Introduction
Atrial fibrillation (AF), the most common sustained
arrhythmia, exhibits a high prevalence associated with ageing,
affecting an estimated 33.5 million individuals globally (1). Typically, AF is associated with a
five-fold increase in strokes, which is more devastating than
stroke caused by cerebrovascular diseases or hypertension. Although
most patients with AF are asymptomatic, stroke often emerges as the
primary symptom of AF. Notably, ≥95% of these thrombi originate
from the left atrial appendage. AF predisposes individuals to
atrial and auricular thrombosis through intricate interactions
among local, systemic and hemodynamic factors, significantly
elevating the likelihood of cerebral and systemic thromboembolic
events, thereby impacting morbidity and mortality rates. Extensive
research over decades has elucidated the formation of the left
atrial appendage thrombosis, primarily linked to structural
abnormities, coagulation mechanisms and endothelial damage.
Consequently, various therapeutic strategies have been developed to
mitigate these risks (2). For
patients with symptomatic AF, radiofrequency ablation is frequently
employed to alleviate symptoms and prevent recurrence. However,
achieving disease control without resorting to radiofrequency
ablation is often the goal. Nevertheless, patients with a history
of left atrial appendage thrombosis are presumed to have a
heightened risk of stroke compared with others. There is increasing
recognition that sole administration of oral anticoagulants after
successful thrombolysis may increase the risk of stroke and death
(2).
Traditionally, systemic anticoagulation has been an
effective strategy for preventing atrial and left atrial
thrombosis. Initially, warfarin and other vitamin K antagonists
were the primary oral anticoagulants used in China until 2009.
Despite their efficacy in preventing thromboembolism, their narrow
therapeutic index necessitated frequent monitoring and dosage
adjustments, posing a substantial risk of bleeding or thrombosis
and often leading to poor patient compliance (3). Therefore, new oral anticoagulants
(NOACs) were developed, demonstrating comparable efficacy and
safety to warfarin in preventing stroke and systemic embolism in
patients with AF. Moreover, these NOACs should not exhibit the
limitations of vitamin K antagonists, especially in terms of
safety. Several NOACs, including dabigatran, rivaroxaban, apixaban
and edoxaban, have been developed and approved by regulatory
agencies for the prevention of stroke in patients with non-valvular
AF. However, their efficacy in thrombus regression remains unclear,
with limited data available on their treatment of left auricular
thrombosis (4). Additionally, the
European Society of Cardiology (ESC) 2020 guidelines recommended
anticoagulation for at least 2 months post-radiofrequency ablation
based on the patient's stroke risk profile, recognising AF itself
as a thrombosis factor. Therefore, reducing the AF burden may
decrease the risk of ischaemic stroke (5). Nevertheless, controversy persists
regarding the necessity of combined radiofrequency ablation
treatment and the choice of anticoagulant therapy in patients with
AF at risk of thrombotic events.
Motivated by these considerations and given the high
propensity for repeat thrombotic events in patients with a history
of left atrial appendage thrombosis, the present study explored the
efficacy and adverse events of radiofrequency ablation combined
with anticoagulation treatment in patients with AF and previous
left auricular thrombosis. Moreover, the efficacy of traditional
anticoagulant warfarin was compared with NOACs while analysing
factors influencing patient mortality and stroke risk.
Consequently, the present study aimed to enhance treatment planning
for patients with AF with previous left atrial appendage thrombosis
and elucidate factors contributing to their poor prognosis. By
focusing on this special population, the present study endeavoured
to explore the relationship between heart rhythm disorders and
recurrent thrombosis, aiming to inform clinical practice
rigorously.
Materials and methods
General information
A total of 92 patients (age, 66.90±8.3 years; 57
males and 35 females) diagnosed with AF and previous left atrial
thrombus, who were treated at Shanghai Jiao Tong University
Affiliated Chest Hospital (Shanghai, China) between March 2018 and
March 2023 were retrospectively enrolled. Successful thrombolysis
was confirmed via trans-esophageal echocardiography (TEE) or
intracardiac ultrasound (ICE). Based on treatment criteria,
patients were divided into two groups: The radiofrequency ablation
(RA) combined anticoagulation group (Group A) and the simple
anticoagulation and antiarrhythmic drug group (Group B). In Group
A, patients underwent RA in conjugation with anticoagulant
medication upon confirmation of thrombus dissolution, while Group B
received oral anticoagulant and antiarrhythmic medications
post-procedure. Oral medication was discontinued after three months
in Group A, whereas patients in Group B continued long-term oral
medication. Subgroup analyses were conducted within Groups A and B,
based on left atrial internal diameter (≤45 mm), duration of AF (≤1
year) and type of anticoagulant. Patients with concomitant valvular
disease were excluded. The present study was approved (approval no.
IS22031) by the Ethics Committee of Shanghai Jiao Tong University
Affiliated Chest Hospital (Shanghai, China) and all patients
provided signed informed consent after family approval. All
procedures involving human participants conformed to the ethical
guidelines of the Declaration of Helsinki (2013.10 revision).
Inclusion and exclusion criteria.
Inclusion criteria
Inclusion criteria were as follows: i) Patients aged
≥55 years; ii) patients diagnosed with non-valvular AF combined
with left atrial thrombus, confirmed by TEE or other means and
successful thrombolysis assessed by TEE or ICE; iii) patients
meeting criteria for RA; and iv) patients having no allergies to
the anticoagulants used in the study and voluntarily participation
with signed informed consent.
Exclusion criteria. Inclusion criteria were
as follows: i) patients with valvular AF, absence of left auricular
thrombus and non-resolution of thrombus post-anticoagulation
therapy; ii) patients with mechanical valve implantation or
moderate-to-severe mitral stenosis; iii) patients with uncontrolled
bleeding disorders; and iv) patients who were prescribed with
anticoagulant drugs other than warfarin and the NOAC selected in
the present study and/or patients with cerebrovascular or other
cardiac-related diseases.
