Introduction
Sintilimab, as a type of immune checkpoint inhibitor
(ICI), represents a notable advancement in the treatment of gastric
cancers. It is a fully recombinant human anti-PD1 IgG4 monoclonal
antibody (1). The adverse events
associated with the use of sintilimab include pneumonitis,
hepatitis and endocrinopathies, as well as myocarditis (2). Despite exhibiting notable treatment
efficacy, this type of agent may be associated with
life-threatening immune-related adverse events (3). Myocarditis together with myositis or
myasthenia gravis overlap syndrome (IM3OS) is one such event.
Although the majority of cases of myocarditis associated with ICI
occur in isolation, 30-40% of cases occur together with myasthenia
gravis and/or myositis (4,5). However, the treatment, clinical
presentation and outcomes remain unclear (2).
IM3OS can be associated with significant mortality
and morbidity (2). Myocarditis
induced by ICI is a life-threatening complication, with mortality
rates ranging from 25 to 50% (5,6). In
order to describe patient clinical features and characteristics,
including biomarker and laboratory data, some authors have
published individual reports, and some authors have published case
series of patients with ICI-related overlap syndrome (7,8).
However, to date, to the best of our knowledge, there have been no
reported cases of patients with gastric cancer with myasthenia
gravis and myocarditis induced by chemoimmunotherapy, who have
survived, and which also detail symptom evolution, the treatment
history and changes in laboratory indices. The present study
reports the case of patient with gastric cancer case with
myasthenia gravis and myocarditis induced by chemoimmunotherapy,
who survived.
In addition, the management strategies of IM3OS
induced by chemoimmunotherapy are full of challenges. Most
strategies are focused on the treatment of extensive cancer, and do
not depend on the cancer species. The majority of strategies
provide a treatment strategyfor a single symptom; however, they do
not provide for an overlap of symptoms, including myasthenia gravis
