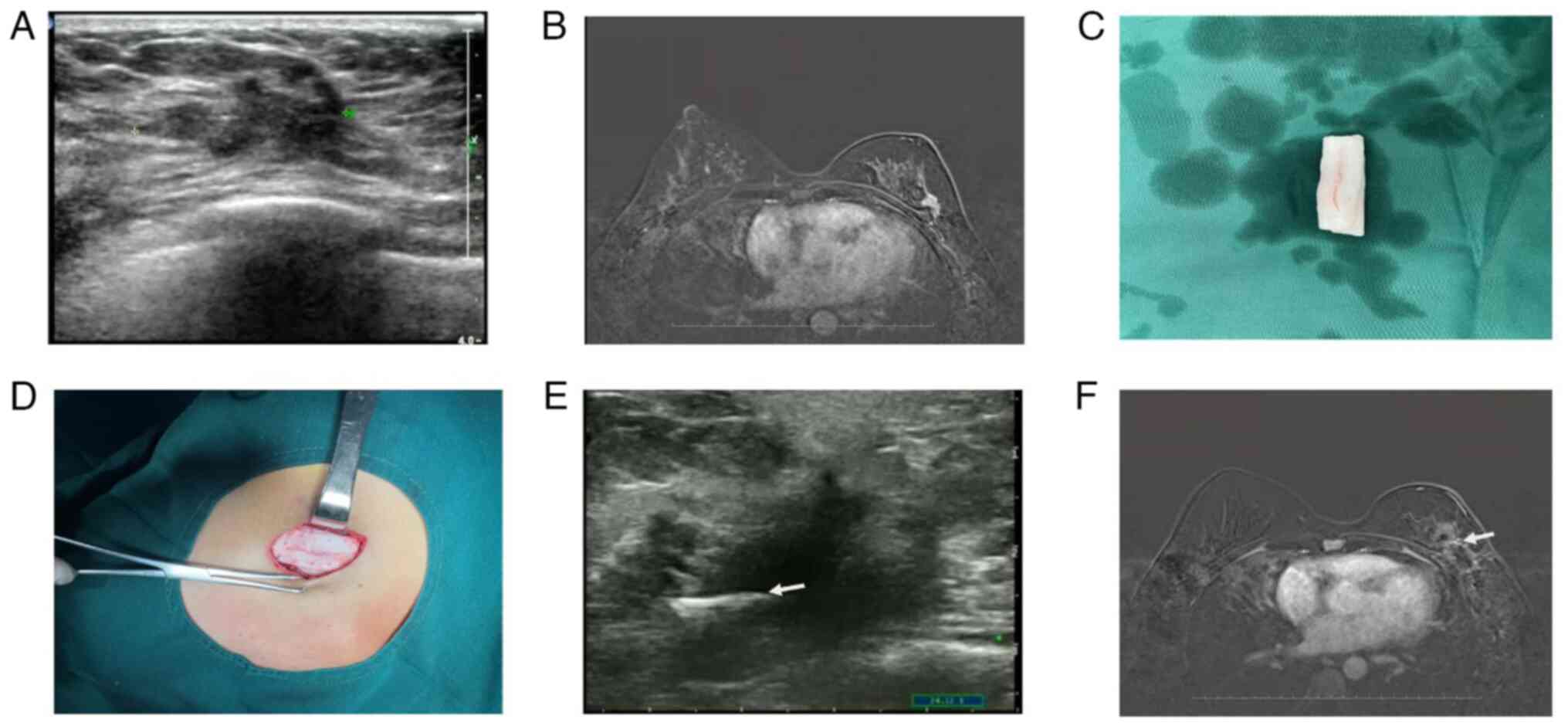
A 40-year-old female patient received a diagnosis of
invasive ductal carcinoma in her left breast following a core
needle biopsy. Her BMI was recorded at 27.3 kg/m². Ultrasound
imaging revealed a complex mass with associated smaller nodules,
measuring ~2.5 cm (Fig. 4A).
Additionally, a contrast-enhanced breast MRI identified an
irregularly shaped mass in the upper outer quadrant of the breast
(Fig. 4B).
The patient underwent partial mastectomy and
axillary lymph node dissection. The excised breast tissue weighed
21 g. A pattern of perforations, spaced 0.5 cm apart, was created
on the ADM material (Fig. 4C).
This ADM was then fashioned into a three-dimensional, grid-like
construct, measuring 1x5 cm (Fig.
4C). The corners of this ADM grid were securely sutured into
the surgical cavity using 3-0 white silk stitches to ensure the
material remained in place (Fig.
