Introduction
Herpes zoster (HZ) is an infectious virus that
mainly affects the nerves and skin. It is caused by the
varicella-zoster virus (VZV) and typically manifests itself as
shingles in adults because the rash appears in blisters and is
distributed in bands (1). The VZV
genome is a linear double-stranded DNA molecule of ~125,000 bp that
encodes at least 71 unique open reading frames (ORFs) and related
promoter sequences. VZV particles are 80-120 nm in diameter
(2). Linear VZV genomes are
packaged into an icosahedral nucleocapsid core that is formed from
proteins encoded by ORF20, ORF21, ORF23, ORF33, ORF40 and ORF41.
Capsids are surrounded by a tegument layer, which is a less
well-defined structure that is made up of proteins with known or
predicted regulatory functions, including the immediate-early (IE)
viral transactivating factors that are encoded by ORF4, ORF62 and
ORF63, those that are encoded by the ORF9-ORF12 gene cluster, and
viral kinases ORF47, ORF66, ORF8, ORF48, ORF59 and ORF13(3).
The outer virion component is a lipid membrane
envelope that is derived from cellular membranes with incorporated
viral glycoproteins, including gB/gH-gL, which forms the minimal
fusion complex. VZV gC and gE have been implicated in membrane
attachment, whereas gB, gH, and gL are the necessary components for
cell entry where the virion must deliver the capsid through the
plasma membrane to initiate infection. The most well-characterized
glycoproteins are those that function in membrane fusion, gB, gH,
and gL (4). In common with all
herpesviruses, after VZV binds to cell surface proteins, the gH-gL
heterodimer is thought to prime gB to enable a gross conformational
change from a prefusion to postfusion structure, leading to fusion
of the virion envelope with the plasma membrane (5).
By contrast, VZV mainly appears as chickenpox in
children following infection, who have no immunity to the virus. A
portion of patients will exhibit no symptoms following infection
but will instead harbor the virus (6). It can remain latent in the dorsal
root ganglion of the spinal cord for long periods of time following
infection due to its neurotropism (7), where it can ‘reactivate’ and
reproduce again when the immune system becomes compromised or
because of diminished cell-mediated immunity to the virus in such
individuals (8). When this occurs,
it moves along the nerve fibers to the skin surface, where they
trigger a potent inflammatory reaction, which usually appears as a
painful or pruritic cutaneous vesicular eruption that occurs in a
characteristic dermatomal distribution (9). This rash is generally unilaterally
and segmentally distributed and consists of clusters of herpes, and
the patient will start to feel pain (neuralgia). It is mostly
distributed along the intercostal nerve, and occurs more naturally
in the chest, and there is no difference between the left and right
sides. The older the patient, the worse the neuralgia tend to be
(10,11). The diagnosis of the disease is
based on the association with neurological symptoms and signs after
the onset of the rash, as well as the presence of VZV virus in the
cerebrospinal fluid as demonstrated by PCR, a 4-fold increase in
the titer of anti-VZV IgG in peripheral blood indicating persistent
viral infection and positive anti-VZV IgM indicating recent
infection/reactivation (12).
Reactivation of VZV may also cause a wide variety of neurological
syndromes, which is treated with corticosteroids and the antiviral
drug acyclovir (13).
VZV infection is particularly common during the
spring and autumn because the air temperature difference is large,
the level of human immunity is low and individuals tend to spend
more time outside in public. The incidence of infected adults tends
to be higher compared with that of children; the global incidence
rate of HZ ranges from 3 to 5/1,000 person-years and from 5.23 to
10.9/1,000 person-years in individuals ≥50 years of age (14,15).
In addition, this incidence increases significantly with age. It
has been found in a prospective study of viral infections in the
CNS in Spain that VZV was considered to be the second most common
cause of infection in adults with meningitis and encephalitis in
2013 (16,17). Both the central nervous system and
peripheral nervous system can be involved following VZV infection
(18,19), However, these two articles only
describe meningitis and myelitis caused by VZV infection, and, to
the best of our knowledge, the present study is the first case
reported where the changes of facial neuritis secondary to
meningitis were found.
Case report
A 59-year-old female patient first presented to
Shengli Oilfield Central Hospital (Dongying, China) with a headache
for 2 days before admission (July 2023). This patient could not
accurately describe the location of this headache, which was
accompanied by nausea and vomiting. There was no eject-like
vomiting (which would have suggested intracranial hypertension and
possible meningitis or encephalitis), fearless of light and sound
(the accompanying symptoms of migraine were photophobia and
phonophobia, and the patient was admitted to the hospital for
headache in order to differentiate it from migraine), no
unconsciousness and confusion, no slurred speech and no numbness or
weakness of limbs. However, the patient did suffer from a fever 1
day before admission, with a maximum temperature of 39˚C,
accompanied by myalgia and diarrhea (with yellow soft stool). The
patient was therefore hospitalized in Hekou Peoples' Hospital
(Shandong, China; July 2023), where and her brain MRI showed no
obvious abnormalities. The novel coronavirus disease-19 nucleic
acid test returned negative. However, there was no improvement
following anti-inflammatory rehydration treatment (normal saline
250 ml + 1.5 g cefuroxime sodium; 0.5 h; twice a day). Therefore,
for further diagnosis and treatment, this patient was referred to
Shengli Oilfield Central Hospital after 1 day of hospitalization in
the local area. However, Shingles appeared on the left anterior
chest, armpit and posterior back 5 days before admission to Shengli
Oilfield Central Hospital. The patient denied any history of other
medical conditions, but had an uncertain history of chickenpox
during childhood and had no history of varicella vaccination.
Physical examination revealed a body temperature of 39˚C, pulse 75
bpm, respiratory 18 bpm and blood pressure of 124/71 mmHg. The
blisters along the intercostal nerve were broken and the skin was
red and crusted. Shingles could still be observed on the skin
surfaces of left anterior chest, armpit and posterior back, which
had been ruptured. However, the skin surfaces had no blood or fluid
seepage.
Nervous system physical examination revealed a clear
consciousness but poor mental state (the patient could answer
questions correctly and speak weakly). The patient exhibited clear
speech and normal advanced intelligence (contains memory,
calculation, understanding, judgement and directional forces). The
eyes had large and round bilateral pupils (with diameters ~3 mm)
and were sensitive to light response as normal, with negative
nystagmus and adequate eye movement. There was also bilateral
nasolabial groove symmetry, with the tongue extending centrally,
bilateral soft palate mobility and centered uvula, where the
pharyngeal reflex was normal. Limb muscle strength was found to be
grade 5 with normal muscle tension, bilateral tendon reflex
symmetry (++) and negative bilateral pathological signs.
Finger-to-nose test and Heel-knee-tibia test could be completed
accurately, bilateral pain and temperature perception were
symmetrical; however, testing revealed neck resistance of 3 fingers
width (when the patient is in a supine position and lifts their
head, the distance between the jaw and sternum is measured. If this
distance can accommodate less than two finger widths, it is
considered unlikely for meningitis to be present) and positive
Kernig sign.
Upon admission, blood routine examination and
C-reactive protein (CRP) tests showed white blood cell counts of
11.7 (3.5-9.5)x109/l, neutrophil counts of 7.55
(1.8-6.3)x109/l, CRP levels of 46.40 (0-10) mg/l and
Tetrad + D-D dimer: DD levels of 1.43 (0-0.5) mg/l. Biochemical
tests revealed total protein levels of 60.4 (65-85) g/l, albumin
levels of 35 (40-55) g/l, glucose concentrations of 9.55
(3.89-6.11) mmol/l, K+ levels of 3.41 (3.5-5.3) mmol/l,
Na+ levels of 132.6 (137-147) mmol/l and
Cl-levels of 98.2 (99-110) mmol/l. Tumor marker testing
found neuron-specific enolase levels to be 16.40 (0-16.3) ng/ml. No
abnormalities were found for hepatitis B, hepatitis C, syphilis and
HIV and the urine test revealed no abnormalities.
According to the blood test results, the patient had
infection, protein and electrolyte deficiencies in the blood and
hypercoagulable states, and the tumor marker neuron-specific
enolase was slightly higher than the normal reference value, which
had little clinical significance.
Lumbar puncture examinations were completed three
times, once in July 2023 and twice in August 2023. No abnormalities
could be found following cerebrospinal fluid (CSF) ink staining and
acid-fast staining, where the routine CSF white blood cell count
was 734 → 329 → 156x106/l. The proportion of leukocyte
mononuclear cells in the CSF was 50 → 98 → 99% and the proportion
of multinucleated cells in the CSF was 50 → 2 → 1%. CSF protein
levels were found to be 253 → 178.6 → 144.7 mg/dl, glucose levels
were 4.18 → 4.2 → 3.68 mmol/l and Cl-levels of 109.1 →
107.7 → 114.3 mmol/l (Table I).
