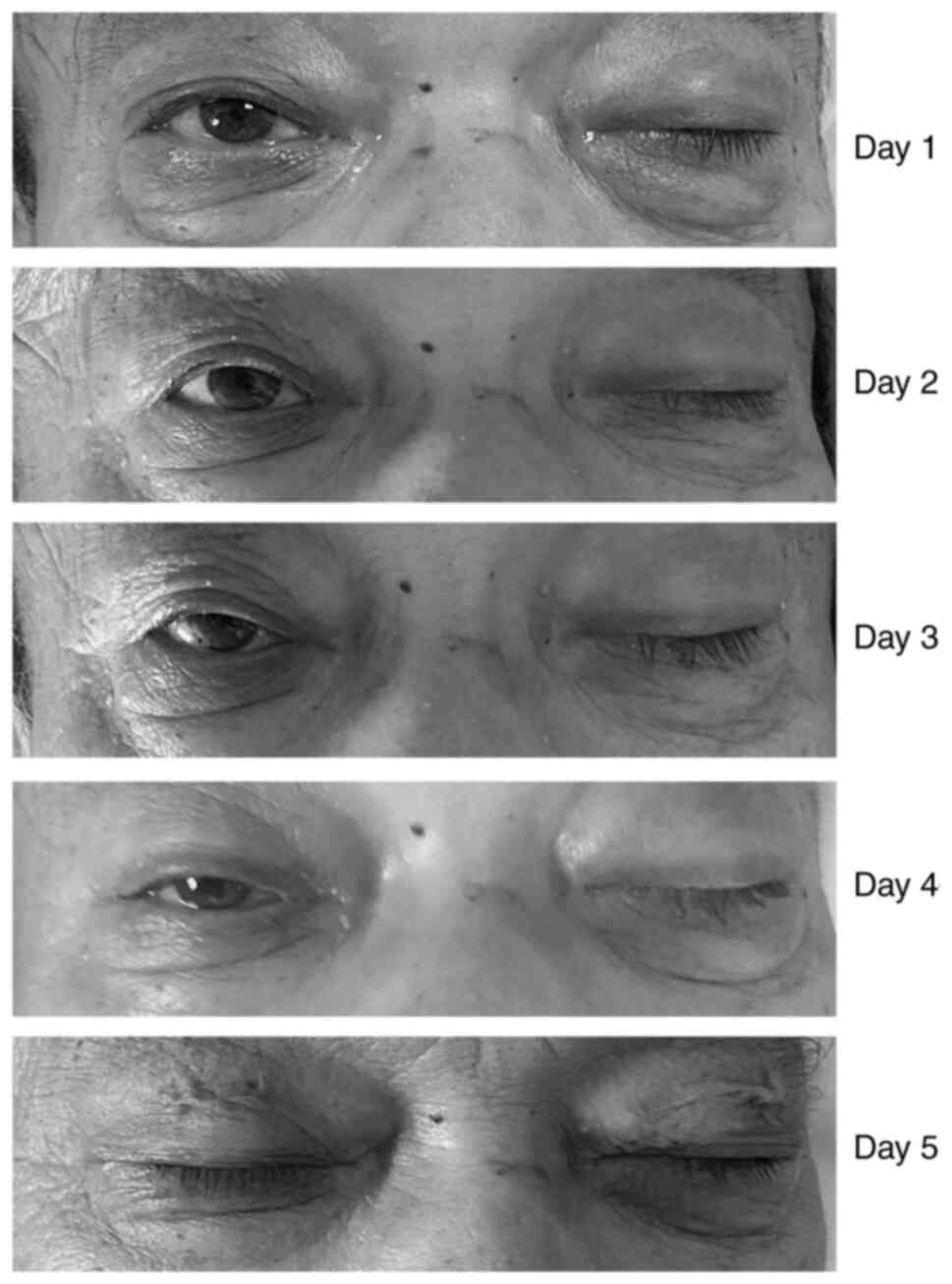
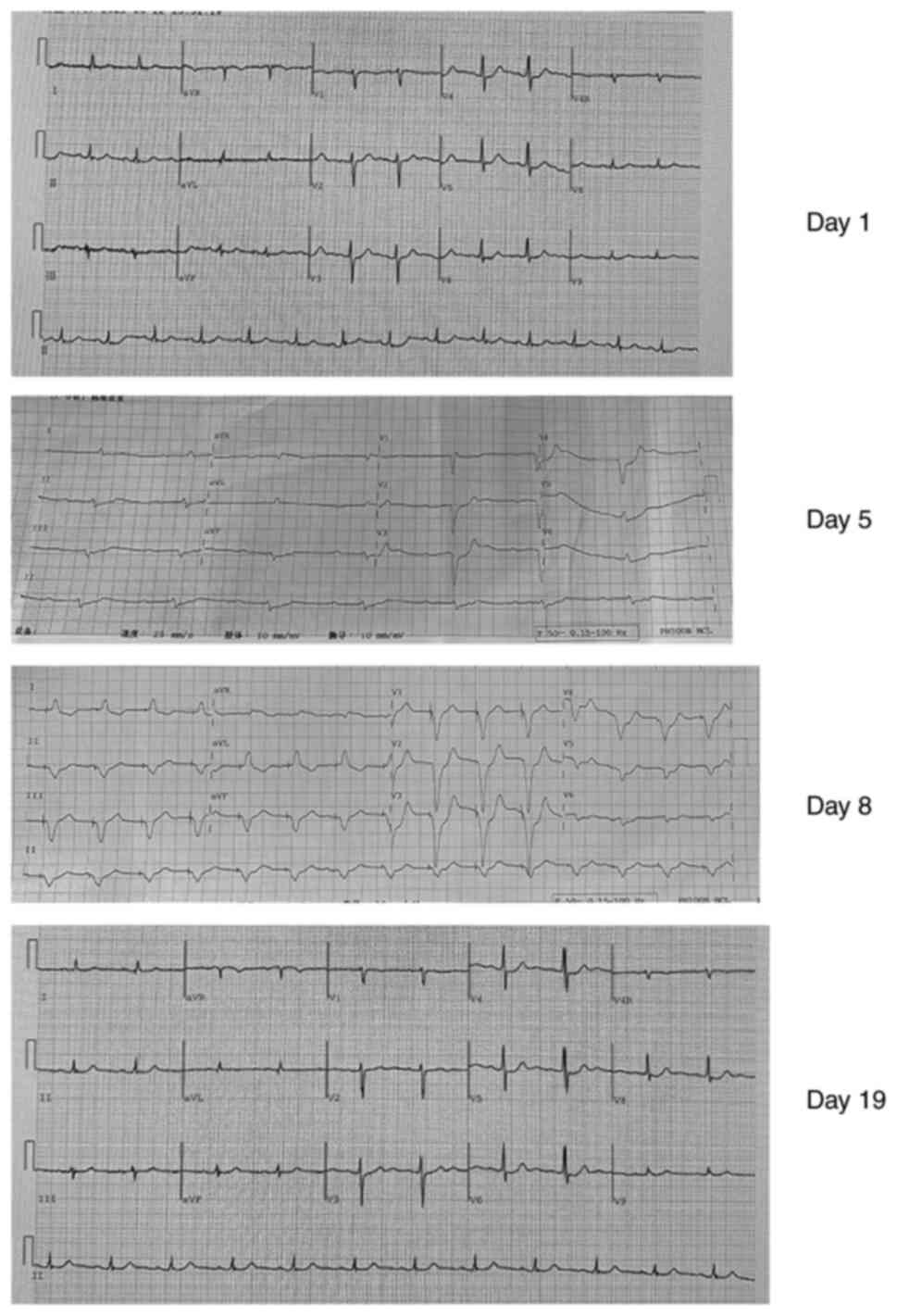
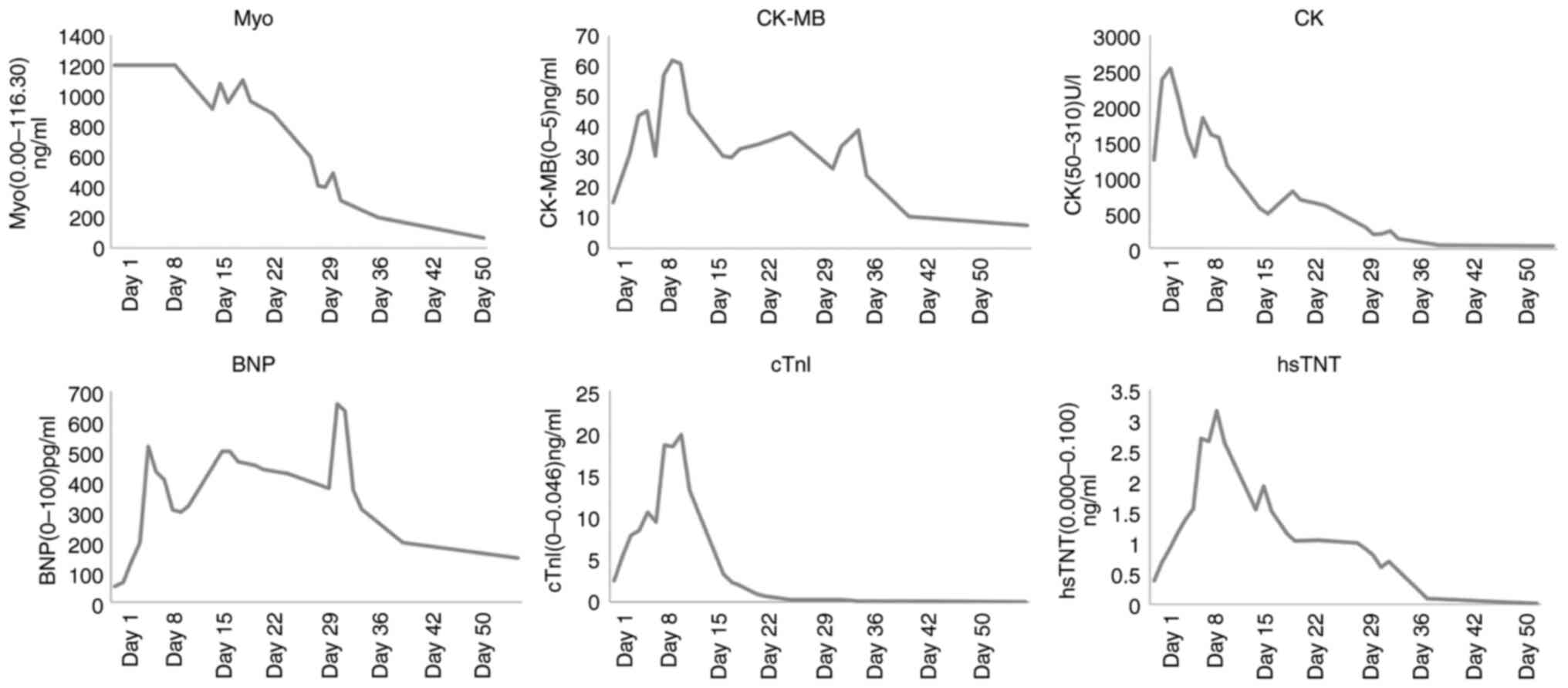
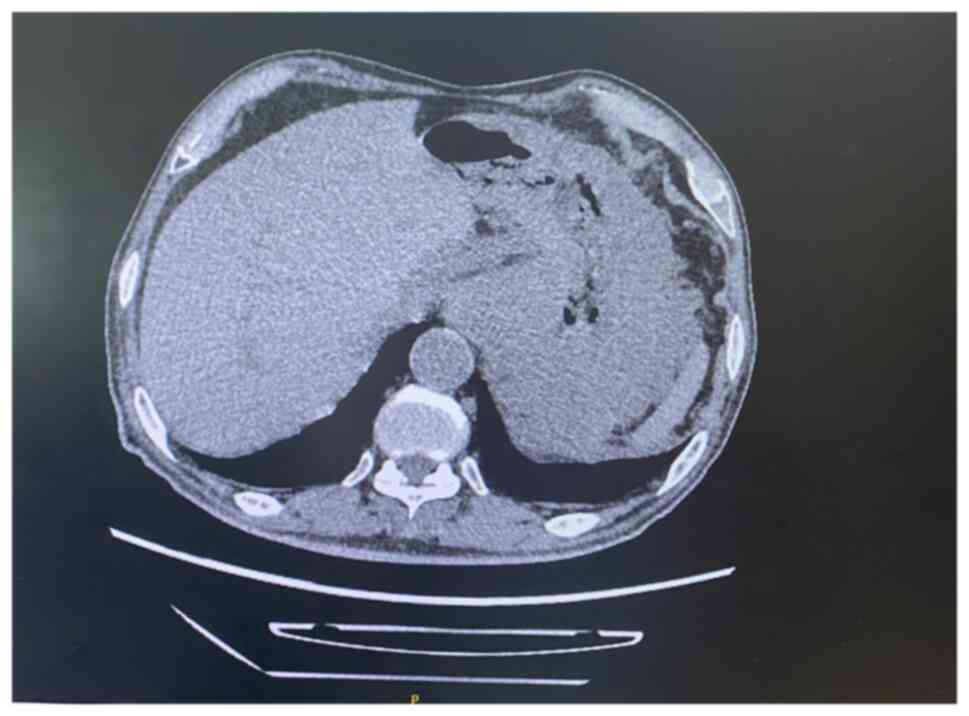
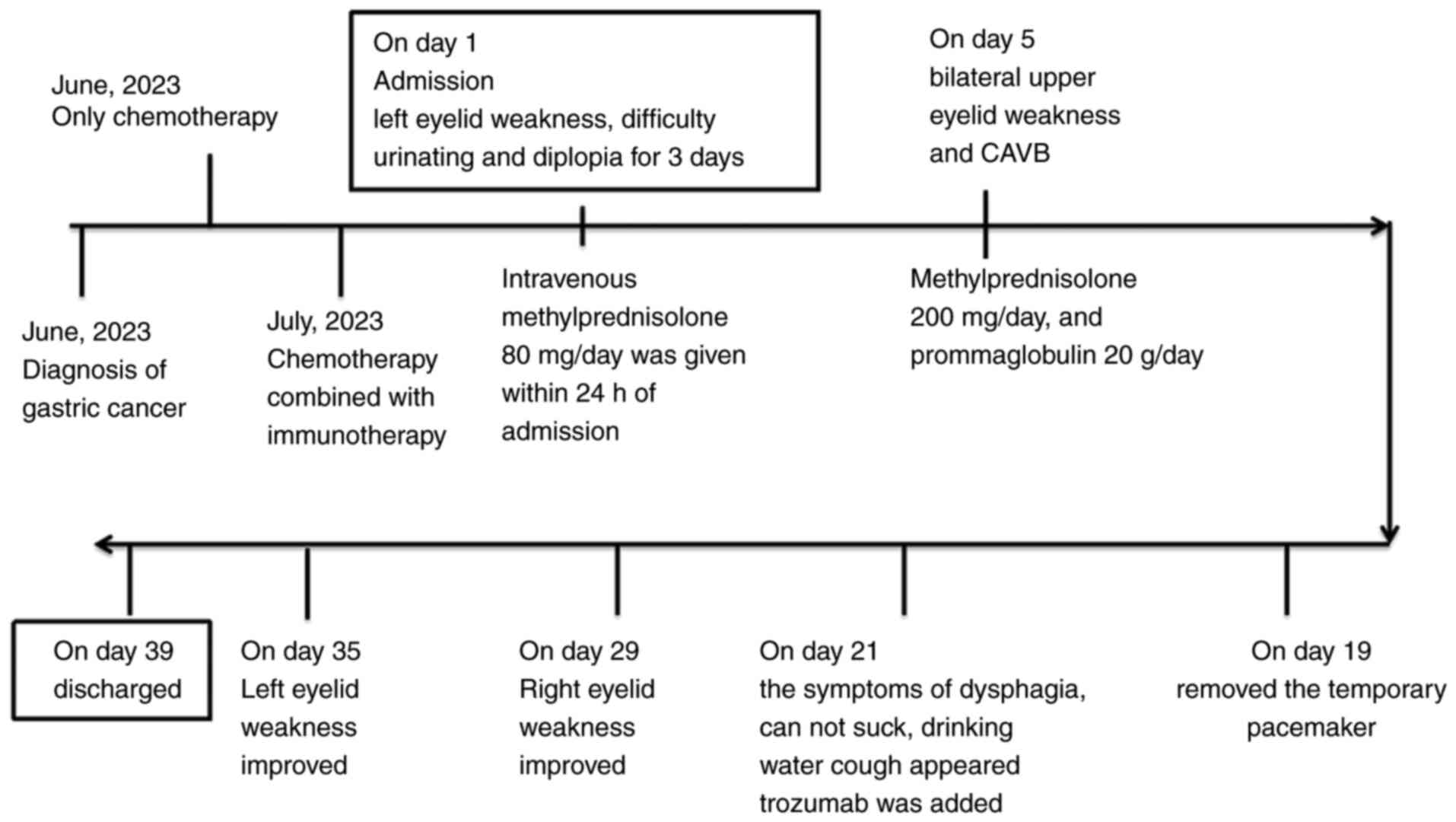
|
1
|
Wang C, Zhong B, He J and Liao X: Immune
checkpoint inhibitor sintilimab-induced lethal myocarditis
overlapping with myasthenia gravis in thymoma patient: A case
report. Medicine (Baltimore). 102(e33550)2023.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Ye Z, Yang W, Xuan B, Li X, He J, Si H and
Ma W: Efficacy and safety evaluation of sintilimab for cancer
treatment: A systematic review and meta-analysis of randomized
controlled trials. Front Pharmacol. 13(895187)2022.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Pathak R, Katel A, Massarelli E, Villaflor
VM, Sun V and Salgia R: Immune checkpoint inhibitor-induced
myocarditis with myositis/myasthenia gravis overlap syndrome: A
systematic review of cases. Oncologist. 26:1052–1061.
