Introduction
As a result of sufficient wear and tear of the knee
joint, patients can suffer from advanced osteoarthrosis, leading to
significant pain and disability; therefore, total knee replacement
(TKR) surgery may be considered. During this surgical procedure,
the femoral and tibial components of the knee are resected and
replaced with artificial metallic elements with a polyethylene
liner between them. The most common indication for TKR is severe
arthrosis causing pain, stiffness, instability and deformity,
leading to disability and a poor quality of life. Arthrosis may be
caused by arthritis or previous trauma (1). TKR can alleviate knee pain, improve
joint function by increasing the range of motion and enhance the
quality of life for patients (2-4).
However, no surgical procedure is without risk, and the most common
side effects after TKR include deep vein thrombosis, infection,
stiffness and osteolysis (5).
Surgical trauma in response to TKR is vast and
activates complex inflammatory pathways crucial to post-surgical
healing (6). Short-term
inflammatory processes, including the release of pro-inflammatory
cytokines (IL-1, IL-8 and TNF), are essential for protecting
against infections, and for tissue repair and remodeling (7). In a balanced physiological system,
anti-inflammatory processes regulate the pro-inflammatory response,
orchestrated by the anti-inflammatory pathway of the vagus nerve
(8-12).
However, a lack of short-term inflammatory response regulation can
cause longer and stronger inflammatory responses, potentially
causing local tissue damage, shock, multiple organ dysfunction
syndromes and even death (13).
The vagus nerve is the main branch of the
parasympathetic nervous system. Its activity is indexed by the
change in regular cardiac interbeat intervals, termed heart-rate
variability (HRV). HRV, derived from electrocardiograms (ECGs), is
strongly correlated with actual vagal nerve activity (r=0.88)
(14). HRV has clinical importance
because cardiac vagal tone is a marker of disease and adaptability.
Low HRV levels predict all-cause mortality (15), worse prognosis after myocardial
infarctions (16), and worse
cancer prognosis (17), as well as
psychopathology, such as stress, anxiety and depression (18), which is essential for postoperative
rehabilitation. For example, several studies have demonstrated a
relationship between preoperative HRV and postoperative outcomes.
Low HRV has been reported to be associated with prolonged
hospitalization (19), worse pain
control (20) and decreased
improvements during the postoperative period (21). However, to the best of our
knowledge, no study has tested the relationship between
preoperative HRV and short-term outcomes following TKR.
In the present study, the relationships between
preoperative vagal activity (HRV) and postoperative outcomes,
including complications, infections, inflammation [indexed by
C-reactive protein (CRP) levels] and postoperative length of stay
(LOS), were examined in patients undergoing TKR.
Materials and methods
Participants
A retrospective study design was used in the present
study. After obtaining approval (approval no. 0082-20-BNZ) from the
Ethics Committee of Bnai-Zion Medical Center (Haifa, Israel),
background and medical information were collected from 156 medical
records of patients who underwent TKR surgery at the Department of
Orthopedic Surgery (Bnai Zion Medical Center) between January 2018
and December 2020. The inclusion and exclusion criteria were as
follows: To be included in the study, participants were required to
be aged >18 years, have a clear preoperative ECG, and possess
recorded CRP measurements on days 1, 2 and 4 after surgery.
Additionally, there was a need for thorough documentation of their
postoperative follow-up. Patients were not excluded based on any
comorbidities. Of the 156 files, 38 had no ECG data in their files,
and 77 of the remaining excluded files had unreadable paper ECG
data. Finally, all data used for the present study were collected
from 41 patients.
Background information
The background information collected included
patient age, sex, comorbidities (hypertension, diabetes, cancer,
heart diseases and dyslipidemia), and operation side. TKR surgery
was not conducted on patients with existing infections; therefore,
no study participants had preoperative infections.
Vagal nerve tone was indexed according to HRV. HRV
was derived from preoperative 10-sec segments of the patients'
ECGs. This included the time-domain HRV markers of standard
deviation of normal-to-normal RR-intervals (SDNN) and root mean
square of successive differences (RMSSD). HRV, as measured by
ultra-short 10-sec ECGs, has been demonstrated to predict heart
failure (22), all-cause mortality
(23) and cancer survival
(24,25). Scanning the ECGs into a digital
file, SDNN and RMSSD were mathematically derived with a specific
code developed using MATLAB software (version R2020a; https://www.mathworks.com/help/releases/R2020a.html).
The MATLAB algorithm collects two vectors of data, one representing
the time in milliseconds and the other the amplitude in millivolts
in the waveforms. From these vectors, the variance of the RR
intervals was measured, from which SDNN and RMSSD were derived
using standard formulas (26,27).
Clinical outcomes
Outcomes were obtained from the patient's electronic
medical files. These included the levels of CRP on postoperative
days 1, 2 and 4, as well as LOS in the hospital. Finally,
information was also obtained about any postoperative
infections.
