Introduction
Elderly patients commonly experience postoperative
cognitive dysfunction (POCD) following anesthesia and surgery. POCD
is commonly characterized by anxiety, confusion, personality
changes and impaired memory (1-3).
Anxiety is a relatively common manifestation in patients
postoperatively, even in the absence of other complications
(4). Therefore, reducing
postoperative anxiety has become a primary goal for preventing
POCD.
Isoflurane is widely used as a maintenance drug for
general anesthesia, due to its good anesthetic effect, easy
adjustment of anesthesia depth, mild circulatory effects, low
toxicity and rapid induction and recovery (5). However, inhalation anesthesia can
exhibit toxic effects on several types of cells, including nerve
cells. It has been reported that isoflurane has significant
toxicity (6-8).
Currently, the research on the effect of isoflurane on anxiety-like
behavior in elderly patients undergoing anesthesia and its
underlying mechanism is limited.
The amygdala is a key structure that processes
anxiety-related information (9).
It is composed of multiple parts, among which the basolateral
amygdala (BLA) and central amygdala are particularly significant
for the treatment of anxiety disorders (10). A previous study demonstrated that
BLA is associated with pathological anxiety, while the excitability
of a subpopulation of excitatory neurons in the BLA continue to
increase during anxiety (11).
Another study also showed that inhibitory neurons in the BLA are
involved in the synaptic plasticity, which can regulate fear
learning in the amygdala (12).
Therefore, in the current study an isoflurane
anesthesia model was established in elderly mice to evaluate the
neuronal status of the BLA and analyze its role in this process,
thus uncovering the possible mechanism underlying the effect of
isoflurane on inducing postoperative anxiety in the elderly.
Materials and methods
Ethics
All experiments were approved by the Laboratory
Animal Committee of The First Affiliated Hospital of Nanchang
University (approval no. CDYFY-IACUC-202205QR015) and conformed to
the National Research Council's Guide for the Care and Use of
Laboratory Animals (13). This
study complied with the Animal Research: Reporting of In
Vivo Experiments guidelines (14). The number of, as well as the
procedures introducing pain to the animals, were minimized
according to the aforementioned regulations.
Experimental grouping and
treatment
A total of 30 13-month-old C57BL/6 male mice
(weight, 30-38 g) were included in the present study. Mice were
given free access to food and water and were randomly allocated
into the control and experimental group (n=15 mice/group). The mice
were housed in groups of six animals per cage under a constant
light-dark cycle (lights on from 08:00-20:00) and fed standard
laboratory food and tap water in an air-conditioned room (23±1˚C
with ~60% humidity). An anxiety model was established after 1.5%
isoflurane anesthesia, as previously described, mice in the
experimental group received 1.5% isoflurane in pure oxygen for 2 h
and then breathed fresh air for 4 h (15,16).
Mice in the control group only received fresh air for 6 h. After
recovering, mice were allowed to eat and drink freely. Behavioral
tests were performed on the following day. After the end of the
behavioral study, mice were injected with 100 mg/kg sodium
pentobarbital into the abdomen, mice were euthanized under deep
anesthesia to remove the brain tissue for slicing, and then brain
slices were isolated for electrophysiological recordings.
Behavioral tests
The elevated plus maze test is used to evaluate
anxiety-like behavior in rodents (17,18).
It consists of four arms, two open and two closed, arranged in a
cross shape with a central area elevated off the ground. In the
present study, the anxiety-like behavior of mice was assessed by
comparing the time spent and distance traveled by the mice in the
open and closed arms. Briefly, each mouse was placed in the central
area of the maze, facing the open arm. The position of each mouse
was consistent throughout the experiment. Subsequently, the number
of entries of each mouse into the open and closed arms and the time
spent in each arm were recorded by a camera for 5 min. The
experiment was conducted in a quiet environment, while the
researcher remained 1 m away from the maze. After recording was
complete, the mouse was returned to its cage. The maze was cleaned
with 5% acetic acid solution or 75% alcohol to eliminate any
residual animal odor. Furthermore, mice were also subjected to open
field test. This test is commonly used to investigate anxiety or
depression in animals (18,19)
by evaluating several behaviors of experimental animals in an open
environment, such as the fear of the animals in a new environment.
Therefore, animals mainly move in the peripheral area and less in
the central one. However, due to their exploratory nature, animals
are motivated to move in the central area, thus resulting in the
development of anxiety symptoms. In the present study, the open
field was set to 50x50 cm with a brightness of 700 lux. The mouse
was placed in the experimental area to adapt for 10 min and then
its behavior was recorded for 20 min. Periphery was defined as the
area within 5 cm of the edge of the field, while the total distance
traveled and the time spent in the center or periphery, measured in
sec, were recorded. The distance traveled by the mouse to the
central area was divided by the total distance covered to obtain
the center distance/total distance ratio, which could be used as an
anxiety index.
Preparation of mice brain slices
After the end of the behavioral study, mice were
injected with 100 mg/kg sodium pentobarbital into the abdomen for
deep anesthesia. Following anesthesia, the brain was quickly
removed and placed in ice-cold sucrose-containing artificial
cerebrospinal fluid [ACSF; containing 100 mM choline-Cl, 13 mM
NaCl, 3 mM KCl, 1 mM NaH2PO4, 25 mM
NaHCO3, 11 mM D-glucose, 1 mM CaCl2 and 5 mM
MgCl2 (pH 7.4 after bubbling with 95% O2 and
5% CO2]. Subsequently, 300 µm-thick horizontal slices
were prepared using a vibratome. The aforementioned slices were
then incubated in standard ACSF at 32˚C for 30 min, followed by
resting at room temperature for 30 min.
In vitro whole-cell patch-clamp
recording
The patch-clamp set-up was performed using the
Olympus BX50WI microscope (Olympus Corporation) equipped with x60
water immersion lens (LUMPlanFL, NA 1.0). Brain slices were
transferred into a recording chamber maintained at 32˚C and were
continuously perfused with standard ACSF at a rate of 2-4 ml/min.
