Introduction
The prognosis of many solid tumors has improved
substantially with the advent of immune checkpoint inhibitors
(ICIs). However, with the new treatment, new toxicities related to
the activation of the immune system have emerged, some of which can
be life-threatening. Some of these potentially serious, albeit
rare, immune-related adverse effects (irAEs) are cytokine release
syndrome (CRS) and immune effector cell-associated neurotoxicity
syndrome (ICANS). Although most frequently described in patients
with hematologic malignancies treated with chimeric antigen
receptor T (CAR-T) cell therapies (1), CRS has also been observed in some
patients treated with ICIs (2-6),
and only one report of ICANS secondary to ICIs was found (7). In the present study, the case of a
patient with resected melanoma who developed clinical symptoms of
CRS and ICANS shortly after initiating adjuvant treatment with
pembrolizumab was reported.
Case report
A 76-year-old Caucasian male patient was admitted in
November 2021 to the Doctor Peset University Hospital (Valencia,
Spain) because of general malaise, chills, arthralgias, progressive
weakness of proximal predominance over the last 10 days and
inability to walk. He also presented with dysarthria, which started
two days before admission. The patient was receiving adjuvant
treatment with anti-programmed death-1 (anti-PD1) monotherapy
pembrolizumab [200 mg every 3 weeks intravenously (iv)] for stage
IIIC melanoma resected 3 months earlier, with the last, 4th dose of
treatment administered 2 weeks before hospital admission. His
medical history was positive for arterial hypertension,
hypercholesterolemia and depressive disorder, for which he was
taking appropriate medication. There was no history of smoking or
alcohol abuse.
On physical examination, the patient was afebrile
with normal blood pressure and oxygen saturation. On initial
neurological examination, the patient was conscious but only
partially oriented, with three failures in the Pfeiffer test, was
bradypsychic, and had difficulty maintaining attention. Meningeal
signs were not observed. Campimetry by confrontation was normal, as
was the exploration of the cranial nerves. He presented with
weakness of proximal predominance in the upper and lower limbs as
well as in the neck flexors. Stretching reflexes were exalted in
the upper limbs and were muted in the lower limbs. There were no
alterations in the sensitivity or cerebellar exploration.
Additionally, on the day after admission, he started to present
with insomnia with psychomotor agitation, nocturnal confusion and
delusional ideas.
On admission, his blood count was normal, however,
C-reactive protein (CRP), aspartate transaminase (AST), alanine
transaminase (ALT), urea and creatinine levels were increased
(Table I). Hormones in the
hypothalamus-pituitary-peripheral gland axis were normal. Central
nervous system (CNS) imaging studies [computed tomography (CT) and
magnetic resonance imaging (MRI) scans] did not reveal
abnormalities nor did body CT scans. A panel of serum and
cerebrospinal fluid (CSF) onco-neuronal and surface antigen
antibodies were within normal limits. The CSF was clear and
transparent with slightly elevated lymphocytes (8/mm3)
and red blood cells (41/mm3). CSF microbiology studies
were negative. Nerve conducting studies (NCS) and electromyography
(EMG) (including single-fiber EMG) were normal. Analytical
screening for other systemic autoimmune or inflammatory processes
was also irrelevant.
 | Table IMaximum alterations of some laboratory
tests observed during two hospital admissions due to CRS and
ICANS. |
Table I
Maximum alterations of some laboratory
tests observed during two hospital admissions due to CRS and
ICANS.
| Serum parameter | Before the onset of
symptoms | 1st admission with
CRS and ICANS symptoms | 2nd admission with
CRS and ICANS symptoms |
|---|
| Creatinine (ULN: 1.20
mg/dl) | 1.29 | 2.0 | 1.59 |
| Urea (ULN: 50
mg/dl) | 42 | 113 | 92 |
| AST (ULN: 34
UI/l) | 26 | 71 | 52 |
| ALT (ULN: 55
UI/l) | 23 | 92 | 77 |
| LDH (ULN: 243
UI/l) | 188 | UNK | 336 |
| CRP (ULN: 10
mg/l) | UNK | 110 | 164 |
| Hb (LLN: 12
g/dl) | 14.1 | 11.9 | 8.5 |
Under the suspicion of immune-related myopathy and
encephalopathy, methylprednisolone at a dose of 1 mg/kg/day iv was
initiated. After 24 h, laboratory tests revealed severe
hyperglycemia without ketosis. There was a marked elevation of
anti-GAD65 antibodies, and the HBA1C level was 6.03%, suggesting
type 1 diabetes secondary to pembrolizumab. The results of the
repeated liver and renal function tests were normal. The patient
was discharged from the hospital with a tapered dose of oral
prednisone over 8 weeks and insulin therapy, with complete recovery
from the neurological symptoms that had led to the hospital
admission. Treatment with pembrolizumab was permanently
discontinued.
