|
1
|
Choi J and Lee SY: Clinical
characteristics and treatment of immune-related adverse events of
immune checkpoint inhibitors. Immune Netw. 20(e9)2020.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Yin Q, Wu L, Han L, Zheng X, Tong R, Li L,
Bai L and Bian Y: Immune-related adverse events of immune
checkpoint inhibitors: A review. Front Immunol.
14(1167975)2023.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Ramos-Casals M, Brahmer JR, Callahan MK,
Flores-Chávez A, Keegan N, Khamashta MA, Lambotte O, Mariette X,
Prat A and Suárez-Almazor ME: Immune-related adverse events of
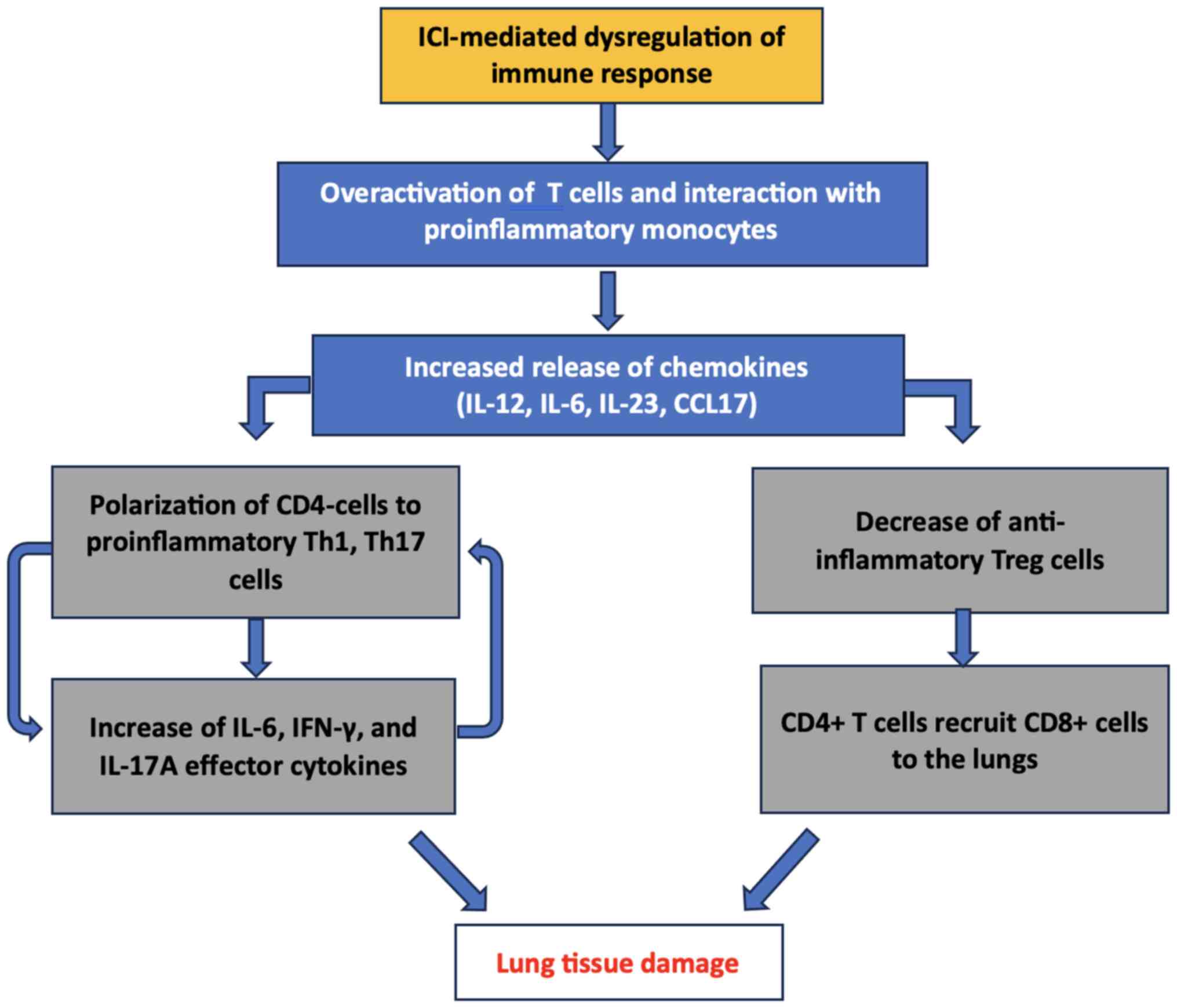
checkpoint inhibitors. Nat Rev Dis Primers. 6(38)2020.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Spain L, Diem S and Larkin J: Management
of toxicities of immune checkpoint inhibitors. Cancer Treat Rev.
