1. Introduction
To date, immune checkpoint inhibitors (ICIs)
represent a cornerstone in treating various cancers as they harness
the potential of the immune system to treat malignancies. However,
their influence on immune pathways can lead to immune-related
adverse events (irAEs). IrAEs are a spectrum of side effects that
can occur because of treatment with ICIs (1). These adverse events arise from the
dysregulation of the immune system by immunotherapeutic drugs,
leading to immune-mediated inflammation and tissue damage in
various organs and tissues throughout the body (2). The spectrum of irAEs is broad and can
involve any organ system, including the skin, gastrointestinal
tract, liver, endocrine glands and lungs (3). Common irAEs include dermatitis,
colitis, hepatitis and thyroid dysfunction, with pneumonitis
standing as a particularly significant concern (4). Pneumonitis, characterized by
inflammation of lung tissue, emerges as one of the notable irAEs
due to its potential for severe morbidity and mortality if not
promptly recognized and managed (5). However, the severity and specific
manifestations of irAEs can significantly vary among patients
depending on the type of immunotherapy, the underlying cancer, and
the immune status of patients (6).
The clinical presentation of irAEs can range from mild,
self-limited symptoms to severe, life-threatening complications
(7). Rapid identification and
management of irAEs are crucial to prevent severe morbidity and
mortality (8). Treatment typically
involves the temporary or permanent discontinuation of
immunotherapy and the initiation of immunosuppressive medications,
such as corticosteroids or other immunomodulators such as
infliximab or vedolizumab related to the severity and nature of the
adverse event (9). The mechanisms
underlying irAEs are complex and need to be fully elucidated.
However, the dysregulation of immune checkpoints and the resultant
immune activation against normal tissues is considered to play a
central role (10). The diversity
of irAEs underscores the need for a multidisciplinary approach to
diagnose and manage these adverse events best, involving
collaboration between oncologists, immunologists, rheumatologists
and other specialists as needed.
2. Incidence
ICI-related pneumonitis is relatively rare compared
with other irAEs, such skin reactions, flu-like- and
gastrointestinal symptoms, yet it is the most fatal complication
associated with programmed cell death protein 1 inhibitors
(PD-1/PD-L1) (1). Studies indicate
that ICI-related pneumonitis is responsible for a substantial
proportion of deaths attributed to PD-1/PD-L1 inhibitor
monotherapy, accounting for ~35% of such fatalities (11-13).
The overall incidence rate of ICI-related pneumonitis in clinical
trials involving ICI monotherapy is 2.5-5.0%, while that in trials
involving ICI combination therapy is higher, at 7-10% (12). Clinical trials typically enroll
patients without underlying lung disease or autoimmune conditions,
and thus, real-world data reveal a broader incidence of 7-19%
(13). Additionally, studies have
demonstrated that PD-1/PD-L1 inhibitors exhibit a greater incidence
and severity of pulmonary toxicity compared with anti-cytotoxic
T-lymphocyte-associated antigen 4 (CTLA-4) agents (14,15).
Specifically, PD-1 inhibitors have been found to have a higher
occurrence and severity of pulmonary toxicity than PD-L1 inhibitors
(14). Moreover, the incidence of
ICI-related pneumonitis tends to be higher in non-small cell lung
cancer compared with other tumor types, such as melanoma and renal
cell carcinoma (15). The overall
incidence of ICI-related pneumonitis for all grades is 1.4-5.8% in
patients with non-small cell lung cancer (NSCLC), 1-4% in patients
with melanoma and 1-4.8% in those with renal cell carcinoma
(15). These statistics underscore
the importance of cautious monitoring and prompt management of
pneumonitis in patients undergoing ICI therapy.
3. Pathophysiology
The pathogenesis of ICI-related pneumonitis is
complex and multifactorial, involving dysregulated immune responses
against pulmonary tissues triggered by the blockade of immune
checkpoints such as CTLA-4 and PD-1 or its ligand PD-L1(16). While the exact mechanisms remain
not fully understood, several vital processes contribute to the
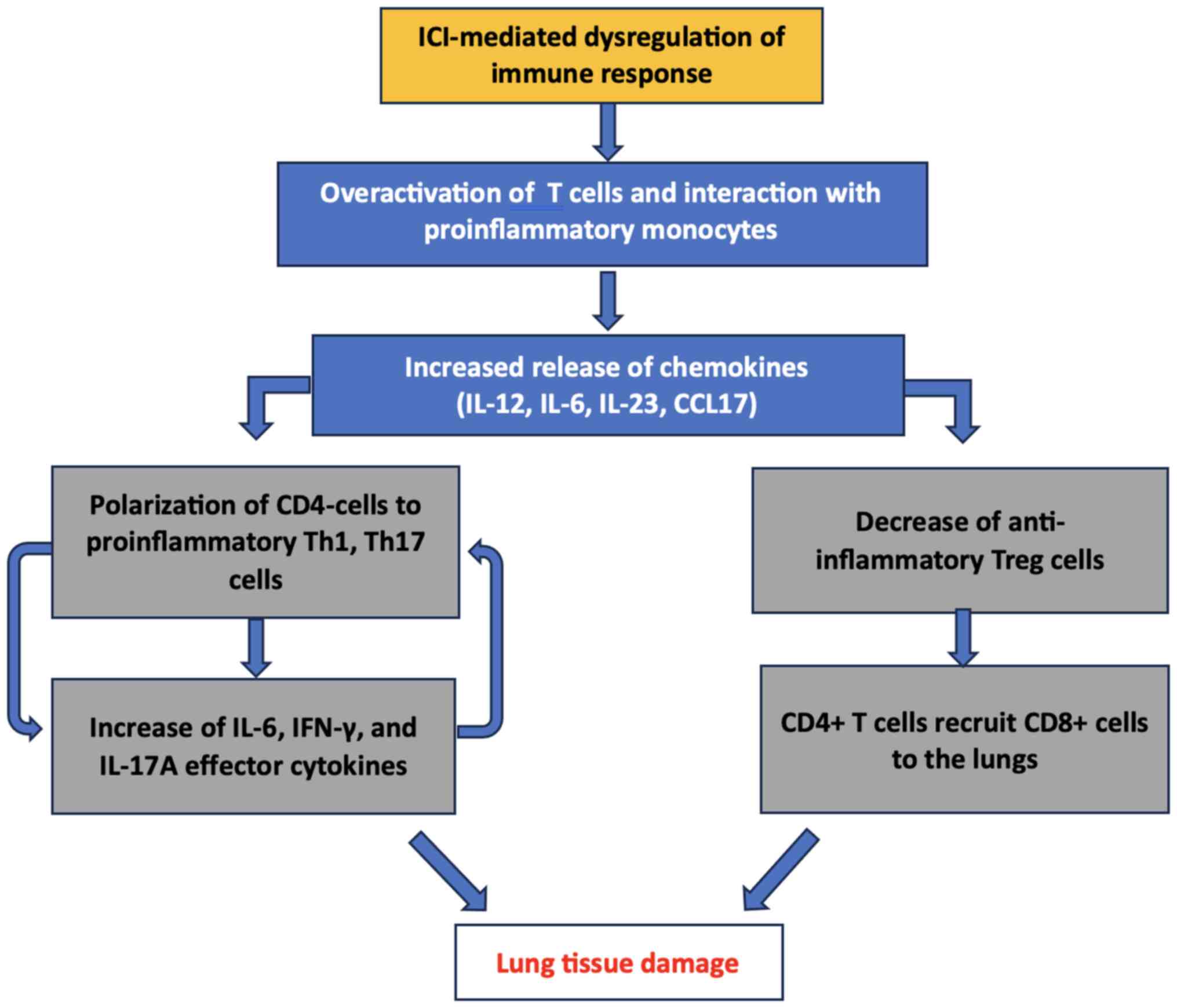
development of pneumonitis in ICI therapy. As depicted in Fig. 1, the disruption of immune tolerance
lies at the core of ICI-related pneumonitis pathogenesis (17). Due to the action of these
immunotherapeutic drugs, including checkpoint inhibition and
blockade, aimed to disrupt immune regulation in tumor tissue, such
alteration, may also occur in normal tissues, such as lungs, even
though a full elucidation of the mechanisms underlying irAEs is
still lacking. Consequently, the irAE mechanism appears similar to
insufficient immune regulation of tissue homeostasis rather than
activation of the immune system. This is supported by the lack of
laboratory confirmation of autoimmunity, since in most cases,
auto-antibodies are absent.
Immune checkpoints are crucial in maintaining
self-tolerance and preventing excessive immune activation against
normal tissues (18). Blockade of