Statistical analysis
SPSS version 26 (IBM Corp.) was utilized for a
comprehensive statistical analysis. Initially, descriptive
statistics, such as percentages, mean and median values, and
standard deviation (SD) values were calculated for all main
variables, with the aim of understanding the fundamental trends of
the data. Subsequently, inferential statistics were employed,
focusing on the relationships between HRV levels, CRP and LOS in
the postoperative phase, using the Pearson correlation
coefficient.
A critical component of the present study was the
mixed-design analysis of variance (ANOVA), which examined the
effects of HRV categories (high or low; patients were divided into
high and low HRV groups by designating them as above or below 15
msec for SDNN or 20 msec for RMSSD, reflecting the sample medians
of each parameter) and time (postoperative days 1, 2 and 4) on CRP
levels. This method was pivotal in determining how HRV influences
the postoperative CRP trajectory. The post hoc analyses included
t-tests on changes in CRP levels between high and low HRV groups.
Sidak's significance level was then calculated, assuming the use of
two post hoc tests due to performing multiple comparisons. This
yielded a critical P-value of 0.025.
Results
Patient demographics and baseline
characteristics
Of the 41 patients, 41.5% were men, and 58.5% were
women. Patient ages ranged from 43 to 84 years, with a mean ± SD of
68.6±10.1 years. Comorbidities included hypertension (65.9%),
hyperlipidemia (41.5%), diabetes (31.7%), heart disease (29.3%) and
cancer (7.3%). A higher percentage of right-sided TKR surgeries was
performed (63.4%) compared with left-sided surgeries (36.6%). The
rate of complications was 12%, including deep vein thrombosis
(2.4%), cellulitis (4.8%), acute myocardial infarction (2.4%) and
cerebrovascular accident (2.4%). Notably, the cohort did not
include any patients with rheumatic diseases. The descriptive
statistics of the two HRV parameters obtained from patient ECGs are
presented in Table I.
 | Table IHeart-rate variability 10-sec time
domain parameters from patients' electrocardiograms. |
Table I
Heart-rate variability 10-sec time
domain parameters from patients' electrocardiograms.
| Variable | Median, msec | Mean, msec | Standard deviation,
msec |
|---|
| SDNN | 15 | 28.08 | 33.69 |
| RMSSD | 20 | 39.56 | 47.09 |
HRV analysis
The patients were then divided into high and low HRV
groups by designating them as above or below 15 msec for SDNN or 20
msec for RMSSD, reflecting the sample medians of each
parameter.
Postoperative clinical outcomes
LOS ranged from 2 to 22 days, with a mean ± SD of
6.22±3.44 days, as shown in Table
II, along with CRP levels on postoperative days 1 (49.00,
SD=26.25), 2 (164.19, SD=85.55) and 4 (146.76, SD=48.98). Notably,
Table I shows that the standard
deviation of HRV exceeded the mean value, suggesting that the data
do not follow a normal distribution and thus cannot be accurately
described by the mean ± SD format. Consequently, a logarithmic
transformation was applied to HRV to normalize its distribution.
Furthermore, the primary analysis focused on categorical HRV
(high/low), rendering the issue of normality moot.
 | Table IIMean and standard deviation of LOS
and CRP levels on postoperative days 1, 2 and 4. |
Table II
Mean and standard deviation of LOS
and CRP levels on postoperative days 1, 2 and 4.
| Variable | Mean | Standard
deviation |
|---|
| LOS, days | 6.22 | 3.44 |
| CRP, mg/l | | |
|
Day 1 | 49.00 | 26.25 |
|
Day 2 | 164.19 | 85.55 |
|
Day 4 | 146.76 | 48.98 |
Statistical analyses and findings
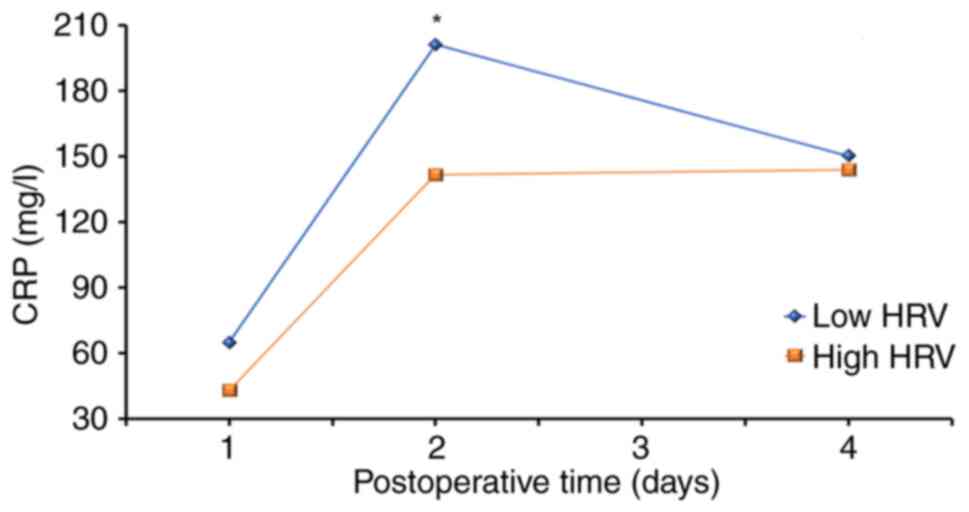
Using a mixed design-ANOVA, a significant effect of
time on CRP levels was determined (P<0.001) and a trend toward a
significant HRV x time interaction (P<0.058) in relation to CRP
was revealed (Fig. 1). This
revealed differences in the CRP levels during postoperative days as
a function of patients' baseline HRV. The low HRV group
demonstrated higher overall CRP levels on all postoperative days.