Two months later, while receiving a daily dose of
prednisone of 5 mg, the patient was readmitted to the hospital for
femoropopliteal deep vein thrombosis and bilateral pulmonary vein
thrombosis. Treatment with low-molecular-weight heparin was
initiated and prednisone was discontinued. A few days later, the
patient started to present with neurological alterations: a
generalized muscular weakness of proximal predominance and in the
extensors of the neck, without sensory alteration or signs of
pyramidal or cerebellar involvement. He also started to present
with symptoms of encephalopathy, being once again bradypsychic,
inattentive, disoriented in time, space, and personal
circumstances, verbose and with a language empty of content and
marked fluctuation throughout the day, with nocturnal aggravation,
and subsequently with visual hallucinations. There was also a
tremor of great amplitude, both at rest and in posture, without
myoclonus. Repeated CNS imaging and NCS once again were normal, and
electroencephalography (EEG) showed a normal path with non-specific
focal irritative signs of little persistence. Laboratory tests
showed elevation of AST, ALT, urea, creatinine, CRP and LDH as well
as rapidly progressive normochromic-normocytic anemia with no signs
of bleeding, iron or vitamin B12 deficiency, or hemolysis (Table I). There was an increased
reticulocyte count and markedly elevated ferritin level;
altogether, the analytical results were compatible with anemia from
chronic disease. Urine tests were negative for proteinuria and
hemorrhagia; consequently, immune-related nephritis was excluded.
Repeated body CT tomography scan showed no liver abnormalities.
Taken together, the patient's symptoms, laboratory
tests and imaging findings were compatible with ICANS in the
context of CRS secondary to anti-PD1 (pembrolizumab) therapy. After