CTLA-4, PD-1, or PD-L1 releases the ‘brakes’ on the immune system,
leading to increased activation of T cells (19). In some cases, this heightened
immune response may result in recognizing self-antigens in lung
tissue, triggering an autoimmune reaction (20). Consequently, the release of
pro-inflammatory cytokines and the infiltration of immune cells
into the lung parenchyma perpetuate tissue damage and contribute to
the development of pneumonitis (21). The activation of effector T cells
plays an essential role in the pathogenesis of lung inflammation.
ICIs promote the activation and proliferation of effector T cells,
crucial for mounting an effective antitumor immune response
(20-22).
However, in the context of pneumonitis, the activation of effector
T cells may lead to their infiltration into the lung parenchyma.
Once within the lung tissue, these activated T cells release a
cascade of pro-inflammatory cytokines and chemokines, including
tumor necrosis factor-α (TNF-α), interleukin-6 (IL-6) and
interferon-γ (IFN-γ) (20-22).
This inflammatory milieu contributes to tissue damage and
exacerbates the immune-mediated lung injury characteristic of
pneumonitis (20-22).
The dysregulated cytokine production (TNF-α, IL-6 and IFN-γ)
orchestrates a cascade of inflammatory responses, recruiting immune
cells to the injury site and amplifying the immune-mediated lung
injury process (22). Moreover,
they can disrupt the delicate balance between pro-inflammatory and
anti-inflammatory signals, further exacerbating tissue damage and
contributing to the severity of pneumonitis (23). Lastly, genetic and environmental
factors play crucial roles in influencing the development of
ICI-related pneumonitis (24).
Specific genetic polymorphisms, such as the gamma-aminobutyric acid
type A receptor subunit pi, the desmocollin and the bromodomain
adjacent to zinc finger domain 2B genes, related to immune
regulation and inflammation have been implicated in the
susceptibility to irAEs, although further research is needed to
elucidate their particular roles in pneumonitis (23,24).
Additionally, factors such as microbiota composition, prior lung
injury, smoking history and concomitant use of other medications
may modulate the risk of developing pneumonitis in patients
receiving ICIs (25,26).
4. Clinical presentation
ICI-related pneumonitis typically presents
nonspecific respiratory symptoms that can mimic other pulmonary
conditions such as infection or malignancy (27). Patients may initially complain of
nonspecific symptoms such as dyspnea, cough, pain and chest
discomfort, which can gradually worsen (28). In more severe cases, respiratory
distress and hypoxia may develop rapidly, warranting urgent medical
attention (29). Constitutional
symptoms such as fatigue, malaise and low-grade fever may accompany
respiratory complaints, further complicating the clinical picture
(Table I) (30). It is important to note that the
onset of symptoms can vary widely, occurring weeks to months after
initiating ICI therapy (31).
Clinical suspicion should be high in patients receiving ICIs who
develop new or worsening respiratory symptoms, especially in the
absence of alternative etiologies and particularly in patients
receiving PD-1/PD-L1 inhibitors (32). Close monitoring for respiratory
symptoms and early intervention is paramount to mitigate the risk
of disease progression and minimize morbidity and mortality
associated with this adverse event.
 | Table IConcise overview of the main clinical
features of ICI-related pneumonitis. |
Table I
Concise overview of the main clinical
features of ICI-related pneumonitis.
| Clinical
features | Description |
|---|
| Respiratory
symptoms | Dyspnea, cough and
chest discomfort |
| Constitutional
symptoms | Fatigue,
generalized weakness or tiredness, malaise and low-grade fever |
| Onset | Variable: Symptoms
may develop weeks to months after initiating ICI therapy |
| Severity | Range: Symptoms can
range from mild to severe, with potential for rapid
progression |
| Progression | • Gradual
worsening: Symptoms may worsen over time, impacting daily
activities and quality of life |
| | • Respiratory
distress: Severe cases may present with acute respiratory distress,
requiring urgent medical attention |
| Associated risk
factors | • Treatment type:
Higher incidence with PD-1/PD-L1 inhibitors compared to anti-CTLA-4
agents |
| | • Tumor type:
Increased risk in non-small cell lung cancer compared with other
malignancies |
| | • Pre-existing lung
disease: History of lung disease may predispose to pneumonitis |
| Management
challenges | • Diagnosis:
Nonspecific symptoms may mimic other pulmonary conditions,
necessitating careful evaluation |
| | • Severity
assessment: Accurate assessment of disease severity is crucial for
appropriate management. |
| Monitoring and
treatment | • Close monitoring:
Regular assessment of respiratory symptoms and clinical status is
essential |
| | • Prompt
intervention: Early recognition and initiation of treatment can
minimize morbidity and mortality |
5. Diagnostic evaluation
Diagnosing ICI-related pneumonitis requires a
comprehensive assessment encompassing clinical, radiological and
laboratory findings (1-3).