and myocarditis (9,10). Therefore, the clinical treatment of
the present patient was challenging. It is hoped that through our
case, similar patients can be provided a reference.
Case report
A 72-year-old male patient with no history of
autoimmune diseases was admitted to the First Hospital of China
Medical University (Shenyang, China) in June 2023 due to weakness
of the gastric wall of the cardia, gastric fundus and gastric body,
rough serosa, uneven enhancement on an enhanced scan, a local mass
protruding into the cavity and enhanced nodules around the stomach.
The greater omentum was slightly thickened, the density was
increased, and the mass could be seen as a locally diffuse
space-occupying lesion in the stomach; the involvement of the
adjacent greater omentum seemed likely; thus, a clinical and
microscopic examination was performed in combination. A gallstone
was found as a localized fatty infiltration in the head of the
pancreas. A bilateral renal cyst was also found, evidenced by left
renal local cortical atrophy. The patient was also found to have
benign prostatic hyperplasia and abdominal and pelvic effusion.
Multiple retroperitoneal lymph nodes were found. The patient had no
conditions that would prevent the use of chemotherapy, and was
administered one cycle of chemotherapy with SOX regimen in June
2023, including oxaliplatin at 180 mg D1 intravenous drip and Tigio
at 60 mgd1-d14 twice a day, once every 3 weeks. The results of gene
detection analysis of the patient suggested that microsatellite
stability, tumor cell proportion score=5% and combined positive
score=40. For the results of gene detection, the patient was
treated with the SOX regimen combined with sintilimab in the second
cycle from July 2023. Specifically, this cycle included:
Oxaliplatin 180 mg D1 via intravenous drip, Tigio at 60 mg d1-d14
was taken orally twice a day and 200 mg sintilimab (one type of ICI
which was widely used in China) was administered intravenously
every 3 weeks. At the same time, symptomatic and supportive
treatments, such as antiemetic drugs and drugs for stomach