4C). This process may take an extra 10 min compared with
traditional breast-conserving surgery. Closure of the surgical site
was achieved through a two-layer technique, encompassing both
glandular and superficial dermal tissues (12). A closed suction drain was placed to
facilitate fluid removal. The incision was meticulously closed in
two layers with interrupted, dissolvable 3-0 and 4-0 monofilament
sutures (Johnson & Johnson). Postoperatively, the site was
dressed with a gently applied elastic bandage to support the breast
structure. Pain levels, monitored by a visual analog scale,
indicated an average pain score of 4 during the inpatient recovery.
The patient was discharged at 3 days post-operation. Pathological
analysis confirmed a 2.0 cm invasive ductal carcinoma, HR-positive,
HER2-positive as the final diagnosis.
At 3 months post-radiotherapy, the three-dimensional
framework of the ADM had established itself as a solid internal
scaffold, with no significant structural alterations evident on
ultrasound and MRI scans of the breast (Fig. 4E-F). Tumor HE staining and
immunohistochemical staining are presented in Fig. 5 (Department of Pathology, Tangshan
People's Hospital, Tangshan City, Hebei Province, China.). The
patient assessed her surgical outcome as favorable initially, and
post-radiotherapy, she reported an excellent aesthetic result,
utilizing a standardized four-point evaluative scale (Fig. 6A-I).
Discussion
Initially investigated for the treatment of
extensive skin repair after severe burns (16), the application of ADM has since
expanded across multiple surgical disciplines. It now serves as a
protective layer when healing wounds, tendons, bones, cartilage and
nerves (12,17), and plays a role in the
reconstruction of various bodily structures (18). In contemporary practices, ADM is
utilized in nipple-sparing and skin-sparing mastectomies for
implant coverage, where the stability of the treatment has been
established. Despite these advancements, its use in reconstructive
BCS remains relatively underexplored (19,20).
Lee et al (10) have explored the synergy of ADM and
ORC in reconstructing partial breast defects. Their findings
suggest that this combination is a viable method with promising
aesthetic results for patients with breast cancer. However, they
noted that while ADM provides structural support, the absorption of
ORC over time can lead to breast shape alterations (10). Additionally, a study from Korea
examined the comparative outcomes of using sheet-like vs.
pellet-like ADM forms in post-breast conservation surgery. The
results underscore the effectiveness of ADM in promptly restoring
breast contour post-surgery. It has been observed that the pellet
form more closely mimics the pre-surgical appearance of the
breasts, although it may pose challenges in terms of stability and
movement within the breast (4).
The present study presents two distinct techniques
of utilizing ADM for volumetric reconstruction in
breast-conservation surgery. For patient 1, the present study
segmented the ADM into slender strips instead of using a single
large piece. This strategy not only accommodates the specific
contours of the surgical cavity but also promotes
re-epithelialization, neovascularization and fibroblast migration.
After a year, the patient demonstrated excellent recovery and
maintained breast contour post-radiotherapy without any
complications. In patient 2, the present study enhanced the
structure of ADM by perforating and folding it to create a
three-dimensional matrix. This approach appeared to provide
improved structural support and aesthetic outcomes as compared with
the strip method, particularly in surgeries where similar volumes
of tissue are excised. These findings lead us to consider the
potential of manufacturing ADM pre-formed into a three-dimensional
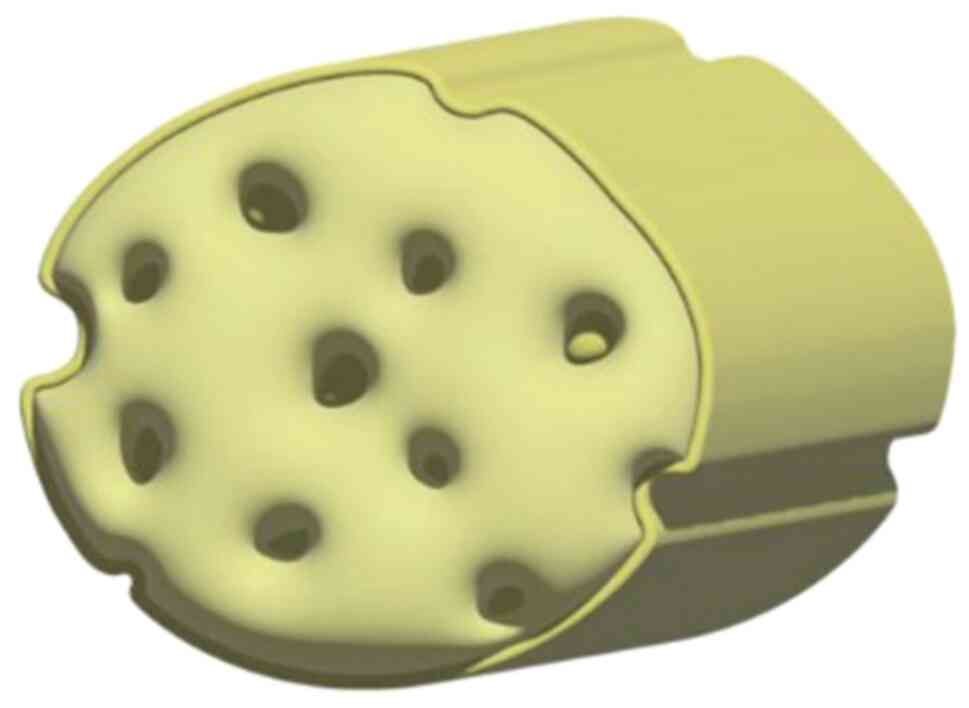
grid for breast-conservation reconstructive surgery (Fig. 7). The exploration of this area is
currently in the clinical trial phase. Since this hypothesis is