restarting oral prednisone (1 mg/kg/day), there was a marked
improvement in neurological symptoms over a few days, except for
the symptoms related to encephalopathy that took longer to resolve.
Rapid normalization of hemoglobin levels and liver and kidney
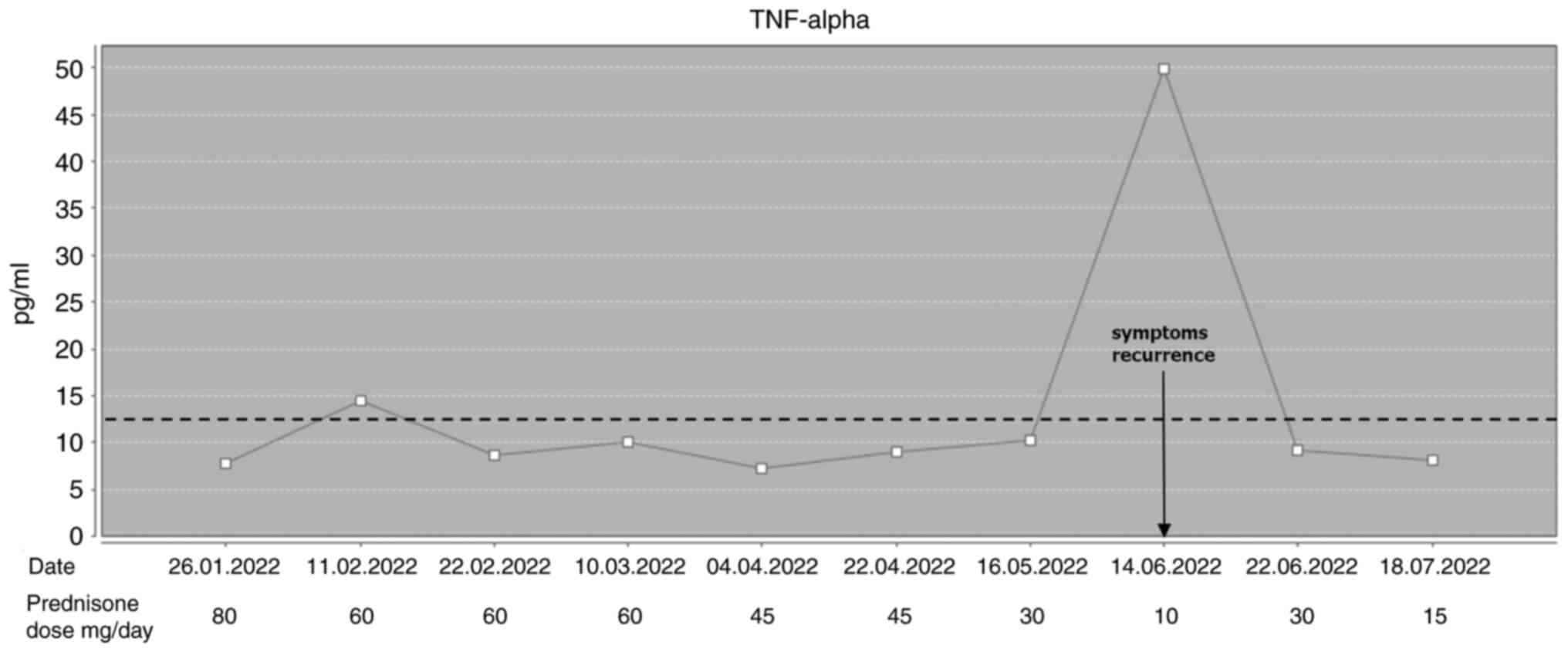
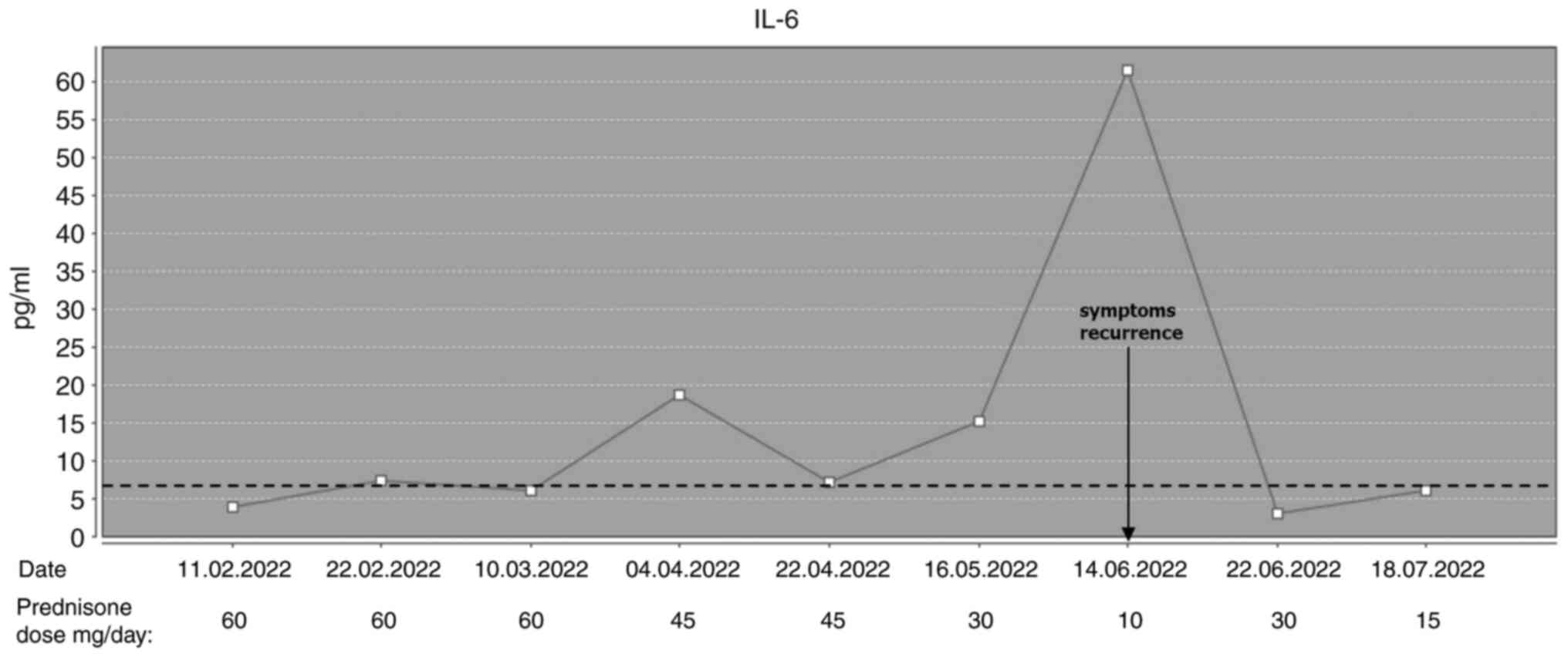
function tests were also observed. Serum cytokine levels [tumor
necrosis factor alpha (TNF-α); and interleukin-6 (IL-6)], which
were evaluated for the first time when the patient was already on
prednisone, were normal. We started to slowly taper down the dose
of steroids; however, a few months later, when the patient was on
10 mg of oral prednisone daily, both TNF-α and IL-6 levels
increased a few times above the upper limit of normal, and the
patient once again started to present symptoms of general malaise
and bradypsychia, which improved rapidly with higher doses of
prednisone (Figs. 1 and 2).
A few months later, a control CT scan showed lung,
liver, and peritoneal metastases, and anti-BRAF and anti-MEK
therapies were initiated. Unfortunately, the patient died a few
weeks later due to respiratory insufficiency secondary to a
syncytial virus infection.
Discussion
ICIs have unquestionably revolutionized the care of
patients with malignant melanoma, in both advanced disease and
adjuvant settings. However, one has to bear in mind that
immunotherapy (IT) is a ‘double-edged sword’ and irAEs can affect
practically any organ system, with some of them being potentially
life-threatening and persisting for a lifetime. Neurological irAEs
represent one of the least frequent AEs related to IT (1-5%), and
although the majority of them are low-grade, some can be fatal
(8,9). Neurological alterations or syndromes
related to ICIs described in the literature include autoimmune
encephalitis, myasthenic syndrome, myasthenia gravis,