protection were initiated.
Initially, almost no adverse events occurred in the
first cycle of treatment [chemotherapy:oxaliplatin combine
S1(Tigio)], and the patient developed fatigue G1 as a single
adverse event after the first cycle of treatment with
chemoimmunotherapy (oxaliplatin + Tigio + sintilimab). However, 3
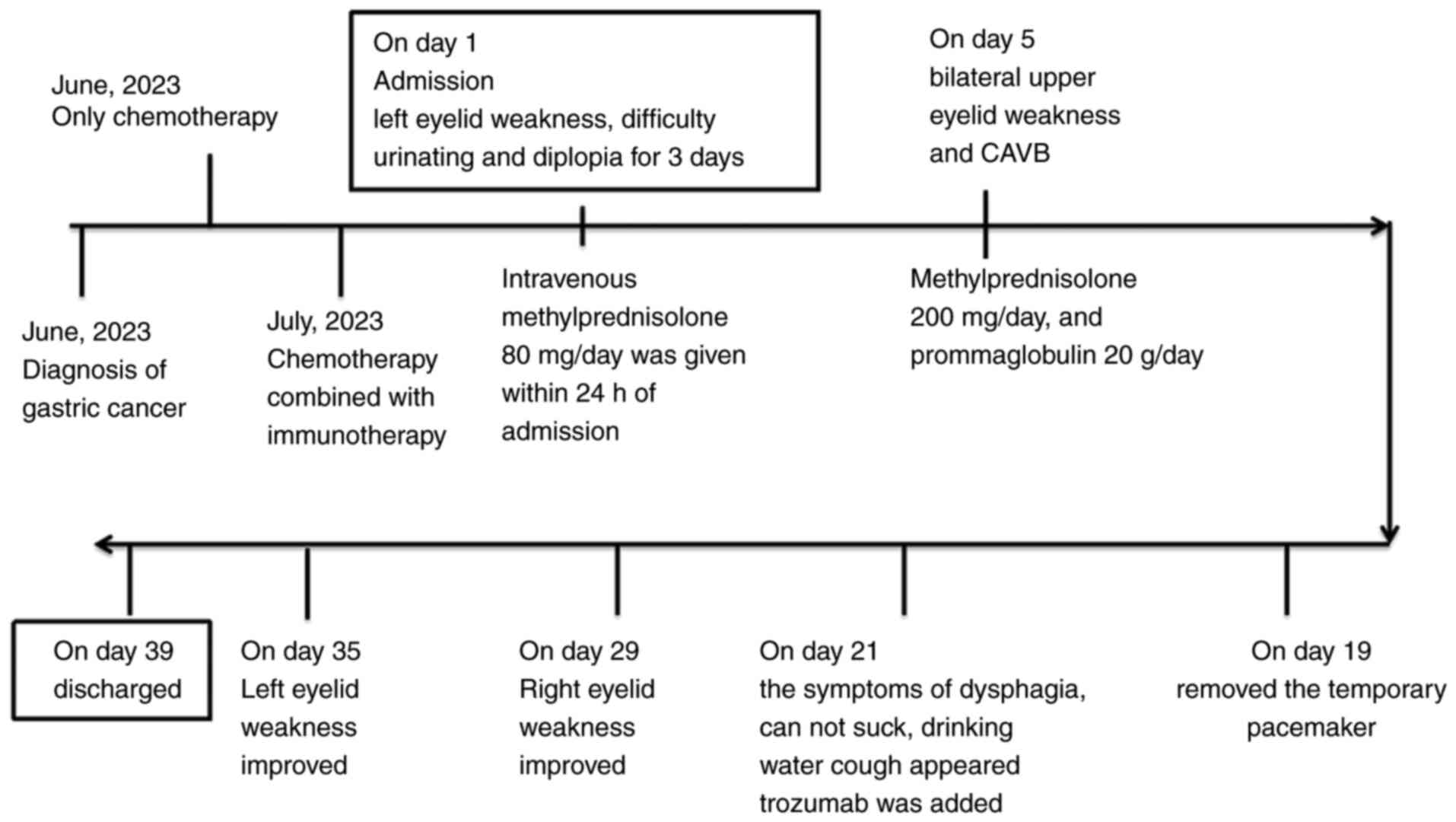
days prior to admission for the second cycle of chemoimmunotherapy,
the patient developed left eyelid weakness, difficulty urinating
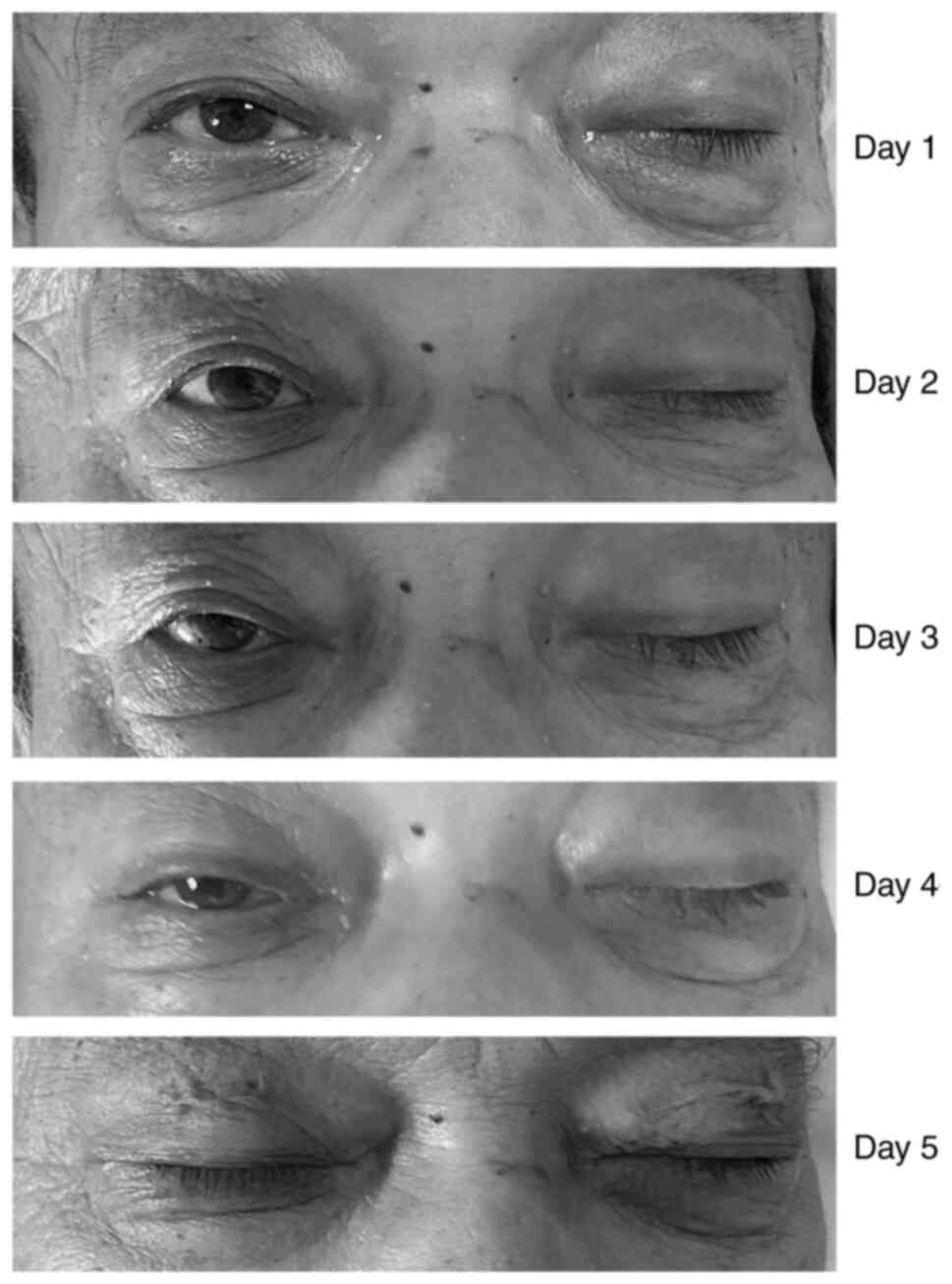
and diplopia (Fig. 1). Following
admission (August 2023), the patient was evaluated according to the
Lovett muscle strength scale (11). The muscle strength of the patient's
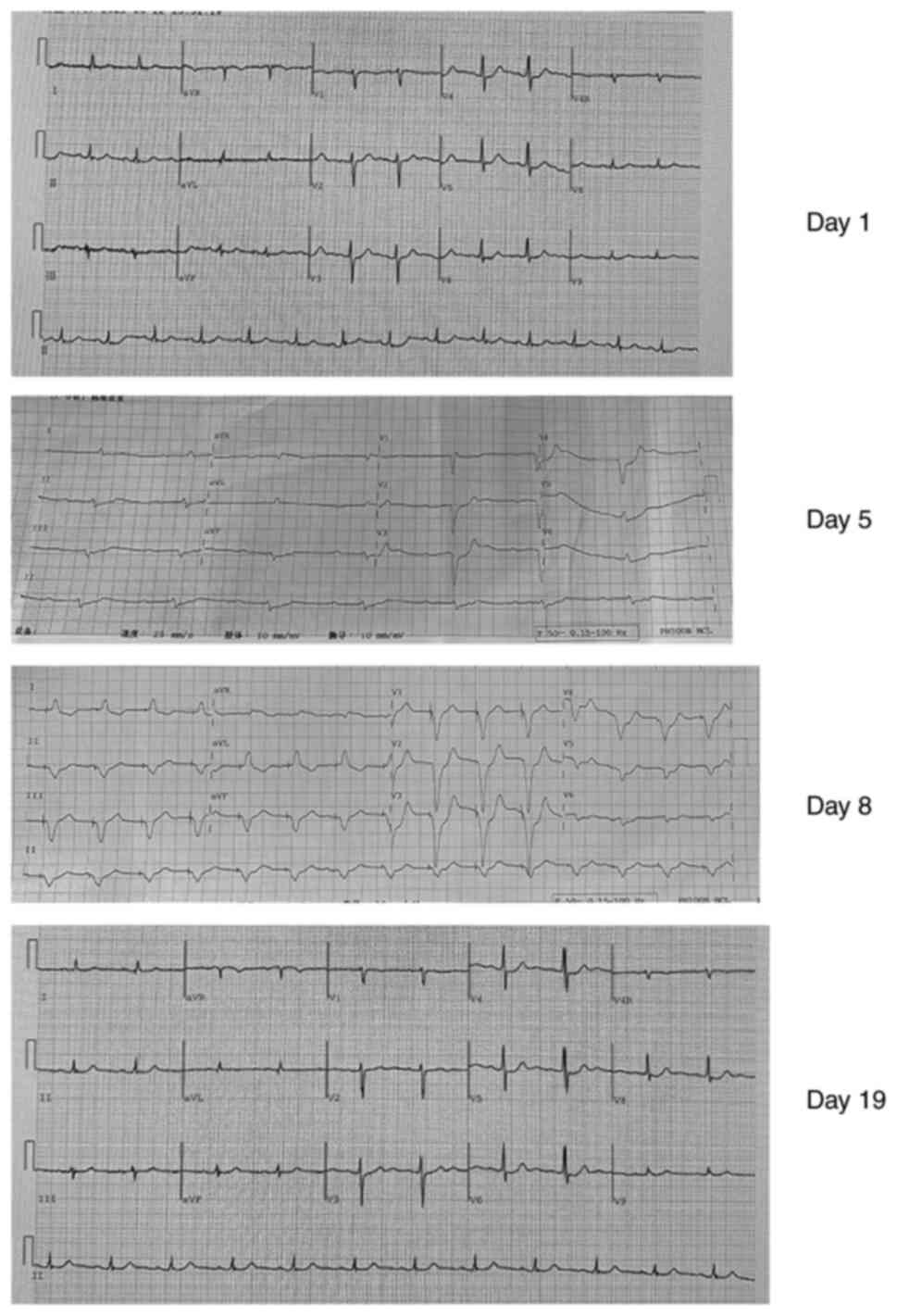
four limbs was grade V. An electrocardiogram revealed sinus
tachycardia (atrial rate 80 beats/min), complete right bundle
branch block, ST change and left axis deviation (Fig. 2). The patient had no dyspnea and
dysphagia, had an occasional mild shortness of breath and clear
articulation; his recent dietary status and sleep quality were
sufficient, he had no obvious black stool and no significant change
in body weight. Following admission, an indwelling catheter was
inserted, and related laboratory and imaging examinations were
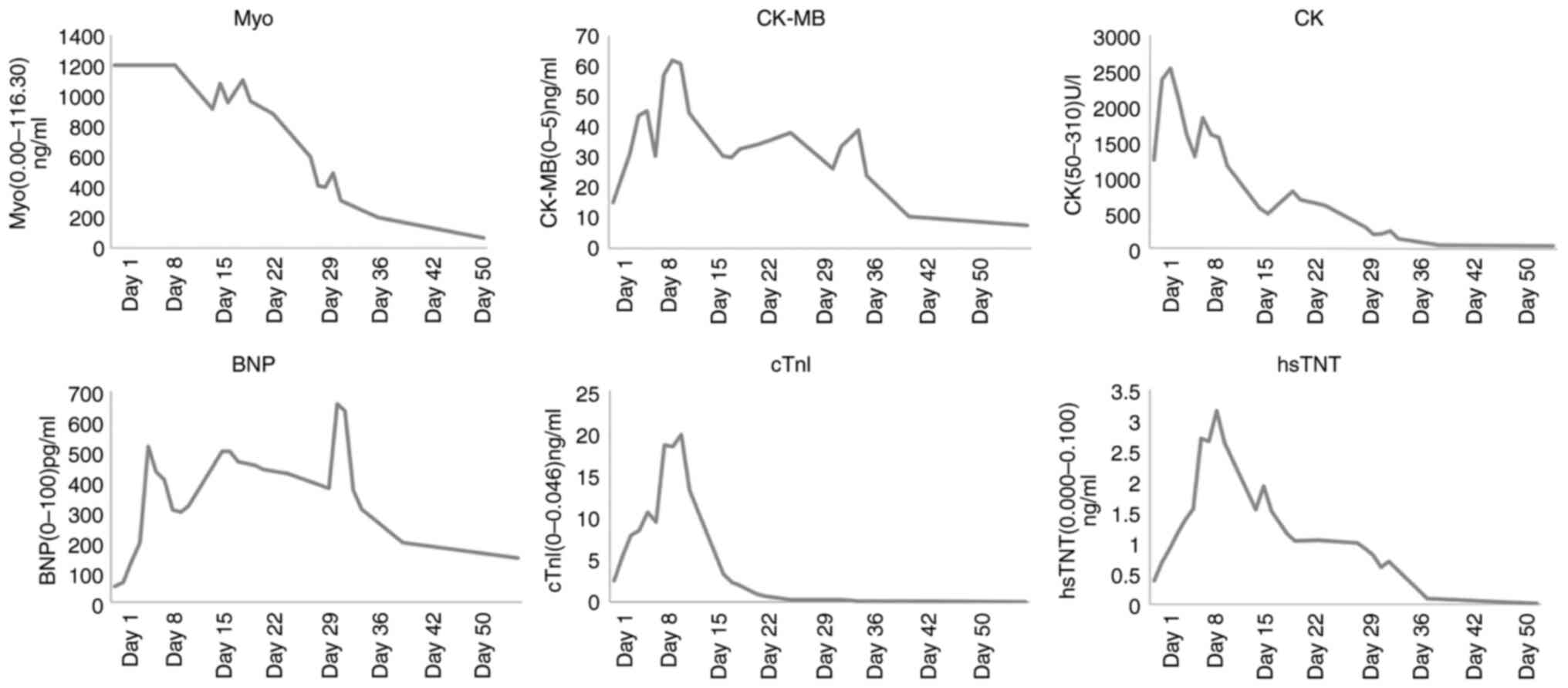
performed at the same time. The results revealed of these tests are
presented in Table I and Fig. 3. The level of creatinine kinase
isoenzyme was 15.03 ng/ml, B-type brain sodium peptide was 52
pg/ml, creatinine kinase was 1,244 U/l, high sensitivity-Troponin T
was 0.379 ng/l, cardiac troponin I was 2.460 ng/ml, lactate
dehydrogenase was 487 U/l and myoglobin was >1,200 ng/ml. The
curative effect was evaluated as stable disease (Fig. 4).
 | Table IChanges in serum cardiac indicators
during the course of the disease. |
Table I
Changes in serum cardiac indicators
during the course of the disease.
| No. of days
post-admission | CK-MB (0-5),
ng/ml | BNP (0-100),
pg/ml | CK (50-310), U/l | hs-TNT (0.000-0.100),
ng/ml | cTnl (0-0.046),
ng/ml | LDH (120-250),