still in the clinical phase, data collection is not yet complete,
so the data cannot be disclosed. However, based on the existing
research, we found that regardless of the method used to apply ADM
for breast defect augmentation, no apparent signs of foreign
objects were observed in the imaging examination at 3 months after
radiotherapy. This indicates that ADM material can integrate well
with the human body, ensuring safety.
The present study envisages a transformation of the
traditional sheet-like ADM into a lattice of filamentous fibers,
reconstituted into a honeycomb-like three-dimensional scaffold with
strategically placed perforations, as depicted in Fig. 7. This re-engineered scaffold is
non-degradable and biocompatible, designed to avoid breast contour
changes over time due to structural failure. The honeycomb design
serves a dual purpose: It minimizes ADM volume and residual foreign
material within the cavity, and it enhances the infusion and
integration of interstitial fluids, encouraging the development of
a fibrovascular matrix. Moreover, the modified spatial
configuration of the ADM not only augments its pliability, offering
a more natural feel to the touch, but also allows surgeons to
tailor the scaffold to the unique contours of the residual breast
cavity, optimizing the aesthetic outcome. Our clinical observations
suggest that this innovative three-dimensional ADM structure can
significantly enhance cosmetic outcomes for patients undergoing
breast-conservation surgery while reducing postoperative
complications.
In conclusion, the techniques explored in the
present study facilitated the preservation of breast tissue even
when excising larger tumors or addressing multifocal lesions within
the same quadrant. The current research research indicated that
employing ADM for such procedures is oncologically sound, with no
elevation in the risk of breast cancer recurrence observed.
Additionally, the postoperative breast contour was well-preserved,
and no significant complications were encountered. In modern
medicine, utilizing ADM fashioned into a honeycomb-like, loosely
structured three-dimensional scaffold was a viable approach,
yielding superior cosmetic outcomes for breast cancer defect repair
compared with traditional breast-conserving surgery.
Acknowledgements
The authors would like to thank Dr. Lei Wang from
the Department of Pathology, Tangshan People's Hospital (Tangshan,
China) for his pathology support. In the study, he identified
tissue-negative margins during breast-conserving surgery.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
YW and SW acquired the data. JH, XL and JM analyzed
and interpreted the data.JM and YW confirm the authenticity of all
the raw data. JH and YW designed the methodology. JH and YW
conceived and designed the study. JM, XL and SW supervised. YW
wrote the original draft. JW reviewed and edited the paper. All
authors have read and approved the final manuscript.
Ethics approval and consent to
participate
This investigation received approval from the ethics
committee at Tangshan People's Hospital (Tangshan, China; approval
no. 2022-005-001). Prior to enrollment, written informed consent
was secured from both patients, adhering to the ethical principles
outlined in the Declaration of Helsinki.
Patient consent for publication
Written informed consent was obtained from the
patients for publication of this case report and any accompanying
images.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Choi JE, Kim Z, Park CS, Park EH, Lee SB,
Lee SK, Choi YJ, Han J, Jung KW, Kim HJ, et al: Breast Cancer
Statistics in Korea, 2019. J Breast Cancer. 26:207–220.
2023.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Bertozzi N, Pesce M, Santi P and Raposio
E: Oncoplastic breast surgery: Comprehensive review. Eur Rev Med
Pharmacol Sci. 21:2572–2585. 2017.PubMed/NCBI
|
|
3
|
Thiessen FEF, Tjalma WAA and Tondu T:
Breast reconstruction after breast conservation therapy for breast
cancer. Eur J Obstet Gynecol Reprod Biol. 230:233–238.