Whole-cell patch-clamp recordings were obtained from the visually
identified neurons in the lateral/basolateral amygdala complex. The
internal solution composed of 130 mM K-gluconate, 5 mM KCl, 10 mM
phosphocreatine, 10 mM HEPES, 0.5 mM EGTA, 2 mM Na2-ATP, 0.3 mM
Na-GTP and 2 mM MgSO4 (pH 7.20-7.30, 290 mosmol/l). Membrane
potential at resting state was recorded within the first 20 sec
after membrane rupture, while input resistance was measured at
resting membrane potential with current pulses (+10 pA; 500 ms).
Additionally, the action potentials were recorded with a series of
1-sec depolarizing current pulses at the resting membrane
potential. There are two main types of neurons in the BLA, namely
the excitatory principal neurons and the local circuit inhibitory
interneurons. Based on the action potential waveform (short
depolarizing process, high membrane potential peak, fast membrane
potential decay and hyperpolarizing afterpotential), the cells were
classified as excitatory neurons. The most common features of
inhibitory neuron action potentials are low peak amplitude,
prolonged depolarization, absence of repolarization process and
absence of after-hyperpolarization. All recordings were obtained
using the Multiclamp 700B amplifier (Molecular Devices, LLC) and
the PowerLab system (ADInstruments Ltd.) with a low-pass filter
frequency of 4 kHz. The signals were digitized at 40 kHz for
computer analysis using WinWCP software (V5.2.6; gift by Dr. John
Dempster, University of Strathclyde). All experiments were carried
out at 32˚C.
Statistical analysis
The results are expressed as the mean ± SD. The
tests for mice were repeated 15 times for each group. All data were
tested for normality by the Kolmogorov-Smirnov test. The animal
behavior, resting membrane potential, input resistance, AP
threshold, AP amplitude and AP half amplitude results between the
two groups were compared by unpaired Student's t-test. One- and
two-way ANOVA followed by Bonferroni's multiple comparison post hoc
test were performed to compare the number of action potentials
evoked by different current steps. All statistical analyses were
carried out with Prism 7 (GraphPad Software, Inc.). P<0.05 was
considered to indicate a statistically significant difference.
Results
Elevated plus maze test
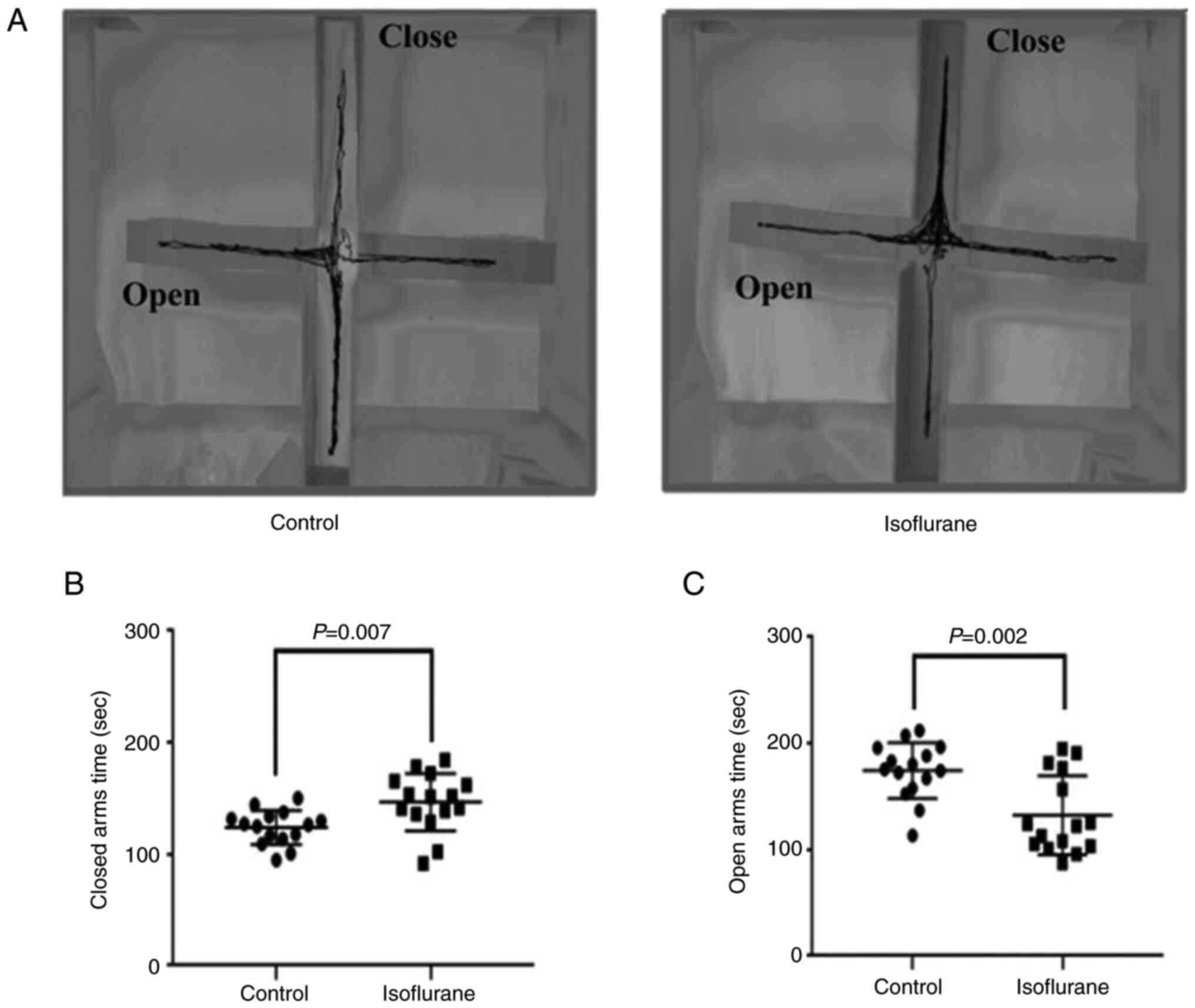
The movement trajectory results showed that mice in
the control group had a particular movement trajectory in both the
open and closed arms, while those in the isoflurane anesthesia
group mainly moved in the closed arms (Fig. 1A). In addition, compared with the
control group, mice in the isoflurane anesthesia group stayed a
significantly longer and shorter time in the closed (P<0.05;
Control vs. Isoflurane, 123.8±15.4 vs. 156.6±25.8 sec; Fig. 1B) and open (P<0.05; Control vs
Isoflurane, 164.3±26.3 vs. 132.5±37.5 sec; Fig. 1C) arms, respectively.
