Introduction
Giant cell tumor of the bone (GCTB) is a common
primary bone tumor that is potentially malignant and rarely
metastasizes, accounting for 20% of benign tumors analyzed by
biopsy (1). Pain is the initial
symptom of the patient, and other manifestations include soft
tissue swelling, skeletal deformity and pathological fractures.
Surgical treatment is the first choice for treating GCTB. When the
surgical plan is not feasible, other treatment methods can be
considered, such as radiotherapy, ablation therapy and embolization
therapy (2). The expression of
RANKL is closely related to that of GCTB, but its specific role is
still poorly understood. GCTB has numerous oval monocytes and
osteoclast-like multinucleated giant cells at the cellular level.
During pathological examination of the resected lesion, different
degrees of cortical bone expansion and destruction, as well as a
relatively complete periosteum, may be observed. (3) The differential diagnosis includes
non-ossifying fibroma, aneurysmal bone cyst, chondroblastoma and
cholesterol granuloma (4). GCTB
characterized by diffuse cholesterol crystal formation is rare and
is, to the best of our knowledge, described in the present case for
the first time.
Case report
A 50-year-old man underwent sigmoidectomy for colon
adenocarcinoma in April 2015. The pathological AJCC stage was
pT2aN0M0, and no treatment was administered after the operation. In
August 2020, the patient underwent surgical exploration at Xijing
Hospital (Xi'an, China) for a left femur lesion, which revealed a
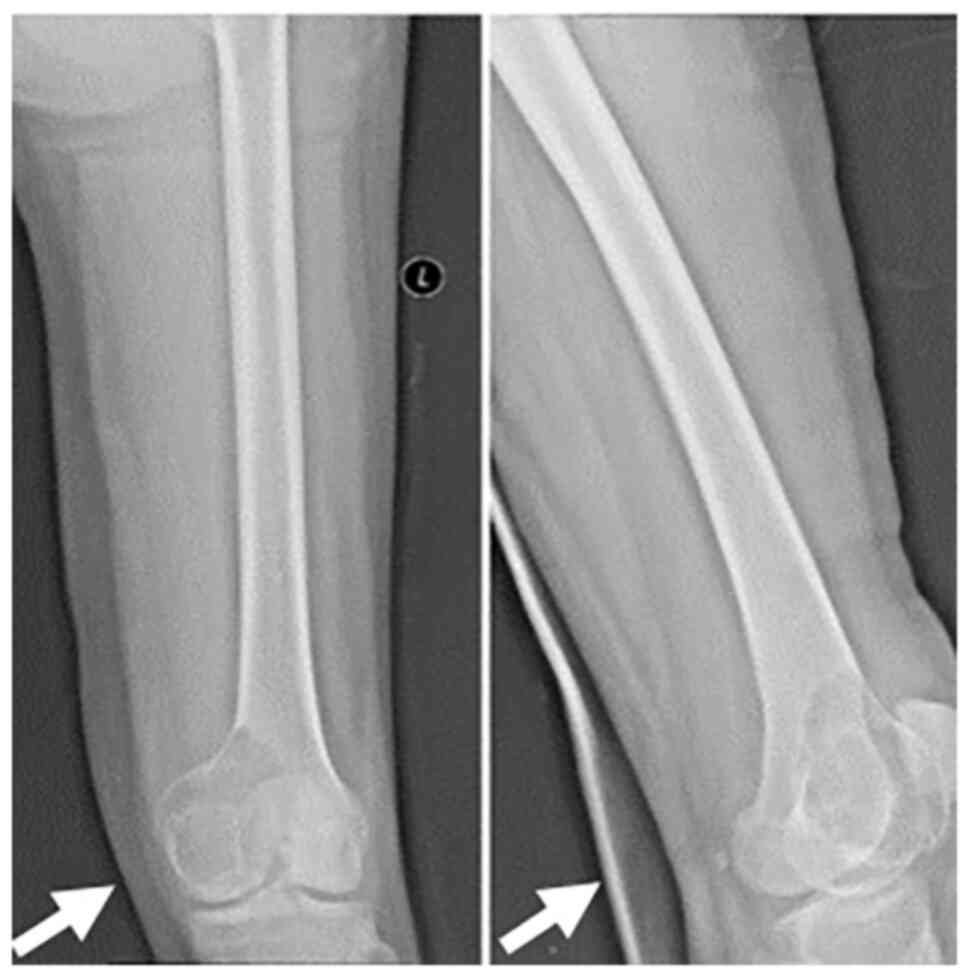
GCTB. X-ray imaging revealed a soluble destructive lesion in the
lateral condyle of the femur (Fig.