Guillain-Barré syndrome, peripheral sensorimotor neuropathies,
posterior reversible encephalopathy syndrome, aseptic meningitis
and transverse myelitis (10).
With the advent of CAR-T cell therapies, new
toxicities have been described; some of the most serious are CRS
and ICANS (1). Initially described
as a systemic inflammatory response following treatment with an
anti-CD3 monoclonal antibody for graft rejection after solid organ
transplants, nowadays the term CRS refers to the immunological
phenomenon triggered by IT (11).
Although CRS is the most common serious AE of CAR-T cell therapy
(about one-half of patients with 10-20% experiencing severe CRS)
(12), it has also been observed,
albeit very rarely (up to 1%), in patients treated with ICIs
(2,4). CRS is a systemic inflammatory disease
characterized by a massive release of cytokines such as IFN-γ,
IL-6, IL-10, TNF-α and GM-CSF, among others (1,2,13),
with IL-6 playing a crucial role in its development (5,13).
It has been revealed that genetic variants in the il6 gene
can cause overexpression of IL-6 through the trans-signaling
pathway, potentially leading to ICI-induced CRS (6). Although higher levels of IL-6 are
generally observed in patients with more severe CRS (6), there have been described patients
with only a relatively small increase of IL-6 and with no
difference in its levels between early and advanced stage CRS
(11). On the other hand, there
are other cytokines, for example, TNF-α, whose levels usually
correlate with the severity of the syndrome (11).
Typical clinical symptoms of CRS include fever,
constitutional symptoms such as fatigue, malaise and anorexia, and
in the most severe cases, hypotension and hypoxia, accompanied by
laboratory abnormalities such as cytopenia, coagulopathy, elevated
liver enzymes and creatinine, and high CRP (2,13).