Treatment and examination. Ablation
protocol
Catheter ablation was performed under the guidance
of an electro-anatomical mapping system (CARTO, Biosense Webster,
Inc.; Johnson & Johnson MedTech) and followed a sequential
approach: i) Circumferential pulmonary vein isolation (PVI): The
radiofrequency energy (RF) was 40-45 W (15-20 ml/min saline
perfusion) with ablation index of 400-500 during PVI. Successful
PVI was defined as eliminating all PV potentials and/or atrium-PV
potential dissociation; ii) Linear ablation: The anterior roof,
lateral mitral isthmus was ablated with linear lesion (RF: 40-45 W,
15-20 ml/min saline perfusion). If bidirectional block could not be
achieved in the mitral isthmus, epicardial ablation within the
coronary sinus or vein of Marshall ethanol infusion was performed
at the operator's discretion with an error margin of ±2% (or
Cronbach's alpha coefficient of 0.85) to indicate the variability
in the procedure. In the RA group, linear ablation at tricuspid
isthmus was performed; iii) Driver ablation: The targeted ablation
regions had electrograms displayed: i) Spatial-temporal dispersion
potentials, which spread over atrial fibrillation cycle length
(AFCL) at a minimum of 3 adjacent bi-pole and associated with local
AFCL ≤ mean AFCL potentials; ii) locally short cycle length
activity; iii) focal activity. Localized ‘patch’ ablation was
applied (40-45 W) at any atrial location except appendage and vena
cava. If two ablated regions were very close (<1 cm), linear
ablation was performed to connect the two regions; and iv) post-AF
treatment: If AF converted to atrial tachycardia during the
procedure, the arrhythmia was mapped and ablated until conversion
to sinus rhythm (SR). After SR conversion, AF re-induction was
tested by burst atrial pacing (Pacing CL: 200-250 msec). If the AF
could not be terminated by ablation, 150-200 J direct current
conversion was performed.
During the RA treatment, 10,000 U of normal heparin
(UH) was administered before the atrial septal puncture.
Furthermore, the activated clotting time (ACT) was checked 15 min
after the administration and every 20 min subsequently. Moreover,
the weight-adjusted UH pushes were performed to maintain ACT for
300-350 sec while the catheter remained in the left atrium.
Medication. In Group A, post-thrombus
dissolution, patients underwent RA and received anticoagulant and
antiarrhythmic medications, including Amiodarone and β-blocker.
Conversely, Group B patients received oral anticoagulant and
antiarrhythmic medications post-procedure. Oral medication was
discontinued after three months in Group A, whereas patients in
Group B continued long-term oral medication.
Follow-up and endpoints. Follow-up was
conducted through outpatient clinical visits every 2-4 weeks for
the first 3 months and every 1-3 months thereafter. Monthly
telephone follow-ups were also performed until the study's
conclusion. The primary endpoint was defined as cerebral embolism
due to cardiogenic thrombus shedding or death. Secondary endpoints
included other thrombotic events such as cerebral infarction, lower
extremity venous thrombosis, mesenteric embolism or transient
ischemic attacks (TIAs). The final follow-up was conducted in
February 2023, with a median follow-up duration of 36.02
months.
Observational indicators
Various observational indicators were recorded,
including age, sex, hypertension, medical history (diabetes
mellitus), diagnostic characteristics, duration of AF, left atrial
internal diameter, left ventricular ejection fraction (LVEF),
dilated left ventricular end-diastolic diameter (LVEDD), CHADS,
B-type natriuretic peptide (BNP), proBNP, auricular opening,
auricular long axis, distal auricular, distal to the opening, type
of anticoagulant and time intervals for repeat ultrasound in Groups
A and B and their respective subgroups (months). Survival outcomes
(stroke and death) were documented during follow-up after
concurrent treatment in Groups A and B and their subgroups. Stroke
diagnosis followed clinical guidelines, including symptoms and
cranial imaging evidence. Univariate and multivariate logistic
analyses were conducted to identify risk factors influencing stroke
occurrence and death in patients with AF and left atrial
thrombus.
Statistical analysis
Due to the exploratory nature of the present study,
formal sample size calculation was not performed. A sample size
exceeding 20 patients per group met the minimum statistical
requirements. The statistical analyses were performed using the
SPSS version 25.0 software (IBM Corp.). Normally distributed
continuous data are expressed as the mean + standard deviation
(SD). The data between the two groups were compared using the
independent samples t-test. Categorical variables were compared
using χ2 tests or Fisher's exact test, as appropriate.
Skewed distribution data were expressed as a median and
interquartile range, with comparisons between groups performed
using the Wilcoxon rank sum test. Additionally, categorical data
were expressed as cases (%) and analysed using the χ2
and Fisher's exact tests. Logistic regression analysis was used to
identify factors influencing stroke and death occurrence. The
sample size was determined based on previous experience. P<0.05
was considered to indicate a statistically significant
difference.
Results
Comparison of baseline characteristics
between the two groups
The baseline characteristics of the two groups are
summarised in Table I.