The detailed description of these examinations extends beyond the
scope of the present review. Pulmonary function tests (PFTs),
including spirometry and diffusion capacity testing, provide
valuable information about lung function and may reveal restrictive
or obstructive patterns indicative of lung involvement (1-3).
However, PFT findings can be nonspecific and should be interpreted
with clinical and radiological findings (3). The European Society for Medical
Oncology and the American Society for Clinical Oncology recommend
bronchoscopy with bronchoalveolar lavage, and, in some cases,
transbronchial lung biopsy may be performed to obtain samples for
analysis (33,34). Bronchoalveolar lavage fluid
analysis, including cell counts, protein level analysis and
microbiological cultures, can evidence a high lymphoid infiltrate
consistent with immune-mediated pneumonitis, helping to exclude
alternative diagnoses and guide therapeutic decisions (35). Transbronchial biopsy may be
utilized in cases of diagnostic uncertainty or severe disease to
obtain histological samples for pathological evaluation (33-35).
Surgical lung biopsy using video-assisted thoracoscopic surgery to
ascertain differential diagnosis from tumor progression may be used
in cases where the suspicion of progressive cancer persists
(33,34). However, these investigations must
be integrated with clinical and radiological findings.
Specifically, CT scanning of the chest serves as the cornerstone in
radiological evaluation, revealing characteristic patterns of
pneumonitis such as ground-glass opacities (GGOs), consolidations
or interstitial infiltrates (1-3,36-38).
6. Radiological findings
Pneumonitis is a rare irAE following ICI therapy,
and it manifests as interstitial lung disease (36). Table
II summarizes the main CT patterns observed in ICI-related
pneumonitis. Pneumonitis secondary to ICI treatment presents in
four distinct patterns: i) Organizing pneumonia (OP); ii)
nonspecific interstitial pneumonia (NSIP); iii) hypersensitivity
pneumonitis (HP); and iv) diffuse alveolar damage (DAD) (36). While chest X-ray findings in
ICI-related pneumonitis may be nonspecific, characteristic
abnormalities can aid the diagnostic process (37). Common radiographic manifestations
include patchy or diffuse opacities, consolidations and