U/l | Myo (0.00-116.30),
ng/ml |
|---|
| Day 1 | 15.03 | 52 | 1244 | 0.379 | 2.460 | 487 | >1200 |
| Day 2 | 23.18 | 65 | 2372 | 0.685 | 5.423 | 473 | >1200 |
| Day 3 | 31.33 | 134 | 2533 | 0.900 | 7.882 | 524 | >1200 |
| Day 4 | 43.50 | 198 | 2083 | 1.160 | 8.438 | 524 | >1200 |
| Day 5 | 45.2 | 516 | 1614 | 1.380 | 10.569 | 597 | >1200 |
| Day 6 | 30.32 | 433 | 1288 | 1.560 | 9.410 | 629 | >1200 |
| Day 7 | 56.99 | 406 | 1841 | 2.700 | 18.514 | 723 | >1200 |
| Day 8 | 61.80 | 306 | 1601 | 2.660 | 18.342 | 876 | >1200 |
| Day 9 | 60.80 | 299 | 1557 | 3.150 | 19.820 | 893 | >1200 |
| Day 10 | 44.50 | 320 | 1166 | 2.630 | 13.140 | 880 | 1143,5 |
| Day 14 | 30.50 | 500 | 568 | 1.540 | 3.285 | 639 | 911.9 |
| Day 15 | 30.00 | 501 | 490 | 1.920 | 2.329 | 600 | 1081.4 |
| Day 16 | 32.80 | 465 | 600 | 1.520 | 1.923 | 610 | 957.5 |
| Day 18 | 34.00 | 455 | 808 | 1.150 | 0.945 | 620 | 1103.5 |
| Day 19 | 35.10 | 441 | 688 | 1.030 | 0.661 | 659 | 964.1 |
| Day 22 | 38.00 | 426 | 609 | 1.040 | 0.269 | 552 | 883.2 |
| Day 27 | 26.13 | 378 | 300 | 1.000 | 0.247 | 540 | 600.0 |
| Day 28 | 33.61 | 658 | 200 | 0.900 | 0.230 | 406 | 408.3 |
| Day 29 | 36.14 | 636 | 209 | 0.800 | 0.176 | 350 | 400.2 |
| Day 30 | 38.90 | 371 | 246 | 0.600 | 0.100 | 300 | 490.0 |
| Day 31 | 23.80 | 309 | 143 | 0.700 | 0.093 | 270 | 312.8 |
| Day 36 | 10.52 | 197 | 54 | 0.100 | 0.079 | 253 | 200.0 |
| Day 50 | 7.57 | 146 | 45 | 0.010 | 0.013 | 120 | 63.8 |
The changes in clinical manifestations and treatment
following admission are as presented in Fig. 5. Upon admission (August 2023),
considering the clinical symptoms, laboratory indicators and
imaging examination results, the patient was diagnosed with
immune-associated myocarditis combined with myasthenia gravis. The
ICIs were discontinued upon admission, and intravenous
methylprednisolone at 80 mg/day, acid suppression and myocardial
protection therapy were administered within 24 h of admission. At 1
day following admission, the left eyelid weakness progressively
worsened; in addition, the patient experienced difficulty in
lifting the other eyelid (Fig. 1).