Statistically significant differences were observed in left atrial
internal diameter,and types of anticoagulants (P<0.05). However,
no significant differences were revealed in other baseline
characteristics.
 | Table IA summary presenting the clinical
baseline data of patients with AF combined with left atrial
thrombus. |
Table I
A summary presenting the clinical
baseline data of patients with AF combined with left atrial
thrombus.
| Variant | Radiofrequency
ablation combined with anticoagulation Group A (n=55) | Anticoagulation alone
Group B (n=37) | t/χ2
valuea | P-value |
|---|
| Age, years | 65.2±10.5 | 68.8±9.0 | 1.694 | 0.094 |
| Male [n (%)] | 33 (60.0) | 24 (64.9) | 0.222 | 0.637 |
| High blood pressure
[n (%)] | 24 (43.6) | 24 (64.9) | 3.995 | 0.056 |
| Diabetes [n (%)] | 9 (16.4) | 13 (35.1) | 4.284 | 0.068 |
| Diagnostic trait [n
(%)] | | | 0.487 | 0.485 |
|
Paroxysmal | 7 (12.7) | 3 (8.1) | | |
|
Sustainability | 48 (87.3) | 34 (91.9) | | |
| AF >1 year [n
(%)] | 22 (40.0) | 20 (54.1) | 1.761 | 0.185 |
| Left atrial internal
diameter >45 mm [n (%)] | 19 (34.5) | 23 (62.2) | 6.799 | 0.009 |
| Concomitant
medications, n (%) | | | 0.481 | 0.476 |
|
Amiodarone | 45 (81.8) | 34 (91.9) | | |
|
β-blocker | 10 (18.1) | 3 (8.1) | | |
| CHADS (Point) | 2.0 (1,4) | 3.0 (1,4) | 0.227 | 0.821 |
| BNP (ng/l) | 580.0 (580,580) | 580.0 (397,785) | 1.172 | 0.248 |
| Heart and ears open
(mm) | 20.3±5.1 | 19.6±3.7 | 0.718 | 0.475 |
| Trunnion (mm) | 28.0±4.4 | 28.2±4.1 | 0.248 | 0.805 |
| The distal part of
the auricle (mm) | 16.1±4.9 | 15.4±3.5 | 0.751 | 0.455 |
| The distal part of
the opening (mm) | 1.38±0.29 | 1.36±0.26 | 0.388 | 0.699 |
| Types of
anticoagulants | | | 20.453 | <0.001 |
|
Traditional
anticoagulants | 9 (16.4) | 23 (62.2) | | |
|
New
anticoagulants | 46 (83.6) | 14 (37.8) | | |
|
Time to
review ultrasound (months) | 3.0 (3,4) | 3.0 (2.5,4) | 0.048 | 0.962 |
Effect of left atrial internal
diameter and duration of AF on survival
Statistically significant differences in stroke and
death rates were observed between Groups A and B (P<0.05).
Stratified analysis revealed no difference in stroke occurrence
related to left atrial internal diameter between the two groups.
However, a significant impact on death rates was noted in Group B
(P<0.05, Table II).
 | Table IIThe data demonstrate the effect of
left atrial internal diameter on the survival of patients with
atrial fibrillation combined with left auricular thrombus in both
groups. |
Table II
The data demonstrate the effect of
left atrial internal diameter on the survival of patients with
atrial fibrillation combined with left auricular thrombus in both
groups.
| | Group A (n=55) | Group B (n=37) | |
|---|
| Variant | Left atrial caliber
≤45 mm | Left atrial caliber
>45 mm | Left atrial caliber
≤45 mm | Left atrial caliber
>45 mm | χ2
valuea | P-value |
|---|
| Stroke [n (%)] | | | | | 6.106 | 0.013 |
|
Clogged | 34 (94.4) | 2 (5.6) | 10 (71.4) | 13 (56.5) | | |
|
Yes | 15 (78.9) | 4 (21.1) | 4 (28.6) | 10 (43.5) | | |
| |
χ2=3.073, P=0.080 | |
χ2=0.822, P=0.365 | | | |
| End up [n (%)] | | | | | 9.195 | 0.002 |
|
Clogged | 30 (93.3) | 11 (57.9) | 10 (71.4) | 10 (43.5) | | |
|
Yes | 6 (16.7) | 8 (42.1) | 4 (28.6) | 13 (56.5) | | |
| |
χ2=4.241, P=0.039 | |
χ2=2.737, P=0.098 | | | |
Stratified analysis of Groups A and B indicated that
the duration of AF significantly impacted patient survival.
Notably, a significant difference was observed between the duration
of AF and the occurrence of stroke in Group B, and an impact on the
occurrence of death in both Groups (P<0.05, Table III).
 | Table IIIInfluence of AF duration on survival
of patients with AF combined with left auricular thrombus in both
groups. |
Table III
Influence of AF duration on survival
of patients with AF combined with left auricular thrombus in both
groups.
| | Group A (n=55) | Group B (n=37) | |
|---|
| Variant | AF ≤1 year | AF >1 year | AF ≤1 year | AF >1 year | χ2
valuea | P-value |
|---|
| Stroke [n (%)] | | | | | 15.947 | <0.001 |
|
Clogged | 31 (93.9) | 18 (81.8) | 16 (94.1) | 7 (35.0) | | |
|
Yes | 2 (6.1) | 4 (18.2) | 1 (5.9) | 13 (65.0) | | |
| |
χ2=1.995, P=0.158 | |
χ2=13.654, P<0.001 | | | |
| End up [n (%)] | | | | | 15.351 | <0.001 |
|
Clogged | 28 (84.8) | 13 (59.1) | 14 (82.4) | 6 (30.0) | | |
|
Yes | 5 (15.2) | 9 (40.9) | 3 (17.6) | 14 (70.0) | | |
| |
χ2=4.615, P=0.032 | |
χ2=10.141, P=0.001 | | | |
Effect of different anticoagulants on
the survival of patients
Groups A and B were further stratified based on the
type of anticoagulant used. No statistically significant difference
was found regarding the impact of different anticoagulants on
stroke and death within Group A (P>0.05). However, in Group B,
traditional anticoagulants (warfarin) demonstrated higher survival
rates and lower stroke rates compared with NOACs (P<0.05,
Table IV).