interstitial infiltrates, which may be bilateral and involve
multiple lung lobes (37,38). Additionally, air bronchograms or a
‘ground-glass’ appearance may suggest alveolar involvement and
inflammatory changes within the lung parenchyma as shown in
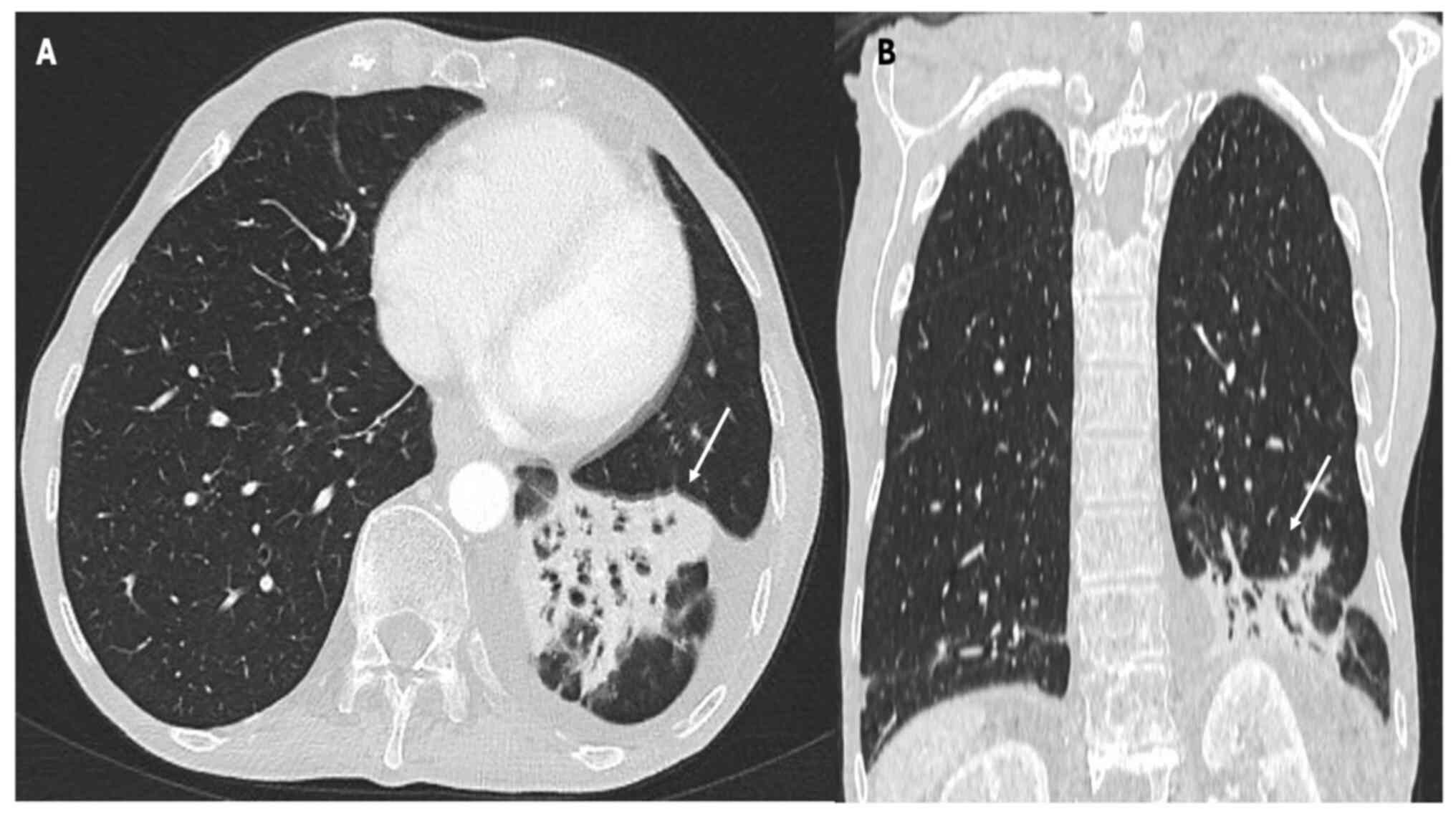
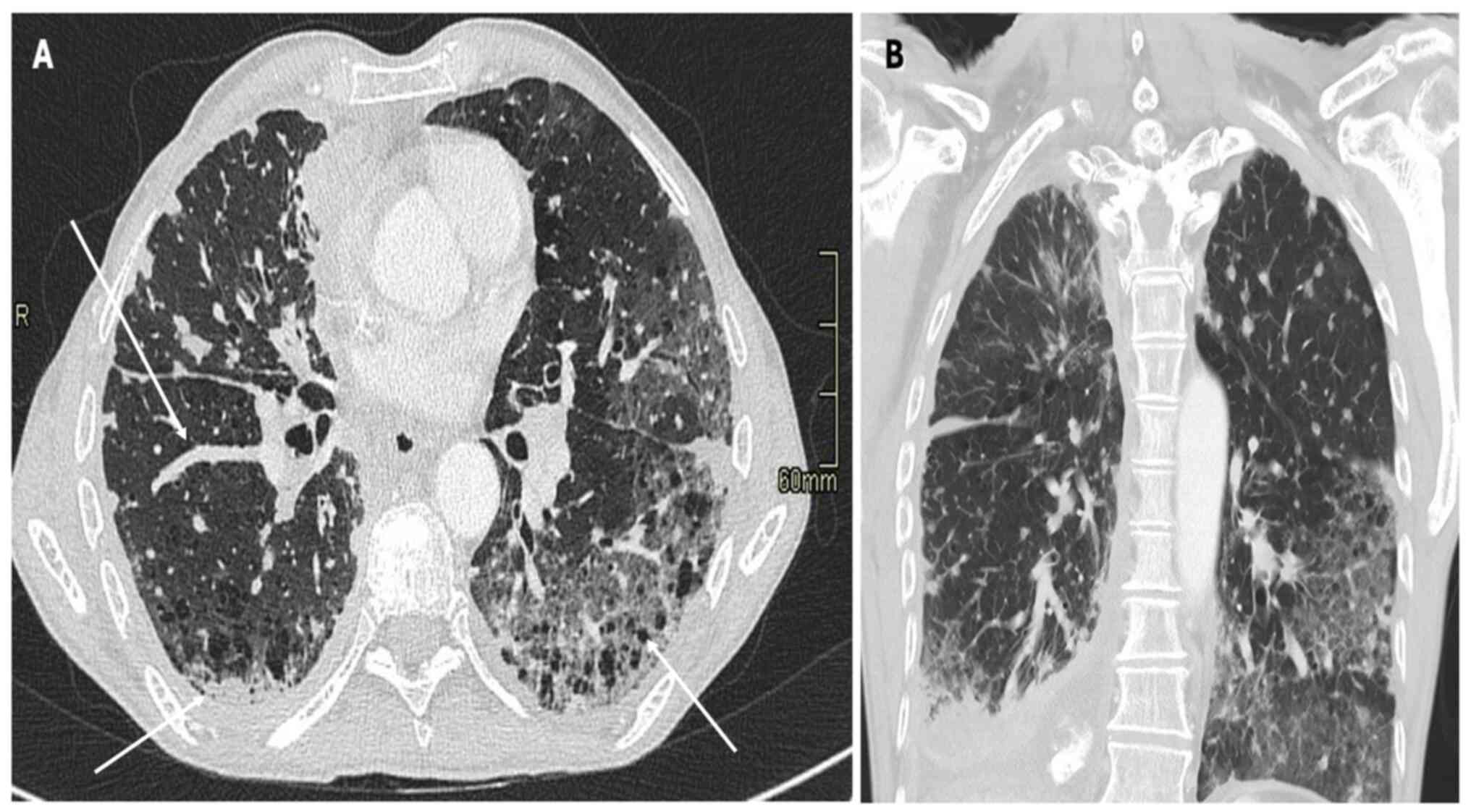
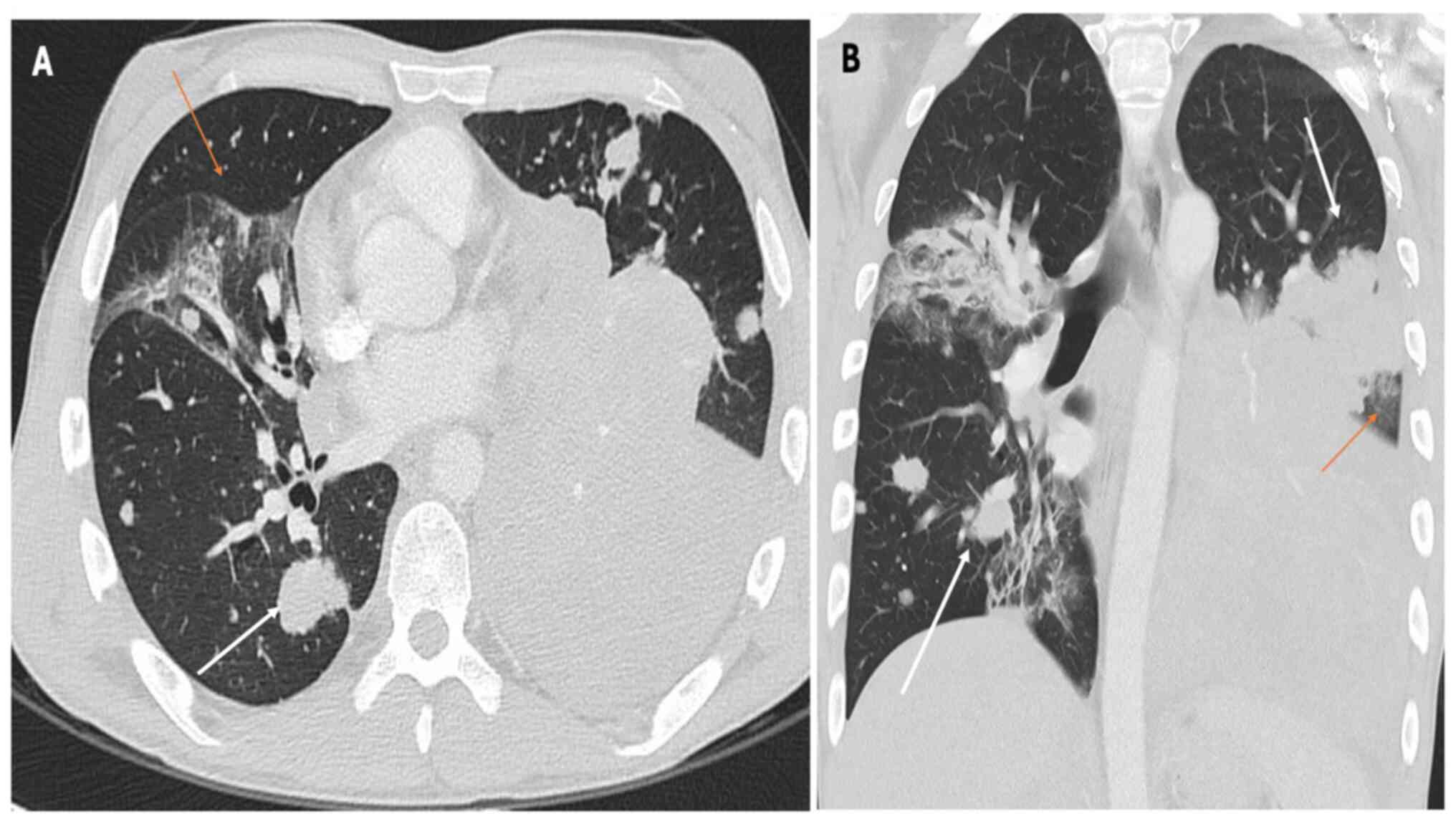
Fig. 2, Fig. 3 and Fig. 4, which report on a case of gastric,
lung and rectal cancer, respectively. However, it is essential to
recognize that chest X-ray findings alone may not be sufficient to
diagnose or exclude pneumonitis (37) definitively. Further imaging with CT
of the chest is often necessary to delineate the extent and nature
of pulmonary abnormalities and guide subsequent management
decisions (38).
 | Table IIMain CT patterns of ICI-related
pneumonitis. |
Table II
Main CT patterns of ICI-related
pneumonitis.
| CT Pattern | Pattern | Features | Histopathology |
|---|
| Organizing
pneumonia | Consolidation with
a peri-bronchial and subpleural distribution, often migrating over
time | GGOs with areas of
consolidation and air bronchograms | Granulation tissue
plugs within airspaces |
| Nonspecific
interstitial pneumonia | Bilateral,
symmetric, peripheral and basal predominant | GGOs with reticular
or linear opacities, traction bronchiectasis, and honeycombing | Uniform
interstitial inflammation and fibrosis |
| Hypersensitivity
pneumonitis | Centrilobular
nodules with a ground-glass or reticular pattern, often with upper
lobe predominance | Centrilobular
nodules, GGOs, mosaic attenuation, and air trapping | Peribronchiolar
inflammation and granuloma formation |
| Diffuse alveolar
damage | Diffuse GGOs and
consolidations with a bilateral and symmetric distribution | Diffuse involvement
of both lungs, often associated with severe respiratory
compromise | Alveolar damage
with hyaline membrane formation |
OP
The characteristic radiographic pattern of OP in
ICI-related pneumonitis often manifests as patchy areas of
consolidation with or without GGOs on high-resolution computed
tomography (HRCT) of the chest (39). OP is characterized by intraluminal
plugs of granulation tissue within the distal airspaces, leading to
consolidative opacities on imaging (37). These consolidations typically have