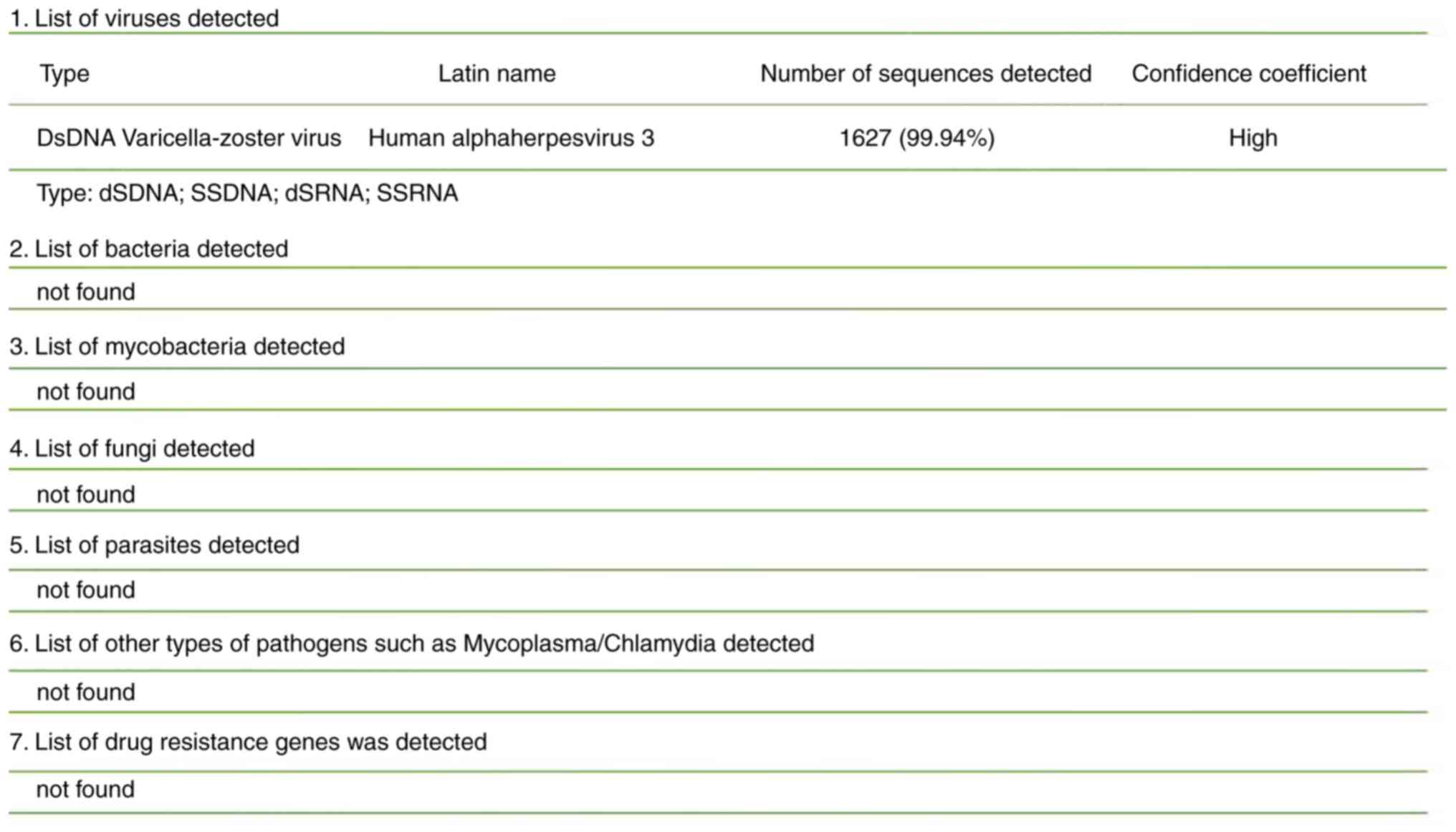
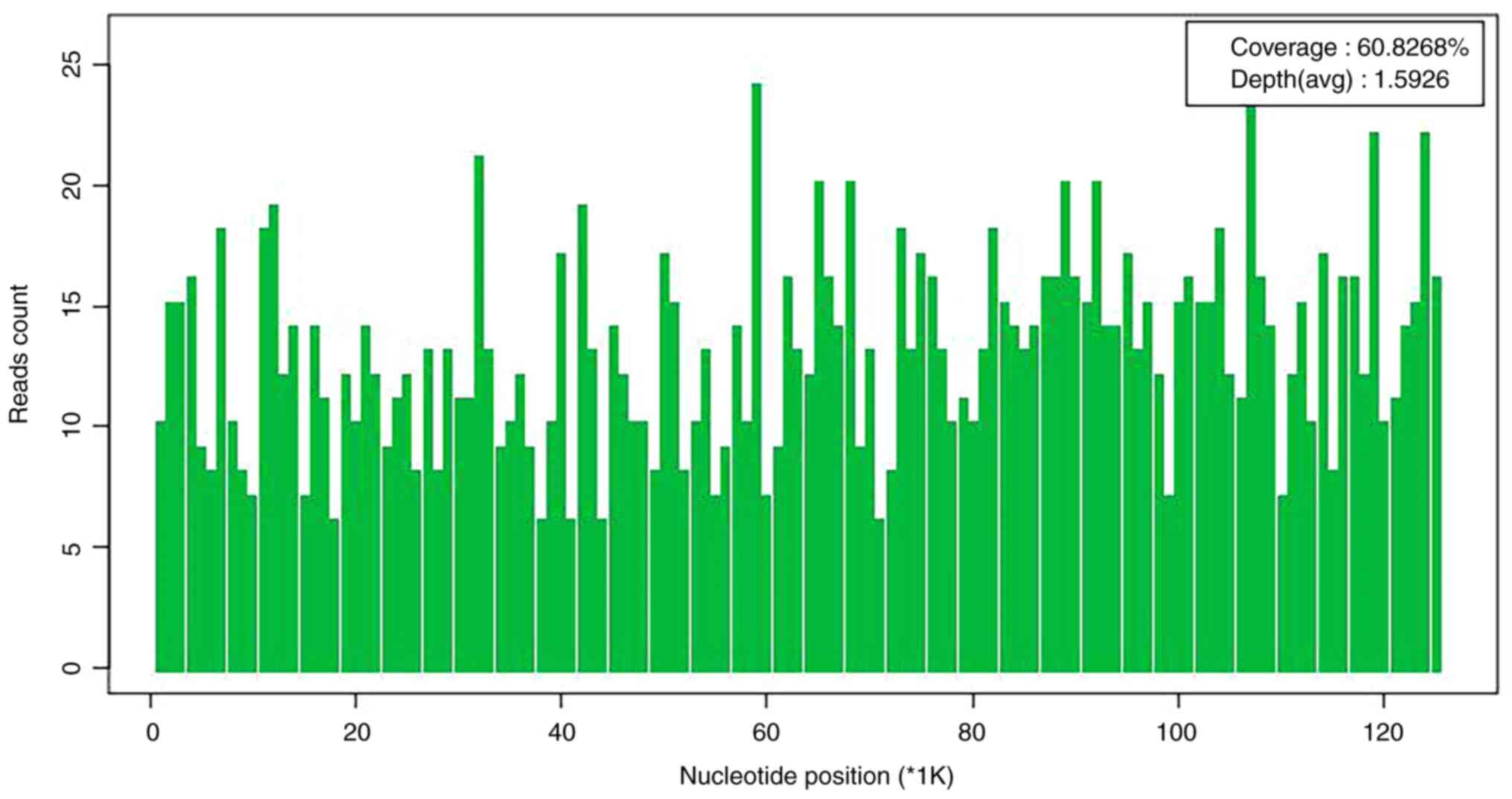
DNA and RNA metagenomic sequencing detection of pathogenic
microorganisms in the CSF (DNA + RNA) by No EU medical laboratory
(Guangzhou, China) revealed VZV (Figs.