44:51–60. 2016.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Rapoport BL, Shannon VR, Cooksley T,
Johnson DB, Anderson L, Blidner AG, Tintinger GR and Anderson R:
Pulmonary toxicities associated with the use of immune checkpoint
inhibitors: An update from the immuno-oncology subgroup of the
neutropenia, infection & myelosuppression study group of the
multinational association for supportive care in cancer. Front
Pharmacol. 12(743582)2021.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Georgakopoulou VE, Garmpis N, Mermigkis D,
Damaskos C, Chlapoutakis S, Mantzouranis K, Gkoufa A, Papageorgiou
C, Garmpi A, Makrodimitri S, et al: Pulmonary adverse events due to
immune checkpoint inhibitors: A literature review. Monaldi Arch
Chest Dis. 92:2021.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Liu YH, Zang XY, Wang JC, Huang SS, Xu J
and Zhang P: Diagnosis and management of immune related adverse
events (irAEs) in cancer immunotherapy. Biomed Pharmacother.
120(109437)2019.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Connolly C, Bambhania K and Naidoo J:
Immune-related adverse events: A case-based approach. Front Oncol.
9(530)2019.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Morgado M, Plácido A, Morgado S and Roque
F: Management of the adverse effects of immune checkpoint
inhibitors. Vaccines (Basel). 8(575)2020.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Casagrande S, Sopetto GB, Bertalot G,
Bortolotti R, Racanelli V, Caffo O, Giometto B, Berti A and Veccia
A: Immune-related adverse events due to cancer immunotherapy:
Immune mechanisms and clinical manifestations. Cancers (Basel).
16(1440)2024.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Wang DY, Salem JE, Cohen JV, Chandra S,
Menzer C, Ye F, Zhao S, Das S, Beckermann KE, Ha L, et al: Fatal
toxic effects associated with immune checkpoint inhibitors: A
systematic review and meta-analysis. JAMA Oncol. 4:1721–1728.
2018.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Naidoo J, Wang X, Woo KM, Iyriboz T,
Halpenny D, Cunningham J, Chaft JE, Segal NH, Callahan MK, Lesokhin
AM, et al: Pneumonitis in patients treated with anti-programmed
death-1/programmed death ligand 1 therapy. J Clin Oncol.
35:709–717. 2017.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Suresh K, Naidoo J, Lin CT and Danoff S:
Immune checkpoint immunotherapy for non-small cell lung cancer:
Benefits and pulmonary toxicities. Chest. 154:1416–1423.
2018.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Sternschein R, Moll M, Ng J and D'Ambrosio
C: Immune checkpoint inhibitor-related pneumonitis. Incidence, risk
factors, and clinical and radiographic features. Am J Respir Crit
Care Med. 198:951–953. 2018.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Ma K, Lu Y, Jiang S, Tang J, Li X and
Zhang Y: The relative risk and incidence of immune checkpoint
inhibitors related pneumonitis in patients with advanced cancer: A
meta-analysis. Front Pharmacol. 9(1430)2018.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Lin MX, Zang D, Liu CG, Han X and Chen J:
Immune checkpoint inhibitor-related pneumonitis: Research advances
in prediction and management. Front Immunol.
15(1266850)2024.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Ando H, Suzuki K and Yanagihara T:
Insights into potential pathogenesis and treatment options for
immune-checkpoint inhibitor-related pneumonitis. Biomedicines.
9(1484)2021.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Okobi TJ, Uhomoibhi TO, Akahara DE, Odoma
VA, Sanusi IA, Okobi OE, Umana I, Okobi E, Okonkwo CC and Harry NM:
Immune checkpoint inhibitors as a treatment option for bladder
cancer: Current evidence. Cureus. 15(e40031)2023.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Guo Z, Yu J, Chen Z, Chen S and Wang L:
Immunological mechanisms behind anti-PD-1/PD-L1 immune checkpoint
blockade: Intratumoral reinvigoration or systemic induction?
Biomedicines. 12(764)2024.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Sakowska J, Arcimowicz Ł, Jankowiak M,
Papak I, Markiewicz A, Dziubek K, Kurkowiak M, Kote S,
Kaźmierczak-Siedlecka K, Połom K, et al: Autoimmunity and
cancer-two sides of the same coin. Front Immunol.
13(793234)2022.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Yin J, Wu Y, Yang X, Gan L and Xue J:
Checkpoint inhibitor pneumonitis induced by anti-PD-1/PD-L1 therapy
in non-small-cell lung cancer: Occurrence and mechanism. Front
Immunol. 13(830631)2022.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Zhao H, Wu L, Yan G, Chen Y, Zhou M, Wu Y
and Li Y: Inflammation and tumor progression: Signaling pathways
and targeted intervention. Signal Transduct Target Ther.