There are different CRS-related coagulopathies, ranging from mild
to severe consumptive coagulopathies to an increased risk of
thrombosis (12). Only one
publication of a case report of ICANS secondary to ICI-related CRS
and only one case series on ICI-related CRS that refers to ICANS as
the neurological toxicity of this entity were found (5,7).
Nearly all patients in these reports presented with fever as a
predominant symptom of CRS and a mild to moderate CRP elevation.
Additionally, in most of them, the laboratory findings were
positive for transaminitis, and only a minority presented with
creatinine elevation and encephalopathy or hypotension according to
the CRS severity. In a study of 25 individuals with ICI-related
CRS, CRS-related toxicities in organ systems varied from
cardiovascular (32.0%), dermatological (28.0%), gastrointestinal
(36.0%), hepatic (40.0%), neurological (16.0%), pulmonary (16.0%),
renal (16.0%) and rheumatic (24.0%) (5). Moreover, patients with more severe
CRS presented with more cardiovascular, neurologic, pulmonary and
rheumatic involvement than less severe cases. The patient in the
present case report presented with chills, fatigue and arthralgias,
but most importantly with neurological symptoms of encephalopathy.
His laboratory findings were also typical for CRS with
transaminitis, creatinine and CRP elevation. Moreover, increased
serum levels of TNF-α and IL-6 have been documented, which, in our
opinion, confirmed CRS in the patient.
ICANS, once considered to be part of CRS, is now
considered a separate entity that can occur in any immune effector
cell engaging therapy, not only CAR-T cells (1). ICANS results from endothelial
dysfunction due to cytokine release, which leads to increased
blood-brain barrier permeability and subsequent invasion of
activated lymphocytes into the CNS with laboratory findings that
may show elevated CSF leukocyte and protein counts (1). The frequency of CRS-related ICANS
depends on the CRS severity, being very rare in low-grade CRS
(0-7%) and affecting up to 45-50% of patients with high-grade CRS
(5,6).
It´s important to distinguish between immune-related
encephalitis secondary to ICIs, and ICANS which are not the same,
although they share some similarities, as both involve the immune
system and can affect the brain. Numerous cases of ICI-related
encephalitis have been described that typically present as focal
limbic or extralimibic encephalitis and meningoencephalitis, and
common symptoms include fever, headache, confusion, seizures,
memory problems and neurological deficits (14). On the other hand, ICANS typically
manifests as toxic encephalopathy, and early findings include
difficulty finding words, confusion, dysphasia, expressive aphasia,
impaired fine motor skills, dysgraphia, tremor and somnolence. In
more severe cases, seizures, motor weakness, global aphasia,
obtundation and coma have been reported (13,15).
Severe ICANS can lead to fatal intracerebral hemorrhage and
malignant cerebral edema (15).
Symptoms of ICANS are variable and can initially be vague,
therefore its diagnosis may represent a great challenge, especially
in the context of ICI therapy where this complication is extremely
rare. It was considered that the patient of the present study
suffered from ICANS and not other clinical conditions described as
complications of treatment with ICIs, such as myositis,
hypophysitis, myasthenic syndrome, or polyneuropathy. In addition,
it was assumed that he did not suffer from encephalitis either
since there were no alterations in any of the three tests
indicative of inflammatory brain involvement: MRI, EEG and CSF
studies were normal.
A workup of patients presenting with symptoms of
ICANS should include CRP, a complete metabolic panel, complete
blood counts and coagulation tests. Brain CT or MRI, as well as an
EEG, should also be performed; while their results are non-specific
for the diagnosis of ICANS, they can detect cerebral edema
(1,16).
The clinical management of CRS and ICANS depends on
the severity of the symptoms; low-grade CRS can be treated with
supportive care and antipyretics, while moderate to severe cases,
especially in patients with comorbidities and elderly, require
immunosuppressive therapy such as the IL-6R-blocking antibody
tocilizumab with or without corticosteroids (5,6).