It took patients with low HRV more time to recover, as seen by the
trajectory of the inflammatory marker CRP, compared with patients
with high HRV (Fig. 1; Table III). The effect of time on CRP
was significant (P<0.001), whereas HRV alone did not have a
significant effect on CRP (P=0.15). When trying to identify the
source of the HRV x time interaction, a t-test was used to compare
between subjects. The change in CRP from day 2 to day 4 after
surgery, in patients with low HRV was significantly larger (47.64)
than that in patients with high HRV [2.24; t(23)=2.44, P=0.023]. This revealed that
the trajectory of postoperative CRP differed according to patients'
baseline HRV levels.
 | Table IIICRP levels on postoperative days 1, 2
and 4, as a function of patients' baseline HRV. |
Table III
CRP levels on postoperative days 1, 2
and 4, as a function of patients' baseline HRV.
| Variable | Vagal tone | Mean, mg/l | Standard deviation,
mg/l |
|---|
| CRP day 1 | Low HRV | 64.78 | 27.98 |
| | High HRV | 43.10 | 24.77 |
| CRP day 2 | Low HRV | 201.08 | 87.79 |
| | High HRV | 141.56 | 68.58 |
| CRP day 4 | Low HRV | 150.25 | 45.16 |
| | High HRV | 143.80 | 54.36 |
Upon initial analysis, none of the confounding
variables (such as age and background illnesses), except sex
(P>0.05), were significantly related to HRV (P>0.05; Table SI). In addition, no significant
correlation was identified between HRV and LOS (for SDNN: r=0.13,
P=0.41; for RMSSD: r=0.10, P=0.52) (data not shown). The changes in
CRP on days 2 and 4 (CRP4-CRP2) were calculated. When examining the
relationship between LOS and change in CRP levels between
postoperative days 2 and 4 (in which HRV groups differed), a
significant correlation was identified between Δ-CRP on days 2 and
4 with LOS (r=-0.61, P=0.034), only in patients with low HRV. These
findings indicated that the greater the decrease in inflammation
between days 2 and 4, the shorter the LOS in the low HRV group. By
contrast, no similar correlation was detected among patients in the
high HRV group, referring to Δ-CRP on days 2 and 4 with LOS
(r=-0.36, P=0.23). When analyzing the results for confounding
variables, it was demonstrated that only pre-existing heart disease
was positively correlated with the patients' LOS (Table SI). After statistically
controlling the effects of heart disease (using a partial
correlation analysis) the correlation between LOS and change in CRP
between days 2 and 4 was no longer significant (but still strong
and negative; r=-0.46, P=0.12) in patients with low HRV. Moreover,
none of the patients' background variables (age, sex or other
comorbid diseases) were related to the inflammatory trajectory
according to the CRP levels (Table
SI).
Finally, no significant association was detected
between HRV and complication rate using t tests (for SDNN,
P>0.05; for RMSSD, P>0.05). Partial η2 for the
ANOVA on CRP levels (time effect) was reported as ~0.749; this
value indicates a large effect size, based on the conventional
benchmarks where η² values of 0.01, 0.06 and 0.14 represent small,
medium and large effects, respectively. Cohen's d for the t-test
between CRP changes in the high and low HRV groups was reported as
~1.021. This value also indicates a large effect size based on
conventional benchmarks where Cohen's d values of 0.2, 0.5 and 0.8
represent small, medium and large effects, respectively.
Discussion
The present study examined the postoperative
inflammatory response after TKR surgery as a function of patients'
preoperative vagal tone, indexed by HRV. The results demonstrated
that patients took a longer time to resume baseline levels of
postoperative HRV if their preoperative HRV levels were low.