1), which was consistent with GCTB. CT revealed a large,
patchy, low-density area on the medial side of the left distal
femur, which was transversely expansive and ~7.1x5.2 cm in size.
The edge contour was blurred, and the density was uneven. Chest
X-ray and other routine examinations showed no abnormalities. The
resected specimen of the left distal femur consisted of grayish
red, grayish yellow and broken tissue that was soft and crunchy,
with a volume of 7x6x2 cm.
The specimens were fixed in 10% neutral formalin at
room temperature for 24 h, dehydrated in a conventional series of
gradient alcohols, made transparent with xylene, dipped in wax,
paraffin embedded into paraffin tissue blocks and sectioned to 4
µm. Staining with hematoxylin for 5 min at room temperature
(20-25˚C) highlighted the nucleus, while eosin staining for 2 min
highlighted the cytoplasm. After gradient dehydration and xylene
transparency, the sections were sealed using neutral resin and then
visualized using a light microscope. Immunohistochemical staining
was performed via the EnVision two-step method (5). Sections of 4-µm thickness were cut
and then immersed in a 10-mM sodium citrate buffer (pH 6) for 20
min at 97˚C for dewaxing and antigen retrieval. An appropriate
amount of 3% endogenous peroxidase blocking agent was added and
incubated at room temperature (20-25˚C) for 10 min. Blocking was
achieved with 10% bovine serum albumin (cat. no. ZLI-9027;
ZSGB-BIO) for 15 min at room temperature (20-25˚C). The following
primary antibodies were used: H3.3 G34W (clone RM263; cat. no.
31-1145-00; RevMab BioSciences), SATB2 (clone EP281; cat. No.
RMA-0750; Fuzhou Maixin Biotech, Co., Ltd.), CD163 (clone MX081;
cat. no. MAB-0869; Fuzhou Maixin Biotech, Co., Ltd.), CD68 (clone
KP1; cat. no. Kit-0026; Fuzhou Maixin Biotech, Co., Ltd.), Ki-67
(clone SP6; cat. no. RMA-0542; Fuzhou Maixin Biotech, Co., Ltd.).
Slice sections were incubated with a primary antibodies (diluted
1:100) at 37˚C for 2 h. Subsequently, the sections were incubated
with HRP-labeled goat anti-mouse IgG and goat anti-rabbit
antibodies secondary antibody (diluted 1:500; cat. no. PV6000;