6(263)2021.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Riondino S, Rosenfeld R, Formica V,
Morelli C, Parisi G, Torino F, Mariotti S and Roselli M:
Effectiveness of immunotherapy in non-small cell lung cancer
patients with a diagnosis of COPD: Is this a hidden prognosticator
for survival and a risk factor for immune-related adverse events?
Cancers (Basel). 16(1251)2024.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Abdel-Wahab N, Diab A, Yu RK, Futreal A,
Criswell LA, Tayar JH, Dadu R, Shannon V, Shete SS and
Suarez-Almazor ME: Genetic determinants of immune-related adverse
events in patients with melanoma receiving immune checkpoint
inhibitors. Cancer Immunol Immunother. 70:1939–1949.
2021.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Vétizou M, Pitt JM, Daillère R, Lepage P,
Waldschmitt N, Flament C, Rusakiewicz S, Routy B, Roberti MP, Duong
CP, et al: Anticancer immunotherapy by CTLA-4 blockade relies on
the gut microbiota. Science. 350:1079–1084. 2015.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Chao Y, Zhou J, Hsu S, Ding N, Li J, Zhang
Y, Xu X, Tang X, Wei T, Zhu Z, et al: Risk factors for immune
checkpoint inhibitor-related pneumonitis in non-small cell lung
cancer. Transl Lung Cancer Res. 11:295–306. 2022.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Postow MA, Sidlow R and Hellmann MD:
Immune-related adverse events associated with immune checkpoint
blockade. N Engl J Med. 378:158–168. 2018.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Cadranel J, Canellas A, Matton L, Darrason
M, Parrot A, Naccache JM, Lavolé A, Ruppert AM and Fallet V:
Pulmonary complications of immune checkpoint inhibitors in patients
with nonsmall cell lung cancer. Eur Respir Rev.
28(190058)2019.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Kumar V, Chaudhary N, Garg M, Floudas CS,
Soni P and Chandra AB: Current diagnosis and management of immune
related adverse events (irAEs) induced by immune checkpoint
inhibitor therapy. Front Pharmacol. 8(49)2017.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Rashdan S, Minna JD and Gerber DE:
Diagnosis and management of pulmonary toxicity associated with
cancer immunotherapy. Lancet Respir Med. 6:472–478. 2018.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Chhabra N and Kennedy J: A review of
cancer immunotherapy toxicity: Immune checkpoint inhibitors. J Med
Toxicol. 17:411–424. 2021.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Martins F, Sofiya L, Sykiotis GP, Lamine
F, Maillard M, Fraga M, Shabafrouz K, Ribi C, Cairoli A,
Guex-Crosier Y, et al: Adverse effects of immune-checkpoint
inhibitors: Epidemiology, management and surveillance. Nat Rev Clin
Oncol. 16:563–580. 2019.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Brahmer JR, Lacchetti C, Schneider BJ,
Atkins MB, Brassil KJ, Caterino JM, Chau I, Ernstoff MS, Gardner
JM, Ginex P, et al: Management of immune-related adverse events in
patients treated with immune checkpoint inhibitor therapy: American
society of clinical oncology clinical practice guideline. J Clin
Oncol. 36:1714–1768. 2018.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Haanen JBAG, Carbonnel F, Robert C, Kerr
KM, Peters S, Larkin J and Jordan K: ESMO Guidelines Committee.
Management of toxicities from immunotherapy: ESMO clinical practice
guidelines for diagnosis, treatment and follow-up. Ann Oncol. 28:
(Suppl 4):iv119–iv142. 2017.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Antoniou KM, Margaritopoulos GA,
Tomassetti S, Bonella F, Costabel U and Poletti V: Interstitial
lung disease. Eur Respir Rev. 23:40–54. 2014.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Sears CR, Peikert T, Possick JD, Naidoo J,
Nishino M, Patel SP, Camus P, Gaga M, Garon EB, Gould MK, et al:
Knowledge gaps and research priorities in immune checkpoint
inhibitor-related pneumonitis. An official American thoracic
society research statement. Am J Respir Crit Care Med. 200:e31–e43.