1 and 2).
 | Table IChanges of cerebrospinal fluid
indexes after lumbar puncture. |
Table I
Changes of cerebrospinal fluid
indexes after lumbar puncture.
| CSF (cerebrospinal
fluid) | White blood cell
count (106/l) | Proportion of
leukocyte monocyte count (%) | Proportion of
multinucleated cells (%) | Protein levels
(mg/dl) | Glucose levels
(mmol/l) | Chloride
concentrations (mmol/l) |
|---|
| 1st lumbar
puncture | 734 | 50 | 50 | 253 | 4.18 | 109.1 |
| 2nd lumbar
puncture | 329 | 98 | 2 | 178.6 | 4.2 | 107.7 |
| 3rd lumbar
puncture | 156 | 99 | 1 | 144.7 | 3.68 | 114.3 |
According to imaging examinations, heart + lower
limb venous ultrasound found mild tricuspid valve regurgitation and
small quantities of pericardial effusion but no venous thrombosis
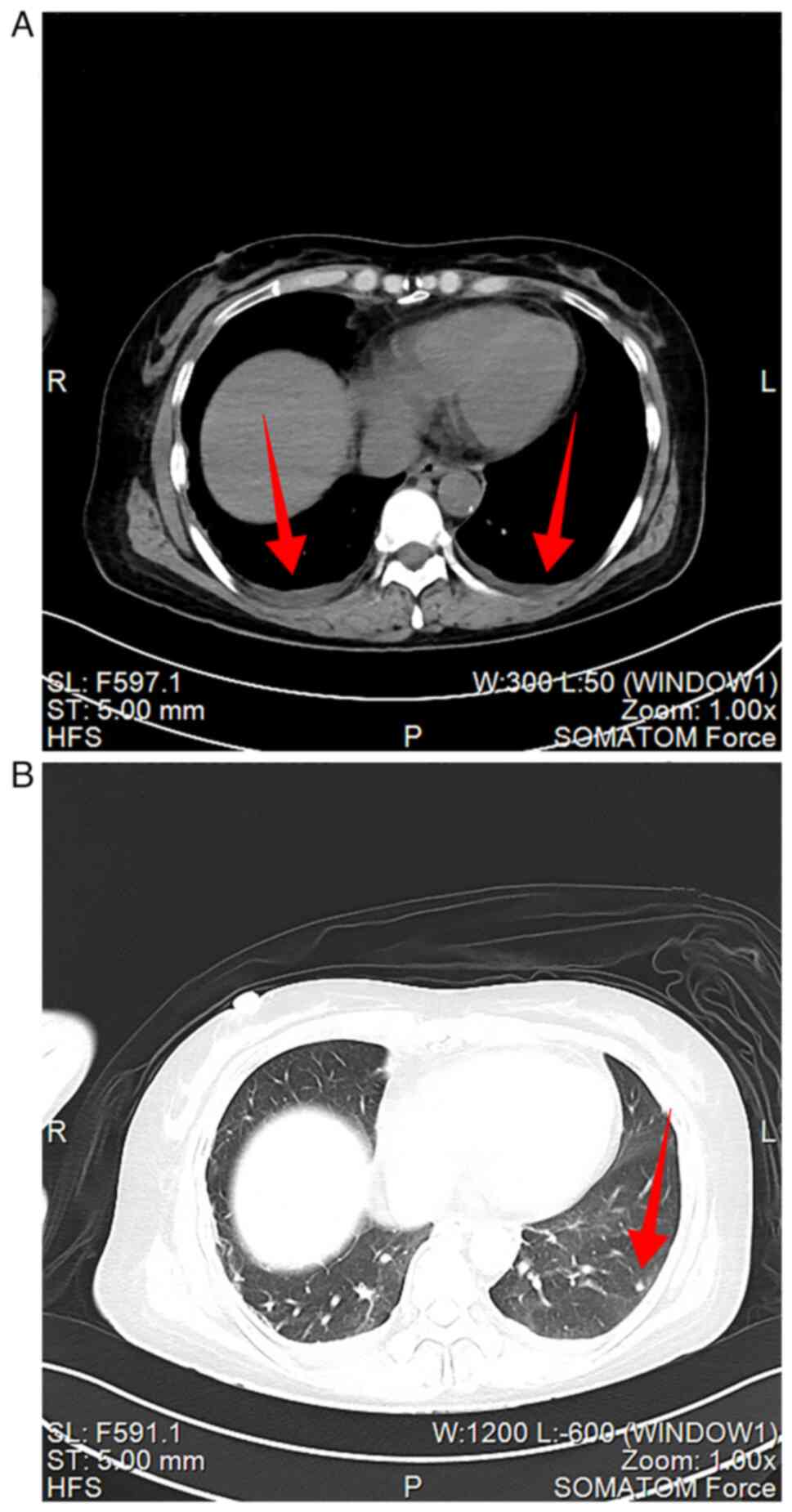
of lower limb. Chest and abdominal CT (Siemens SOMATOM Force;
Imaging parameters: layer thickness 5 mm, layer spacing 5 mm,
voltage 120 KV, current 225 mA, matrix 512x512) found multiple
small nodules in both lungs, where mild edema in bilateral pleural
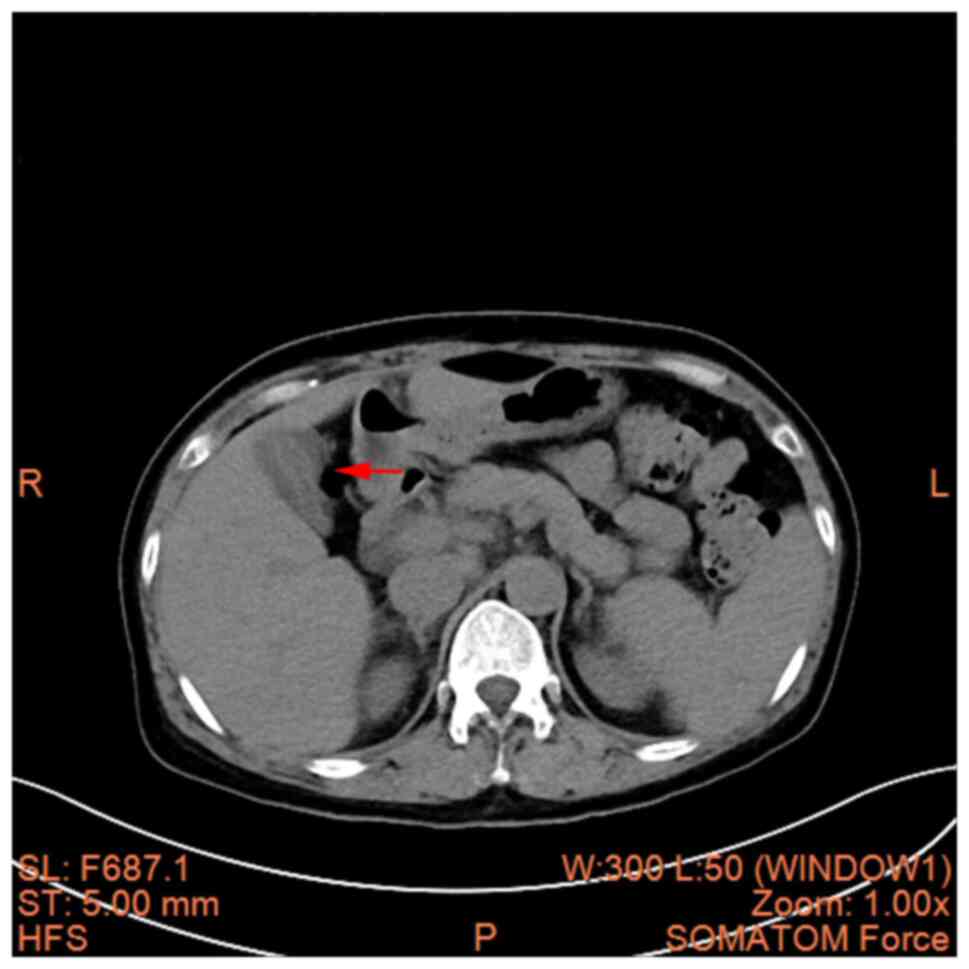
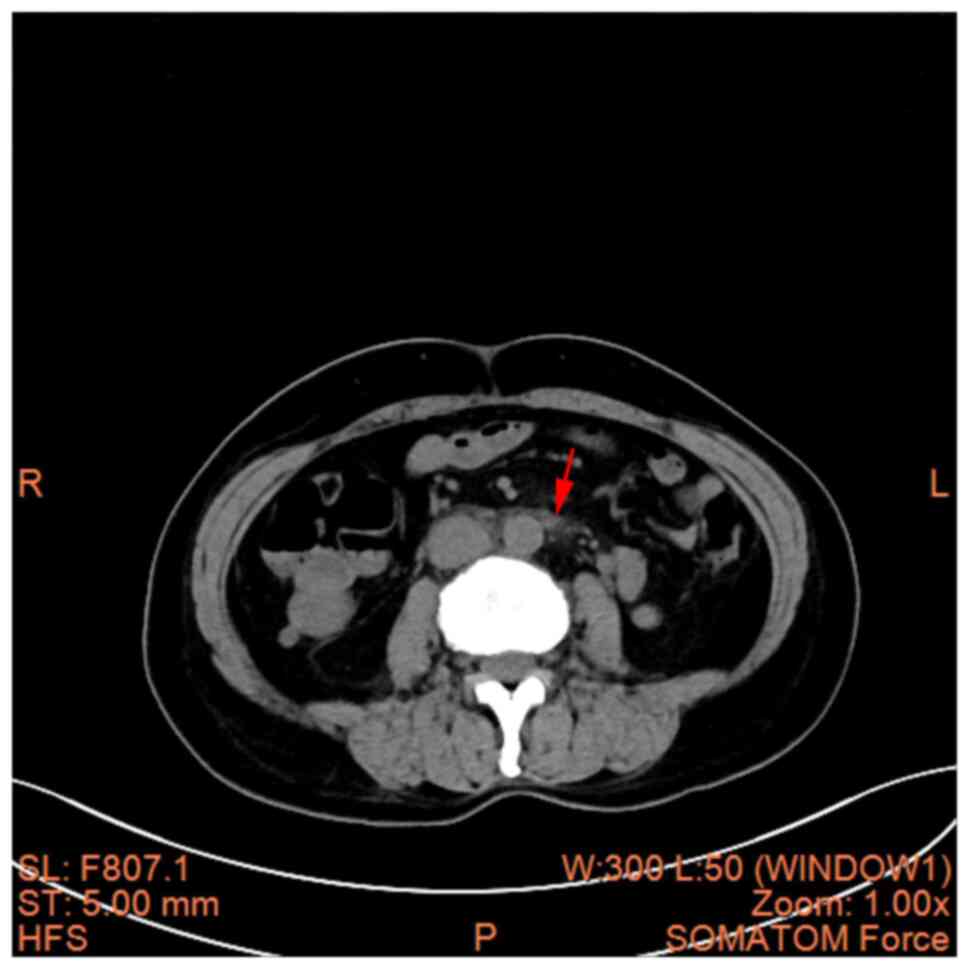