Open field test
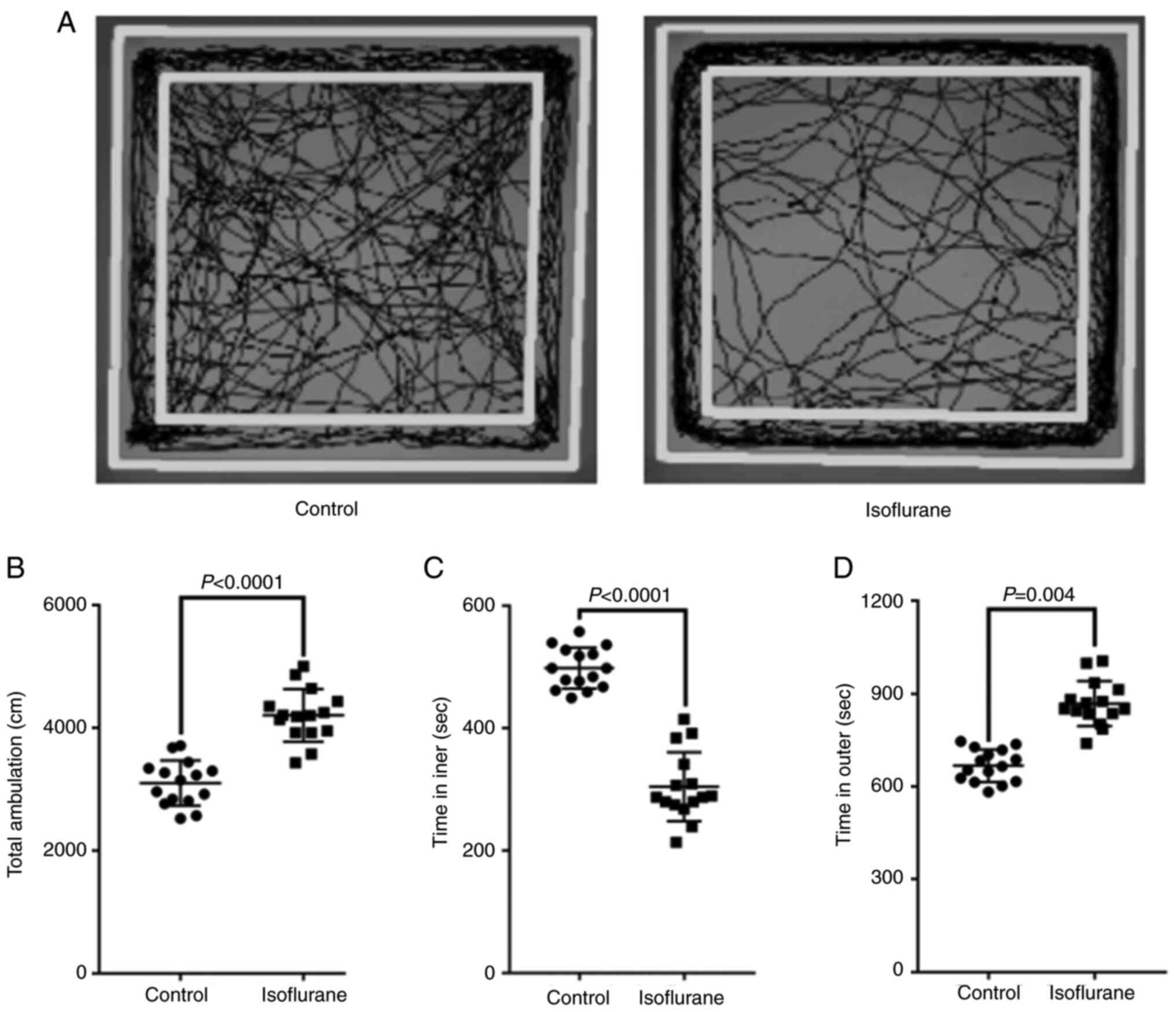
The results of movement trajectory revealed that
mice in the control group displayed a certain movement trajectory
in both the central and peripheral areas, while mice in the
isoflurane anesthesia group mainly moved in the peripheral area
(Fig. 2A). Furthermore, mice in
the isoflurane anesthesia group traveled a significantly longer
total distance (P<0.05; Control vs. Isoflurane, 3040.3±338.2 vs.
4069.6±419.3 cm; Fig. 2B), spent a
significantly shorter time in the central area (P<0.05; Control
vs. Isoflurane, 507.3±48.2 vs. 318.5±59.3 sec; Fig. 2C) and markedly longer time in the
peripheral area (P<0.05; Control vs. Isoflurane, 687.2±59.3 vs.
871.6±89.3 sec; Fig. 2D), compared
with the control group.