Moreover, a significant correlation was revealed between
inflammatory change across recovery days and LOS. Patients with
lower vagal tone demonstrated a longer inflammatory recovery time,
with a more significant decrease in CRP between postoperative days
2 and 4, when compared to patients with higher vagal tone.
Moreover, in the low HRV group only, the magnitude of CRP decline
between postoperative days 2 and 4 was correlated with their LOS,
revealing the clinical significance of the CRP trajectory. The
level of CRP before surgery may also be an important factor, but it
was not analyzed because the present study focused on routinely
measured data only, and no patients in the present cohort had
undergone preoperative CRP measurements.
To gather the two time-domain measurements of HRV:
SDNN and RMSSD, preoperative ECGs were used, since ECG tests are
mandatory before TKR surgery. Reduced HRV is known to be associated
with several chronic diseases and poor outcomes, such as
hypertension (28,29), systematic inflammation (30,31),
diabetes (32), and increasing
age. Studies have shown abundant evidence for the significant
prognostic value of HRV in conditions such as myocardial infarction
(16) and cancer (17,24).
However, this broad spectrum of associated diseases and influencing
factors suggests that low HRV may have low specificity in the
distinction between specific illnesses. The mean values of HRV
observed in the present cohort were in line with the normal range
of HRVs concerning the influence of age and medical history. The
median of RMSSD and SDNN (20 and 15 msec, respectively)
corresponded to low levels in the normal range of HRVs referenced
in the literature (33,34), that are mainly collected from
cohorts with no significant medical conditions, which could affect
HRV. By contrast, the present cohort exhibited several
comorbidities, including hypertension, diabetes, heart disease and
cancer. Therefore, given the known information and the medical
history of the patients, the median HRVs observed in the present
study appeared similar to the low cut-off of the normal range of
HRVs, but well within the normative data concerning ultra-short
measurements of 10-sec ECGs (33,34).
CRP is an acute-phase protein used as a marker of
inflammation or infection in orthopedic surgery, including TKR.
After infectious or inflammatory stimuli, serum CRP levels may rise
abruptly, reaching up to 1,000 times the baseline value in 48 h
(35). It has received particular
attention due to its predictive role in several diseases and its
relation to HRV indices (34,36).
Several studies have examined the relationship between HRV and CRP
across age groups (31,37-39),
and have revealed that inflammatory parameters are strongly
associated with decreased HRV. Moreover, a unique prospective study
supporting the cholinergic anti-inflammatory role of the vagal
nerve has been performed (40),
which demonstrated that HRV levels at baseline can predict CRP
levels 4 years later. CRP also has a vital role in orthopedics, and
can be used in the diagnosis and follow-up of infections (41).
The vagus nerve regulates peripheral inflammation
via two mechanisms. First, after peripheral monocytes secrete IL-1,
that signal binds to IL-1 receptors on the vagal paraganglia. This
is then translated to a cholinergic signal and reaches the
hypothalamus, where it activates the hypothalamic-pituitary-adrenal
axis, resulting in cortisol secretion, which reduces inflammation.
Second, the efferent vagus converts at the celiac ganglion to a
sympathetic branch, which enters the spleen. There, a sub-type of
residing T cells express the β-adrenergic receptor, which upon
stimulation secretes acetylcholine. That neurotransmitter then
binds to the α-7 nicotinic acetylcholine receptor on residing
macrophages, and this inhibits their production of pro-inflammatory
cytokines (8-12).
The results of the present study demonstrated that
the behavior of the inflammatory index CRP among the patients
assessed, exhibited similarities to the existing data in the
literature among patients following TKR. Several studies have
demonstrated a specific pattern for CRP change following TKR and
have shown that CRP levels typically peak within 48 h following
surgery and decrease significantly by postoperative day 4 (42-44).
It has also been reported that rising CRP levels after
postoperative day 3 may indicate a complication of surgery, such as
infection. The rise in CRP following surgical trauma is used
post-operatively to quantify the degree of tissue damage and deduce
the operative stress experienced by the patient. The present
results demonstrated the known increases and decreases in CRP over
4 postoperative days (42-44).
Moreover, when examining the CRP dynamics as a
function of patients' baseline HRV, two distinct profiles of CRP
dynamics emerged. Patients with low vagal tone exhibited a higher
inflammatory peak, which was associated with a more extended
inflammatory recovery period than patients with high vagal tone.
This finding expands existing knowledge about the relationship
between the autonomic nervous system and inflammatory processes
after surgical trauma. These results could also help orthopedic
surgeons know in advance which patients may display postoperative
inflammatory complications.
A limitation of the present study is the relatively
small number of patients included in the final analysis, which
arises from the retrospective nature of the study in which
preoperative paper ECGs were collected. Often, these ECGs were
degraded to the point where HRV could not be reliably analyzed.
