The human gut microbiota constitutes a highly
intricate community comprised of ~500-1,000 bacterial species
(1). Despite this diversity, a
substantial discrepancy exists in the abundance of each bacterial
type. Notably, a mere 30-40 species contribute to >99% of the
total bacterial population, with the remaining species collectively
constituting less than 1% (2).
Crucially, the gut microbiota plays a pivotal role in essential
physiological functions including immunity (3), metabolism (4), inflammation (5) and cell proliferation (6). These microorganisms not only interact
extensively with the intestinal epithelium but also engage in
communication with diverse organ systems. It is estimated that the
cumulative weight of gut bacteria in an adult can range between
1.0-1.5 kg, housing an immense population of ~1014
bacteria (7). The intestinal
genome contains millions of genes, 100 times more than the human
genome and contains various gene clusters associated with metabolic
processes, immune responses and multiple signaling pathways
(8). Beyond their influence on
body mass and metabolism, the gut microbiota is intricately linked
to variety of diseases, including, but not limited to, inflammatory
bowel disease, diabetes, obesity, gastrointestinal cancer,
cirrhosis, hyperuricemia and autoimmune disorders (9-11).
The significance of a healthy gut microbiota as a crucial
determinant of overall health cannot be understated, as alterations
in its composition strongly correlate with changes in an
individual's health status. The National Institutes of Health (NIH)
has undertaken a substantial commitment to human microbiome
research, investing a substantial $15 billion since 2008. This
extensive investment has facilitated comprehensive population
studies focusing on microbial populations across various tissues
such as the skin, respiratory tract, gastrointestinal tract,
urinary tract and vaginal tract (12). Since the commencement of the NIH
Human Microecology Program and the EU Intestinal Microecology
Program, gut microbiota has attracted much attention and has become
an international research hotspot. Now, a number of countries in
the world have launched human microbiome projects, including
Canada, Australia, France, North Korea and Ireland (13).
Advancements in molecular biology techniques have
greatly expanded our understanding of gut microbiota. Techniques
such as fluorescence in situ hybridization, polymerase chain
reaction denaturing gradient gel electrophoresis, gene chips and
sequencing have played a vital role. These techniques have enabled
more detailed and accurate analyses of gut microbiota.
Specifically, next-generation sequencing and macrogenomics have
been revolutionary, notably advancing the study of the gut
microbiome. These advances mark a significant leap forward in this
field of research (14,15).
The morbidity and mortality of cardiovascular
diseases have been increasing globally, posing a serious threat to
human health. Consequently, there is a pressing need for early
preventive measures and innovative treatment strategies for
cardiovascular diseases. This has become a focal point of research
in the field. Recent studies exploring the relationship between gut
microbiota and various diseases have shed light on the crucial role
these microbes play in human health (16-18).
Recent investigations have highlighted a connection between gut
microbiota and cardiovascular disease (19,20).
Modulating gut microbiota is anticipated as a potential emerging
therapeutic approach for managing cardiovascular conditions
(21,22).
Due to of the multiple functions of the gut
microbiota, scientists have focused on comprehensive studies of
this intricate ecosystem. The traditional approach to study
microorganisms is to analyze the physiological functions of
individual strains or groups of bacteria using in vitro
culture. However, numerous species within the gut microbiota can
only thrive within the human body and are challenging to culture
in vitro. Consequently, traditional methods face limitations
when addressing the complexity of the gut microbiota. However, with
the advancement of sequencing technology, especially the increasing
maturity of macrogenomics, the composition and complexity of gut
microbiota were clarified. This breakthrough enables a more
profound exploration of the relationship between the gut microbiota
and human health, overcoming the constraints of traditional
methodologies.