Electrophysiological changes of the
BLA excitatory neurons in aged mice after isoflurane
anesthesia
The activity of excitatory neurons were recorded by
whole-cell patch-clamp in both the control (Fig. 3A) and isoflurane (Fig. 3B) groups. To detect the basic
electrophysiological properties of the BLA principal neurons, the
activity of neurons (n=24) located in the BLA were recorded using a
whole-cell patch-clamp. The results demonstrated that, compared
with the control group, the resting membrane potential of the
excitatory neurons in the isoflurane anesthesia group was enhanced
(P<0.05; Control vs. Isoflurane, -66.7±6.5 vs. -62.3±5.2;
Fig. 3C). However, no significant
difference was recorded in input resistance between the isoflurane
and control groups (P>0.05; Control vs. Isoflurane, 177.9±42.4
vs. 182.3±40.3; Fig. 3D). As the
input current was increased, the number of action potentials
generated by the excitatory neurons of mice in the isoflurane
anesthesia group was notably elevated compared with the control
group (P<0.05; Fig. 3E).
Additionally, the action potential threshold was higher in the
control group compared with the isoflurane anesthesia group
(P<0.05; Control vs. Isoflurane, -39.4±5.3 vs. -36.6±7.9;
Fig. 3F). However, there was no
difference in the amplitude of the action potential or the
half-amplitude of the action potential between the two groups
(P>0.05; Fig. 3G and H).
Electrophysiological changes of BLA
inhibitory neurons in aged mice following isoflurane
anesthesia
The activity of inhibitory neurons were recorded by
whole-cell patch-clamp in both the control (Fig. 4A) and isoflurane (Fig. 4B) groups. To investigate the basic
electrophysiological properties of the BLA inhibitory neurons, the
activity of 20 neurons in the BLA was recorded using the whole-cell
patch-clamp technique. The results showed that compared with the
control group, the resting potential of the isoflurane anesthesia
group was significantly lower (P<0.05; Control vs. Isoflurane,
-61.2±5.2 vs. -67.3±6.5; Fig. 4C).
Consistently, the input resistance was also markedly reduced
(P<0.05; Control vs. Isoflurane, 172.5±19.7 vs. 157.1±23.7;
Fig. 4D). As the input current was
elevated, the number of evoked action potentials also gradually
increased. However, notably fewer action potentials were recorded
in the isoflurane anesthesia group compared with the control group
(P<0.05; Fig. 4E).
Additionally, the threshold of action potential was also
significantly enhanced (P<0.05; Control vs. Isoflurane,
-43.8±6.1 vs. -36.9±5.4; Fig. 4F).
However, no difference between the two groups was obtained in terms
of the amplitude of action potential and action potential
half-duration (P>0.05; Fig. 4G
and H).
Effect of diazepam on the BLA
inhibitory neurons in aged mice following isoflurane
anesthesia
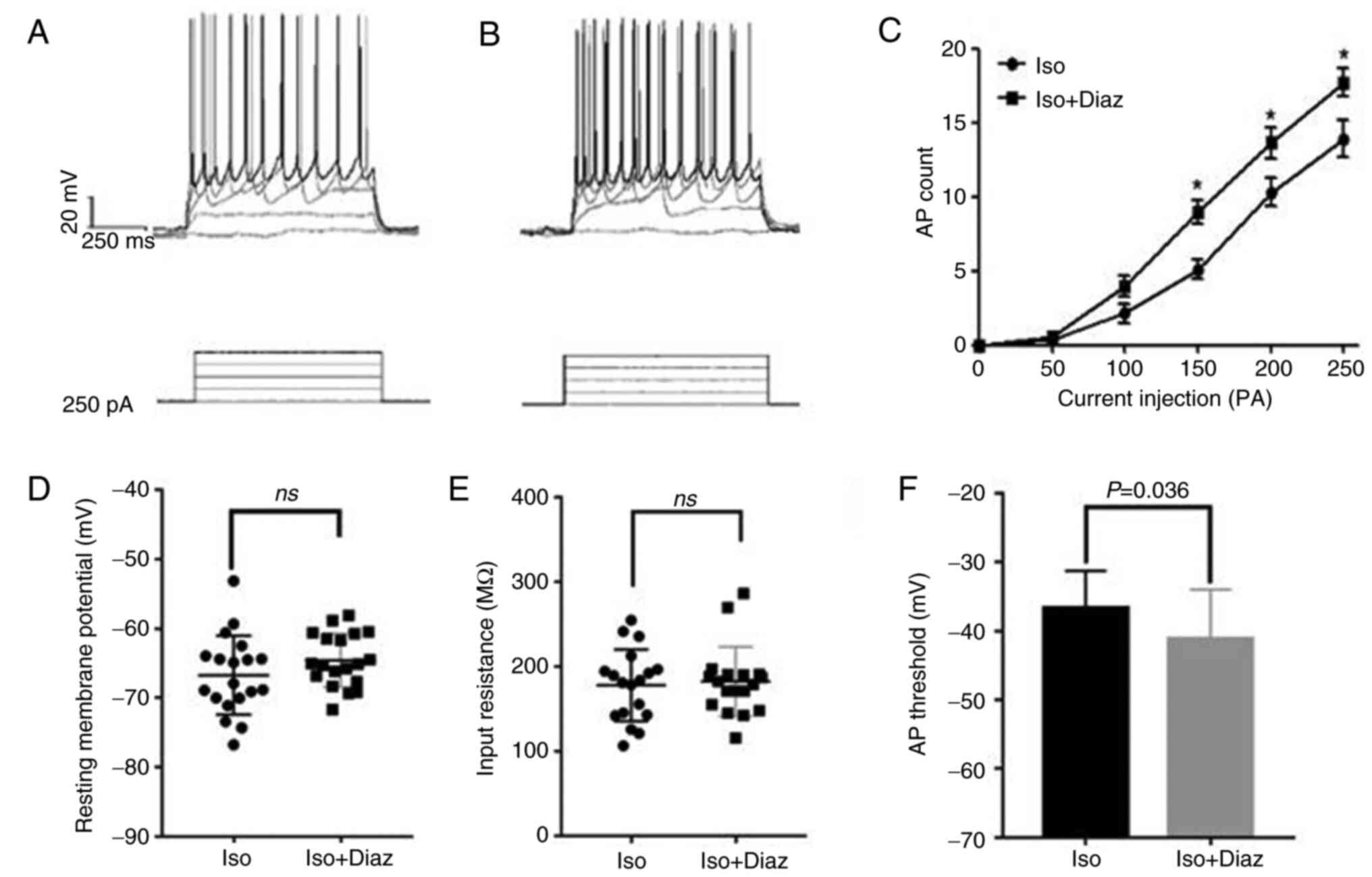
To further investigate the effects of isoflurane
anesthesia on BLA neurons, the ACSF perfusion solution was
supplemented with diazepam (500 ng/ml) (18), to enhance the excitability of
inhibitory neurons (20).