a subpleural and peri broncho vascular distribution (37). They may show a migratory or
‘reverse halo’ pattern, where a rim of consolidation surrounds a
central area of ground-glass opacity but is not pathognomonic
(40).
NSIP
The HRCT pattern of NSIP in ICI-related pneumonitis
typically manifests as bilateral, patchy ground-glass and reticular
opacities with a basal and peripheral distribution (37,38).
These findings may be accompanied by traction bronchiectasis and
architectural distortion (37,38).
Additionally, unlike usual interstitial pneumonia, NSIP in
ICI-related pneumonitis tends to spare the subpleural regions and
show less honeycombing (41).
HP
The CT pattern of HP can resemble certain features
observed in ICI-related pneumonitis, particularly with NSIP. The
characteristic pattern typically includes diffuse GGOs,
centrilobular nodules and mosaic attenuation patterns, reflecting
the characteristic involvement of the airspaces and small airways
(12,41,42).
Findings such as bronchial wall thickening, air trapping, and
mosaic perfusion on perfusion imaging may also indicate HP
(12). Conversely, NSIP typically
presents with bilateral and symmetric reticular opacities, GGOs, or
a combination of both, with a predominantly basal and subpleural
distribution (12). Honeycombing,
traction bronchiectasis and architectural distortion are more
commonly observed in advanced stages of NSIP and may aid in
distinguishing it from HP (42).
DAD
On CT scans, DAD manifests as diffuse bilateral GGOs
with or without associated consolidations and interstitial
thickening, often accompanied by traction bronchiectasis (43). These findings reflect the
underlying pathophysiology of pneumonitis, characterized by
widespread injury to the alveolar epithelium and capillary
endothelium, leading to alveolar flooding, interstitial edema and
inflammatory cell infiltration (44). This pattern has been observed in
patients treated with EGFR-TKIs, anaplastic lymphoma kinase
inhibitors and ICIs in various stages of their disease (45-47).
Its severity increases over time and is associated with severe
clinical outcomes.
7. Management strategies
At present, there are no validated recommendations
for ICI-related pneumonitis treatment; patient management relies on
clinical experience and a multidisciplinary approach (3,35).