2019.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Beigelman-Aubry C and Schmidt S: Pulmonary
infections: Imaging with CT. In: Schoepf U, Meinel F (eds)
Multidetector-Row CT of the Thorax. Medical Radiology. Springer,
Cham, pp131-161, 2016.
|
|
38
|
Cordier JF: Cryptogenic organising
pneumonia. Eur Respir J. 28:422–446. 2006.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Cherian SV, Patel D, Machnicki S, Naidich
D, Stover D, Travis WD, Brown KK, Naidich JJ, Mahajan A, Esposito
M, et al: Algorithmic approach to the diagnosis of organizing
pneumonia: A correlation of clinical, radiologic, and pathologic
features. Chest. 162:156–178. 2022.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Godoy MCB, Viswanathan C, Marchiori E,
Truong MT, Benveniste MF, Rossi S and Marom EM: The reversed halo
sign: Update and differential diagnosis. Br J Radiol. 85:1226–1235.
2012.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Hashisako M and Fukuoka J: Pathology of
idiopathic interstitial pneumonias. Clin Med Insights Circ Respir
Pulm Med. 9 (Suppl 1):S123–S133. 2016.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Brixey AG, Oh AS, Alsamarraie A and Chung
JH: Pictorial review of fibrotic interstitial lung disease on
high-resolution CT scan and updated classification. Chest.
165:908–923. 2024.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Cozzi D, Cavigli E, Moroni C, Smorchkova
O, Zantonelli G, Pradella S and Miele V: Ground-glass opacity
(GGO): A review of the differential diagnosis in the era of
COVID-19. Jpn J Radiol. 39:721–732. 2021.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Swenson KE and Swenson ER: Pathophysiology
of acute respiratory distress syndrome and COVID-19 lung injury.
Crit Care Clin. 37:749–776. 2021.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Nishino M, Sholl LM, Hodi FS, Hatabu H and
Ramaiya NH: Anti-PD-1-related pneumonitis during cancer
immunotherapy. N Engl J Med. 373:288–290. 2015.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Min JH, Lee HY, Lim H, Ahn MJ, Park K,
Chung MP and Lee KS: Drug-induced interstitial lung disease in
tyrosine kinase inhibitor therapy for non-small cell lung cancer: A
review on current insight. Cancer Chemother Pharmacol.
68:1099–1109. 2011.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Rossi SE, Erasmus JJ, McAdams HP, Sporn TA
and Goodman PC: Pulmonary drug toxicity: Radiologic and pathologic
manifestations. Radiographics. 20:1245–1259. 2000.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Haanen J, Obeid M, Spain L, Carbonnel F,
Wang Y, Robert C, Lyon AR, Wick W, Kostine M, Peters S, et al:
Management of toxicities from immunotherapy: ESMO clinical practice
guideline for diagnosis, treatment and follow-up. Ann Oncol.
33:1217–1238. 2022.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Mohammed N, Zhou RR and Xiong Z: Imaging
evaluation of lung cancer treated with PD-1/PD-L1 inhibitors. Br J
Radiol. 94(20210228)2021.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Vaddepally R, Doddamani R, Sodavarapu S,
Madam NR, Katkar R, Kutadi AP, Mathew N, Garje R and Chandra AB:
Review of immune-related adverse events (irAEs) in non-small-cell
lung cancer (NSCLC)-their incidence, management, multiorgan irAEs,
and rechallenge. Biomedicines. 10(790)2022.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Ferrara R, Mezquita L, Texier M, Lahmar J,
Audigier-Valette C, Tessonnier L, Mazieres J, Zalcman G, Brosseau
S, Le Moulec S, et al: Hyperprogressive disease in patients with
advanced non-small cell lung cancer treated with PD-1/PD-L1
inhibitors or with single-agent chemotherapy. JAMA Oncol.
4:1543–1552. 2018.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Kanjanapan Y, Day D, Wang L, Al-Sawaihey
H, Abbas E, Namini A, Siu LL, Hansen A, Razak AA, Spreafico A, et
al: Hyperprogressive disease in early-phase immunotherapy trials:
Clinical predictors and association with immune-related toxicities.
Cancer. 125:1341–1349. 2019.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Kim CG, Kim KH, Pyo KH, Xin CF, Hong MH,
Ahn BC, Kim Y, Choi SJ, Yoon HI, Lee JG, et al: Hyperprogressive
disease during PD-1/PD-L1 blockade in patients with non-small-cell
lung cancer. Ann Oncol. 30:1104–1113. 2019.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Naidoo J, Nishino M, Patel SP, Shankar B,
Rekhtman N, Illei P and Camus P: Immune-related pneumonitis after
chemoradiotherapy and subsequent immune checkpoint blockade in
unresectable stage III non-small-cell lung cancer. Clin Lung
Cancer. 21:e435–e444. 2020.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Delaunay M, Prévot G, Collot S,
Guilleminault L, Didier A and Mazières J: Management of pulmonary
toxicity associated with immune checkpoint inhibitors. Eur Respir
Rev. 28(190012)2019.PubMed/NCBI View Article : Google Scholar
|