Subsequently, the action potentials of 19 inhibitory neurons at
resting potential was recorded using the whole-cell patch-clamp
technique (Fig. 5A and B). Therefore, compared with the control
group, there was no significant difference in the resting potential
(Fig. 5D) or input resistance
(Fig. 5E) of the BLA inhibitory
neurons in the isoflurane anesthesia group (P>0.05). As the
input current increased, the number of evoked action potentials
also gradually enhanced. However, significantly fewer action
potentials were recorded following treatment with diazepam
(P<0.05; Fig. 5C), while the
threshold of action potential was significantly reduced (P<0.05;
Iso vs. Iso + Diaz, -36.7±5.4 vs. -41.2±7.2; Fig. 5F).
Effect of diazepam on the BLA
excitatory neurons in aged mice following isoflurane
anesthesia
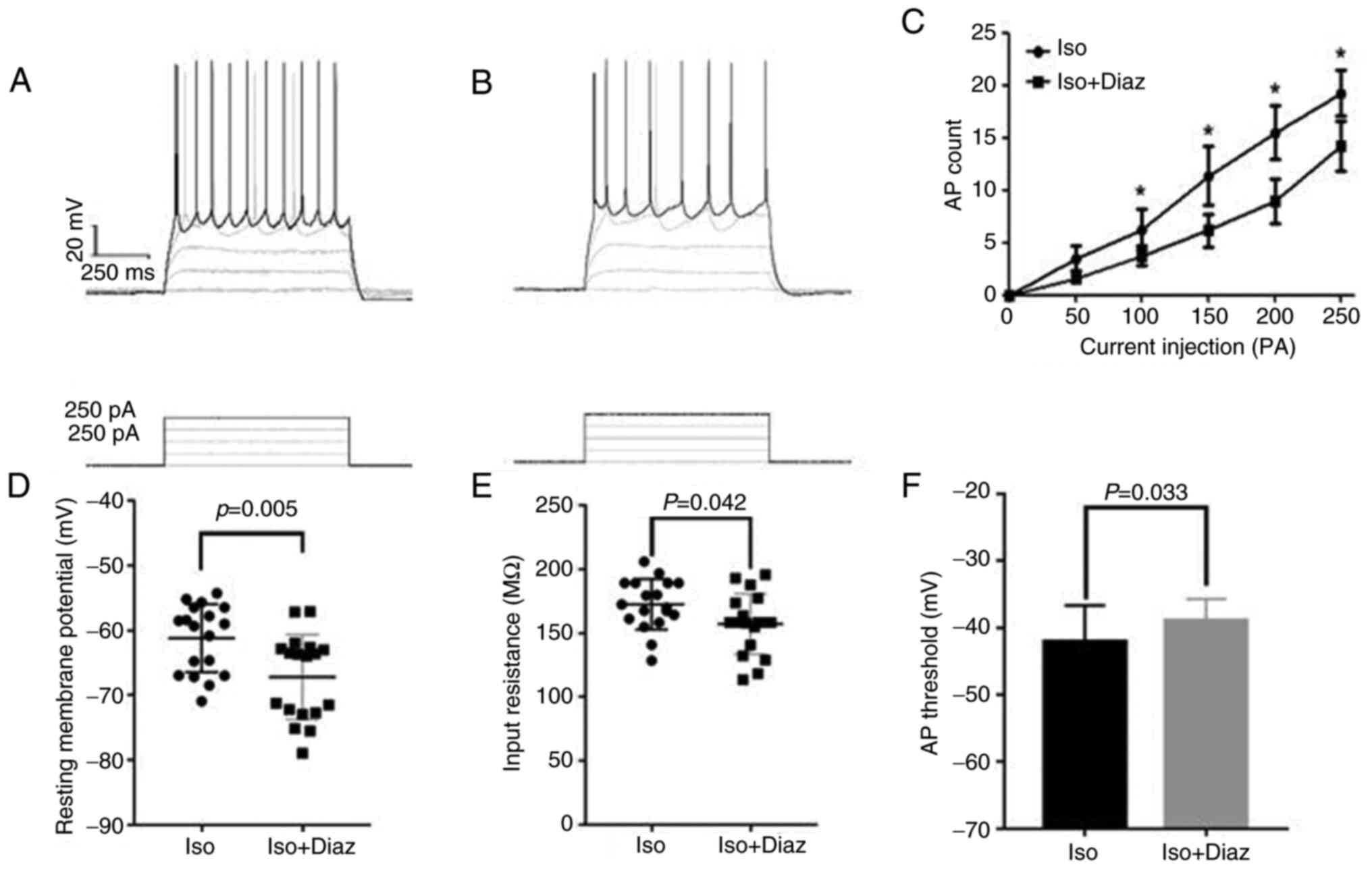
The action potentials of 18 excitatory neurons after
injecting different currents at resting potential were recorded
using the whole-cell patch-clamp technique (Fig. 6A and B). Therefore, compared with the
isoflurane anesthesia group, the resting potential of excitatory
neurons in the diazepam group was significantly lower (P<0.05;
Iso vs. Iso + Diaz, 172.5±19.8 vs. 157.1±23.7; Fig. 6D). In addition, the input
resistance was also notably reduced in the isoflurane anesthesia
group compared with the diazepam group (P<0.05; Iso vs. Iso +
Diaz, -61.2±5.2 vs. -67.3±6.5; Fig.