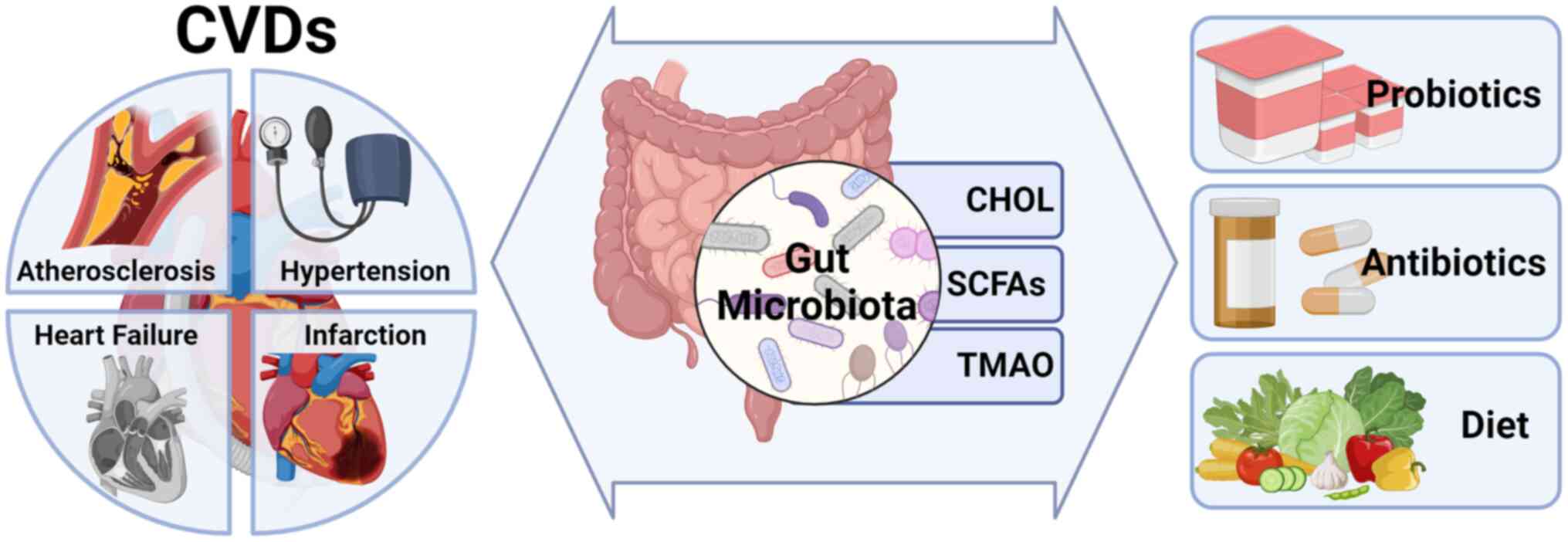
The present review provided a systematic exploration
of the link between imbalances in gut microbiota and various
cardiovascular diseases, such as atherosclerosis, hypertension,
heart failure and myocardial infarction. Drawing from recent
clinical research and biological studies, it delineates the
association between dysbiosis of gut microbiota and the onset, as
well as the advancement, of cardiovascular diseases. Emphasizing
this connection, the present review underscores the potential of
modulating gut microbiota as a viable therapeutic avenue for
managing cardiovascular conditions.
To generate the initial list of studies in this
review, literature searches were conducted on PubMed using specific
keywords such as ‘gut microbiome’ or ‘gut microbiota’ combined with
terms in the category of cardiovascular diseases such as
‘Atherosclerosis’, ‘Hypertension’, ‘Heart failure’ and ‘Cardiac
infarction’. These searches were performed to identify the most
recent research papers published within the last decade (10 years).
Papers published within the past 5 years were prioritized to ensure
the inclusion of the most current findings in the present review.
Additionally, the inclusion of the papers published in high impact
journals and those with higher citation numbers were prioritized.
Through these specific criteria, the literature collection process
collected ~200 reviews and research papers. To ensure the relevance
of the information presented in the present review, papers
published within the last 10 years were excluded if they had
subsequent updates. Research articles older than 10 years were
included if they keep having recent citations or ongoing
significance within the field. This selection process helped us
compile a comprehensive and up-to-date set of literature for the
present review.
The gut microbiota is composed of four main phyla:
Firmicutes, Bacteroidetes, Actinobacteria and Proteobacteria.
Saprophytes and Bacteroidetes constitute the majority of the
intestinal community in healthy adults and the ratio of
Firmicutes:Bacteroidetes is considered an indicator of the health
of the gut microbiota (23,24).
However, microbial composition varies from person to person and is
dynamically sensitive to host factors and environmental parameters
(25). Notably, increased
abundance of host opportunistic pathogens (e.g., E. coli,
Clostridium lambda, C. carinii and Eggerthella
lenta) and decreased short-chain fatty acid (SCFA)-producing
bacteria (e.g., Roseburia, Faecalibacterium and
Eubacterium rectale) are both associated with an increased
risk of cardiovascular diseases (26). Thus, both the type of microorganism
and their relative abundance are potential risk factors that may
alter susceptibility to developing cardiovascular diseases.
Atherosclerosis is the most important and common
group of atherosclerotic vascular diseases and the pathogenesis of
this disease has been discussed in various theories (27), including the lipid infiltration
theory, thrombosis theory, smooth muscle cell cloning theory,
inflammation theory and infection theory. In recent years, most
researchers have supported the endothelial injury response theory,
suggesting that the main risk factors for the disease ultimately
damage the intima and that the formation of atherosclerotic lesions
is the result of the inflammatory response of the arteries to
intimal damage (28). Inflammation
plays a key role in the onset and progression of atherosclerosis
(29). Currently, bacteria are
considered to be a contributing factor to the disease.