Tocilizumab is a humanized, immunoglobin G1κ anti-human
interleukin-6 receptor monoclonal antibody that inhibits the action
of IL-6, thereby reducing the inflammatory response associated with
CRS. By binding to the IL-6 receptor, tocilizumab prevents IL-6
from activating its signaling pathways within cells, which are
responsible for the production of pro-inflammatory molecules, and
subsequently reduces the severity and duration of CRS symptoms
(17). Tocilizumab selectively
blocks the IL-6-related pathway of inflammation and unlike
corticosteroids, it does not appear to suppress T-cell function
and/or induce T-cell apoptosis, thereby preserving the efficacy of
IT (17). Despite this,
tocilizumab is underutilized in the treatment of CRS owing to
diagnostic difficulties because the initial symptoms of CRS are
non-specific and can be similar to those of sepsis or other irAEs
(4,18). Additionally, there have been
described patients with CRS and a relatively small increase of IL-6
in which other members of the IL-6 cytokine family induce this
reaction. It is important to be aware of this as IL-6 targeting
therapy may not be the optimal treatment for these patients
(11). Indeed, in one case series,
out of 6 patients with high-grade CRS treated with tocilizumab, 3
experienced fatal outcomes (5).
However, because ICANS results from the invasion of activated
lymphocytes into the CNS, it is postulated that tocilizumab has no
role in its treatment and paradoxically can even worsen its
symptoms (15). Therefore,
corticosteroids are usually used to manage this complication
because their immunosuppressive properties are necessary to quiet
down overactive immune cells that invade the CNS due to cytokine
storms (13,15). Tanaka et al (7) described a patient with advanced lung
cancer treated with nivolumab and ipilimumab who suffered severe
CRS and severe ICANS with generalized convulsions due to brain
edema; after initiating steroids, the laboratory abnormalities
improved rapidly, however, because of fever persistence, further
immunosuppressive treatment with intravenous cyclophosphamide and
immunoglobulin was required (7).
In the case of the patient of the present report, we decided to
start treatment with steroids as the most prominent symptoms that
led to various hospitalizations were neurologic and we showed that
corticosteroids are very effective in the treatment of both CRS and
ICANS; however, the duration of the treatment and the optimal dose
are yet to be established.
Finally, a concern regarding prolonged use of
steroids which may negatively impact IT efficacy in patients with
cancer remains. Numerous data showed that patients who experience
irAEs due to ICIs generally have improved outcomes compared with
patients without irAEs, although data are conflicting regarding the
type of irAEs, tumor type and ICIs schedule (19). The possibility that the prolonged
steroid use in the patient contributed to the tumor recurrence
cannot be ruled out; nevertheless, treating CRS and ICANs was the
priority as both are potentially life-threatening
complications.
In conclusion, CRS and ICANS are potential
toxicities of IT. Although most frequently observed in the
treatment of hematologic malignancies with CAR-T cells and
bi-specific T-cell engager antibodies, CRS has also been described
in some patients treated with ICIs. The case of the patient of the
present study proved that ICANS secondary to CRS may also develop
in patients with solid tumors treated with ICIs. Therefore,
clinicians must be aware of these potentially life-threatening
complications, the importance of their emergent management and most
importantly, they should always consider the risks and benefits of
any IT.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study are included
in the figures and tables of this article.
Authors' contributions
SO conceptualized the study, conducted comprehensive
literature search, analyzed and interpreted the data and wrote and
critically reviewed the manuscript. LL, DCM, AGF, CF, MDT and IMM
analyzed and interpreted the data, wrote and critically reviewed
the manuscript. SO and LL confirm the authenticity of all the raw
data. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Written informed consent to publish this report was
obtained from the patient's wife.
Competing interests
The authors declare that they have no competing
interests.
Use of artificial intelligence tools
During the preparation of this work, the authors
used AI tools in order to improve readability and language. After
using these tools, the authors reviewed and edited the content as
needed and take full responsibility for the content of the
publication.
References
|
1
|
Wesley SF, Haggiagi A, Thakur KT and De
Jager PL: Neurological immunotoxicity from cancer treatment. Int J
Mol Sci. 22(6716)2021.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Ceschi A, Noseda R, Palin K and Verhamme
K: Immune checkpoint inhibitor-related cytokine release Syndrome:
Analysis of WHO global pharmacovigilance database. Front Pharmacol.
11(557)2020.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Liu LL, Skribek M, Harmenberg U and
Gerling M: Systemic inflammatory syndromes as life-threatening side
effects of immune checkpoint inhibitors: Case report and systematic
review of the literature. J Immunother Cancer.