6E). As the input current increased, the number of evoked
action potentials was also gradually enhanced. However, the number
of action potentials evoked by excitatory neurons in the diazepam
group was markedly reduced compared with the isoflurane anesthesia
group (P<0.05; Fig. 6C).
Finally, the threshold of action potential was significantly higher
in mice in the isoflurane anesthesia group compared with the
diazepam group (P<0.05; Iso vs. Iso + Diaz, -42.1±5.4 vs.
-38.9±3.2; Fig. 6F).
Discussion
POCD is a type of cognitive impairment that occurs
after surgery and is characterized by decreased memory, lack of
concentration and impaired executive function (21,22).
It has been suggested that anxiety can be a major factor associated
with the onset of POCD after surgery, particularly in elderly
patients (3). Anxiety is a
physiological mechanism that is crucial for survival. However,
anxiety circuit dysregulation caused by chronic stress, traumatic
brain injury or drugs, can result in pathological anxiety (23).
In the present study, an aged mouse model of
isoflurane anesthesia was established and elevated plus maze and
open field tests were performed to assess anxiety behavior. The
results indicated that aged mice displayed anxiety-like behavior
after receiving isoflurane anesthesia. More particularly, the
results demonstrated that mice spent more time in the closed arms,
showed wall-hugging behavior and traveled longer distances, thus
indicating fear and avoidance behavior towards new environments and
objects. Additionally, a previous study revealed that volatile
anesthetics can cause neurodevelopmental toxicity in rodents and
primates and lead to more exaggerated anxiety-like behavior in
response to future stress (24).
The processing of anxiety-related information
involves a widespread network of brain areas, with the amygdala
being a key structure in this network (25). Among the multiple branches of the
amygdala, the BLA and central amygdala (CeA) serve a significant
role in anxiety processing (26,27).
The BLA is a cortical structure predominantly composed of
excitatory principal projection neurons and local inhibitory
interneurons, which not only modulate the output of the CeA, but
also play multiple roles in shaping information flow through the
amygdala circuits (28). It has
been reported that the overactivity of the BLA is associated with
pathological anxiety. Previous studies also showed that a subset of
inhibitory interneurons in the BLA continued to increase their
firing rate during anxiety-like behavior (29,30).
Inhibitory interneurons in the BLA can regulate the output of
excitatory principal projection neurons to limit the magnitude of
anxiety behaviors.
In the present study, whole-cell patch clamp
electrophysiology showed that the excitability of the BLA
excitatory neurons in aged mice was significantly increased after
isoflurane anesthesia, as evidenced by the significantly higher
resting membrane potential and input resistance, lower action
potential threshold and the markedly increased number of action
potentials fired. By contrast, the excitability of inhibitory
neurons was markedly decreased, as evidenced by the notably lower
resting membrane potential and input resistance, the higher action
potential threshold and the significant decrease in the number of
action potentials fired compared with the control group.
It has been reported that isoflurane and other
anesthetics can affect postsynaptic γ-aminobutyric acid sub-type A
(GABAA) receptors and increase their inhibitory function via
allosteric modulation (31,32).
Therefore, when isoflurane is present, the GABAA receptor-mediated
charge transfer is increased, primarily due to the prolongation of
the inhibitory current decay. The aforementioned effect has been
observed in evoked inhibitory postsynaptic potentials in the BLA
(33). Therefore, a previous study
demonstrated that repeated exposure to isoflurane promotes a
long-term increase in spontaneous GABAA receptor-mediated synaptic
transmission (34). Inhibitory
interneurons in the amygdala regulate the output of excitatory
principal projection neurons to prevent overt behavioral responses
to anxiety-provoking stimuli. Therefore, it was hypothesized that
they could serve a critical role in defining the valence of
incoming sensory stimuli (20). In
the present study, to further investigate the role of inhibitory
neurons in the increased excitability of the BLA excitatory neurons
following isoflurane anesthesia, the perfusate of the brain slices
was supplemented with diazepam. Diazepam is the most commonly used
psychotropic medication for the treatment of anxiety disorders
(35). It enhances the
excitability of central inhibitory neurons primarily by enhancing
the inhibitory effects of GABA at the GABA A receptor (36). Diazepam binds to specific sites on
the GABA A receptor, thus inducing the inhibitory effects of GABA
(37). In turn, the aforementioned
process facilitates the opening of chloride ion channels, allowing
more chloride ions to enter the neurons, thus strengthening the
inhibitory effects of GABA (36).
The aforementioned enhanced inhibitory activity can reduce neuronal
excitability, thus resulting in sedative, anxiolytic and
anticonvulsant effects (38). In
the present study, co-treatment of isoflurane anesthesia-treated
aged mice with diazepam significantly increased the excitability of
the BLA inhibitory neurons, while that of excitatory neurons was
notably decreased. This finding suggested that the reduced
excitability of inhibitory neurons in aged mice following
isoflurane anesthesia could lead to attenuated inhibition of
excitatory neurons, thus resulting in the increased excitability
and electrical activity of excitatory neurons, ultimately leading
to anxiety-like behaviors.
Interneurons in the BLA can form local circuits,
thus promoting feedforward and feedback inhibition to projection
neurons and other interneurons (28). These interneurons can be classified
into different subgroups based on the expression of calcium binding
proteins and neuropeptides, such as parvalbumin, somatostatin,
cholecystokinin, calbindin and calretinin. The aforementioned
interneurons can differ in soma size and dendritic tree shape,
while they can target distinct compartments of their postsynaptic
targets within the BLA (28).
Therefore, emerging evidence has suggested that inhibition of
interneurons in the BLA plays a crucial role in regulating
anxiety.