 | Table IVA summary demonstrating the effects
of different anticoagulants on the survival of patients with atrial
fibrillation combined with left auricular thrombus in both
groups. |
Table IV
A summary demonstrating the effects
of different anticoagulants on the survival of patients with atrial
fibrillation combined with left auricular thrombus in both
groups.
| | Group A (n=55) | Group B (n=37) | |
|---|
| Variant | Traditional
anticoagulants | New
anticoagulants | Traditional
anticoagulants | New
anticoagulants | χ2
valuea | P-value |
|---|
| Stroke [n (%)] | | | | | 1.078 | 0.299 |
|
Clogged | 8 (88.9) | 41 (89.1) | 19 (82.6) | 4 (28.6) | | |
|
Yes | 1 (11.1) | 5 (10.9) | 4 (17.4) | 10 (71.4) | | |
| |
χ2=0.001, P=0.983 | |
χ2=10.804, P<0.001 | | | |
| End up [n (%)] | | | | | 1.661 | 0.198 |
|
Clogged | 6 (66.7) | 35 (76.1) | 18 (78.3) | 2 (14.3) | | |
|
Yes | 3 (33.3) | 11 (23.9) | 5 (21.7) | 12 (85.7) | | |
| |
χ2=0.352, P=0.553 | |
χ2=14.342, P<0.001 | | | |
Univariate analysis affecting patient
survival
Univariate analysis revealed that age, hypertension,
diabetes mellitus, RA, LVEF, CHADS, BNP and proBNP were significant
factors affecting stroke in patients with AF and previous thrombus
(P<0.05). Nevertheless, no statistical differences were noted
for the remaining factors (Table
V).
 | Table VThe data presenting the unifactorial
analysis of factors affecting stroke in patients with atrial
fibrillation combined with left auricular thrombus. |
Table V
The data presenting the unifactorial
analysis of factors affecting stroke in patients with atrial
fibrillation combined with left auricular thrombus.
| | Stroke | |
|---|
| Variant | Clogged (n=72) | Yes (n=20) | t/χ2
valuea | P-value |
|---|
| Age, years | 65.1±10.4 | 72.3±6.2 | 3.903 | <0.001 |
| Male [n (%)] | 47 (65.3) | 10 (50.0) | 1.550 | 0.213 |
| High blood pressure
[n (%)] | 32 (44.4) | 16 (80.0) | 7.930 | 0.005 |
| Diabetes [n
(%)] | 9 (12.5) | 13 (65.0) | 23.711 | <0.001 |
| Radiofrequency
ablation [n (%)] | 49 (68.1) | 6 (30.0) | 9.428 | 0.002 |
| Diagnostic trait [n
(%)] | | | 0.020 | 0.888 |
|
Paroxysmal | 8 (11.1) | 2 (10.0) | | |
|
Sustainability | 64 (88.9) | 18 (90.0) | | |
| LVEF (%) | 54.8±11.7 | 47.3±10.3 | 2.590 | 0.011 |
| LVEDD (mm) | 51.4±7.6 | 50.3±8.4 | 0.564 | 0.574 |
| CHADS (Point) | 2 (1,3) | 3.5 (3,5) | 3.542 | 0.001 |
| BNP (ng/l) | 580 (405,580) | 794
(769,845.5) | 1.997 | 0.049 |
| proBNP (ng/l) | 2324
(1812,2324) | 26052
(2324,2880) | 2.004 | 0.048 |
| Heart and ears open
(mm) | 19.7±4.2 | 21.1±5.9 | 1.166 | 0.247 |
| Trunnion (mm) | 28.1±4.4 | 28.2±3.6 | 0.049 | 0.961 |
| The distal part of
the auricle (mm) | 15.5±4.1 | 17.3±5.1 | 1.648 | 0.103 |
| The distal part of
the opening (mm) | 1.4±0.3 | 1.3±0.2 | 1.038 | 0.302 |
Additionally, the univariate analysis indicated that
RA, trunnion and the distal part of the auricle were significant
factors influencing death in patients with AF and left auricle
thrombus (P<0.05). However, no statistical differences were
observed for other factors (Table
VI).
 | Table VIA summary demonstrates the univariate
analysis of factors affecting death in patients with atrial
fibrillation combined with left auricular thrombus. |
Table VI
A summary demonstrates the univariate
analysis of factors affecting death in patients with atrial
fibrillation combined with left auricular thrombus.