Therefore, only ECGs that were readable and allowed for accurate
HRV analysis were used, and no degraded files were used. Another
important issue is that the present study did not gather
information on the intake of medications, such as β-blockers,
although they are known to influence vagal tone (41). Another problem is that each
preoperative ECG came from a different clinic, and hence they were
not standardized. Nevertheless, the finding that baseline HRV does
predict the postoperative trajectory of CRP shows the robustness of
this relationship. In future studies, these results need to be
replicated by implementing a prospective study design using a
single ECG machine to generate digital ECGs. Conducting a
prospective study will also allow for further data collection via
patient self-report questionnaires (e.g., to assess stress levels),
long-term follow-up and vagal tone interventions (45) that may lead to better
patient-specific preoperative preparation.
Personalized medicine with the goal of ‘the right
treatment to the right patient at the right time’ (46) is defined as the prevention of
chronic diseases, early intervention and personalized treatment
planning (47). Patient HRV is
known to be affected by numerous parameters, such as sex and age
(48,49), circadian rhythms (50,51),
mental and physical activity, and ethnicity (52-54).
Hence, knowledge of patients' HRV should allow for better
patient-specific preoperative and postoperative treatment plans
resulting in improved recovery, fewer complications and greater
patient satisfaction.
Knowledge of patient preoperative vagal tone allows
for behavioral and biofeedback interventions as part of surgical
preparation. Recognizing the presurgical stress and psychopathology
of patients via their HRV (18),
can enable clinicians to provide them with important information
and valuable coping skills. Psychological screening and evaluation
could address stress and anxiety before the surgical procedure. It
also assists in altering risky personal habits that adversely
affect vagal tone and surgery outcomes, such as smoking cessation,
developing adapted eating habits, promoting physical activity and
acknowledging the candidate's concerns regarding the expected
surgical results.
However, surgical recovery may also be possibly
influenced by activating the vagus via biofeedback. Biofeedback is
based on the collection and visualization of biological activities
and teaching patients to modify a certain biological parameter.
This can empower users to control their physiological processes in
conjunction with changes to their psychological state. Biofeedback
has demonstrated effectiveness in managing different diseases by
increasing vagal activity and improving the autonomic nervous
system balance. Specifically, HRV biofeedback (HRVB) involves the
modulation of the autonomic nervous system through slow-paced
breathing. HRVB is considered a learned skill that maximizes HRV,
and facilitates autonomic and cardiorespiratory homeostasis
(55). Typically, HRVB is guided
by a visual and auditory representation of one's heartbeat or HRV
in real-time to support the process of increasing the amplitude of
HRV (56). Several systemic
reviews have examined the effects of biofeedback on stress and
anxiety, depression, emotional and physical health, and the
applicability of the HRVB intervention among populations of
patients with chronic diseases (57-61).
These reviews show that HRVB training is associated with a
considerable reduction in self-reported stress and anxiety,
improves depressive symptoms, improves behavioral symptoms (such as
arousal) and functioning (such as avoidance) and various patient
clinical outcomes, including hypertension and cardiovascular
prognosis, inflammatory states and pain.
In conclusion, the present study highlighted the
significant impact of presurgical vagal activity on postoperative
inflammatory response following TKR surgery. Patients with lower
vagal activity experienced worse outcomes, while the magnitude of
inflammatory decline was correlated with hospital stay. This
knowledge may improve presurgical interventions to enhance surgical
outcomes and patient well-being. Future research should explore
behavioral and biofeedback interventions to enhance presurgical
vagal tone, potentially reducing stress, improving recovery and
increasing patient satisfaction. These findings hold great promise
for personalized medicine and transformative advancements in
surgical practice.
Supplementary Material
Correlation analysis.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
AG and ADL were involved in study conception and
design, data collection, analysis and interpretation of the
results, and manuscript writing. AEAA and AGM were involved in data
collection. OBL was involved in study conception and design, and
performed clinical supervision. YG was involved in study conception
and design, and analysis and interpretation of results. AG and ADL
confirm the authenticity of all the raw data. All authors reviewed
the results, and read and approved the final manuscript.
Ethics approval and consent to
participate
The research was performed in accordance with the
Declaration of Helsinki and was approved by the Ethics Committee of
Bnai-Zion Medical Center (approval no. 0082-20-BNZ).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Lespasio MJ, Piuzzi NS, Husni ME, Muschler
GF, Guarino A and Mont MA: Knee osteoarthritis: A primer. Perm J.