In conclusion, aged mice displayed anxiety-like
behavior after receiving isoflurane anesthesia, possibly due to the
decreased excitability of the inhibitory neurons in the BLA area.
This process resulted in an enhanced excitability and electrical
activity of excitatory neurons, eventually leading to anxiety-like
behavior. However, the mechanism involved was not clarified, and
further animal experiments are required to elucidate the effects of
isoflurane anesthesia on anxiety-like behavior. Anesthesia-induced
consciousness disturbances are usually short-term, with
anxiety-like behaviors in aged mice following isoflurane anesthesia
being most prominent 2 to 3 days post-anesthesia, gradually
resolving even without drug intervention. Therefore, the present
study only explored behavioral changes in aged mice after
isoflurane anesthesia and electrophysiological alterations in the
BLA region, thereby providing a potential direction for future
research on anxiety-like behavior changes in elderly patients
following anesthesia.
Acknowledgements
Not applicable.
Funding
Funding: All funds used for experiments were supported by the
Foundation of Science and Technology of Jiangxi Provincial Health
and Health Commission (grant no. 202130211).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
LQ conceived and designed this study. ML, RZ, SW, LC
and HF performed the experiments. ML, RZ, SW, LC, and HF
contributed reagents, materials or analysis tools. LQ and ML
confirm the authenticity of all the raw data. ML, RZ, SW, LC, HF
and LQ wrote the paper. Critical revision of the manuscript was
given by all authors. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
All experiments were approved by the Laboratory
Animal Committee of the First Affiliated Hospital of Nanchang
University (approval no. CDYFY-IACUC-202205QR015) and conformed to
the National Research Council's Guide for the Care and Use of
Laboratory Animals. This study was reported in accordance with
ARRIVE guidelines.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Beloeil H, Garot M, Lebuffe G, Gerbaud A,
Laviolle B, Dubout E, Oger S, Nadaud J, Becret A, et al: Balanced
opioid-free anesthesia with dexmedetomidine versus balanced
anesthesia with remifentanil for major or intermediate noncardiac
surgery. Anesthesiology. 134:541–551. 2021.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Wang CM, Chen WC, Zhang Y, Lin S and He
HF: Update on the mechanism and treatment of sevoflurane-induced
postoperative cognitive dysfunction. Front Aging Neurosci.
13(702231)2021.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Lin X, Chen Y, Zhang P, Chen G, Zhou Y and
Yu X: The potential mechanism of postoperative cognitive
dysfunction in older people. Exp Gerontol.
130(110791)2020.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Bruce SL, Ching THW and Williams MT:
Pedophilia-themed obsessive-compulsive disorder: Assessment,
differential diagnosis, and treatment with exposure and response
prevention. Arch Sex Behav. 47:389–402. 2018.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Kozu F, Shirahama-Noda K, Araki Y, Kira S,
Niwa H and Noda T: Isoflurane induces Art2-Rsp5-dependent
endocytosis of Bap2 in yeast. FEBS Open Bio. 11:3090–3100.
2021.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Hao X, Ou M, Zhang D, Zhao W, Yang Y, Liu
J, Yang H, Zhu T, Li Y and Zhou C: The effects of general
anesthetics on synaptic transmission. Curr Neuropharmacol.
18:936–965. 2020.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Yamamoto T, Iwamoto T, Kimura S and Nakao
S: Persistent isoflurane-induced hypotension causes hippocampal
neuronal damage in a rat model of chronic cerebral hypoperfusion. J
Anesth. 32:182–188. 2018.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Chírico MTT, Guedes MR, Vieira LG, Reis
TO, Dos Santos AM, Souza ABF, Ribeiro IML, Noronha SISR, Nogueira
KO, Oliveira LAM, et al: Lasting effects of ketamine and isoflurane
administration on anxiety- and panic-like behavioral responses in
Wistar rats. Life Sci. 276(119423)2021.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Feinstein JS, Gould D and Khalsa SS:
Amygdala-driven apnea and the chemoreceptive origin of anxiety.
Biol Psychol. 170(108305)2022.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Morel C, Montgomery SE, Li L, Durand-de
Cuttoli R, Teichman EM, Juarez B, Tzavaras N, Ku SM, Flanigan ME,
Cai M, et al: Midbrain projection to the basolateral amygdala
encodes anxiety-like but not depression-like behaviors. Nat Commun.
13(1532)2022.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Puggioni P, Pelko M, Rossum MV and Duguid
I: Behavioral state differentially regulates input sensitivity and
firing rates of motor cortex pyramidal neurons. Bmc Neurosci. 14
(Suppl 1)(P114)2013.
|
|
12
|
Perumal MB and Sah P: Inhibitory circuits
in the basolateral amygdala in aversive learning and memory. Front
Neural Circuits. 15(633235)2021.PubMed/NCBI View Article : Google Scholar
|
|
13
|
National Research Council (US): Committee
for the Update of the Guide for the Care and Use of Laboratory
Animals. Guide for the Care and Use of Laboratory Animals, 8th
edition. National Academies Press, Washington, DC, 2011.