| Variant | Existence
(n=61) | End up (n=31) | t/χ2
valuea | P-value |
|---|
| Age, years | 66.3±10.3 | 67.3±9.7 | 0.462 | 0.646 |
| Male [n (%)] | 39 (63.9) | 18 (58.1) | 0.300 | 0.584 |
| High blood pressure
[n (%)] | 29 (47.5) | 19 (61.3) | 1.557 | 0.212 |
| Diabetes [n
(%)] | 12 (19.7) | 10 (32.3) | 1.789 | 0.181 |
| Radiofrequency
ablation [n (%)] | 41 (67.2) | 14 (45.2) | 4.157 | 0.041 |
| Diagnostic trait
[n(%)] | | | 0.069 | 0.793 |
|
Paroxysmal | 7 (11.5) | 3 (9.7) | | |
|
Sustainability | 54 (88.5) | 28 (90.3) | | |
| LVEF (%) | 53.1±12.7 | 53.2±9.8 | 0.033 | 0.974 |
| LVEDD (mm) | 51.9±8.1 | 49.7±7.0 | 1.275 | 0.206 |
| CHADS (Point) | 2 (1,4) | 3 (2,4) | 1.421 | 0.159 |
| BNP (ng/l) | 580 (580,580) | 580 (580,794) | 0.122 | 0.903 |
| proBNP (ng/l) | 2324
(1900,2324) | 2324
(2324,2677) | 1.717 | 0.094 |
| Heart and ears open
(mm) | 19.5±4.2 | 20.9±5.3 | 1.461 | 0.148 |
| Trunnion (mm) | 27.5±4.4 | 29.4±3.6 | 2.093 | 0.039 |
| The distal part of
the auricle (mm) | 15.1±4.0 | 17.3±4.7 | 2.326 | 0.022 |
| The distal part of
the opening (mm) | 1.4±0.3 | 1.3±0.2 | 1.337 | 0.185 |
Multifactorial logistic regression
analysis affecting patient survival
Multivariate logistic regression analysis included
several attributes such as age, sex, hypertension, diabetes
mellitus, diagnostic characteristics, duration of AF. Using stroke
occurrence as the dependent variable (yes=‘1’, no=‘0’), factors
such as LVEF, LVEDD, CHADS, BNP, proBNP, auricular opening, the
long axis of the auricle, distal auricle, distal part of the
opening, type of anticoagulant and time to repeat ultrasound
(months) were analysed through logistic stepwise regression. The
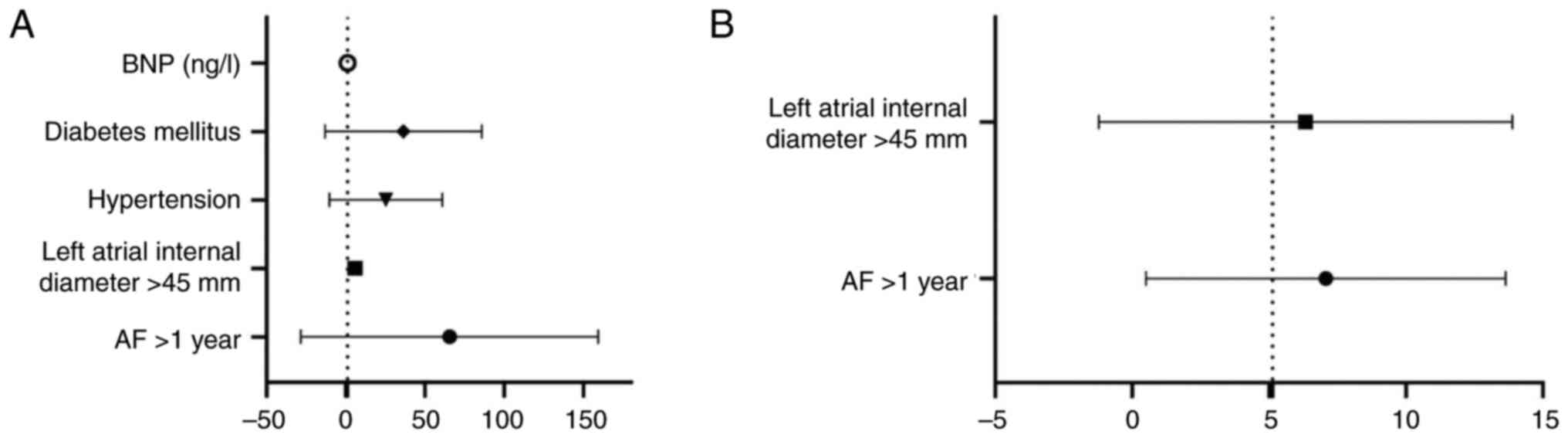
regression analysis identified that an AF of >1 year, a left
atrial internal diameter >45 mm, a history of hypertension,
diabetes mellitus and high BNP levels were significant risk factors
for stroke (P<0.05; Table VII
and Fig. 1A).
 | Table VIIThe data present the logistic
regression analysis affecting stroke in patients with AF combined
with left auricular thrombus. |
Table VII
The data present the logistic
regression analysis affecting stroke in patients with AF combined
with left auricular thrombus.
| | Univariate
regression analyses | Multivariate
regression analysis |
|---|
| Variables | B | S.E. | Wald | P | OR | 95% CI | B | S.E. | Wald | P | B | S.E. |
|---|
| AF >1 year | 3.025 | 1.086 | 7.755 | 0.005 | 20.594 | 2.45-173.12 | 1.617 | 0.536 | 9.095 | 0.003 | 5.039 | |
| Left atrial
internal diameter >45 mm | 2.092 | 0.926 | 5.109 | 0.024 | 8.101 | 1.32-49.699 | 1.634 | 0.549 | 8.849 | 0.003 | 5.126 | 1.746-15.047 |
| Hypertension | 2.137 | 1.048 | 4.159 | 0.041 | 8.474 | 1.087-66.079 | | | | | | |
| Diabetes
mellitus | 2.628 | 0.972 | 7.314 | 0.007 | 13.849 | 2.062-93.036 | | | | | | |
| Coronary heart
disease | 2.183 | 0.925 | 5.571 | 0.018 | 8.875 | 1.448-54.387 | | | | | | |
| BNP (ng/l) | 0.001 | 0.001 | 2.767 | 0.096 | 1.001 | 1.000-1.003 | | | | | | |
| Constant | -8.691 | 2.193 | 15.707 | | | | -2.034 | 0.516 | 15.563 | | | |
Similarly, multifactorial logistic regression
analysis considered factors such as age, sex, hypertension,
diabetes mellitus, diagnostic characteristics, duration of AF and
left atrial internal diameter. Using death as the dependent
variable (yes=‘1’, no=‘0’), LVEF, LVEDD, CHADS, BNP, proBNP,
auricular opening, the long axis of the auricle, distal auricle,
distal part of the opening, type of anticoagulant and time to
repeat ultrasound (months) were analysed through logistic stepwise
regression analysis. The logistic stepwise regression analysis
revealed that an AF duration >1 year and a left atrial internal
diameter >45 mm were significant risk factors for death
(P<0.05; Table VII and
Fig. 1B).