21:16–183. 2017.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Lenssen TAF, Van Steyn MJA, Crijns YHF,
Waltjé EMH, Roox GM, Geesink RJT, van den Brandt PA and De Bie RA:
Effectiveness of prolonged use of continuous passive motion (CPM),
as an adjunct to physiotherapy, after total knee arthroplasty. BMC
Musculoskelet Disord. 9(60)2008.PubMed/NCBI View Article : Google Scholar
|
|
3
|
McCaffrey R and Locsin R: The effect of
music on pain and acute confusion in older adults undergoing hip
and knee surgery. Holist Nurs Pract. 20:218–226. 2006.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Zhang W, Moskowitz RW, Nuki G, Abramson S,
Altman RD, Arden N, Bierma-Zeinstra S, Brandt KD, Croft P, Doherty
M, et al: OARSI recommendations for the management of hip and knee
osteoarthritis, Part II: OARSI evidence-based, expert consensus
guidelines. Osteoarthritis Cartilage. 16:137–162. 2008.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Medical Advisory Secretariat. Total knee
replacement: An evidence-based analysis. Ont Health Technol Assess
Ser. 5:1–51. 2005.PubMed/NCBI
|
|
6
|
Máca J, Burša F, Ševčík P, Sklienka P,
Burda M and Holub M: Alarmins and clinical outcomes after major
abdominal surgery-a prospective study. J Invest Surg. 30:152–161.
2017.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Barbul A, Lazarou SA, Efron DT, Wasserkrug
HL and Efron G: Arginine enhances wound healing and lymphocyte
immune responses in humans. Surgery. 108:331–337. 1990.PubMed/NCBI
|
|
8
|
Tracey KJ: The inflammatory reflex.
Nature. 420:853–859. 2002.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Pavlov VA and Tracey KJ: The vagus nerve
and the inflammatory reflex-linking immunity and metabolism. Nat
Rev Endocrinol. 8:743–754. 2012.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Bonaz B, Sinniger V and Pellissier S:
Anti-inflammatory properties of the vagus nerve: Potential
therapeutic implications of vagus nerve stimulation. J Physiol.
594:5781–5790. 2016.PubMed/NCBI View
Article : Google Scholar
|
|
11
|
Kim KN, Yao Y and Ju SY: Heart rate
variability and inflammatory bowel disease in humans: A systematic
review and meta-analysis. Medicine (Baltimore).
99(e23430)2020.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Sorski L and Gidron Y: The vagal nerve,
inflammation, and diabetes-a holy triangle. Cells.
12(1632)2023.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Pavlov VA, Wang H, Czura CJ, Friedman SG
and Tracey KJ: The cholinergic anti-inflammatory pathway: A missing
link in neuroimmunomodulation. Mol Med. 9:125–134. 2003.PubMed/NCBI
|
|
14
|
Kuo TBJ, Lai CJ, Huang YT and Yang CCH:
Regression analysis between heart rate variability and
baroreflex-related vagus nerve activity in rats. J Cardiovasc
Electrophysiol. 16:864–869. 2005.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Tsuji H, Venditti FJ Jr, Manders ES, Evans
JC, Larson MG, Feldman CL and Levy D: Reduced heart rate
variability and mortality risk in an elderly cohort. The Framingham
heart study. Circulation. 90:878–883. 1994.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Buccelletti E, Gilardi E, Scaini E,
Galiuto L, Persiani R, Biondi A, Basile F and Silveri NG: Heart
rate variability and myocardial infarction: Systematic literature
review and metanalysis. Eur Rev Med Pharmacol Sci. 13:299–307.
2009.PubMed/NCBI
|
|
17
|
Zhou X, Ma Z, Zhang L, Zhou S, Wang J,
Wang B and Fu W: Heart rate variability in the prediction of
survival in patients with cancer: A systematic review and
meta-analysis. J Psychosom Res. 89:20–25. 2016.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Beauchaine TP and Thayer JF: Heart rate
variability as a transdiagnostic biomarker of psychopathology. Int
J Psychophysiol. 98:338–350. 2015.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Stein PK, Schmieg RE, El-Fouly A,
Domitrovich PP and Buchman TG: Association between heart rate
variability recorded on postoperative day 1 and length of stay in
abdominal aortic surgery patients. Crit Care Med. 29:1738–1743.
2001.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Powezka K, Adjei T, von Rosenberg W,
Normahani P, Goverdovsky V, Standfield NJ, Mandic DP and Jaffer U:
A pilot study of preoperative heart rate variability predicting
pain during local anesthetic varicose vein surgery. J Vasc Surg
Venous Lymphat Disord. 7:382–386. 2019.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Grote V, Levnajić Z, Puff H, Ohland T,
Goswami N, Frühwirth M and Moser M: Dynamics of vagal activity due
to surgery and subsequent rehabilitation. Front Neurosci.
13(1116)2019.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Rautaharju PM, Kooperberg C, Larson JC and
LaCroix A: Electrocardiographic predictors of incident congestive
heart failure and all-cause mortality in postmenopausal women: The
women's health initiative. Circulation. 113:481–489.