|
|
14
|
Kilkenny C, Browne W, Cuthill IC, Emerson
M and Altman DG: NC3Rs Reporting Guidelines Working Group. Animal
research: reporting in vivo experiments: The ARRIVE guidelines. J
Gene Med. 12:561–563. 2010.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Zuo CL, Wang CM, Liu J, Shen T, Zhou JP,
Hao XR, Pan YZ, Liu HC, Lian QQ and Lin H: Isoflurane anesthesia in
aged mice and effects of A1 adenosine receptors on cognitive
impairment. CNS Neurosci Ther. 24:212–221. 2018.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Wang Z, Meng S, Cao L, Chen Y, Zuo Z and
Peng S: Critical role of NLRP3-caspase-1 pathway in age-dependent
isoflurane-induced microglial inflammatory response and cognitive
impairment. J Neuroinflammation. 15(109)2018.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Wang J, Zhu S, Lu W, Li A, Zhou Y, Chen Y,
Chen M, Qian C, Hu X, Zhang Y and Huang C: Varenicline improved
laparotomy-induced cognitive impairment by restoring mitophagy in
aged mice. Eur J Pharmacol. 916(174524)2022.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Rabat Y, Henkous N, Corio M, Nogues X and
Beracochea D: Baclofen but not diazepam alleviates alcohol-seeking
behavior and hypothalamic-pituitary-adrenal axis dysfunction in
stressed withdrawn mice. Front Psychiatry. 10(238)2019.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Rana T, Behl T, Sehgal A, Singh S, Sharma
N, Abdeen A, Ibrahim SF, Mani V, Iqbal MS, Bhatia S, et al:
Exploring the role of neuropeptides in depression and anxiety. Prog
Neuropsychopharmacol Biol Psychiatry. 114(110478)2022.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Jones SK, McCarthy DM, Vied C, Stanwood
GD, Schatschneider C and Bhide PG: Transgenerational transmission
of aspartame-induced anxiety and changes in glutamate-GABA
signaling and gene expression in the amygdala. Proc Natl Acad Sci
USA. 119(e2213120119)2022.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Wang S, Cardieri B, Mo Lin H, Liu X, Sano
M and Deiner SG: Depression and anxiety symptoms are related to
pain and frailty but not cognition or delirium in older surgical
patients. Brain Behav. 11(e02164)2021.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Hem S, Albite R, Loresi M, Rasmussen J,
Ajler P, Yampolsky C, Chabot JD, Gerszten PC and Goldschmidt E:
Pathological changes of the hippocampus and cognitive dysfunction
following frontal lobe surgery in a rat model. Acta Neurochir
(Wien). 158:2163–2171. 2016.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Craske MG and Stein MB: Anxiety. Lancet.
388:3048–3059. 2016.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Zhong J, Li C, Peng L, Pan Y, Yang Y, Guo
Q and Zhong T: Repeated neonatal isoflurane exposure facilitated
stress-related fear extinction impairment in male mice and was
associated with ΔFosB accumulation in the basolateral amygdala and
the hippocampal dentate gyrus. Behav Brain Res.
446(114416)2023.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Liu WZ, Zhang WH, Zheng ZH, Zou JX, Liu
XX, Huang SH, You WJ, He Y, Zhang JY, Wang XD and Pan BX:
Identification of a prefrontal cortex-to-amygdala pathway for
chronic stress-induced anxiety. Nat Commun. 11(2221)2020.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Zheng ZH, Tu JL, Li XH, Hua Q, Liu WZ, Liu
Y, Pan BX, Hu P and Zhang WH: Neuroinflammation induces anxiety-
and depressive-like behavior by modulating neuronal plasticity in
the basolateral amygdala. Brain Behav Immun. 91:505–518.
2021.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Tye KM, Prakash R, Kim SY, Fenno LE,
Grosenick L, Zarabi H, Thompson KR, Gradinaru V, Ramakrishnan C and
Deisseroth K: Amygdala circuitry mediating reversible and
bidirectional control of anxiety. Nature. 471:358–362.
2011.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Babaev O, Piletti Chatain C and
Krueger-Burg D: Inhibition in the amygdala anxiety circuitry. Exp
Mol Med. 50:1–16. 2018.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Prager EM, Bergstrom HC, Wynn GH and Braga
MFM: The basolateral amygdala γ-aminobutyric acidergic system in
health and disease. J Neurosci Res. 94:548–567. 2016.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Lee SC, Amir A, Haufler D and Pare D:
Differential recruitment of competing valence-related amygdala
networks during anxiety. Neuron. 96:81–88.e5. 2017.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Hemmings HC Jr, Akabas MH, Goldstein PA,
Trudell JR, Orser BA and Harrison NL: Emerging molecular mechanisms
of general anesthetic action. Trends Pharmacol Sci. 26:503–510.
2005.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Krasowski MD and Harrison NL: General
anaesthetic actions on ligand-gated ion channels. Cell Mol Life
Sci. 55:1278–1303. 1999.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Ranft A, Kurz J, Deuringer M, Haseneder R,
Dodt HU, Zieglgänsberger W, Kochs E, Eder M and Hapfelmeier G:
Isoflurane modulates glutamatergic and GABAergic neurotransmission
in the amygdala. Eur J Neurosci. 20:1276–1280. 2004.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Long Ii RP, Aroniadou-Anderjaska V, Prager
EM, Pidoplichko VI, Figueiredo TH and Braga MF: Repeated isoflurane
exposures impair long-term potentiation and increase basal
GABAergic activity in the basolateral amygdala. Neural Plast.
2016(8524560)2016.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Wesołowska A: Potential role of the 5-HT6
receptor in depression and anxiety: An overview of preclinical
data. Pharmacol Rep. 62:564–577. 2010.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Tong X, Zhang Z, Zhu J, Li S, Qu S, Qin B,
Guo Y and Chen R: A comparison of epileptogenic effect of status
epilepticus treated with diazepam, midazolam, and pentobarbital in
the mouse pilocarpine model of epilepsy. Front Neurol.
13(821917)2022.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Kasaragod VB, Malinauskas T, Wahid AA,
Lengyel J, Knoflach F, Hardwick SW, Jones CF, Chen WN, Lucas X, El
Omari K, et al: The molecular basis of drug selectivity for α5
subunit-containing GABAA receptors. Nat Struct Mol Biol.
30:1936–1946. 2023.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Courtney CD, Sobieski C, Ramakrishnan C,
Ingram RJ, Wojnowski NM, DeFazio RA, Deisseroth K and
Christian-Hinman CA: Optoα1AR activation in astrocytes modulates
basal hippocampal synaptic excitation and inhibition in a
stimulation-specific manner. Hippocampus. 33:1277–1291.
2023.PubMed/NCBI View Article : Google Scholar
|