At 2 days following admission, the diplopia continued and eyelid
weakness became more severe; therefore, the methylprednisolone dose
was increased to 200 mg/day, and gammaglobulin at 20 g/day was
added; the patient then experienced difficulty in speaking and
complete atrioventricular block (CAVB) on day 5 after admission
(Fig. 2). As the patient also
experienced heart rate fluctuations between 30 and 40 beats/min, a
temporary transvenous pacemaker was inserted.
A coronary computed tomographic angiography scan
revealed no significant coronary artery stenosis. The
intraoperative physician considered that the patient had
significant myocardial edema. On day 10, the serum creatine kinase
and troponin I level steadily decreased. On day 14, the patient was
administered an intravenous injection of 80 mg methylprednisolone
sodium succinate, and the gammaglobulin dose was reduced to 20
g/day. On day 19 (2 weeks following the symptom of CAVB), an
electrocardiogram revealed an improvement in atrioventricular
conduction (as shown in Fig. 2),
and the temporary pacemaker was removed. On day 20, the patient
exhibited symptoms of dysphagia, was unable to suck and coughed
when drinking water. On day 21, the patient was treated with
trozumab (80 mg/day). On day 22, an attempt to pull out the
catheter failed. Gammaglobulin and methylprednisolone treatment was
continued on days 25-29. From days 30-31, the symptoms of right
eyelid weakness and inarticulation improved, while the symptoms of
dysphagia and an occasional episode of coughing persisted. The
myocardial index had also improved. The dose of gammaglobulin was
adjusted to 5 g/day, and methylprednisolone was continued at 80
mg/day; at the same time, the respiratory function was exercised.
On day 32 the dysphagia was attenuated. The right eyelid movement
began to improve and the patient was able to slightly open his
right eye. On day 35, the patient was able to half-open his right
eyelid and could slightly open his left eyelid. Liquid food and
intravenous nutrition were provided. The dose of piperacillin
tazobactam sodium was adjusted to 4.5 b.i.d. The urinary catheter
was also successfully removed. On day 36, there was an improvement
in all symptoms, and the dose of methylprednisolone was changed to
40 mg/day orally. On day 39, the patient was discharged. After 1
month, the levels of myocardial enzyme indices decreased to normal
levels (Table I and Fig. 3). After a period of 2 months (in
October, 2023), the patient returned to the hospital for
conventional chemotherapy, and, to date, at follow-up, there has
been no recurrence of previous symptoms.
Discussion
In recent years, ICIs have become a novel therapy
for advanced-stage tumors (12).
Sintilimab, a type of ICI, is widely used in China, and was
previously extensively used for the treatment of lung cancer or
malignant melanoma for ~2 years before it was used for the
treatment of gastric cancer. Pneumonitis, endocrinopathies,
hepatitis, gastrointestinal toxicity, myositis and dermatological
toxicity are common side-effects associated with its use (13). In recent years, cardiovascular
toxicity, as a rare immune related adverse event, has been commonly
reported (5,14). The fatality rate is as high as 67%
with combination immunotherapy and as high as 50% with monotherapy;
however, the incidence of myocarditis induced by immune therapy
ranges only from 0.09 to 1.14% (5).
It has been widely studied in numerous types of
cancer, including gastric cancer (15). However, patients with gastric
cancer presenting with immuno-related ocular type myasthenia gravis
and myocarditis, without limp weakness, without severe dyspnea, but
combined with urination difficulties and symptoms of third-degree
atrioventricular block, have not been previously reported to the
best of our knowledge. ICI-related myocarditis has been mostly
reported in patients with melanoma and lung cancer (5). Likewise, patients with lung cancer,
melanoma, or other types of cancer are the ones who most commonly
experience immune-related myasthenia gravis (16). This suggests no differences in the
incidence of ICI-related adverse events by cancer type (17). However, patients with unresectable
primary lesions or positive fecal occult blood are at a high risk
of developing gastrointestinal bleeding. Notably, there is no
consensus yet available on whether hormones can be used for such
patients, or the dose to be used, or the duration of medication and
points for attention during the course of treatment; these issues
have not been previously reported or studied and an established
theory on this matter is still lacking.
The present case presented is, to the best of our
knowledge, the first reported case of a patient with locally
advanced gastric cancer who developed immune-related myasthenia
gravis and myocarditis and survived. The patient was treated with
sintilimab together with chemotherapy with the written consent of
himself and his family. The patient was admitted to hospital due to
left eyelid weakness, difficulty urinating and diplopia at 19 days
following only one cycle of chemoimmunotherapy. The initial
symptoms were dysuria and weakness of the left eyelid, and the
clinical manifestations progressed rapidly at 1 week following
admission. A third-degree atrioventricular block occurred on 5th
day following admission; a pacemaker was thus inserted and this
pacemaker was removed on the 19th day. Coronary computed
tomographic angiography revealed no significant coronary artery
stenosis. Methylprednisolone was administered upon admission;
however, treatment the effect was not satisfactory, and the
symptoms continued progress. Gammaglobulin was added to the
treatment regimen on the 3rd day following admission. Following the
use of gammaglobulin, eyelid weakness and dysuria did not improve;
however, there was an improvement in the third-degree
atrioventricular block and the pacemaker was thus successfully
removed. For the symptoms of dysphagia, inability to suck and
coughing when drinking water, trozumab was added to the treatment
regimen on day 21. The symptoms improved on the 29th day and the
catheter was removed on the 35th day. The diagnosis of ICI-induced
myasthenia gravis and myocarditis reached following careful
consideration. For example, primary myasthenia and fatal
emergencies, such as acute and aortic dissection were excluded.