Discussion
The present study investigated the effects of RA
combined with anticoagulant therapy in patients with AF and left
atrial appendage thrombosis post-thrombolysis. The findings of the
present study demonstrated a significant reduction in the risk of
stroke and mortality when RA was combined with anticoagulant
therapy compared with anticoagulant therapy alone. Notably,
parameters such as left atrial internal diameter and duration of AF
significantly impacted mortality risk. Subgroup analyses revealed
no significant difference in survival rates between patients
treated with NOACs and those treated with warfarin. Multivariate
logistic regression identified AF duration exceeding 1 year, left
atrial internal diameter >45 mm, history of hypertension,
diabetes mellitus and elevated BNP levels as stroke risk factors.
Similarly, AF duration exceeding 1 year and left atrial internal
diameter >45 mm emerged as significant risk factors for
mortality. Therefore, considering RA in conjunction with
anticoagulants is advisable for patients with AF and left atrial
appendage thrombosis. Particularly, tailored management strategies
are necessary for individuals with prolonged AF duration and
enlarged left atrial diameter to mitigate the risks of stroke and
mortality.
Recently, RA has emerged as the primary approach for
preventing AF recurrence. Despite its recognised safety and
efficacy over the past decade, RA still carries potential risks of
thrombotic events and bleeding. A previous study reported that
~4.5% of patients with AF treated with RA may experience serious
complications, including a haemorrhage incidence of ~2.8% and
thrombotic events of ~0.94% (6).
Hence, the use of anticoagulation therapy during the perioperative
and postoperative phases of RA is recommended. Perioperative
anticoagulant administration can significantly mitigate the risks
of thrombotic events and major bleeding (7). Furthermore, the 2020 ESC guidelines
emphasise that patients with AF should receive anticoagulants for a
minimum of 2 months following RA, depending on their stroke
risk.
However, there are contrasting findings regarding
the efficacy of RA for the treatment of AF (8). A meta-analysis of 11 randomised
controlled studies revealed that, while RA improved the quality of
life in patients with AF compared with antiarrhythmic drugs, no
significant advantage was observed in terms of all-cause mortality,
stroke and transient ischaemic attack (9). By contrast, Saglietto et al
(10) conducted a meta-analysis of
nine studies, demonstrating that RA significantly reduced the risk
of heart failure-related death, stroke and hospitalisation in
patients with AF compared with medication alone. Mansour et
al (11) indicated that
patients with AF treated with RA had a lower risk of thromboembolic
events and cardiovascular hospitalisation compared with those on
antiarrhythmic medications. Similarly, Srivatsa et al
(12) found that RA was associated
with lower mortality, ischaemic stroke and haemorrhagic stroke in
patients with AF. A propensity-matched study by Saliba et al
(13) utilising a computerised
database from the largest health maintenance organisation in
Israel, revealed that radiofrequency CA was linked to reduced
stroke/TIA and mortality in patients with high CHA2DS2-Vasc scores.
Kim et al (4), utilising
the Korean National Health Insurance database, reported that RA
significantly reduced the risk of ischaemic stroke and major
haemorrhage compared with pharmacological treatment alone.
Consistent with these findings (13), the present study suggested that
combining anticoagulants with RA lowers the risk of stroke and
death compared with anticoagulants alone in patients with AF with
left atrial appendage thrombosis. This suggests that the ability of
RA to address AF at its source may reduce the recurrence of left
atrial appendage thrombosis and subsequent stroke-related deaths.
Therefore, early consideration of ablation for patients with AF is
advisable as delayed treatment may decrease the success rate of
anticoagulation.
In a previous study, thrombosis was treated using
low molecular heparin bridged with oral anticoagulants until the
international normalised ratio reached a therapeutic range of
2.0-3.0, maintained for at least 3 weeks (14). Oral anticoagulants act by
inhibiting the hepatic synthesis of certain factors in the
coagulation cascade, such as factors II, VII, IX and X. However,
sustained effective anticoagulation may not entirely prevent
thromboembolic events in patients with permanent (15) AF and generalised left atrial
thrombosis (16). A retrospective
series of 43 patients with AF and left atrial thrombosis revealed
an increased risk of cerebral embolism and/or death was observed,
even in patients under effective anticoagulant therapy with
phenprocoumon. Moreover, left atrial thrombus persisted in up to
40% of patients receiving anticoagulants (17). Notably, anticoagulant
administration appeared to be less effective in resolving large
intracardiac thrombi. Thus, careful selection of effective
anticoagulants is crucial for treating patients with AF combined
with left auricular thrombus. It was previously suggested that NOAC
might be associated with a lower incidence of stroke in the broader
AF population compared with warfarin due to fewer haemorrhagic
strokes and improved survival (18). However, the comprehensive safety
and efficacy of NOAC therapy post-cardiac surgery remain
underexplored, resulting in low adoption rates. A previous survey
conducted by the European Heart Rhythm Association across 16
centres in 14 countries/regions revealed a low and significantly
variable overall adoption of NOACs for AF post-cardiac surgery,
with 25% of centres exclusively using warfarin (19). Concerns regarding an increased risk
of haemorrhagic pericardial effusion were cited as the primary
reason for avoiding NOACs. Furthermore, bleeding complications
after cardiac surgery may occur during hospitalisation or shortly
after discharge. A study documenting the experience with 72
patients (27 on NOAC vs. 45 on warfarin) found no difference in the
incidence rates of short-term stroke or bleeding complications
based on the type of oral anticoagulation therapy (20). The results indicated a higher
survival rate with oral conventional anticoagulant (warfarin)
compared with oral NOACs, potentially attributed to complications
associated with NOACs. However, oral warfarin alone may still not
be recommended for patients with AF and atrial appendage
thrombosis.