The ASCO expert consensus developed clinical practice guidelines
according to The Common Terminology Criteria for Adverse Events
(35). The grading was as follows:
i) Grade 1, asymptomatic disease; ii) grade 2, symptomatic disease
with limited instrumental activities of daily living (ADL) needing
medical intervention; iii) grade 3, severe symptoms limiting
self-care ADL requiring oxygen; and iv) grade 4, life-threatening
respiratory compromise requiring urgent intervention (35). Managing ICI-related pneumonitis
necessitates a tailored approach balancing immunosuppression with
oncologic efficacy (48,49). Treatment algorithms involve prompt
cessation of ICIs and initiation of corticosteroids, typically
prednisone or methylprednisolone, at a dose of 1-2 mg/kg/day
(48). Close monitoring of
clinical and radiological response is paramount, with gradual
tapering of corticosteroids guided by symptom resolution and
radiographic improvement (49). In
refractory cases or those with severe pneumonitis, additional
immunosuppressive agents such as infliximab or mycophenolate
mofetil may be considered, although their efficacy remains
uncertain (50). Infliximab or
cyclophosphamide have been proposed in clinical trials and
initially approved by the US FDA for patients receiving ipilimumab,
especially for severe, immune-mediated gastrointestinal side
effects (33,50). Anecdotal reports have suggested the
use of interleukin-17 blockade may provide relief from
immune-mediated skin and gastrointestinal toxic effects (33,50).
Collaborative efforts between oncologists, pulmonologists and
rheumatologists are essential in optimizing patient outcomes and
minimizing treatment-related complications (33).
8. Differential diagnosis from disease
progression in lung cancer
Distinguishing between ICI-related pneumonitis and
disease progression in lung cancer demands a comprehensive
evaluation incorporating multiple criteria, including variations in
tumor growth rate and tumor growth kinetics (51-53).
It typically represents a diagnosis of exclusion, necessitating the
comprehensive exclusion of alternative etiologies such as infection
and radiation-related pneumonitis (54). Given the overlapping clinical and
radiological features of these conditions, a thorough evaluation is
essential to differentiate ICI-related pneumonitis from other
potential causes of pulmonary symptoms (Table III). Firstly, the clinical
context is crucial, as recent initiation or ongoing treatment with
ICIs raises suspicion for pneumonitis (51-53).
At the same time, a history of lung cancer or evidence of disease
progression suggests tumor advancement (51-53).
Secondly, the temporal relationship plays a pivotal role, with
ICI-related pneumonitis typically manifesting weeks to months after
therapy initiation, contrasting with the more gradual progression
of lung cancer (33).
Symptomatology, including respiratory and constitutional symptoms,
offers additional insight, as does the response to treatment;
improvement with corticosteroids favors pneumonitis, while lack
thereof may indicate disease progression (16). Radiographic findings, mainly CT,
provide further differentiation, with characteristic patterns such
as GGOs favoring pneumonitis and progressive nodules suggesting
tumor growth (44).
 | Table IIIFeatures of ICI-related pneumonitis
vs. cancer progression. |
Table III
Features of ICI-related pneumonitis
vs. cancer progression.
| Feature | ICI-related
pneumonitis | Disease
progression |
|---|
| Onset | Variable, can occur
weeks to months after initiating ICI therapy | Typically gradual,
may occur at any stage of cancer progression |
| Symptoms | Respiratory
symptoms including dyspnea and cough; constitutional symptoms
including fatigue and malaise | May be asymptomatic
and respiratory symptoms may worsen gradually |
| Radiographic
findings | Ground-glass
opacities, consolidations, interstitial infiltrates and air
bronchograms | Progression of
known or new pulmonary nodules, infiltrative or cavitary lesions
and pleural effusions (in advanced stages) |
| Progression of
symptoms | May fluctuate with
treatment and symptoms and may improve with corticosteroid
therapy | Progressive
worsening of symptoms over time and symptoms may not respond to
corticosteroids |
| Response to
treatment | Improvement in
symptoms and radiographic findings with corticosteroid therapy | Lack of improvement
or worsening despite treatment |
| Clinical
context | Recent initiation
or ongoing treatment with immune checkpoint inhibitors | Known history of
lung cancer or evidence of disease progression on imaging |
| Multidisciplinary
approach | Collaboration among
oncologists, pulmonologists and radiologists is essential for
diagnosis and management | Collaboration among
oncologists, radiologists and pathologists is crucial for accurate
staging and treatment decisions |
9. Concluding statement
ICI-related pneumonitis represents a clinically
significant irAE associated with immunotherapy, necessitating
timely recognition and management to mitigate morbidity and
mortality. A systematic diagnostic approach integrating clinical,
radiological and laboratory findings is imperative for accurate
diagnosis and appropriate therapeutic interventions (55). Future research endeavors should
focus on elucidating the underlying mechanisms of pneumonitis and
identifying predictive biomarkers to guide personalized treatment
strategies in this evolving field of oncology.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
Not applicable.
Authors' contributions
SL, MRV and EM equally participated in the
conceptualization of the paper. EM and VG wrote and edited the
manuscript. AA, GS and DS critically revised the literature and
manuscript. All authors read and approved the final version of the
manuscript. Data authentication is not applicable.
Ethics approval and consent to
participate
Not applicable.
Patient consent to publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Choi J and Lee SY: Clinical
characteristics and treatment of immune-related adverse events of
immune checkpoint inhibitors. Immune Netw. 20(e9)2020.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Yin Q, Wu L, Han L, Zheng X, Tong R, Li L,
Bai L and Bian Y: Immune-related adverse events of immune
checkpoint inhibitors: A review. Front Immunol.
14(1167975)2023.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Ramos-Casals M, Brahmer JR, Callahan MK,
Flores-Chávez A, Keegan N, Khamashta MA, Lambotte O, Mariette X,
Prat A and Suárez-Almazor ME: Immune-related adverse events of
checkpoint inhibitors. Nat Rev Dis Primers. 6(38)2020.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Spain L, Diem S and Larkin J: Management
of toxicities of immune checkpoint inhibitors. Cancer Treat Rev.
44:51–60. 2016.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Rapoport BL, Shannon VR, Cooksley T,
Johnson DB, Anderson L, Blidner AG, Tintinger GR and Anderson R:
Pulmonary toxicities associated with the use of immune checkpoint
inhibitors: An update from the immuno-oncology subgroup of the
neutropenia, infection & myelosuppression study group of the
multinational association for supportive care in cancer. Front
Pharmacol. 12(743582)2021.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Georgakopoulou VE, Garmpis N, Mermigkis D,
Damaskos C, Chlapoutakis S, Mantzouranis K, Gkoufa A, Papageorgiou
C, Garmpi A, Makrodimitri S, et al: Pulmonary adverse events due to
immune checkpoint inhibitors: A literature review. Monaldi Arch
Chest Dis. 92:2021.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Liu YH, Zang XY, Wang JC, Huang SS, Xu J
and Zhang P: Diagnosis and management of immune related adverse
events (irAEs) in cancer immunotherapy. Biomed Pharmacother.