2018.PubMed/NCBI View Article : Google Scholar
|
|
4
|
An J, Kwon H, Lim W, Moon BI and Paik NS:
The comparison of breast reconstruction using two types of
acellular dermal matrix after breast-conserving surgery. J Clin
Med. 10(3430)2021.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Hallberg H, Rafnsdottir S, Selvaggi G,
Strandell A, Samuelsson O, Stadig I, Svanberg T, Hansson E and
Lewin R: Benefits and risks with acellular dermal matrix (ADM) and
mesh support in immediate breast reconstruction: A systematic
review and meta-analysis. J Plast Surg Hand Surg. 52:130–147.
2018.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Gravina PR, Pettit RW, Davis MJ, Winocour
SJ and Selber JC: Evidence for the use of acellular dermal matrix
in implant-based breast reconstruction. Semin Plast Surg.
33:229–235. 2019.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Wazir U and Mokbel K: The evolving role of
pre-pectoral ADM-assisted implant-based immediate breast
reconstruction following skin-sparing mastectomy. Am J Surg.
216:639–640. 2018.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Cabalag MS, Rostek M, Miller GS, Chae MP,
Quinn T, Rozen WM and Hunter-Smith DJ: Alloplastic adjuncts in
breast reconstruction. Gland Surg. 5:158–173. 2016.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Sorkin M, Qi J, Kim HM, Hamill JB, Kozlow
JH, Pusic AL and Wilkins EG: Acellular dermal matrix in immediate
expander/implant breast reconstruction: A multicenter assessment of
risks and benefits. Plast Reconstr Surg. 140:1091–1100.
2017.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Lee J, Yang JD, Lee JW, Li J, Jung JH, Kim
WW, Park CS, Lee JS and Park HY: Acellular dermal matrix combined
with oxidized regenerated cellulose for partial breast
reconstruction: two case reports. Medicine (Baltimore).
99(e21217)2020.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Fadaeizadeh L, Emami H and Samiei K:
Comparison of visual analogue scale and faces rating scale in
measuring acute postoperative pain. Arch Iranian Med. 12:73–75.
2009.PubMed/NCBI
|
|
12
|
Lee J and Bae Y: The use of absorbable
interceed® pouch with double-layer skin closure for partial defect
of breast. Breast J. 20:414–419. 2014.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Araujo AM, Gómez M, Pascual J, Castañeda
M, Pezonaga L and Borque JL: Treatment of pain in the oncology
patient. An Sist Sanit Navar. 27 (Suppl 3):S63–S75. 2004.PubMed/NCBI(In Spanish).
|
|
14
|
Rhodes LE, de Rie MA, Leifsdottir R, Yu
RC, Bachmann I, Goulden V, Wong GA, Richard MA, Anstey A and Wolf
P: Five-year follow-up of a ran domized, prospective trial of
topical methyl aminolevulinate photody namic therapy vs surgery for
nodular basal cell carcinoma. Arch Dermatol. 143:1131–1136.
2007.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Mosterd K, Arits AH, Nelemans PJ and
Kelleners-Smeets NW: Aesthetic evaluation after non-invasive
treatment for superficial basal cell carcinoma. J Eur Acad Dermatol
Venereol. 27:647–650. 2013.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Stone Ii R, Natesan S, Kowalczewski CJ,
Mangum LH, Clay NE, Clohessy RM, Carlsson AH, Tassin DH, Chan RK,
Rizzo JA and Christy RJ: Advancements in regenerative strategies
through the continuum of burn care. Front Pharmacol.
9(672)2018.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Cooper J and Mirzayan R: Acellular dermal
matrix in rotator cuff surgery. Am J Orthop (Belle Mead NJ).
45:301–305. 2016.PubMed/NCBI
|
|
18
|
Haney NM, Huang MM, Liu JL, Hawksworth DJ
and Burnett AL: Acellular dermal matrix tissues in genitourinary
reconstructive surgery: A review of the literature and case
discussions. Sex Med Rev. 9:488–497. 2021.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Gwak H, Jeon YW, Lim ST, Park SY and Suh
YJ: Volume replacement with diced acellular dermal matrix in
oncoplastic breast-conserving surgery: A prospective single-center
experience. World J Surg Oncol. 18(60)2020.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Schwartz JD: Use of a bioabsorbable
implant-acellular dermal matrix construct to facilitate oncoplastic
breast-conserving surgery. Plast Reconstr Surg Glob Open.
9(e3356)2021.PubMed/NCBI View Article : Google Scholar
|