Patients with AF and left atrial thrombus are at a
high risk of stroke and death (15), underscoring the importance of
identifying risk factors for early prevention and prognostic
improvement. Previous investigations have highlighted stroke risk
factors such as hypertension and diabetes mellitus (15). Elevated plasma BNP levels predict
the risk of ischaemic stroke within men from the general population
(HR=2.38; 95% CI=1.07-5.29). Left atrial diameter (OR: 1.13; 95%
CI: 1.07-1.19) is associated with a heightened risk of future AF
following ischaemic stroke (21).
However, the left atrial diameter assessed by M-mode
echocardiography did not predict stroke (22). Furthermore, it was found that AF
with a disease duration of >1 year and left atrial internal
diameter >45 mm were risk factors for stroke development in
patients. Notably, the risk of death was higher in patients with AF
lasting >1 year and left atrial internal diameter >45 mm.
Enhanced therapeutic management is often warranted for patients
with a combination of these risks.
In conclusion, the present study has investigated
the efficacy and adverse events associated with combining RA with
anticoagulation treatment in patients with AF and previous left
auricular thrombosis. The findings of the present study indicated
that the combination of RA with anticoagulants for treating AF with
left atrial appendage thrombosis post-thrombolysis yields improved
outcomes compared with that of anticoagulants alone. Furthermore,
factors such as AF duration (>1 year) and left atrial diameter
(>45 mm) may influence patient outcomes. By uniquely examining
the efficacy and safety of this combined treatment approach, the
present study offers valuable insights into the potential benefits
of RA in conjunction with anticoagulation therapy over
anticoagulation therapy alone. Nevertheless, there are certain
limitations to the present study. The retrospective and
non-randomised design of the present study may introduce selection
bias and hinder the establishment of causal relationships. Despite
statistical adjustments, confirmation of these findings through
randomised controlled trials (RCTs) is necessary. While the sample
size of 92 patients was adequate for the statistical analyses of
the present study, its limitation in generalizability underscores
the need for larger, multicentre studies to validate the results
obtained from the present study. Multicentre studies could provide
a more comprehensive understanding of this topic. Despite employing
multivariate logistic regression and propensity score matching,
residual confounding due to unmeasured variables remains a
possibility. Future research will focus on larger, multicentre RCTs
to corroborate the efficacy and safety of combining RA with
anticoagulation therapy. Additionally, while the therapeutic effect
of administered warfarin appeared higher for those receiving only
anticoagulants, further investigation is warranted to confirm these
results.
Acknowledgements
Not applicable.
Funding
Funding: The study was supported by The Fund of Traditional
Chinese Medicine Science and Technology Project of Shandong
Province (grant no. 2020M020).
Availability of data and materials
The data generated in the present study are included
in the figures and/or tables of this article.
Authors' contributions
YS, XH and CX designed the study, wrote the first
draft of the manuscript and conducted the statistical analysis. MZ
participated in the initial experimental design. SW performed the
data collection and took part in statistical analysis. MQ provided
critical input into the data analysis and interpretation of the
results. XL and YD participated in conception and design of the
study and revised it critically for important intellectual content.
YS, XH and CX confirm the authenticity of all the raw data. All
authors read and approved the final version of the manuscript.
Ethics approval and consent to
participate
The present study was approved (approval no.
IS22031) by the Research Ethics Committee at Shanghai Jiao Tong
University Affiliated Chest Hospital (Shanghai, China). All
procedures involving human participants conformed to the ethical
guidelines of the Declaration of Helsinki. Written informed consent
was obtained from all the patients included in the present
study.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Chew DS, Black-Maier E, Loring Z,
Noseworthy PA, Packer DL, Exner DV, Mark DB and Piccini JP:
Diagnosis-to-Ablation time and recurrence of atrial fibrillation
following catheter ablation: A systematic review and meta-analysis
of observational studies. Circ Arrhythm Electrophysiol.
13(e008128)2020.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Virani SS, Alonso A, Aparicio HJ, Benjamin
EJ, Bittencourt MS, Callaway CW, Carson AP, Chamberlain AM, Cheng
S, Delling FN, et al: Heart disease and stroke statistics-2021
update: A report from the American Heart Association. Circulation.
143:e254–e743. 2021.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Rong B, Han W, Lin M, Hao L, Zhang K, Chen
T, Sha R, Wang J, Wang R and Zhong J: Thromboembolic risk of
cessation of oral anticoagulation post catheter ablation in
patients with and without atrial fibrillation recurrence. Am J
Cardiol. 137:55–62. 2020.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Kim M, Yu HT, Kim J, Kim TH, Uhm JS, Joung
B, Lee MH and Pak HN: Atrial fibrillation and the risk of ischaemic
strokes or intracranial haemorrhages: Comparisons of the catheter
ablation, medical therapy, and non-atrial fibrillation population.
Europace. 23:529–538. 2021.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Inoue K, Hirao K, Kumagai K, Kimura M,
Miyauchi Y, Tsushima E, Ohishi M, Kimura K, Yasaka M, Yamaji H, et
al: Long-term efficacy and safety of anticoagulation after atrial
fi brillation ablation: Data from the JACRE registry. J Cardiol.
77:263–270. 2021.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Kaplon-Cieslicka A, Budnik M, Gawalko M,
Peller M, Gorczyca I, Michalska A, Babiarz A, Bodys A, Uliński R,
Żochowski M, et al: Atrial fibrillation type and renal dysfunction
as important predictors of left atrial thrombus. Heart.