ZSGB-BIO) at room temperature for 30 min for labeling (H3.3, G34W,
SATB2, CD163, CD68, Ki-67), followed by staining with DAB substrate
at room temperature (20-25˚C) for 8 min and counterstaining with
hematoxylin for 20 sec. After dehydration through a graded alcohol
series and clearing in xylene, the sections were mounted with
neutral resin. Under the light microscope (magnification, x10 and
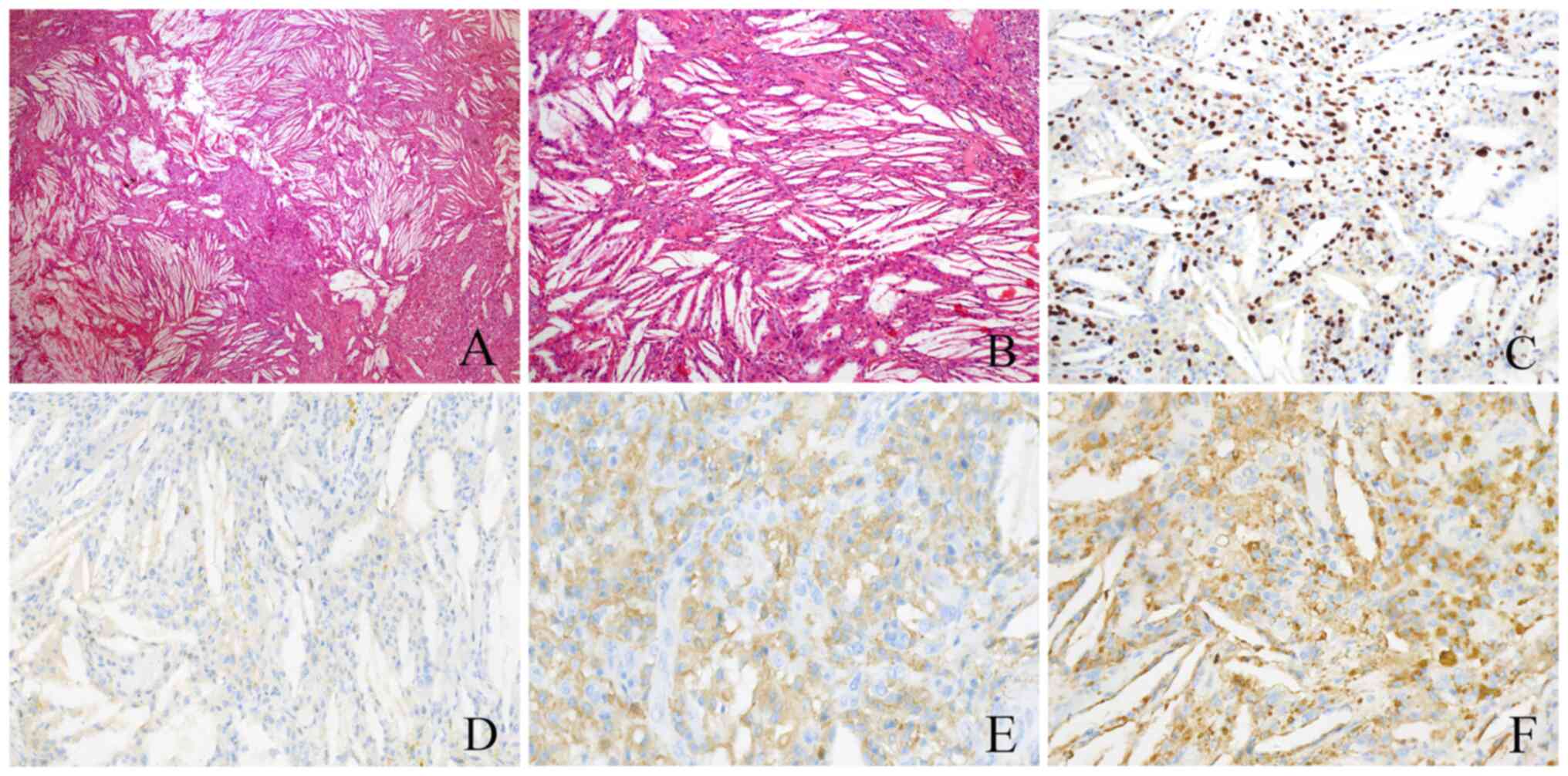
x40), the tumor cells were oval or spindle-shaped, with diffuse
cholesterol crystals (Fig. 2A) and
macrophage infiltration but no obvious osteoclast-like giant cells
(Fig. 2B). Immunohistochemistry
(IHC) showed that the tumor cells were positive for H3.3G34W
(Fig. 2C) and negative for SATB2.
The Ki-67 proliferation index was ~10%. Macrophage cells were
positive for cluster of differentiation (CD)163 (Fig. 2E) and CD68 (KP1) (Fig. 2F).
In summary, the patient was diagnosed with GCTB
(left femur) with diffuse cholesterol crystal formation. After
surgical excision treatment, the patient recovered well. During his
3-year follow-up, the patient reported that his physical condition
had remained normal without any abnormalities.
Discussion
GCTB is usually composed of numerous osteoclast-type
multinucleated giant cells and mononuclear interstitial cells.
Fibrosis, cystic degeneration, hemorrhage and hemosiderin
deposition can be observed in GCTB, which is characterized by the
uniform distribution of oval mononuclear tumor cells between large
giant cell-like osteoblasts (6).
While GCTB is a common primary bone tumor, GCTB containing fewer
multinucleated giant cells and numerous cholesterol crystals has,
to the best of our knowledge, not been previously reported in the
literature.
The genetic characteristics of GCTB include highly
periodic and specific mutations in the H3F3A gene, which encodes
histone H3.3(7). The H3F3A
mutation on chromosome 1 can be detected in 69-96% of patients with
GCTB. As a specific molecular pathological change in GCTB, the
H3F3A mutation has good specificity for distinguishing GCTB from
other giant cell bone tumors (8).