cavity was revealed (Fig. 3A and
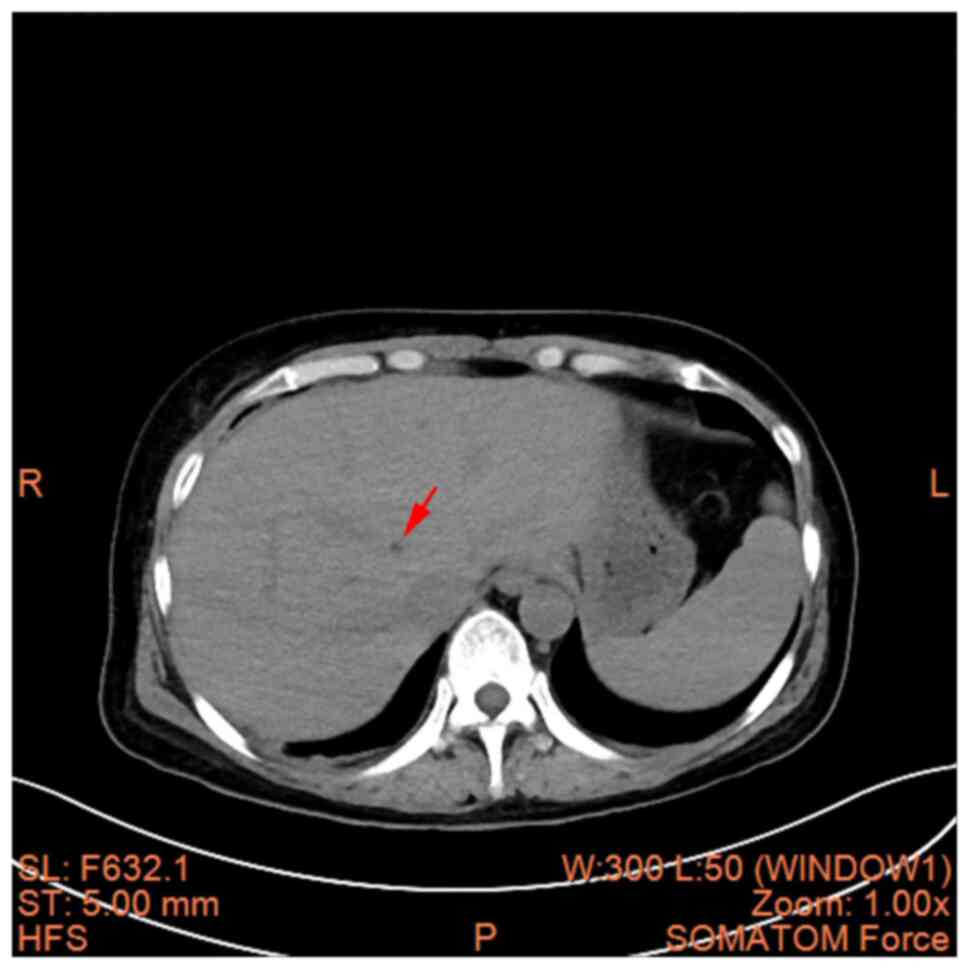
B). A hepatic cyst, cholecystitis
and mesenteric panniculitis were also revealed (Fig. 4, Fig.
5 and Fig. 6). The pancreas
was a normal shape and size, with no discernible abnormalities in
its parenchyma. The pancreatic duct did not display any signs of
dilation; the spleen demonstrated a normal shape and size, without
any abnormalities in its parenchyma; there was no significant
expansion or fluid accumulation observed in the stomach and
intestines, and there were no evident masses present; the kidneys
and adrenal glands exhibited normal size and shape, with no
abnormalities detected in their parenchyma; there were no
abnormalities found in the renal pelvis or ureters on both
sides.
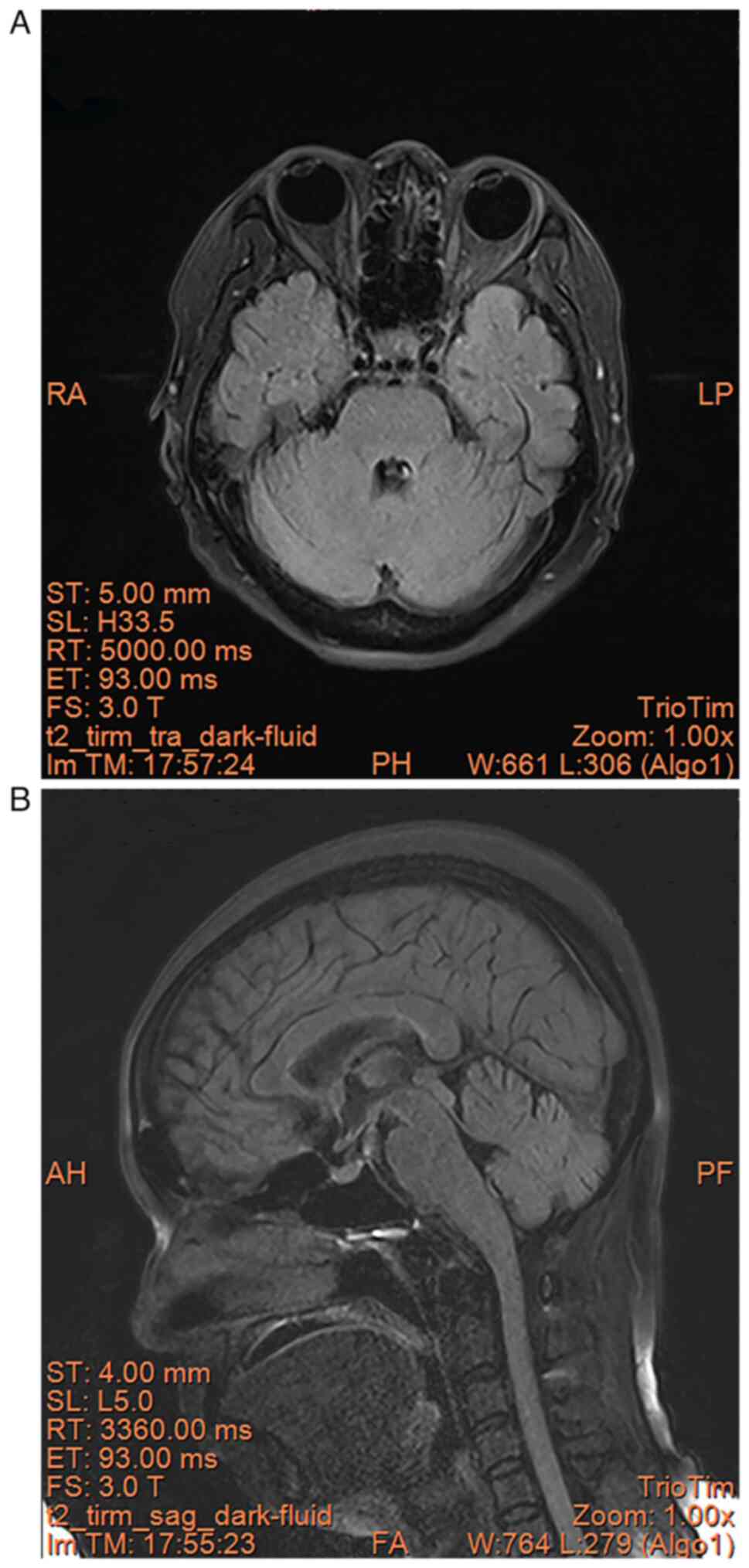
Reexamination of the brain MRI (Siemens Trio Tim
3.0T; Scanning parameters: repetition time (TR)/echo time (TE)
5,860 ms/93 ms, repetition time (Ti) 2,003 ms, layer thickness 5
mm, layer spacing 1 mm, field of view 26x26 cm, matrix 256x256) in
August 2023 showed no significant abnormality (Fig. 7A and B).