2006.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Dekker JM, Schouten EG, Klootwijk P, Pool
J, Swenne CA and Kromhout D: Heart rate variability from short
electrocardiographic recordings predicts mortality from all causes
in middle-aged and elderly men: The Zutphen study. Am J Epidemiol.
145:899–908. 1997.PubMed/NCBI View Article : Google Scholar
|
|
24
|
De Couck M, Maréchal R, Moorthamers S, Van
Laethem JL and Gidron Y: Vagal nerve activity predicts overall
survival in metastatic pancreatic cancer, mediated by inflammation.
Cancer Epidemiol. 40:47–51. 2016.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Kloter E, Barrueto K, Klein SD, Scholkmann
F and Wolf U: Heart rate variability as a prognostic factor for
cancer survival-a systematic review. Front Physiol.
9(623)2018.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Georgieva-Tsaneva G: Application of
mathematical methods for analysis of digital ECG data. Inf Technol
Control. 14:34–43. 2016.
|
|
27
|
Vollmer M: A robust, simple and reliable
measure of heart rate variability using relative RR intervals. In
2015 Computing in Cardiology Conference (CinC). IEEE, pp609-612,
2015.
|
|
28
|
Huikuri HV, Ylitalo A, Pikkujämsä SM,
Ikäheimo MJ, Airaksinen KE, Rantala AO, Lilja M and Kesäniemi YA:
Heart rate variability in systemic hypertension. Am J Cardiol.
77:1073–1077. 1996.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Singh JP, Larson MG, Tsuji H, Evans JC,
O'Donnell CJ and Levy D: Reduced heart rate variability and
new-onset hypertension: insights into pathogenesis of hypertension:
The Framingham heart study. Hypertension. 32:293–297.
1998.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Huston JM and Tracey KJ: The pulse of
inflammation: Heart rate variability, the cholinergic
anti-inflammatory pathway and implications for therapy. J Intern
Med. 269:45–53. 2011.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Sajadieh A, Nielsen OW, Rasmussen V, Hein
HO, Abedini S and Hansen JF: Increased heart rate and reduced
heart-rate variability are associated with subclinical inflammation
in middle-aged and elderly subjects with no apparent heart disease.
Eur Heart J. 25:363–370. 2004.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Benichou T, Pereira B, Mermillod M,
Tauveron I, Pfabigan D, Maqdasy S and Dutheil F: Heart rate
variability in type 2 diabetes mellitus: A systematic review and
meta-analysis. PLoS One. 13(e0195166)2018.PubMed/NCBI View Article : Google Scholar
|
|
33
|
O'Neal WT, Chen LY, Nazarian S and Soliman
EZ: Reference ranges for short-term heart rate variability measures
in individuals free of cardiovascular disease: The multi-ethnic
study of atherosclerosis (MESA). J Electrocardiol. 49:686–690.
2016.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Van den Berg ME, Rijnbeek PR, Niemeijer
MN, Hofman A, van Herpen G, Bots ML, Hillege H, Swenne CA,
Eijgelsheim M, Stricker BH and Kors JA: Normal values of corrected
heart-rate variability in 10-sec electrocardiograms for all ages.
Front Physiol. 9(424)2018.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Barretto JM, Loures FB, Albuquerque RS,
Bezerra FD, Faro RV and Cavanellas NT: Evaluation of serum levels
of C-reactive protein after total knee arthroplasty. Rev Bras
Ortop. 52:176–181. 2017.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Madsen T, Christensen JH, Toft E and
Schmidt EB: C-reactive protein is associated with heart rate
variability. Ann Noninvasive Electrocardiol. 12:216–222.
2007.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Aeschbacher S, Schoen T, Dörig L,
Kreuzmann R, Neuhauser C, Schmidt-Trucksäss A, Probst-Hensch NM,
Risch M, Risch L and Conen D: Heart rate, heart rate variability
and inflammatory biomarkers among young and healthy adults. Ann
Med. 49:32–41. 2017.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Araújo F, Antelmi I, Pereira AC, Latorre
Mdo R, Grupi CJ, Krieger JE and Mansur AJ: Lower heart rate
variability is associated with higher serum high-sensitivity
C-reactive protein concentration in healthy individuals aged 46
years or more. Int J Cardiol. 107:333–337. 2006.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Lampert R, Bremner JD, Su S, Miller A, Lee
F, Cheema F, Goldberg J and Vaccarino V: Decreased heart rate
variability is associated with higher levels of inflammation in
middle-aged men. Am Heart J. 156:759.e1–e7. 2008.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Jarczok MN, Koenig J, Mauss D, Fischer JE
and Thayer JF: Lower heart rate variability predicts increased
level of C-reactive protein 4 years later in healthy, nonsmoking
adults. J Intern Med. 276:667–671. 2014.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Parvizi J, Gehrke T and Chen AF:
Proceedings of the international consensus on periprosthetic joint
infection. Bone Joint J. 95-B:1450–1452. 2013.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Maniar RN, Navaneedhan G, Ranvir S, Maniar
AR, Dhiman A and Agrawal A: What Is the normal trajectory of
interleukin-6 and C-reactive protein in the hours and days
immediately after TKA? Clin Orthop Relat Res. 477:41–46.