H3.3G34W mutant-specific antibodies are valuable surrogate markers
for the H3F3A genotype, aiding in the diagnosis of GCTB and its
variants (9,10). CD163 and CD68 are markers commonly
used in immunohistochemical detection to confirm the presence and
activity of macrophages. However, these two markers lack
specificity between diseases such as GCTB and chondroblastoma
(11,12). In the present case, there were no
typical histological features of GCTB, but the tumor could be
clearly identified as GCTB according to the imaging findings
combined with H3.3G34W positivity on IHC.
GCTB can be differentiated from non-ossifying
fibroma (NOF), aneurysmal bone cyst (ABC), chondroblastoma and
cholesterol granuloma (CG) as follows: i) NOF is a benign tumor
composed of spindle cells with an early onset age that often
manifests as single lesions with typical radiological features. It
is more common in the metaphysis of the mandible and exhibits round
or oval eccentric expansion, a clear boundary and a thin cortex; it
is often accompanied by a sclerotic margin on the medullary side of
the tumor and no periosteal reaction, with an irregular shape and a
fan-shaped, sclerotic margin. Microscopically, spindle cells, foam
cells and focally aggregated osteoclasts are arranged in a
spoke-like pattern. However, the genetic background is still
unclear. KRAS or FGFR1 activation mutations are often found in
sporadic cases of NOF (13-15).
GCTB is composed of neoplastic monocytes, some spindle cells and
evenly distributed multinucleated giant cells. It is mostly found
in individuals with mature bones. The incidence of GCTB before
epiphyseal closure is low. It often manifests as different degrees
of pain at the ends of the long bones of the limbs, with local
tumors and limited activity. The lesions are located at the
metaphysis end and exhibit eccentric, osteolytic and expansive bone
destruction; clear boundaries; hemosiderin deposition; and bleeding
(3).
ii) ABC is composed of fibroblasts, spindle cells,
multinucleated osteoclasts and osteoblasts and has a moderate
proliferation density. It mostly manifests as a single lesion and
causes no obvious pain. It is more likely to occur in the vertebrae
and the epiphysis of the flat shaft in adolescents (under 20 years
old) before epiphyseal closure (16). Computed tomography and magnetic
resonance imaging typically reveal multiple liquid-liquid planes,
with symmetrically distributed lesions with obvious hardened edges,
fewer solid components and less separation. Histology typically
reveals multiple blood-filled cavities separated by a capsule wall
composed of fibroblasts and osteoclasts, with small multinucleated
giant cells distributed around the capsule wall (17). Molecular pathology has shown that
patients with ABC often exhibit USP6 gene translocation (18). When GCTB occurs in older
individuals, imaging shows fewer liquid-liquid planes, clear lesion
boundaries and often complete and continuous cortical bone around
the lesion, generally with no marginal localized sclerosis. The
multinucleated giant cells of GCTB are large and evenly
distributed. During the process of diagnosis, ABCs should be
carefully distinguished from other bone cysts with cystic changes
(3).
iii) Chondroblastoma is composed of obvious
polygonal cells and occurs in individuals aged 10 to 20 years. It
is often accompanied by pain and limited activity at the site of
onset and often occurs in the epiphysis. The boundary of the lesion
is clear, showing map-like bone destruction, eccentric growth and,
commonly, calcification. The lace-like calcification area is
referred to as 'chicken wire' calcification, and is capable of
penetrating the bone cortex to form soft tissue masses and
periosteal reactions (19,20). The surrounding bone is slightly
sclerotic. Generally, T1- and T2-weighted images show enhancement
and uneven signals, with a smooth interface (21). Tumors proliferate and are formed by
chondroblast-like cells (20). The
nucleus of the tumor is lobulated, the nuclear membrane is thick,
mitotic figures are rare, there is relatively little cartilage
matrix and some multinucleated giant cells are scattered among the
cells. Immunohistochemistry shows positivity for SOX9, S100 and
discovered on GIST 1 and specific H3.3 protein mutations, with
strong, diffuse K36M expression (22). By contrast, GCTB can invade the
surrounding soft tissue and the lesions are mainly soft tissue with
uneven density. Cystic degeneration, hemorrhage and hemosiderin
deposition can be observed, but there are no calcification points
in the lesions and the number of multinucleated giant cells is
large and evenly distributed (6).
iv) CG is a benign tumor-like lesion that is common
in the middle ear or mastoid in patients with chronic
inflammation-related diseases such as cholesteatoma and otitis
media. It consists of fibrous granulation tissue filled with
cholesterol crystals, and hemosiderin deposition and keratotic
substances can appear around it (23).