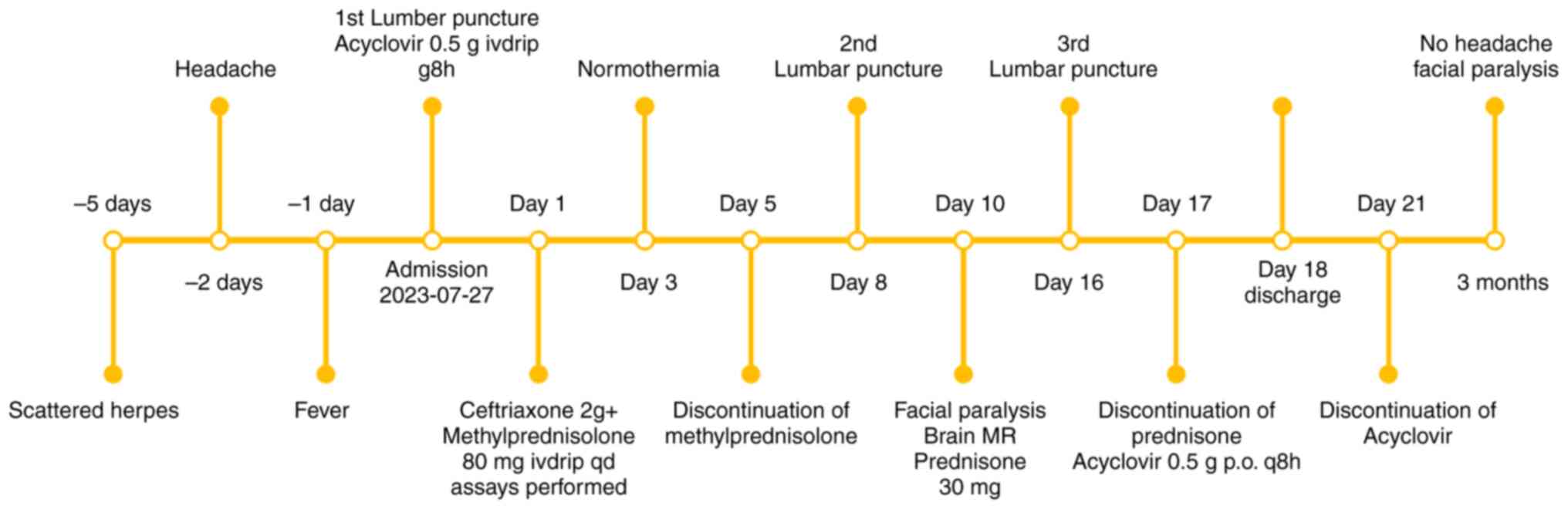
As a result, the patient was treated with the
following regimen (13): i)
Mannitol 125 ml intravenous (IV) drip q12 h (for 6 days) + 125 ml
IV drip qday (Mannitol was tapered for an additional 2 days); ii)
normal saline (NS) 100 ml + 2 g ceftriaxone (Rocephin) IV drip qday
(for 7 days); iii) NS 250 ml + 0.5 g acyclovir IV drip q8 h [for 16
days, followed by sequential oral therapy (0.5 g q8 h) until day
21]; and iv) NS 100 ml + methylprednisolone sodium succinate 80 mg
IV drip qday (for 5 days). The patient presented with fever and
headache upon admission. Physical examination revealed neck
stiffness of three fingers, positive Kernig's sign and herpes on
the anterior chest and back in the distribution of intercostal
nerves, consistent with HZ. Combined with the results of lumbar
puncture at admission, these findings suggested the presence of VZV
meningitis and intracranial hypertension in the patient. Therefore,
the present study initiated treatment upon admission including
mannitol for dehydration and reduction of intracranial pressure,
ceftriaxone for antibiotic therapy, acyclovir for antiviral
treatment and methylprednisolone for anti-inflammatory purposes. On
the third day of treatment, the temperature of the patient returned
to within normal range, and her mental status exhibited significant
improvement compared to prior assessment, transitioning from a
state of lethargy to heightened alertness. Consequently,
methylprednisolone was discontinued on day 5 of treatment while
ceftriaxone was ceased and mannitol dosage reduced after 1 week of
therapy, taking into consideration the effective control of
meningitis. Acyclovir monotherapy was continued (Fig. 8).
At 10 days after admission (August 2023), the
patient developed right peripheral facial paralysis, but no
significant abnormality was found in brain MRI. After
reexamination, prednisone acetate 30 mg qday, mecobalamine 0.5 mg
tid (20), vitamin B1 10 mg tid,
potassium chloride tablet 0.5 g tid and calcium carbonate 300 mg qd
(for 7 days) were treated orally (21). However, on the tenth day of
hospitalization, the patient presented with symptoms of facial
paralysis, prompting resumption of oral prednisone for
anti-inflammatory treatment. Additionally, methylcobalamin and
vitamin B1 were administered for nerve nutrition therapy, potassium
chloride was supplemented to mitigate hormone-induced electrolyte
imbalance side effects and calcium carbonate was provided as a
preventive measure against hormone-induced osteoporosis (Fig. 8).
Based on the findings of high fever, dizziness, neck
resistance and positive Kernig's sign on physical examination
which, combined with the results of lumbar puncture and CSF and
meta-genomics sequencing results, meningitis was considered. After
a 10-day treatment period, the patient exhibited several symptoms
suggestive of right peripheral facial paralysis. The afferent nerve
of the corneal reflex is the ophthalmic branch of the trigeminal
nerve, the efferent nerve is the facial nerve, and the facial nerve
innervates the orbicularis oculi muscle. If the facial nerve has
inflammation, then its frontal branch, temporal branch, zygomatic
branch, buccal branch, and mandibular branch will have dysfunction
(22). These branches are all
motor function nerves, patients will have frontal wrinkles that are
shallower than the opposite side, their eyes will not close, the
nasolabial groove will be shallower than the opposite side and they
will be leaking air while puffing up cheeks (23). Specifically, in the present
patient, the right frontal lines became shallow, the sclera of the
right eye became visible when the eye was closed, the right
nasolabial fold appeared shallow and there was leakage air on the
right side of the cheek. Therefore, the patient was diagnosed with
meningitis in addition to peripheral facial palsy caused by VZV
reactivation.
On the 17th day of admission, the patient received a
1 week course of prednisone acetate for Bell's palsy; however,
there was no significant improvement in facial paralysis symptoms
at that time. The efficacy of prednisone acetate and B-vitamins in
reducing post-paralysis sequelae could only be confirmed 2 weeks
later. Considering the significant improvement in the third lumbar
puncture results on the 17th day of admission, with notable
reductions in cerebrospinal fluid white blood cells and protein
levels indicating effective control of VZV virus infection,
intravenous acyclovir antiviral treatment was sequentially followed
by oral administration as the patient entered into the recovery
phase. Consequently, the patient was discharged from the hospital
on the 18th day of admission. Following discharge, the patient
continued to receive oral acyclovir antiviral treatment until the
21st day (Fig. 8).
At 3 months after discharge (November 2023), a
follow-up was be conducted (meningitis was considered on admission
and was discharged after 21 days of treatment), the patient
described that the headache was significantly relieved. The fever
had subsided, but the sequelae of the right facial nerve paralysis
persisted. The eyes were not tight (the sclera was visible when the
eyes were closed) on the right side, air leaked from the right
corner of the mouth when puffing out cheeks and the right corner of
the mouth drooped when smiling. These were sequelae of facial
neuritis, rehabilitation can be opted for improving the aesthetics
of the face, but the chances of fully recovering are slim (24). The present study suggested further
acupuncture treatment, but the patient and their family felt that
the current symptoms of facial paralysis only affected appearance
and not daily activities or life, so the patient was unwilling to
continue with rehabilitation treatment. The recent follow-up
results (March 2024) showed that there were no more symptoms of
headache and fever, and compared to 3 months after discharge, there
was no significant change in the symptoms of right facial
paralysis.
Discussion
The probability of central nervous system injury
after VZV infection is only between 0.1 and 0.3% in patients with
healthy immune systems (25).
However, to the best of our knowledge, facial neuritis secondary to
meningitis after VZV infection has not been reported before. The
present case documented a patient who contracted meningitis 5 days
after VZV infection and unilateral facial neuritis 2 weeks later.