2021.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Aldrich J, Pundole X, Tummala S, Palaskas
N, Andersen CR, Shoukier M, Abdel-Wahab N, Deswal A and
Suarez-Almazor ME: Inflammatory myositis in cancer patients
receiving immune checkpoint inhibitors. Arthritis Rheumatol.
73:866–874. 2021.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Moslehi JJ, Salem JE, Sosman JA,
Lebrun-Vignes B and Johnson DB: Increased reporting of fatal immune
checkpoint inhibitor-associated myocarditis. Lancet.
391(933)2018.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Mahmood SS, Fradley MG, Cohen JV, Nohria
A, Reynolds KL, Heinzerling LM, Sullivan RJ, Damrongwatanasuk R,
Chen CL, Gupta D, et al: Myocarditis in patients treated with
immune checkpoint inhibitors. J Am Coll Cardiol. 71:1755–1764.
2018.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Kadowaki H, Akazawa H, Ishida J and Komuro
I: Mechanisms and management of immune checkpoint inhibitor-related
cardiac adverse events. JMA J. 4:91–98. 2021.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Zhang L, Reynolds KL, Lyon AR, Palaskas N
and Neilan TG: The evolving immunotherapy landscape and the
epidemiology, diagnosis, and management of cardiotoxicity: JACC:
CardioOncology primer. JACC CardioOncol. 3:35–47. 2021.PubMed/NCBI View Article : Google Scholar
|
|
9
|
The NCCN Clinical Practice Guidelines in
Oncology. Management of Immunot therapy-Related Toxicity. Version
2.2023.[2023-05-09]. Journal.
|
|
10
|
The Chinese Society of Clinical Oncology
immune checkpoint inhibitor related toxicity management guidelines
2023. http://www.csco.org.cn/cn/index.aspx. Journal.
|
|
11
|
Andrejeva J, Grisanina A, Sniepienė G,
Mockiene A and Strazdauskaite D: The effect of TRX suspension
trainer and BOSU platform after reconstruction of anterior cruciate
ligament of the knee joint. Pedagogy Phys Cult Sports. 26:47–56.
2022.
|
|
12
|
Guven DC, Stephen B, Sahin TK, Cakir IY,
Erul E and Aksoy S: The efficacy of immune checkpoint inhibitors in
rare tumors: A systematic review of published clinical trials. Crit
Rev Oncol Hematol. 174(103700)2022.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Chen Y, Chen T, Zhu W, Li L, Fang C and
Zhang H: Rare primary intrapulmonary malignant peripheral nerve
sheath tumor showing significant response to sintilimab: A case
report and literature review. Oncol Lett. 28(423)2024.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Schneider BJ, Naidoo J, Santomasso BD,
Lacchetti C, Adkins S, Anadkat M, Atkins MB, Brassil KJ, Caterino
JM, Chau I, et al: Management of immune-related adverse events in
patients treated with immune checkpoint inhibitor therapy: ASCO
guideline update. J Clin Oncol. 39:4073–4126. 2021.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Gong J, Chehrazi-Raffle A, Reddi S and
Salgia R: Development of PD-1 and PD-L1 inhibitors as a form of
cancer immunotherapy: A comprehensive review of registration trials
and future considerations. J Immunother Cancer. 6(8)2018.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Suzuki S, Ishikawa N, Konoeda F, Seki N,
Fukushima S, Takahashi K, Uhara H, Hasegawa Y, Inomata S, Otani Y,
et al: Nivolumab-related myasthenia gravis with myositis and
myocarditis in Japan. Neurology. 89:1127–1134. 2017.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Shimozaki K, Sukawa Y, Beppu N, Kurihara
I, Suzuki S, Mizuno R, Funakoshi T, Ikemura S, Tsugaru K, Togasaki
K, et al: Multiple immune-related adverse events and anti-tumor
efficacy: Real-world data on various solid tumors. Cancer Manag
Res. 12:4585–4593. 2020.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Gao L, Li X, Guo Z, Tang L, Peng J and Liu
B: Immune checkpoint inhibitor-induced myocarditis with myasthenia
gravis overlap syndrome: A case report and literature review.