105:1310–1315. 2019.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Conen D, Rodondi N, Muller A, Beer JH,
Ammann P, Moschovitis G, Auricchio A, Hayoz D, Kobza R, Shah D, et
al: Relationships of overt and silent brain lesions with cognitive
function in patients with atrial fibrillation. J Am Coll Cardiol.
73:989–999. 2019.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Yang WY, Du X, Jiang C, He L, Fawzy AM,
Wang L, Liu C, Xia SJ, Chang SS, Guo XY, et al: The safety of
discontinuation of oral anticoagulation therapy after apparently
successful atrial fibrillation ablation: A report from the Chinese
Atrial Fibrillation Registry study. Europace. 22:90–99.
2020.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Chang SS, Dong JZ, Ma CS, Du X, Wu JH,
Tang RB, Xia SJ, Guo XY, Yu RH, Long DY, et al: Current status and
time trends of oral anticoagulation use among Chinese Patients with
nonvalvular atrial fibrillation: The Chinese Atrial fibrillation
registry study. Stroke. 47:1803–1810. 2016.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Saglietto A, De Ponti R, Di Biase L, Matta
M, Gaita F, Romero J, De Ferrari GM and Anselmino M: Impact of
atrial fibrillation catheter ablation on mortality, stroke, and
heart failure hospitalizations: A meta-analysis. J Cardiovasc
Electrophysiol. 31:1040–1047. 2020.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Mansour M, Heist EK, Agarwal R, Bunch TJ,
Karst E, Ruskin JN and Mahapatra S: Stroke and cardiovascular
events after ablation or antiarrhythmic drugs for treatment of
patients with atrial fibrillation. Am J Cardiol. 121:1192–1199.
2018.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Srivatsa UN, Danielsen B, Amsterdam EA,
Pezeshkian N, Yang Y, Nordsieck E, Fan D, Chiamvimonvat N and White
RH: CAABL-AF (California Study of Ablation for Atrial
Fibrillation): Mortality and Stroke, 2005 to 2013. Circ Arrhythm
Electrophysiol. 11(e005739)2018.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Saliba W, Schliamser JE, Lavi I,
Barnett-Griness O, Gronich N and Rennert G: Catheter ablation of
atrial fibrillation is associated with reduced risk of stroke and
mortality: A propensity score-matched analysis. Heart Rhythm.
14:635–642. 2017.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Schaeffer B, Ruden L, Salzbrunn T,
Pinnschmidt HO, Akbulak RO, Moser JM, Jularic M, Meyer C, Eickholt
C, Sultan A, et al: Incidence of intracardiac thrombus formation
prior to electrical cardioversion in respect to the mode of oral
anticoagulation. J Cardiovasc Electrophysiol. 29:537–547.
2018.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Ganesan AN, Chew DP, Hartshorne T,
Selvanayagam JB, Aylward PE, Sanders P and McGavigan AD: The impact
of atrial fibrillation type on the risk of thromboembolism,
mortality, and bleeding: A systematic review and meta-analysis. Eur
Heart J. 37:1591–1602. 2016.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Hindricks G, Potpara T, Dagres N, Arbelo
E, Bax JJ, Blomström-Lundqvist C, Boriani G, Castella M, Dan GA,
Dilaveris PE, et al: 2020 ESC Guidelines for the diagnosis and
management of atrial fibrillation developed in collaboration with
the European Association for Cardio-Thoracic Surgery (EACTS): The
Task Force for the diagnosis and management of atrial fibrillation
of the European Society of Cardiology (ESC) Developed with the
special contribution of the European Heart Rhythm Association
(EHRA) of the ESC. Eur Heart J. 42:373–498. 2021.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Uzieblo-Zyczkowska B, Krzesinski P, Jurek
A, Budnik M, Gorczyca I, Kapłon-Cieślicka A, Kiliszek M, Wójcik A,
Gawałko M, Jelonek O, et al: Prevalence and risk factors of left
atrial thrombus in patients with atrial fibrillation and lower
class (IIa) recommendation to anticoagulants. Cardiovasc Diagn
Ther. 10:717–724. 2020.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Heeger CH, Rillig A, Geisler D, Wohlmuth
P, Fink T, Mathew S, Tilz RR, Reissmann B, Lemes C, Maurer T, et
al: Left atrial appendage isolation in patients not responding to
pulmonary vein isolation. Circulation. 139:712–715. 2019.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Huang J, Wu SL, Xue YM, Fei HW, Lin QW,
Ren SQ, Liao HT, Zhan XZ, Fang XH and Xu L: Association of CHADS(2)
and CHA(2)DS(2)-VASc scores with left atrial thrombus with
nonvalvular atrial fibrillation: A single center based
retrospective study in a cohort of 2695 Chinese Subjects. Biomed
Res Int. 2017(6839589)2017.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Nauffal V, Trinquart L, Osho A, Sundt TM,
Lubitz SA and Ellinor PT: Non-Vitamin K antagonist oral
anticoagulant vs warfarin for post cardiac surgery atrial
fibrillation. Ann Thorac Surg. 112:1392–1401. 2021.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Sezenoz B, Yalcin Y, Caglayan HB, Yazgan
E, Kiziltunc E, Ünlü S, Altıparmak T, Nazlıel B and Özdemir HM:
Predictive value of supraventricular short runs for new-onset
atrial fibrillation in patients with ischemic stroke. Ann Indian
Acad Neurol. 26:902–907. 2023.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Echocardiographic predictors of stroke in
patients with atrial fibrillation: A prospective study of 1066
patients from 3 clinical trials. Arch Intern Med. 158:1316–1320.
1998.PubMed/NCBI View Article : Google Scholar
|