11(e005841)2023.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Hamida O, Karlsson F, Lundqvist A, Gerling
M and Liu LL: Cytokine release syndrome after treatment with immune
checkpoint inhibitors: An observational cohort study of 2672
patients from Karolinska University Hospital in Sweden.
Oncoimmunology. 13(2372875)2024.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Tay SH, Toh MMX, Thian YL, Vellayappan BA,
Fairhurst AM, Chan YH, Aminkeng F, Bharwani LD, Huang Y, Mak A and
Wong ASC: Cytokine release syndrome in cancer patients receiving
immune checkpoint inhibitors: A case series of 25 patients and
review of the literature. Front Immunol. 13(807050)2022.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Zhang Y, Wen X, OuYang Y, Hu Y, Fang X,
Zhang J and Yuan Y: Severe cytokine release syndrome induced by
immune checkpoint inhibitors in cancer patients-A case report and
review of the literature. Heliyon. 10(e24380)2024.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Tanaka T, Taoka M, Makimoto G, Ninomiya K,
Higo H, Fujii M, Ichihara E, Ohashi K, Hotta K, Tabata M and Maeda
Y: Severe cytokine release syndrome and immune effector
cell-associated neurotoxicity syndrome in a man receiving immune
checkpoint inhibitors for lung cancer: A case report. Intern Med.
63:1261–1267. 2024.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Kennedy LB and Salama AKS: A review of
cancer immunotherapy toxicity. CA Cancer J Clin. 70:86–104.
2020.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Haanen JBAG, Carbonnel F, Robert C, Kerr
KM, Peters S, Larkin J and Jordan K: ESMO Guidelines Committee.
Management of toxicities from immunotherapy: ESMO Clinical Practice
Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 28
(suppl_4):iv119–iv142. 2017.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Puzanov I, Diab A, Abdallah K, Bingham CO
III, Brogdon C, Dadu R, Hamad L, Kim S, Lacouture ME, LeBoeuf NR,
et al: Managing toxicities associated with immune checkpoint
inhibitors: Consensus recommendations from the Society for
Immunotherapy of Cancer (SITC) Toxicity Management Working Group. J
Immunother Cancer. 5(95)2017.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Tvedt THA, Vo AK, Bruserud Ø and Reikvam
H: Cytokine release syndrome in the immunotherapy of hematological
malignancies: The biology behind and possible clinical
consequences. J Clin Med. 10(5190)2021.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Wang J and Doran J: The many faces of
cytokine release syndrome-related coagulopathy. Clin Hematol Int.
3:3–12. 2021.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Morris EC, Neelapu SS, Giavridis T and
Sadelain M: Cytokine release syndrome and associated neurotoxicity
in cancer immunotherapy. Nat Rev Immunol. 22:85–96. 2022.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Velasco R, Villagrán M, Jové M, Simó M,
Vilariño N, Alemany M, Palmero R, Martínez-Villacampa MM, Nadal E
and Bruna J: Encephalitis induced by immune checkpoint inhibitors:
A systematic review. JAMA Neurol. 78:864–873. 2021.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Siegler EL and Kenderian SS: Neurotoxicity
and cytokine release syndrome after chimeric antigen receptor T
cell therapy: Insights into mechanisms and novel therapies. Front
Immunol. 11(1973)2020.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Rice J, Nagle S, Randall J and Hinson HE:
Chimeric antigen receptor T cell-related neurotoxicity: Mechanisms,
clinical presentation, and approach to treatment. Curr Treat
Options Neurol. 21(40)2019.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Si S and Teachey DT: Spotlight on
tocilizumab in the treatment of CAR-T-Cell-induced cytokine release
syndrome: Clinical evidence to date. Ther Clin Risk Manag.
16:705–714. 2020.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Ohira J, Kawamoto M, Sugino Y and Kohara
N: A case report of fulminant cytokine release syndrome complicated
by dermatomyositis after the combination therapy with immune
checkpoint inhibitors. Medicine (Baltimore).
99(e19741)2020.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Das S and Johnson DB: Immune-related
adverse events and anti-tumor efficacy of immune checkpoint
inhibitors. J Immunother Cancer. 7(306)2019.PubMed/NCBI View Article : Google Scholar
|