Subsequently, diseases that cause oculomotor nerve palsy were also
excluded. Considering the onset time of eyelid weakness, and the
time of sintilimab usage, ICI-associated myasthenia gravis was
highly suspected. Unfortunately, indicators of negative/positive
acetylcholine receptor antibody and EMG results were not available
for the patient. Subsequently, considering that the coronary
computed tomographic angiography revealed no significant coronary
artery stenosis, third-degree atrioventricular block caused by
acute myocardial infarction was excluded. The patient did not
express chest pain and gasping; thus, acute pulmonary embolism and
aortic dissection were excluded. The symptoms of the patient and
the examination results did not support these emergencies.
Immuno-induced myasthenia gravis combined with
myocarditis is a rare, yet critical disease. It is often overlooked
due to tumor-related symptoms. Thus, it is even more critical to
identify the predictive factors and provide early treatment for
patients. The increase in myoglobin levels may be a good predictive
factor. In the in the present study (Table I and Fig. 3), myoglobin levels reached peak
levels much earlier than other myocardial enzyme indicators and
clinical symptoms. The authors searched all similar cases on
PubMed; however, no studies reporting patterns of myoglobin and
several other myocardial enzymes were identified. To the best of
our knowledge, only three reports have mentioned specific
myocardial enzyme indices. For example, Gao et al (18) reported a patient with hs-cTnT 3,015
pg/ml, NT-proBNP 5,671 pg/ml and CK 1,419 U/l, whose main symptoms
were chest tightness, shortness of breath and the progressive
aggravation of limb weakness. Kondo et al (19) reported two cases of ICI-induced
myocarditis with CAVB with different clinical outcomes. In the
first case, who finally succumbed, the complete atrioventricular
block did not improve. By contrast, in the second case, who
survived, intravenous mPSL pulse therapy at 1,000 mg/day was used
within 24 h and the level of troponin I decreased rapidly. Wang
et al (20) presented a
report that the level of cTnI was 13.5767 ng/ml, that of α-HBD was
1,104.93 U/l and the pro-BNP level was 2,164.4 pg/ml. Following
plasmapheresis, the level of cTnI decreased to 10.4138 ng/ml and
that of pro-BNP decreased to 910.4 pg/ml. After 1 day, the cTnI
level increased to 18.0849 ng/ml and that of pro-BNP rapidly
increased to 1,227.1 pg/ml. That patient also finally succumbed.
However, the aforementioned three studies did not point out the
changing trend in myoglobin and its predictability. During the
monitoring process of clinical immunotherapy, the myoglobin index
may be improved, thus requiring testing only once a week. The
earlier application of hormones may achieve better efficacy.
The elucidation of the mechanisms of myasthenia
gravis combined with myocarditis induced by ICIs is the key for
clinical treatment. However, the mechanisms involved are currently
unclear. Some possible mechanisms have been previously mentioned,
the first one being that cardiac myocytes may share the same
targeted antigens with tumor cells (1,3). The
second is that ICI-induced myocarditis may be identical to
lymphocytic myocarditis (21).
Third, some of the reported cases containing EMB have revealed that
PD1+, CD8+ and granzyme B+
T-lymphocytes constitute the main lymphocytic infiltration
(22,23). Fourth, mouse models have revealed
that the peripheral immune tolerance may be broken by the blockade
or absence of the PD1or PDL1 axis (1,24).
These findings are consistent with those of patients with an
injured myocardium (24). As is
commonly known, ICIs weaken immune tolerance and enhance the
release of specific T-cells from tumors, thus leading to heart
tissue damage and activation (1,25).
It is worth noting that Weaver et al (26) demonstrated that an overlap syndrome
of myositis, myocarditis and myasthenia represents the most common
presentation; when one of these symptoms occurs, the other two
symptoms should also be paid more attention. Recently, the authors
reviewed The Christie Hospital (Manchester, UK) database of
immunotherapy-related toxicity from 2017 to 2020 and 10 patients
with ICI-related MG were identified (26). In their cohort, they demonstrated
good outcomes associated with early intensive immunosuppressive
treatment with IVIG and IVMP. An agreed national treatment protocol
or clinical discussion forum would be beneficial. However, the
arrhythmia was not mentioned in their study. The relationship
between arrhythmia and the aforementioned overlap syndrome is not
clear, which may need further attention.
In order to combat the adverse effects induced by
ICIs, immunosuppressive therapy is the key treatment (14). The two commonly used management
strategies are the NCCN Clinical Practice Guidelines in Oncology,
Management of Immunotherapy-Related Toxicity (9) and the Chinese Society of Clinical
Oncology immune checkpoint inhibitor related toxicity management
guidelines (10). However, both
guidelines indicate that: Immediately after the diagnosis of
immune-related side effects, 1 to 2 mg/kg/day of methylprednisolone
should be administered for hemodynamically stable patients. For
patients with myocarditis of grade 3 to 4, particularly those with
fulminant malignant arrhythmia or myocarditis, intravenous
methylprednisolone at 1,000 mg/day should be administered and
maintained for 3 to 5 days (9,10,14,23).
Nguyen et al (27) reported a patient who was
successfully treated using 1,000 mg/day methylprednisolone form the
beginning. Liang et al (25) also reported a patient treated with
160 mg/q8 h methylprednisolone for 5 days. If patients do not
respond well to hormone shock, tacrolimus, azathioprine,
methotrexate and mycophenolate mofetil may be used as
immunomodulatory chemical drugs; infliximab, rituximab,
ruxolitinib, abatacept and tocilizumab may be used as biological
agents; and antithymocyte globulin as immunoglobulin (9,10,14,21).
Moreover, lymphocyte depletion, plasmapheresis and ECLS could also
be proposed (21). The common
hemodynamic and respiratory support treatment could also be
employed at the same time (14).
However, the patients in the aforementioned studies
mainly consisted of patients with renal cancer, melanoma, lung
cancer and colorectal cancer, but not gastric cancer. Although
these patients did not have gastric cancer, they are cancer
patients who received immunochemotherapy before, and they incurred
side effects caused by immunochemotherapy, including both of
myocarditis and myasthenia gravis.