2019.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Neumaier M, Metak G and Scherer MA:
C-reactive protein as a parameter of surgical trauma: CRP response
after different types of surgery in 349 hip fractures. Acta Orthop.
77:788–790. 2006.PubMed/NCBI View Article : Google Scholar
|
|
44
|
White J, Kelly M and Dunsmuir R:
C-reactive protein level after total hip and total knee
replacement. J Bone Joint Surg Br. 80:909–911. 1998.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Liu L, Zhao M, Yu X and Zang W:
Pharmacological modulation of vagal nerve activity in
cardiovascular diseases. Neurosci Bull. 35:156–166. 2019.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Redekop WK and Mladsi D: The faces of
personalized medicine: A framework for understanding its meaning
and scope. Value Health. 16 (6 Suppl):S4–S9. 2013.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Poon CCY and Zhang YT: Perspectives on
high technologies for low-cost healthcare. IEEE Eng Med Biol Mag.
27:42–47. 2008.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Umetani K, Singer DH, McCraty R and
Atkinson M: Twenty-four hour time domain heart rate variability and
heart rate: Relations to age and gender over nine decades. J Am
Coll Cardiol. 31:593–601. 1998.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Liao D, Barnes RW, Chambless LE, Simpson
RJ Jr, Sorlie P and Heiss G: Age, race, and sex differences in
autonomic cardiac function measured by spectral analysis of heart
rate variability-the ARIC study. Atherosclerosis risk in
communities. Am J Cardiol. 76:906–912. 1995.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Huikuri HV, Kessler KM, Terracall E,
Castellanos A, Linnaluoto MK and Myerburg RJ: Reproducibility and
circadian rhythm of heart rate variability in healthy subjects. Am
J Cardiol. 65:391–393. 1990.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Massin MM, Maeyns K, Withofs N, Ravet F
and Gérard P: Circadian rhythm of heart rate and heart rate
variability. Arch Dis Child. 83:179–182. 2000.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Tobaldini E, Nobili L, Strada S, Casali
KR, Braghiroli A and Montano N: Heart rate variability in normal
and pathological sleep. Front Physiol. 4(294)2013.PubMed/NCBI View Article : Google Scholar
|
|
53
|
McCraty R, Atkinson M, Tiller WA, Rein G
and Watkins AD: The effects of emotions on short-term power
spectrum analysis of heart rate variability. Am J Cardiol.
76:1089–1093. 1995.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Lane RD, McRae K, Reiman EM, Chen K, Ahern
GL and Thayer JF: Neural correlates of heart rate variability
during emotion. Neuroimage. 44:213–222. 2009.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Burch JB, Ginsberg JP, McLain AC, Franco
R, Stokes S, Susko K, Hendry W, Crowley E, Christ A, Hanna J, et
al: Symptom management among cancer survivors: Randomized pilot
intervention trial of heart rate variability biofeedback. Appl
Psychophysiol Biofeedback. 45:99–108. 2020.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Lehrer PM and Gevirtz R: Heart rate
variability biofeedback: How and why does it work? Front Psychol.
5(756)2014.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Goessl VC, Curtiss JE and Hofmann SG: The
effect of heart rate variability biofeedback training on stress and
anxiety: A meta-analysis. Psychol Med. 47:2578–2586.
2017.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Lehrer P, Kaur K, Sharma A, Shah K, Huseby
R, Bhavsar J, Sgobba P and Zhang Y: Heart rate variability
biofeedback improves emotional and physical health and performance:
A systematic review and meta-analysis. Appl Psychophysiol
Biofeedback. 45:109–129. 2020.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Pizzoli SFM, Marzorati C, Gatti D, Monzani
D, Mazzocco K and Pravettoni G: A meta-analysis on heart rate
variability biofeedback and depressive symptoms. Sci Rep.
11(6650)2021.PubMed/NCBI View Article : Google Scholar
|
|
60
|
Fournié C, Chouchou F, Dalleau G, Caderby
T, Cabrera Q and Verkindt C: Heart rate variability biofeedback in
chronic disease management: A systematic review. Complement Ther
Med. 60(102750)2021.PubMed/NCBI View Article : Google Scholar
|
|
61
|
Gitler A, Vanacker L, De Couck M, De Leeuw
I and Gidron Y: Neuromodulation applied to diseases: The case of
HRV biofeedback. J Clin Med. 11(5927)2022.PubMed/NCBI View Article : Google Scholar
|