GCTB is a locally invasive tumor with a high
recurrence rate. The distal femur is the most common site of this
tumor, and the treatment strategy varies according to the stage of
the tumor. Intralesional curettage is the most commonly used
treatment (24). Denosumab is a
human monoclonal antibody that inhibits RANKL and has been approved
for the treatment of adults and skeletally mature adolescents with
GCTB (25). Decreased tumor size
and calcification are observed after treatment with denosumab,
which may reduce blood loss, promote tumor resection and reduce
surgical difficulty. Denosumab is used not only for patients who
plan to undergo curettage but also for patients who plan to undergo
resection (26). The introduction
of denosumab therapy represents a milestone in the treatment of
GCTB.
In conclusion, understanding the unique histological
features of GCTB in the present case report is helpful for
pathologists to correctly diagnose GCTB and avoid misdiagnosis.
Acknowledgements
Not applicable.
Funding
Funding: This study was funded by the National Natural Science
Foundation of China (grant no. 82260319).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
CW and QY made substantial contributions to the
conception and design of the study. CW, YG, and ZZN were primarily
responsible for writing the manuscript. YG, LW, ZN, JZ and QY were
responsible for collecting the patient's clinical data and data
analysis. CW and QY confirm the authenticity of all the raw data.
All authors have read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Written informed consent was obtained from the
patient for the publication of anonymized data and any accompanying
images.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Bansal K, Singh J, Gupta P and Singh S,
Kumar R and Singh S: Giant cell tumor: A case series of seven
patients with GCT at atypical sites. J Orthop Case Rep. 13:171–179.
2023.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Todi N, Hiltzik DM and Moore DD: Giant
cell tumor of bone and secondary osteoarthritis. Heliyon.
10(e30890)2024.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Jha Y and Chaudhary K: Giant cell tumor of
bone: A comprehensive review of pathogenesis, diagnosis, and
treatment. Cureus. 15(e46945)2023.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Ratnagiri R and Uppin S: H3F3A mutation as
a marker of malignant giant cell tumor of the bone: A case report
and review of literature. J Cancer Res Ther. 19:832–834.
2023.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Xu S, Huang S, Li D, Zou Q, Yuan Y and
Yang Z: Negative expression of DSG1 and DSG2, as prognostic
biomarkers, impacts on the overall survival in patients with
extrahepatic cholangiocarcinoma. Anal Cell Pathol (Amst).
2020(9831646)2020.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Yang L, Zhang H, Zhang X, Tang Y, Wu Z,
Wang Y, Huang H, Fu X, Liu J, Hogendoorn PCW, et al:
Clinicopathologic and molecular features of denosumab-treated giant
cell tumor of bone (GCTB): Analysis of 21 cases. Ann Diagn Pathol.
57(151882)2022.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Yoshida KI, Nakano Y, Honda-Kitahara M,
Wakai S, Motoi T, Ogura K, Sano N, Shibata T, Okuma T, Iwata S, et
al: Absence of H3F3A mutation in a subset of malignant giant cell
tumor of bone. Mod Pathol. 32:1751–1761. 2019.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Leinauer B, Wolf E, Werner M, Baumhoer D,
Breining T, Luebke AM, Maas R, Schultheiß M, von Baer A,
Sufi-Siavach A, et al: H3F3A-mutated giant cell tumor of bone
without giant cells-clinical presentation, radiology and histology
of three cases. Histopathology. 79:720–730. 2021.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Amary F, Berisha F, Ye H, Gupta M,
Gutteridge A, Baumhoer D, Gibbons R, Tirabosco R, O'Donnell P and
Flanagan AM: H3F3A (Histone 3.3) G34W Immunohistochemistry: A
reliable marker defining benign and malignant giant cell tumor of
bone. Am J Surg Pathol. 41:1059–1068. 2017.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Yamamoto H, Iwasaki T, Yamada Y, Matsumoto
Y, Otsuka H, Yoshimoto M, Kohashi K, Taguchi K, Yokoyama R,
Nakashima Y and Oda Y: Diagnostic utility of histone H3.3 G34W,
G34R, and G34V mutant-specific antibodies for giant cell tumors of
bone. Hum Pathol. 73:41–50. 2018.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Behzatoglu K: Osteoclasts in tumor
biology: Metastasis and epithelial-mesenchymal-myeloid transition.