At present, the pathogenesis of this condition remains unclear,
both of which are presented in the present case. Namely, the nerve
injury caused by meningeal inflammation after VZV infection and the
potential secondary mechanism of nerve damage caused by
immune-mediated inflammatory response, similar to the pathogenesis
of Guillain-Barre syndrome (26).
The pathogenesis of VZV-associated neuroinflammation involves a
complex interplay between viral replication within sensory ganglia
and immune-mediated responses that contribute to tissue damage and
dysfunction. Upon primary infection, VZV gains access to sensory
ganglia, establishing latent infection within neurons. During
reactivation, the virus can spread along sensory nerves, triggering
a cascade of inflammatory mediators, chemokines, and immune cell
infiltration in the affected neural tissues (27). This then promotes VZV penetration
through the blood-brain barrier to reach the intracranial secondary
meninges (28). Since the facial
nerve can reach into the leptomeninges and extracranial regions,
Ottaiano et al (29)
describes in detail the process of the facial nerve exiting from
the brainstem nucleus and passing through the dura mater; the
inflammation may then spread to the cranial nerves through the
meninges. Furthermore, the aberrant activation of the natural
defense mechanism, characterized by the dysregulated production of
immunomodulatory proteins and chemokines, has been implicated in
the pathogenesis of VZV-induced neurological disorders, such as
encephalitis, myelitis and vasculopathy (30). Liu et al (30) divided 28 patients with VZV
infection complicated with meningitis into a good prognosis group
and a poor prognosis group. After analysis, it was found that
cerebrospinal fluid IL-18 may be an important reference index for
the prognosis of patients. In the proteomic analysis of
cerebrospinal fluid, proteins (CXCL10, ELANE, IL-1RN, MPO, PRTN3,
WARS1, TYMP) related to inflammation and immune cell activation are
upregulated, while proteins (CKMT1B, SLITRK3, Synaptotagmin-3,
KIF5B) related to nerve function and energy metabolism are
downregulated (31).
In the present case, the patient was given 80 mg/day
of methylprednisolone for 5 days, which was stopped after the
patient's body temperature returned to normal. However, facial
neuritis occurred 5 days after the glucocorticoid was stopped, and
30 mg/day of prednisolone acetate was provided as oral treatment,
which was sustained for 1 week. There have also been reports on the
dosage and course of glucocorticoid used after VZV infection
(32,33).
In a previous Japanese patient with Hunt syndrome
secondary to polycranial neuritis and meningitis, the patient was
given 1 g/day methylprednisolone shock treatment for 3 days and
changed to 40 mg/day oral treatment with prednisolone acetate and
then gradually reduced to 5 mg/day within 7 weeks. The patient was
discharged from hospital upon recovery; however, the patient's
weight was not taken into consideration during the administration
of a cortisone shock treatment (34). The standard dosage for
corticosteroid shock therapy is 1,000 mg. However, upon reviewing
the patient's weight, which was ~70 kg, the given dosage was closer
to 14.3 mg/kg (34). The use of
high-dose corticosteroids can lead to secondary infections and
osteoporosis with femoral head fractures. After careful
consideration, the present patient had a body temperature of 39˚C
when she was admitted to the hospital, but the ECG monitoring
showed that the heart rate, blood pressure, respiration, and oxygen
saturation were stable, and the laboratory indicators were not
critical value. After careful consideration, the patient was given
~1.5 mg/kg methylprednisolone. The patient received the normal
adult dose (35), without
undergoing steroid pulse therapy like the Japanese patient who
recovered and was discharged. However, in this case, the patient
still had residual facial neuritis upon discharge. We hypothesize
that this may be related to insufficient steroid dosage. For
patients with VZV-induced meningitis, it might be advisable to
initially opt for corticosteroid shock therapy. Additional
attention to this treatment regimen under similar circumstances
should be paid in the future.
The body temperature of the present patient
fluctuated at 38-39˚C during the first 3 days where her
consciousness began to be blurred (between drowsiness and lethargy,
on the day of admission, the patient's consciousness went from
being alert to drowsy, and on the second day of admission it
progressed from drowsiness to lethargy. After treatment, on the
third day of admission, it shifted from lethargy to clear
consciousness). In combination with the color of the CSF, white
blood cell count and neutrophil ratio, ceftriaxone 2 g/day
anti-inflammatory treatment was given to the patient. The family
members of the patient strongly requested for the replacement of
ordinary ceftriaxone with Rocephin treatment after 1 day of
anti-inflammatory medication. Patient consciousness gradually
improved and the peak temperature gradually returned to normal,
rendering stoppage of the anti-inflammatory treatment after 1 week.
Administration of ceftriaxone (Rocephin) for VZV-associated
meningitis has been previously reported. Shahkarami et al
previously reported a young male patient with VZV infection
complicated with intracranial streptococcal infection, who was
given ceftriaxone 2 g (IV drip; bid) for 2 weeks and was discharged
from hospital after a complete recovery (36). Therefore, the use of ceftriaxone
should be individualized according to the patient's situation. At
the beginning of the treatment, this patient was administered
domestically produced ceftriaxone and indeed experienced persistent
high fever, gradually progressing to a state of drowsiness and
eventually lethargy. The present study promptly switched to
imported Rocephin for treatment, resulting in a reduction in peak
body temperature and improvement in consciousness. Considering the
reported case by Shahkrarami, we will consider administering
Rocephin directly for future cases of VZV-induced meningitis.
A majority of children suffering from stroke have
been documented for this to be attributed to VZV infection
(37). VZV enters the brain
through the reactivation of the latent virus in the trigeminal
ganglion and by transaxonal migration to infect the cerebral
arteries. In the pediatric population, VZV mostly affects the
larger arteries, and in the adult population, it affects both
medium- and large-sized arteries, with increased risk among
immunocompromised (37,38). VZV infection can cause cerebral
artery lesions, promoting the risk of stroke (37). Therefore, in clinical practice, it
is necessary to screen for the causes of stroke in children with
VZV infection, where lumbar puncture examination should be improved
(39). Although the present
patient was not a child with stroke, her experience was similar to
numerous cases of teenagers who have had strokes after upper
respiratory tract infections, and we considered that lumbar
puncture was needed to rule out viral infection in this uncommon
population of patients with cerebral infarction.
After admission, the fasting blood glucose levels of
the present patient was 9.55 mmol/l and no increase in fasting
blood glucose or 2 h after three meals were observed in the
follow-up surveillance about the finger blood glucose, which was
considered to be associated with the application of glucocorticoids
in the course of treatment. However, previous studies have found
that the increase in blood glucose can increase the risk of VZV
infection by 20%, the risk of HZ increases in the diabetes group
compared with the non-diabetes group (RR 1.2; 95% credibility
interval, 1.17-1.22) (40,41). Due to the low innate immune
response of polymorphonuclear cells and mononuclear/macrophages in
patients with diabetes (42), it
is necessary to improve the screening for diabetes for such
patients. It has been previously reported that VZV infection was
associated with ‘invisible’ diabetes (abnormal glucose tolerance or
impaired fasting blood glucose regulation) (43). The susceptibility to VZV infection
is associated with a number of risk factors, including age, immune
status and a number of chronic underlying diseases, including
diabetes mellitus, chronic obstructive pulmonary disease,
rheumatoid arthritis and systemic lupus erythematosus (44).
The present study also examined indicators related
to rheumatic immunity, and there were no abnormal indicators
related to disease. Chest CT was completed in this patient, and no
COPD was found. Detailed screening for some of the aforementioned
risk factors was not performed in the treatment of the present
patient. According to a previous meta-analysis, the overall risk of
developing shingles in patients with diabetes mellitus is 1.6X
higher compared with that in patients without diabetes mellitus
(45). Therefore, monitoring and
control of blood sugar is necessary. Patients with diabetes
mellitus bring difficulties to treatment. The present patient's
blood glucose monitoring did not meet the criteria for diabetes. If
a VZV infected patient has diabetes while taking glucocorticoids it
will raise blood glucose levels, then the increase in blood glucose
will decrease the effect of treatment. For the treatment of this
disease, glucocorticoids are necessary, and it would promote the
increase of blood glucose. This forms a vicious circle, whereby if
the increase in blood glucose is severe, glucose-lowering
medications may be added as the next step for faster recovery. For
patients with VZV infection and diabetes, it is recommended to
perform regular monitoring of fasting and postprandial 2-h finger
blood glucose levels upon admission. Based on the results of
glucose monitoring, insulin preparations should be administered to
correct the condition and ensure that the patient's blood glucose
remains within a normal range, thereby preventing any impediment in
the recovery process caused by abnormal blood glucose levels.
It has also been reported that CSF protein and serum
procalcitonin levels are potential markers for differentiating
bacterial from viral meningitis, where their combinations conferred
higher predictive accuracy to bacterial meningitis. No one marker
is better than the other, but it is easier to determine the nature
of the disease if these two test results either increase together
or decrease together (46).