Medicine (Baltimore). 101(e32240)2022.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Kondo H, Kirigaya J, Matsuzawa Y and Hibi
K: Two cases of immune checkpoint inhibitor-induced myocarditis
with complete atrioventricular block. Cureus.
15(e36446)2023.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Wang DY, Salem JE, Cohen JV, Chandra S,
Menzer C, Ye F, Zhao S, Das S, Beckermann KE, Ha L, et al: Fatal
toxic effects associated with immune checkpoint inhibitors: A
systematic review and meta-analysis. JAMA Oncol. 4:1721–1728.
2018.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Nguyen LS, Bretagne M, Arrondeau J, Zahr
N, Ederhy S, Abbar B, Pinna B, Allenbach Y, Mira JP, Moslehi J, et
al: Reversal of immune-checkpoint inhibitor fulminant myocarditis
using personalized-dose-adjusted abatacept and ruxolitinib: Proof
of concept. J Immunother Cancer. 10(e004699)2022.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Sobol I, Chen CL, Mahmood SS and Borczuk
AC: Histopathologic characterization of myocarditis associated with
immune checkpoint inhibitor therapy. Arch Pathol Lab Med.
144:1392–1396. 2020.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Zhong P, Zhang C, Guan H, Yan J, He M and
Zhou X: Myocarditis and myasthenia gravis induced by immune
checkpoint inhibitor in a patient with relapsed thymoma: A case
report. Clin Case Rep. 11(e7039)2023.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Wei SC, Meijers WC, Axelrod ML, Anang NAS,
Screever EM, Wescott EC, Johnson DB, Whitley E, Lehmann L, Courand
PY, et al: A genetic mouse model recapitulates immune checkpoint
inhibitor-associated myocarditis and supports a mechanism-based
therapeutic intervention. Cancer Discov. 11:614–625.
2021.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Liang S, Yang J, Lin Y, Li T, Zhao W, Zhao
J and Dong C: Immune myocarditis overlapping with myasthenia gravis
due to anti-PD-1 treatment for a chordoma patient: A case report
and literature review. Front Immunol. 12(682262)2021.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Weaver JM, Dodd K, Knight T, Chaudhri M,
Khera R, Lilleker JB, Roberts M, Lorigan P and Cooksley T: Improved
outcomes with early immunosuppression in patients with
immune-checkpoint inhibitor induced myasthenia gravis, myocarditis
and myositis: A case series. Support Care Cancer.
31(518)2023.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Nguyen BHV, Kuo J, Budiman A, Christie H
and Ali S: Two cases of clinical myasthenia gravis associated with
pembrolizumab use in responding melanoma patients. Melanoma Res.
27:152–154. 2017.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Komatsu M, Hirai M, Kobayashi K, Hashidate
H, Fukumoto J, Sato A, Usuda H, Tanaka K, Takahashi K and Kuwabara
S: A rare case of nivolumab-related myasthenia gravis and
myocarditis in a patient with metastatic gastric cancer. BMC
Gastroenterol. 21(333)2021.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Hamada N, Maeda A, Takase-Minegishi K,
Kirino Y, Sugiyama Y, Namkoong H, Horita N, Yoshimi R and Nakajima
H: YCU irAE Working Group. Incidence and distinct features of
immune checkpoint inhibitor-related myositis from idiopathic
inflammatory myositis: A single-center experience with systematic
literature review and meta-analysis. Front Immunol.
12(803410)2021.PubMed/NCBI View Article : Google Scholar
|