In the intestine of patients with symptomatic
atherosclerosis, there are changes in some genera, such as an
increase in Corynebacterium, and in healthy controls, there
were more Rhodobacter and Eubacterium. This
difference suggests that changes in gut microbiota may be
associated with atherosclerosis (30). In addition, some researchers have
identified Aeromonas, Veronella and
Streptococcus in atherosclerotic plaques in both animal and
patient studies (31-33).
In an animal study, it was discovered that the gut
microbiota's role in metabolizing and absorbing bile acids and
cholesterol significantly influences blood cholesterol levels.
These cholesterol levels, in turn, represent crucial factors
contributing to the development of atherosclerosis (34). The mechanisms that affect
cholesterol metabolism include the production of cholesterol
oxidase, inhibition of hepatic lipid synthase activity, regulation
of cholesterol redistribution in blood and liver and influence on
bile salt hepato-intestinal circulation (35). In addition, the generation of gut
microbiota-dependent trimethylamine N-oxide (TMAO) alters the main
pathway of cholesterol removal in the body (36). TMAO is a substance that is
metabolized in the liver after trimethylamine (TMA) is absorbed in
the intestine (37). Trenteseaux
et al (38) found that in
mice, TMAO could cause intracellular accumulation of cholesterol by
increasing the expression of proatherosclerotic CD36 and scavenger
receptor A. TMAO also decreases Cyp7a1 expression, which is an
important enzyme for bile acid synthesis, inhibits cholesterol
transport and causes intracellular cholesterol accumulation and
foam cell formation, thus becoming a risk factor for
atherosclerosis (38).
In addition to correlation analyses, numerous
studies are also investigating potential mechanisms that establish
a connection between atherosclerosis and gut microbiota. An animal
study showed that the ability of gut microbiota to reduce
cholesterol levels (45). The
mechanism involves: i) Co-precipitation; bacteria produce bile salt
hydrolases, which break down conjugated bile salts into free bile
salts. At a pH of 5.5, this process leads to the co-precipitation
of cholesterol and bile acids, thereby reducing the ability of
cholesterol entering the bloodstream (46); ii) Cholesterol uptake by bacteria;
bacterial cells uptake cholesterol, representing the primary ways
of cholesterol removal by these microorganisms. While the capacity
for cholesterol uptake varies among bacterial strains, this process
contributes to reducing serum cholesterol concentrations (47). These mechanisms associated with gut
microbiota and atherosclerosis also suggest potential therapeutic
targets for the prevention and treatment of atherosclerosis.
Hypertension is a progressive cardiovascular
syndrome with both genetic and environmental causes, leading to
changes in cardiac and vascular function and structure (48). Except for genetic causes and
environmental factors, gut microbiota also contributes to the
progression of hypertension (49).
The first evidence suggesting the role of the gut microbiota in
development of hypertension was the effect of antibiotic treatment
on blood pressure observed in rats (50). In 2015, Yang et al (51) reported that dysbiosis of gut flora
may be associated with hypertension with the finding of increasing
of fecal microbiota variability in both rat model and patient. It
has been reported that the microbial profiles of the
pre-hypertensive and hypertensive populations were similar, with
significant overgrowth of Prevotella and Klebsiella
in both groups. These findings were established through experiments
involving the transplantation of fecal microbiota from patients
with hypertension into germ-free mice (52). The abundance of butyrate-producing
Odoribacter spp. and butyrate production were inversely
correlated with blood pressure levels in women at higher risk of
developing pregnancy-induced hypertension and preeclampsia
(53,54).
Antibiotics for hypertension are a new discovery in
the cardiovascular field and are not yet well supported by clinical
studies (55). An effective animal
study conducted on rats demonstrated the potential use of
broad-spectrum antibiotics, including vancomycin, rifampicin and
levofloxacin, in aiding the treatment of resistant hypertension,
suggesting indirect evidence that antibiotics can improve gut
microbiota and initiate potential blood pressure regulatory
mechanisms to decreasing blood pressure (56). However, the mechanism of antibiotic
against hypotension has yet to be investigated.
The mechanisms by which gut microbiota affects
hypertension are complex. Studies found that short chain fatty
acids (SCFAs) produced by the gut microbiota regulate blood
pressure through various receptors and pathways (57,58).
Additional animal research conducted on rats indicated that the
inhibition of intestinal sodium transporters might offer potential
benefits in ameliorating cardiorenal damage associated with
essential hypertension (59).