Komatsu et al (28) reported the first metastatic gastric
cancer case of nivolumab-related myasthenia gravis and myocarditis;
despite plasma exchange, methylprednisolone and immunoglobulin
administration, the patient finally succumbed. When comparing the
case in the present study with others, the patient was administered
immunosuppressive therapy with methylprednisolone at 200 mg/day,
gammaglobulin (20 g/day) and trozumab (80 mg/day). For hormone
resistance, gammaglobulin total dosing should be 2 g/kg,
administered in divided doses per package insert (11,23).
Other immunosuppressive agents are also available such as
Alemtuzumab, but the dosage is not mentioned (23).
Trozumab as an immunosuppressant, is recommended in
the CSCO guidelines (24), but for
the dosage is also not clear. Therefore, the present study used the
clinical empirical safe dosage of gammaglobulin and tuzumab:
Gammaglobulin (20 g/day) and trozumab (80 mg/day). Although these
two doses are safe and within the scope of instructions, whether it
is the most reasonable amount of immune-related toxicity therapy is
still a question worth further study. Although this the present
patient survived, it is not known which one of these three types of
medicine was the key and considering that patients with gastric
cancer have a higher risk of gastrointestinal bleeding compared
with other tumors, intravenous methylprednisolone at 1,000 mg/day
was not initiated. It remains unknown whether trozumab can be used
in advance and the reasonable withdrawal time of hormones; it is
hoped that there will be more research data to guide the treatment.
Moreover, it continues to remain unclear whether tracing the
medical history of patients, previous health conditions or regular
exercise may prove useful, or whether long-term exercise is
associated with increased immunity, whether there is any
association between the lymphocyte count and the side-effects of
ICIs. It is hoped that further research will shed light on this
matter in the future.
There is a lack of clinical data on the long-term
prognosis of such patients as they are often lost to follow-up or
due to mortalities in the short term. Although, with the wide
application of immunosuppressants, some researchers have reported a
series of related side reactions. A single-center experience with
systematic literature review and meta-analysis pointed out that
most of the patients died in the short term due to critical illness
(29). There is no prospective
study to follow up such patients, and even the present case is the
first reported case of a patient with locally advanced gastric
cancer who developed immune-related myasthenia gravis and
myocarditis and survived. Weaver et al (26) reported on 10 patients with
ICI-related MG and myocarditis. Among the 10 patients, none of the
patients died within 30 days of initial presentation. One patient
died in the ICU at 6 months post initial presentation, while eight
patients were still alive. Subsequently, one patient died from
progressive cancer. At 12 months, a further four patients had died
(2 due to progressive cancer, 1 due to sepsis and 1 due to
aspiration pneumonia following relapsed myasthenia gravis). None of
the patients were re-challenged with ICI therapy. However, unlike
in the present study, none of these patients were noted to have
cardiac arrhythmia.
In conclusion, the present study reports a rare case
of patient with locally advanced gastric cancer who developed
immuno-related myasthenia gravis and myocarditis, who survived. The
present case report demonstrates the importance of improving the
current understanding of ICI-induced myasthenia gravis with
concomitant myocarditis following the administration of sintilimab
in order to improve clinical outcomes. The predictive value of
myoglobin in this disease warrants further research. There are a
number of urgent problems to solve, such as the few management
strategies for chemoimmunotherapy-induced myasthenia gravis and
myocarditis in locally advanced gastric cancer and the long-term
follow-up and care of such patients.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
YuW, FL, YiW, QW and SD made substantial
contributions to conception and design of the manuscript. XW and ZS
contributed to acquisition and interpretation of data, and wrote
the abstract. YuW and FL confirm the authenticity of all the raw
data. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
The patient provided written informed consent for
the publication of their data.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Wang C, Zhong B, He J and Liao X: Immune
checkpoint inhibitor sintilimab-induced lethal myocarditis
overlapping with myasthenia gravis in thymoma patient: A case
report. Medicine (Baltimore). 102(e33550)2023.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Ye Z, Yang W, Xuan B, Li X, He J, Si H and
Ma W: Efficacy and safety evaluation of sintilimab for cancer
treatment: A systematic review and meta-analysis of randomized
controlled trials. Front Pharmacol. 13(895187)2022.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Pathak R, Katel A, Massarelli E, Villaflor
VM, Sun V and Salgia R: Immune checkpoint inhibitor-induced
myocarditis with myositis/myasthenia gravis overlap syndrome: A
systematic review of cases. Oncologist. 26:1052–1061.
2021.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Aldrich J, Pundole X, Tummala S, Palaskas
N, Andersen CR, Shoukier M, Abdel-Wahab N, Deswal A and
Suarez-Almazor ME: Inflammatory myositis in cancer patients
receiving immune checkpoint inhibitors. Arthritis Rheumatol.
73:866–874. 2021.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Moslehi JJ, Salem JE, Sosman JA,
Lebrun-Vignes B and Johnson DB: Increased reporting of fatal immune
checkpoint inhibitor-associated myocarditis. Lancet.
391(933)2018.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Mahmood SS, Fradley MG, Cohen JV, Nohria
A, Reynolds KL, Heinzerling LM, Sullivan RJ, Damrongwatanasuk R,
Chen CL, Gupta D, et al: Myocarditis in patients treated with
immune checkpoint inhibitors. J Am Coll Cardiol. 71:1755–1764.
2018.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Kadowaki H, Akazawa H, Ishida J and Komuro
I: Mechanisms and management of immune checkpoint inhibitor-related
cardiac adverse events. JMA J. 4:91–98. 2021.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Zhang L, Reynolds KL, Lyon AR, Palaskas N
and Neilan TG: The evolving immunotherapy landscape and the
epidemiology, diagnosis, and management of cardiotoxicity: JACC:
CardioOncology primer. JACC CardioOncol. 3:35–47. 2021.PubMed/NCBI View Article : Google Scholar
|
|
9
|
The NCCN Clinical Practice Guidelines in
Oncology. Management of Immunot therapy-Related Toxicity. Version
2.2023.[2023-05-09]. Journal.
|
|
10
|
The Chinese Society of Clinical Oncology
immune checkpoint inhibitor related toxicity management guidelines
2023. http://www.csco.org.cn/cn/index.aspx. Journal.
|
|
11
|
Andrejeva J, Grisanina A, Sniepienė G,
Mockiene A and Strazdauskaite D: The effect of TRX suspension
trainer and BOSU platform after reconstruction of anterior cruciate
ligament of the knee joint. Pedagogy Phys Cult Sports. 26:47–56.