120(109437)2019.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Connolly C, Bambhania K and Naidoo J:
Immune-related adverse events: A case-based approach. Front Oncol.
9(530)2019.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Morgado M, Plácido A, Morgado S and Roque
F: Management of the adverse effects of immune checkpoint
inhibitors. Vaccines (Basel). 8(575)2020.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Casagrande S, Sopetto GB, Bertalot G,
Bortolotti R, Racanelli V, Caffo O, Giometto B, Berti A and Veccia
A: Immune-related adverse events due to cancer immunotherapy:
Immune mechanisms and clinical manifestations. Cancers (Basel).
16(1440)2024.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Wang DY, Salem JE, Cohen JV, Chandra S,
Menzer C, Ye F, Zhao S, Das S, Beckermann KE, Ha L, et al: Fatal
toxic effects associated with immune checkpoint inhibitors: A
systematic review and meta-analysis. JAMA Oncol. 4:1721–1728.
2018.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Naidoo J, Wang X, Woo KM, Iyriboz T,
Halpenny D, Cunningham J, Chaft JE, Segal NH, Callahan MK, Lesokhin
AM, et al: Pneumonitis in patients treated with anti-programmed
death-1/programmed death ligand 1 therapy. J Clin Oncol.
35:709–717. 2017.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Suresh K, Naidoo J, Lin CT and Danoff S:
Immune checkpoint immunotherapy for non-small cell lung cancer:
Benefits and pulmonary toxicities. Chest. 154:1416–1423.
2018.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Sternschein R, Moll M, Ng J and D'Ambrosio
C: Immune checkpoint inhibitor-related pneumonitis. Incidence, risk
factors, and clinical and radiographic features. Am J Respir Crit
Care Med. 198:951–953. 2018.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Ma K, Lu Y, Jiang S, Tang J, Li X and
Zhang Y: The relative risk and incidence of immune checkpoint
inhibitors related pneumonitis in patients with advanced cancer: A
meta-analysis. Front Pharmacol. 9(1430)2018.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Lin MX, Zang D, Liu CG, Han X and Chen J:
Immune checkpoint inhibitor-related pneumonitis: Research advances
in prediction and management. Front Immunol.
15(1266850)2024.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Ando H, Suzuki K and Yanagihara T:
Insights into potential pathogenesis and treatment options for
immune-checkpoint inhibitor-related pneumonitis. Biomedicines.
9(1484)2021.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Okobi TJ, Uhomoibhi TO, Akahara DE, Odoma
VA, Sanusi IA, Okobi OE, Umana I, Okobi E, Okonkwo CC and Harry NM:
Immune checkpoint inhibitors as a treatment option for bladder
cancer: Current evidence. Cureus. 15(e40031)2023.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Guo Z, Yu J, Chen Z, Chen S and Wang L:
Immunological mechanisms behind anti-PD-1/PD-L1 immune checkpoint
blockade: Intratumoral reinvigoration or systemic induction?
Biomedicines. 12(764)2024.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Sakowska J, Arcimowicz Ł, Jankowiak M,
Papak I, Markiewicz A, Dziubek K, Kurkowiak M, Kote S,
Kaźmierczak-Siedlecka K, Połom K, et al: Autoimmunity and
cancer-two sides of the same coin. Front Immunol.
13(793234)2022.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Yin J, Wu Y, Yang X, Gan L and Xue J:
Checkpoint inhibitor pneumonitis induced by anti-PD-1/PD-L1 therapy
in non-small-cell lung cancer: Occurrence and mechanism. Front
Immunol. 13(830631)2022.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Zhao H, Wu L, Yan G, Chen Y, Zhou M, Wu Y
and Li Y: Inflammation and tumor progression: Signaling pathways
and targeted intervention. Signal Transduct Target Ther.
6(263)2021.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Riondino S, Rosenfeld R, Formica V,
Morelli C, Parisi G, Torino F, Mariotti S and Roselli M:
Effectiveness of immunotherapy in non-small cell lung cancer
patients with a diagnosis of COPD: Is this a hidden prognosticator
for survival and a risk factor for immune-related adverse events?
Cancers (Basel). 16(1251)2024.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Abdel-Wahab N, Diab A, Yu RK, Futreal A,
Criswell LA, Tayar JH, Dadu R, Shannon V, Shete SS and
Suarez-Almazor ME: Genetic determinants of immune-related adverse
events in patients with melanoma receiving immune checkpoint
inhibitors. Cancer Immunol Immunother. 70:1939–1949.
2021.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Vétizou M, Pitt JM, Daillère R, Lepage P,
Waldschmitt N, Flament C, Rusakiewicz S, Routy B, Roberti MP, Duong
CP, et al: Anticancer immunotherapy by CTLA-4 blockade relies on
the gut microbiota. Science. 350:1079–1084. 2015.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Chao Y, Zhou J, Hsu S, Ding N, Li J, Zhang
Y, Xu X, Tang X, Wei T, Zhu Z, et al: Risk factors for immune
checkpoint inhibitor-related pneumonitis in non-small cell lung
cancer. Transl Lung Cancer Res. 11:295–306. 2022.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Postow MA, Sidlow R and Hellmann MD:
Immune-related adverse events associated with immune checkpoint
blockade. N Engl J Med. 378:158–168. 2018.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Cadranel J, Canellas A, Matton L, Darrason
M, Parrot A, Naccache JM, Lavolé A, Ruppert AM and Fallet V:
Pulmonary complications of immune checkpoint inhibitors in patients
with nonsmall cell lung cancer. Eur Respir Rev.
28(190058)2019.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Kumar V, Chaudhary N, Garg M, Floudas CS,
Soni P and Chandra AB: Current diagnosis and management of immune
related adverse events (irAEs) induced by immune checkpoint
inhibitor therapy. Front Pharmacol. 8(49)2017.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Rashdan S, Minna JD and Gerber DE:
Diagnosis and management of pulmonary toxicity associated with
cancer immunotherapy. Lancet Respir Med. 6:472–478. 2018.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Chhabra N and Kennedy J: A review of
cancer immunotherapy toxicity: Immune checkpoint inhibitors. J Med
Toxicol. 17:411–424. 2021.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Martins F, Sofiya L, Sykiotis GP, Lamine
F, Maillard M, Fraga M, Shabafrouz K, Ribi C, Cairoli A,
Guex-Crosier Y, et al: Adverse effects of immune-checkpoint
inhibitors: Epidemiology, management and surveillance. Nat Rev Clin
Oncol. 16:563–580. 2019.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Brahmer JR, Lacchetti C, Schneider BJ,
Atkins MB, Brassil KJ, Caterino JM, Chau I, Ernstoff MS, Gardner
JM, Ginex P, et al: Management of immune-related adverse events in
patients treated with immune checkpoint inhibitor therapy: American
society of clinical oncology clinical practice guideline. J Clin
Oncol. 36:1714–1768. 2018.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Haanen JBAG, Carbonnel F, Robert C, Kerr
KM, Peters S, Larkin J and Jordan K: ESMO Guidelines Committee.