However, the present study only tested CRP without testing
procalcitonin (PCT). PCT would usually be tested if the fever lasts
for >5 days (47), whereas the
present patient had only been running a fever for 3 days after
admission and we felt that the cost of measuring procalcitonin was
relatively high. Moreover, it would not significantly impact the
treatment plan. Therefore, in order to save costs for the patient,
we did not proceed with further testing of procalcitonin. Both CRP
and PCT should be tested in subsequent clinical analyses of
patients exhibiting similar characteristics. In addition, it is
also necessary to see the cerebrospinal fluid color, cell number
and the proportion of neutrophils. Generally, the cerebrospinal
fluid color of viral encephalitis is colorless and transparent, and
the color of bacterial infection will have turbidity or color
change. If the cerebrospinal fluid white blood cell number is
>10x106/l, and the proportion of neutrophils is
>50%, then bacterial infection or secondary bacterial infection
after viral infection should be suspected (48). Although cerebrospinal fluid NGS did
not find bacterial infection, we should not rely too much on the
auxiliary examination results and antimicrobial treatment should be
given in time.
At present, most of the literature on the treatment
of VZV central nervous system infection still discusses antiviral
and glucocorticoid therapy (13,49),
Antiviral drugs (50) include
acyclovir, valacyclovir and famciclovir (FDA-approved drug for the
treatment of VZV infection), brivudine (used in some European
countries), and anamivir (helicase-primer inhibitor, approved in
Japan). These antiviral drugs have a certain effect, but the effect
on postherpetic pain is poor, and new antiviral drugs still need to
be further developed (51).
Prevention of VZV infection by vaccination or passive immunization
is well established in medical practice (49). The present patient was not
vaccinated, and novel antiviral agents were not available at this
institution, which contributed to the treatment we did not expect
for this patient.
VZV infection may be associated with some potential
confounding factors, which will affect the clinical manifestations
and outcomes of the disease. A Japanese study showed that patients
with a history of herpes zoster for <10 years have half the risk
of infection compared with the general population (52). The present patient did not have a
history of herpes zoster infection in the last 10 years, so she was
at higher risk of infection. Another study showed that hot and
humid weather increases hospital visits for HZ infections, that the
onset of the disease is more common in women >40 years old and
the season of this patient's onset is more often in a hot and humid
environment, and the age and sex of the present patient was
consistent with this report (53).
Further research on confounding factors for VZV infection is
needed, this can prevent the occurrence of such diseases.
In conclusion, the present case documented a patient
with meningitis after VZV infection, followed by peripheral facial
paralysis. The clinical manifestations of VZV infection are complex
and varied, which requires the clinician to have an accurate
understanding of disease progression and treatment. In addition,
changes in blood sugar in the patient require constant monitoring,
because the incidence of herpes zoster infection is higher in
patients with diabetes mellitus compared with that in general
patients. If we see such patients again, we need to reduce the
hormone dosage slowly for about 3 weeks, and choose the right brand
of ceftriaxone. A Chinese specialist in the diagnosis and treatment
of herpes zoster (35) recommends
a 3-day course of corticosteroids for patients with VZV-induced
meningitis, similar to the treatment received by the patient in
Japan who was given 1 g of methylprednisolone intravenously. The
normal protocol involves tapering off the dosage slowly to prevent
exacerbation of the underlying condition. After 3 days of using 1
g, the dosage is reduced to 500 mg for another 3 days, then further
reduced to 250 mg for another 3 days, followed by a reduction to
125 mg for another 3 days. After that, oral administration begins
at a dose of 60 mg and is gradually decreased over a period of
three days until reaching a dose of only 15 mg before stopping
completely (54). This is the
standard procedure. The recommended dosage and treatment plan for
methylprednisolone suggested by Chinese experts did not align with
the patients' conditions, the prednisolone instructions have
already stated that shock therapy should only be used for patients
with severe disease deterioration or those who are unresponsive to
conventional treatments such as non-steroidal anti-inflammatory
drugs, gold salts and penicillamine (55). The present patient showed
improvement with regular treatment, resulting in no fever and a
change from unconsciousness to alertness. The present study
considered this treatment to be effective, so there is no need for
shock therapy with methylprednisolone. There are also a number of
adverse reactions associated with shock therapy, such as masking
infections, electrolyte imbalances and avascular necrosis of the
femoral head. As doctors, we should always consider treatment plans
that provide more benefits than risks for our patients.
Nevertheless, we are inclined to believe that this protocol holds
potential for treating individuals afflicted with VZV-induced
meningitis. As for the use of ceftriaxone sodium (Rocephin), the
present study found it to be more effective than other brands in
treating high fever associated with this type of infection. Hence,
we hypothesize that, for severe cases, after an initial high-dose
corticosteroid treatment followed by gradual tapering off,
ceftriaxone sodium could be chosen as an effective option
specifically targeting signs of intracranial infection.
Acknowledgements
Not applicable.
Funding
Funding: The present case report was supported by Medical and
Health Science and Technology Project of Shandong Province (grant
no. 202303071517).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author. The corresponding datasets
have been submitted to a public database (National Center of
Biotechnology Information; accession no. PRJNA1123841; https://dataview.ncbi.nlm.nih.gov/object/PRJNA1123841?reviewer=8i43p9hgrn6gpvf0rim02k5uac).
Authors' contributions
YH, MZ, MH and LZ contributed to the conception of
this article. Discussion and analysis were performed by LZ. YH and
MZ wrote the first draft of the manuscript. MH and YH confirm the
authenticity of all the raw data. All authors have read and
approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
The patient was informed that data concerning the
case would be submitted for publication and consented.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Gnann JW and Whitley J: Neurologic
manifestations of varicella and herpes zoster. Scheld WM, Whitley
RJ and Marra CM, (eds): In: Infections of the central nervous
system. 3rd edition. Lippincott Williams & Wilkins,
Philadelphia, pp145-157, 2004.
|
|
2
|
Cohen JI: The varicella-zoster virus
genome. Curr Top Microbiol Immunol. 342:1–14. 2010.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Arvin AM: Varicella-zoster virus. Clin
Microbiol Rev. 9:361–381. 1996.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Vleck SE, Oliver SL, Brady JJ, Blau HM,
Rajamani J, Sommer MH and Arvin AM: Structure-function analysis of
varicella-zoster virus glycoprotein H identifies domain-specific
roles for fusion and skin tropism. Proc Natl Acad Sci USA.
108:18412–18417. 2011.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Zerboni L, Sen N, Oliver SL and Arvin AM:
Molecular mechanisms of varicella zoster virus pathogenesis. Nat
Rev Microbiol. 12:197–210. 2014.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Ayoade F and Kumar S: Varicella-Zoster
Virus (Chickenpox). In: StatPearls. StatPearls Publishing, Treasure
Island, FL, 2022.
|
|
7
|
Gershon M and Gershon A: Varicella-Zoster
virus and the enteric nervous system. J Infect Dis. 218 (Suppl
2):S113–S119. 2018.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Cunningham AL and Heineman T: Vaccine
profile of herpes zoster (HZ/su) subunit vaccine. Expert Rev
Vaccines. 16:1–10. 2017.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Kennedy PGE and Gershon AA: Clinical
features of Varicella-Zoster virus infection. Viruses.
10(609)2018.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Tiwaskar M and Vora A: Unveiling the
uncertainties: Exploring the utility of herpes zoster vaccines. J
Assoc Physicians India. 71:11–12. 2023.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Patil A, Goldust M and Wollina U: Herpes
zoster: A review of clinical manifestations and management.
Viruses. 14(192)2022.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Nagel MA, Forghani B, Mahalingam R,
Wellish MC, Cohrs RJ, Russman AN, Katzan I, Lin R, Gardner CJ and
Gilden DH: The value of detecting anti-VZV IgG antibody in CSF to
diagnose VZV vasculopathy. Neurology. 68:1069–1073. 2007.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Nagel MA, Niemeyer CS and Bubak AN:
Central nervous system infections produced by varicella zoster
virus. Curr Opin Infect Dis. 33:273–278. 2020.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Harbecke R, Cohen JI and Oxman MN: Herpes
Zoster vaccines. J Infect Dis. 224 (Suppl 2):S429–S442.
2021.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Werner RN and Ghoreschi K: Herpes
zoster-prevention, diagnosis, and treatment. Hautarzt. 73:442–451.
2022.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Shikova E, Kumanova A, Tournev I,
Zhelyazkova S, Vassileva E, Ivanov I and Pishmisheva M: Varicella
zoster virus infection in neurological patients in Bulgaria. J
Neurovirol. 27:272–278. 2021.PubMed/NCBI View Article : Google Scholar
|
|
17
|
de Ory F, Avellón A, Echevarría JE,
Sánchez-Seco MP, Trallero G, Cabrerizo M, Casas I, Pozo F, Fedele
G, Vicente D, et al: Viral infections of the central nervous system
in Spain: A prospective study. J Med Virol. 85:554–562.