2022.
|
|
12
|
Guven DC, Stephen B, Sahin TK, Cakir IY,
Erul E and Aksoy S: The efficacy of immune checkpoint inhibitors in
rare tumors: A systematic review of published clinical trials. Crit
Rev Oncol Hematol. 174(103700)2022.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Chen Y, Chen T, Zhu W, Li L, Fang C and
Zhang H: Rare primary intrapulmonary malignant peripheral nerve
sheath tumor showing significant response to sintilimab: A case
report and literature review. Oncol Lett. 28(423)2024.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Schneider BJ, Naidoo J, Santomasso BD,
Lacchetti C, Adkins S, Anadkat M, Atkins MB, Brassil KJ, Caterino
JM, Chau I, et al: Management of immune-related adverse events in
patients treated with immune checkpoint inhibitor therapy: ASCO
guideline update. J Clin Oncol. 39:4073–4126. 2021.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Gong J, Chehrazi-Raffle A, Reddi S and
Salgia R: Development of PD-1 and PD-L1 inhibitors as a form of
cancer immunotherapy: A comprehensive review of registration trials
and future considerations. J Immunother Cancer. 6(8)2018.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Suzuki S, Ishikawa N, Konoeda F, Seki N,
Fukushima S, Takahashi K, Uhara H, Hasegawa Y, Inomata S, Otani Y,
et al: Nivolumab-related myasthenia gravis with myositis and
myocarditis in Japan. Neurology. 89:1127–1134. 2017.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Shimozaki K, Sukawa Y, Beppu N, Kurihara
I, Suzuki S, Mizuno R, Funakoshi T, Ikemura S, Tsugaru K, Togasaki
K, et al: Multiple immune-related adverse events and anti-tumor
efficacy: Real-world data on various solid tumors. Cancer Manag
Res. 12:4585–4593. 2020.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Gao L, Li X, Guo Z, Tang L, Peng J and Liu
B: Immune checkpoint inhibitor-induced myocarditis with myasthenia
gravis overlap syndrome: A case report and literature review.
Medicine (Baltimore). 101(e32240)2022.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Kondo H, Kirigaya J, Matsuzawa Y and Hibi
K: Two cases of immune checkpoint inhibitor-induced myocarditis
with complete atrioventricular block. Cureus.
15(e36446)2023.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Wang DY, Salem JE, Cohen JV, Chandra S,
Menzer C, Ye F, Zhao S, Das S, Beckermann KE, Ha L, et al: Fatal
toxic effects associated with immune checkpoint inhibitors: A
systematic review and meta-analysis. JAMA Oncol. 4:1721–1728.
2018.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Nguyen LS, Bretagne M, Arrondeau J, Zahr
N, Ederhy S, Abbar B, Pinna B, Allenbach Y, Mira JP, Moslehi J, et
al: Reversal of immune-checkpoint inhibitor fulminant myocarditis
using personalized-dose-adjusted abatacept and ruxolitinib: Proof
of concept. J Immunother Cancer. 10(e004699)2022.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Sobol I, Chen CL, Mahmood SS and Borczuk
AC: Histopathologic characterization of myocarditis associated with
immune checkpoint inhibitor therapy. Arch Pathol Lab Med.
144:1392–1396. 2020.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Zhong P, Zhang C, Guan H, Yan J, He M and
Zhou X: Myocarditis and myasthenia gravis induced by immune
checkpoint inhibitor in a patient with relapsed thymoma: A case
report. Clin Case Rep. 11(e7039)2023.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Wei SC, Meijers WC, Axelrod ML, Anang NAS,
Screever EM, Wescott EC, Johnson DB, Whitley E, Lehmann L, Courand
PY, et al: A genetic mouse model recapitulates immune checkpoint
inhibitor-associated myocarditis and supports a mechanism-based
therapeutic intervention. Cancer Discov. 11:614–625.
2021.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Liang S, Yang J, Lin Y, Li T, Zhao W, Zhao
J and Dong C: Immune myocarditis overlapping with myasthenia gravis
due to anti-PD-1 treatment for a chordoma patient: A case report
and literature review. Front Immunol. 12(682262)2021.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Weaver JM, Dodd K, Knight T, Chaudhri M,
Khera R, Lilleker JB, Roberts M, Lorigan P and Cooksley T: Improved
outcomes with early immunosuppression in patients with
immune-checkpoint inhibitor induced myasthenia gravis, myocarditis
and myositis: A case series. Support Care Cancer.
31(518)2023.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Nguyen BHV, Kuo J, Budiman A, Christie H
and Ali S: Two cases of clinical myasthenia gravis associated with
pembrolizumab use in responding melanoma patients. Melanoma Res.
27:152–154. 2017.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Komatsu M, Hirai M, Kobayashi K, Hashidate
H, Fukumoto J, Sato A, Usuda H, Tanaka K, Takahashi K and Kuwabara
S: A rare case of nivolumab-related myasthenia gravis and
myocarditis in a patient with metastatic gastric cancer. BMC
Gastroenterol. 21(333)2021.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Hamada N, Maeda A, Takase-Minegishi K,
Kirino Y, Sugiyama Y, Namkoong H, Horita N, Yoshimi R and Nakajima
H: YCU irAE Working Group. Incidence and distinct features of
immune checkpoint inhibitor-related myositis from idiopathic
inflammatory myositis: A single-center experience with systematic
literature review and meta-analysis. Front Immunol.
12(803410)2021.PubMed/NCBI View Article : Google Scholar
|