Pathol Oncol Res. 27(609472)2021.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Zheng BW, Yang ML, Huang W, Zheng BY,
Zhang TL, Li J, Lv GH, Yan YG and Zou MX: Prognostic significance
of tumor-associated macrophages in chondroblastoma and their
association with response to adjuvant radiotherapy. J Inflamm Res.
14:1991–2005. 2021.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Corsi A, Remoli C, Riminucci M, Ippolito E
and Dimitriou J: A unique case of multiple non-ossifying fibromas
with polyostotic monomelic distribution and aggressive clinical
course. Skeletal Radiol. 46:233–236. 2017.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Heitkötter B and Hartmann W:
Riesenzell-haltige tumoren des knochens und differenzialdiagnosen.
Pathologe. 43:174–182. 2022.PubMed/NCBI View Article : Google Scholar : (In German).
|
|
15
|
Khandaitkar S, Lamba G, Kolte V, Shenoi R
and Shukla D: Non-ossifying fibroma of mandible in a four-year-old
girl: A case report. Cureus. 15(e36470)2023.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Maximen J, Robin F, Tronchot A, Rossetti
A, Ropars M and Guggenbuhl P: Denosumab in the management of
aneurysmal bone cyst. Joint Bone Spine. 89(105260)2022.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Bakarman KA: Diagnosis and current
treatment of aneurysmal bone cysts. Cureus.
16(e53587)2024.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Spinnato P, Colangeli M, Pedrini E,
Parmeggiani A, Papalexis N, Crombé A, Gambarotti M and Bazzocchi A:
Aneurysmal bone cyst-like changes developed in melorheostosis with
epiphyseal osteopoikilosis. Skeletal Radiol. 53:1437–1441.
2023.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Vinoth A, Kumar DB and Manivannan S: A
novel technique of approach in a skeletally immature case of
chondroblastoma-a case report. J Orthop Case Rep. 11:67–71.
2021.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Radzinsky E, Bateni C, Theriault R, Thorpe
SW and Bindra J: A rare case of chondroblastoma involving the
distal phalanx of the ring finger. Radiol Case Rep. 18:2441–2446.
2023.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Muhammed A, Meshneb M, Saro H, Elnakib N
and Elnakib E: Management of cranial chondroblastoma in adults; a
pooled analysis. Am J Otolaryngol. 41(102486)2020.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Murphy J, Patel A, Hughes S, Rehousek P,
Drake J, Sumathi V, Botchu R and Davies AM: Bone metastases from
chondroblastoma: A rare pattern of metastatic disease in an adult.
Skeletal Radiol. 53:1219–1224. 2023.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Abdelkarim AZ, Fereir A, Elzayat AM,
Lozanoff S and Paudyal S: Cholesterol granuloma in the maxillary
sinus: A rare presentation associated with an odontogenic cyst.
Cureus. 15(e43041)2023.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Mohaidat ZM, Al-jamal HZ, Bany-Khalaf AM,
Radaideh AM and Audat ZA: Giant cell tumor of bone: Unusual
features of a rare tumor. Rare Tumors.
11(2036361319878894)2019.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Chawla S, Henshaw R, Seeger L, Choy E,
Blay JY, Ferrari S, Kroep J, Grimer R, Reichardt P, Rutkowski P, et
al: Safety and efficacy of denosumab for adults and skeletally
mature adolescents with giant cell tumor of bone: Interim analysis
of an open-label, parallel-group, phase 2 study. Lancet Oncol.
14:901–908. 2013.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Mattei TA, Ramos E, Rehman AA, Shaw A,
Patel SR and Mendel E: Sustained long-term complete regression of a
giant cell tumor of the spine after treatment with denosumab. Spine
J. 14:e15–e21. 2014.PubMed/NCBI View Article : Google Scholar
|