2013.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Nagel MA and Gilden D: Neurological
complications of varicella zoster virus reactivation. Curr Opin
Neurol. 27:356–360. 2014.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Liu Q, Zhou X and Li Z: Acute myelitis
with multicranial neuritis caused by Varicella zoster virus: A case
report. BMC Neurol. 22(45)2022.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Heckmann JG, Lang C, Urban P, Glocker FX,
Weder BJ, Reiter G, Bischoff C, Meier U and Guntinas-Lichius O:
Therapie der idiopathischen Fazialisparese (Bell's palsy). Akt
Neurol. 44:712–727. 2017.
|
|
21
|
Heckmann JG, Urban PP, Pitz S,
Guntinas-Lichius O and Gágyor I: The diagnosis and treatment of
idiopathic facial paresis (Bell's Palsy). Dtsch Arztebl Int.
116:692–702. 2019.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Holman AE and Puglia MP II: Loss of
corneal reflex in children undergoing spinal anesthesia: A case
series. A A Pract. 14:9–11. 2020.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Warner MJ, Hutchison J and Varacallo M:
Bell Palsy. In: StatPearls. StatPearls Publishing, Treasure Island,
FL, 2023.
|
|
24
|
Kim SJ and Lee HY: Acute peripheral facial
palsy: Recent guidelines and a systematic review of the literature.
J Korean Med Sci. 35(e245)2020.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Takami K, Kenzaka T, Kumabe A, Fukuzawa M,
Eto Y, Nakata S, Shinohara K and Endo K: Varicella-zoster
virus-associated meningitis, encephalitis, and myelitis with
sporadic skin blisters: A case report. World J Clin Cases.
10:717–724. 2022.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Muñoz-Sellart M, García-Vidal C,
Martínez-Yelamos S, Niubó J and Fernández-Viladrich P: Peripheral
facial palsy after varicella. Report of two cases and review of the
literature. Enferm Infecc Microbiol Clin. 28:504–508.
2010.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Hakami MA, Khan FR, Abdulaziz O,
Alshaghdali K, Hazazi A, Aleissi AF, Abalkhail A, Alotaibi BS,
Alhazmi AYM, Kukreti N and Binshaya AS: Varicella-zoster
virus-related neurological complications: From infection to
immunomodulatory therapies. Rev Med Virol. 34(e2554)2024.PubMed/NCBI View
Article : Google Scholar
|
|
28
|
Tavazzi E, Minoli L, Ferrante P, Scagnelli
P, Del Bue S, Romani A, Ravaglia S and Marchioni E: Varicella
zoster virus Meningo-encephalo-myelitis in an immunocompetent
patient. Neurol Sci. 29:279–283. 2008.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Ottaiano AC, Gomez GD and Freddi TAL: The
facial nerve: Anatomy and pathology. Semin Ultrasound CT MR.
44:71–80. 2023.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Liu H, Wang J, Zhang Y, Gu J, Wang Y, Yan
Y, Pan D and Sun Z: Cerebrospinal fluid proteomics in meningitis
patients with reactivated varicella zoster virus. Immun Inflamm
Dis. 11(e1038)2023.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Lind L, Eriksson K and Grahn A: Chemokines
and matrix metalloproteinases in cerebrospinal fluid of patients
with central nervous system complications caused by varicella-
zoster virus. J Neuroinflammation. 16(42)2019.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Tunkel AR, Glaser CA, Bloch KC, Sejvar JJ,
Marra CM, Roos KL, Hartman BJ, Kaplan SL, Scheld WM and Whitley RJ:
Infectious Diseases Society of America. The management of
encephalitis: Clinical practice guidelines by the infectious
diseases society of America. Clin Infect Dis. 47:303–327.
2008.PubMed/NCBI View
Article : Google Scholar
|
|
33
|
Siciliano V, Rosà T, Del Vecchio P,
D'Angelillo A, Brigida M, Longhitano Y, Zanza C, Santoro MC,
Candelli M, Franceschi F and Piccioni A: Viral encephalitis in
adults: A narrative review. Rev Recent Clin Trials. 17:259–267.
2022.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Hongo T, Ikeda F, Fujioka S, Akatsuka R,
Fujiwara T and Yamamoto K: Intravenous Vitamin C as ancillary
treatment for cranial polyneuritis and meningitis due to varicella
zoster virus reactivation. Acta Med Okayama. 74:257–260.
2020.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Chinese Medical Association
Dermatologists' Subcommittee on Herpes Zoster Expert Consensus
Working Group. Chinese consensus on the diagnosis and management of
herpes zoster (2022). Chin J Dermatol. 55:1033–1040.
2022.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Shahkarami F, Fallah Tafti M, Alizadeh M,
Foroughi A and Bayati R: An unusual case of Group B streptococcal
meningitis with concomitant varicella-zoster virus infection in a
previously healthy male. Cureus. 14(e32134)2022.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Amlie-Lefond C and Gilden D: Varicella
zoster virus: A common cause of stroke in children and adults. J
Stroke Cerebrovasc Dis. 25:1561–1569. 2016.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Murala S, Nagarajan E and Bollu PC:
Infectious causes of stroke. J Stroke Cerebrovasc Dis.
31(106274)2022.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Gilden D: Varicella-Zoster virus
infections. Continuum (Minneap Minn). 21:1692–1703. 2015.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Muñoz-Quiles C, López-Lacort M,
Ampudia-Blasco FJ and Díez-Domingo J: Risk and impact of herpes
zoster on patients with diabetes: A population-based study,
2009-2014. Hum Vaccin Immunother. 13:2606–2611. 2017.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Papagianni M, Metallidis S and Tziomalos
K: Herpes zoster and diabetes mellitus: A review. Diabetes Ther.
9:545–550. 2018.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Verma VK, Ram VS, Singh PS, Kumar M,
Awasthi S and Kela D: Herpes zoster as a presentation of diabetes
mellitus. Int J Res Med Sci. 5:1878–1881. 2017.
|
|
43
|
Odhaib SA and Mansour A: Herpes zoster
infection as a presentation for hidden diabetes mellitus. Cureus.
12(e7363)2020.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Koshy E, Mengting L, Kumar H, Somagond YM,
Priyadarsini S, Kuniyal A, Prakash V and Sahoo A: Epidemiology,
treatment and prevention of herpes zoster: A comprehensive review.
Indian J Dermatol Venereol Leprol. 84:251–262. 2018.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Lai SW, Liu CS, Kuo YH, Lin CL, Hwang BF
and Liao KF: The incidence of herpes zoster in patients with
diabetes mellitus: A meta-analysis of cohort studies. Medicine
(Baltimore). 100(e25292)2021.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Alnomasy SF, Alotaibi BS, Mujamammi AH,
Hassan EA and Ali ME: Microbial aspects and potential markers for
differentiation between bacterial and viral meningitis among adult
patients. PLoS One. 16(e0251518)2021.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Hu L, Shi Q, Shi M, Liu R and Wang C:
Diagnostic value of PCT and CRP for detecting serious bacterial
infections in patients with fever of unknown origin: A systematic
review and Meta-analysis. Appl Immunohistochem Mol Morphol.
25:e61–e69. 2017.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Shahan B, Choi EY and Nieves G:
Cerebrospinal fluid analysis. Am Fam Physician. 103:422–428.
2021.PubMed/NCBI
|
|
49
|
Sauerbrei A: Diagnosis, antiviral therapy,
and prophylaxis of varicella-zoster virus infections. Eur J Clin
Microbiol Infect Dis. 35:723–734. 2016.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Yaldiz M, Solak B, Kara RO, Cosansu N and
Erdem MT: Comparison of famciclovir, valaciclovir, and brivudine
treatments in adult immunocompetent patients with herpes zoster. Am
J Ther. 25:e626–e634. 2018.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Andrei G and Snoeck R: Advances and
perspectives in the management of varicella-zoster virus
infections. Molecules. 26(1132)2021.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Kawahira K, Imano H, Yamada K, Mori Y,
Asada H, Okuno Y, Yamanishi K and Iso H: Risk of herpes zoster
according to past history in the general population: The Japanese
Shozu herpes zoster study. J Dermatol. 50:1140–1144.
2023.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Lv X, Fang X, Qian T, Cai Y, Gao P, Chen
H, Wu Q, Wu J, Fan Y and Ye D: Association between meteorological
factors and outpatient visits for herpes zoster in Hefei, China: A
Time-Series analysis. Int J Environ Res Public Health.
20(2097)2023.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Sinha A and Bagga A: Pulse steroid
therapy. Indian J Pediatr. 75:1057–1066. 2008.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Pikkel YY and Pikkel J: Acute retinal
necrosis in childhood. Case Rep Ophthalmol. 5:138–143.
2014.PubMed/NCBI View Article : Google Scholar
|