Management of toxicities from immunotherapy: ESMO clinical practice
guidelines for diagnosis, treatment and follow-up. Ann Oncol. 28:
(Suppl 4):iv119–iv142. 2017.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Antoniou KM, Margaritopoulos GA,
Tomassetti S, Bonella F, Costabel U and Poletti V: Interstitial
lung disease. Eur Respir Rev. 23:40–54. 2014.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Sears CR, Peikert T, Possick JD, Naidoo J,
Nishino M, Patel SP, Camus P, Gaga M, Garon EB, Gould MK, et al:
Knowledge gaps and research priorities in immune checkpoint
inhibitor-related pneumonitis. An official American thoracic
society research statement. Am J Respir Crit Care Med. 200:e31–e43.
2019.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Beigelman-Aubry C and Schmidt S: Pulmonary
infections: Imaging with CT. In: Schoepf U, Meinel F (eds)
Multidetector-Row CT of the Thorax. Medical Radiology. Springer,
Cham, pp131-161, 2016.
|
|
38
|
Cordier JF: Cryptogenic organising
pneumonia. Eur Respir J. 28:422–446. 2006.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Cherian SV, Patel D, Machnicki S, Naidich
D, Stover D, Travis WD, Brown KK, Naidich JJ, Mahajan A, Esposito
M, et al: Algorithmic approach to the diagnosis of organizing
pneumonia: A correlation of clinical, radiologic, and pathologic
features. Chest. 162:156–178. 2022.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Godoy MCB, Viswanathan C, Marchiori E,
Truong MT, Benveniste MF, Rossi S and Marom EM: The reversed halo
sign: Update and differential diagnosis. Br J Radiol. 85:1226–1235.
2012.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Hashisako M and Fukuoka J: Pathology of
idiopathic interstitial pneumonias. Clin Med Insights Circ Respir
Pulm Med. 9 (Suppl 1):S123–S133. 2016.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Brixey AG, Oh AS, Alsamarraie A and Chung
JH: Pictorial review of fibrotic interstitial lung disease on
high-resolution CT scan and updated classification. Chest.
165:908–923. 2024.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Cozzi D, Cavigli E, Moroni C, Smorchkova
O, Zantonelli G, Pradella S and Miele V: Ground-glass opacity
(GGO): A review of the differential diagnosis in the era of
COVID-19. Jpn J Radiol. 39:721–732. 2021.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Swenson KE and Swenson ER: Pathophysiology
of acute respiratory distress syndrome and COVID-19 lung injury.
Crit Care Clin. 37:749–776. 2021.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Nishino M, Sholl LM, Hodi FS, Hatabu H and
Ramaiya NH: Anti-PD-1-related pneumonitis during cancer
immunotherapy. N Engl J Med. 373:288–290. 2015.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Min JH, Lee HY, Lim H, Ahn MJ, Park K,
Chung MP and Lee KS: Drug-induced interstitial lung disease in
tyrosine kinase inhibitor therapy for non-small cell lung cancer: A
review on current insight. Cancer Chemother Pharmacol.
68:1099–1109. 2011.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Rossi SE, Erasmus JJ, McAdams HP, Sporn TA
and Goodman PC: Pulmonary drug toxicity: Radiologic and pathologic
manifestations. Radiographics. 20:1245–1259. 2000.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Haanen J, Obeid M, Spain L, Carbonnel F,
Wang Y, Robert C, Lyon AR, Wick W, Kostine M, Peters S, et al:
Management of toxicities from immunotherapy: ESMO clinical practice
guideline for diagnosis, treatment and follow-up. Ann Oncol.
33:1217–1238. 2022.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Mohammed N, Zhou RR and Xiong Z: Imaging
evaluation of lung cancer treated with PD-1/PD-L1 inhibitors. Br J
Radiol. 94(20210228)2021.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Vaddepally R, Doddamani R, Sodavarapu S,
Madam NR, Katkar R, Kutadi AP, Mathew N, Garje R and Chandra AB:
Review of immune-related adverse events (irAEs) in non-small-cell
lung cancer (NSCLC)-their incidence, management, multiorgan irAEs,
and rechallenge. Biomedicines. 10(790)2022.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Ferrara R, Mezquita L, Texier M, Lahmar J,
Audigier-Valette C, Tessonnier L, Mazieres J, Zalcman G, Brosseau
S, Le Moulec S, et al: Hyperprogressive disease in patients with
advanced non-small cell lung cancer treated with PD-1/PD-L1
inhibitors or with single-agent chemotherapy. JAMA Oncol.
4:1543–1552. 2018.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Kanjanapan Y, Day D, Wang L, Al-Sawaihey
H, Abbas E, Namini A, Siu LL, Hansen A, Razak AA, Spreafico A, et
al: Hyperprogressive disease in early-phase immunotherapy trials:
Clinical predictors and association with immune-related toxicities.
Cancer. 125:1341–1349. 2019.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Kim CG, Kim KH, Pyo KH, Xin CF, Hong MH,
Ahn BC, Kim Y, Choi SJ, Yoon HI, Lee JG, et al: Hyperprogressive
disease during PD-1/PD-L1 blockade in patients with non-small-cell
lung cancer. Ann Oncol. 30:1104–1113. 2019.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Naidoo J, Nishino M, Patel SP, Shankar B,
Rekhtman N, Illei P and Camus P: Immune-related pneumonitis after
chemoradiotherapy and subsequent immune checkpoint blockade in
unresectable stage III non-small-cell lung cancer. Clin Lung
Cancer. 21:e435–e444. 2020.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Delaunay M, Prévot G, Collot S,
Guilleminault L, Didier A and Mazières J: Management of pulmonary
toxicity associated with immune checkpoint inhibitors. Eur Respir
Rev. 28(190012)2019.PubMed/NCBI View Article : Google Scholar
|