Angiotensin-converting enzyme 2 (ACE2) plays a significant role in
the pathophysiology of hypertension (60). It has been demonstrated that ACE2
serves as a regulator of gut microbiota homeostasis, inflammation
and genetic susceptibility to colitis (61,62).
A growing number of studies support the role of the
gut in the pathogenesis of heart failure (63,64).
Under normal circumstances, the gut microbiota maintains a balanced
state and performs a crucial role in supporting the integrity and
functionality of the intestinal barrier (65). However, in patients with heart
failure, the intestinal tract function is altered and dysbiosis of
the gut microbiota occurs due to factors such as congestion of the
visceral circulation and immune deficiency of the host (66). It has been shown that patients with
chronic heart failure may have excessive growth of pathogenic gut
microbiota and increased intestinal permeability, which is related
to the severity of heart failure (67).
In addition to the clear link between TMAO and risk
of atherosclerotic cardiovascular disease, TMAO levels have
recently been associated with the development and poor prognosis of
patients with heart failure (35).
Previous studies have shown that circulating TMAO levels are higher
in patients with heart failure compared with age- and sex-matched
subjects without heart failure (68,69).
In addition, the study observed significantly strong adverse
prognostic values associated with elevated plasma TMAO levels in a
group of stable patients with heart failure, which were incremental
to traditional risk factors, cardiac and renal indices and systemic
inflammatory markers (68).
However, the mechanisms explaining the elevated TMAO levels in
patients with heart failure remain to be elucidated.
Compared to normal subjects, patients with
myocardial infarction have high levels of phosphatidylcholine
metabolites, which are plasma choline, TMAO and betaine, and these
three substances are significantly associated with the development
of cardiovascular diseases (70).
In both patients and mice, their metabolism, which involves the
dietary phosphatidylcholine pathway, relies on the active
participation of gut microbiota (71). In addition, an association between
gut microbiota and myocardial infarct severity has been reported in
rats (71-73).
The use of broad-spectrum antibiotics has been shown to affect the
levels of analytes produced during leptin and aromatic amino acid
catabolism, which are associated with a reduction in myocardial
infarct size. In addition, in rodent model studies, delivery of
Lactobacillus plantarum was correlated with a significant
infarct size reduction following myocardial infarction relief and
improvement in left ventricular function. Another study showed that
administration of Lactobacillus rhamnosus GR-1 attenuated
left ventricular hypertrophy and heart failure after myocardial
infarction in rats (74). These
observations not only demonstrate the relationship between gut
microbiota and myocardial infarction, but also suggest that
altering the composition of the flora can be a potential
therapeutic strategy.
The gut microbiota can influence the regulation of
cholesterol metabolism in the liver (75,76)
and plays a role in altering bile acids, which can affect systemic
cholesterol levels (77). Bile
acids, catalyzed by the rate-limiting enzyme cholesterol
7-α-hydroxylase (78), are the
main metabolites of cholesterol in the liver and contribute to the
absorption of fats and lipophilic vitamins (79), as well as to the regulation of
lipid, glucose and energy metabolism (80,81).
Primary bile acids are conjugated with the amino acids taurine or
glycine to form bile salts, which are secreted into the bile and
stored in the gallbladder until they are released into the small
intestine, where they emulsify fats and form micelles that are
absorbed by enterocytes (79). In
the intestine, primary bile acids such as cholic acid and
chenodeoxycholic acid (CDCA) are deconjugated by the gut microbiota
and bile salt hydrolase to form secondary bile acids including
deoxycholic acid, lithodeoxycholic acid and ursodeoxycholic acid
(82). The ratio of primary to
secondary bile acids may be associated with the development of
hypercholesterolemia and cardiovascular diseases. For example, one
study (83) found that patients
with heart failure had reduced plasma primary bile acids and a
higher ratio of secondary to primary bile acids. Bile acids may
also play a role in cardiovascular function by modulating channel
conductance and calcium dynamics in sinoatrial and ventricular
cardiomyocytes and by regulating vascular tone to reduce heart rate
(83). Furthermore, it has been
proposed that an unbalanced and unhealthy gut microbiota, which
regulates the bile acid ratio, may lead to a decrease in secondary
bile acids, which may increase primary bile acids, such as CDCA,
activating farnesoid X receptor and downregulating bile acid
secretion, thereby increasing cholesterol and cardiovascular
diseases development. Therefore, the gut microbiota and the
underlying mechanisms involved require further study.
It has long been known that certain gut microbiota
has the ability to convert absorbable cholesterol into
coprostanols, which are reduced non-absorbable sterols that are
excreted in the feces (84-86).
Coprostanol production in humans begins at 6 months after birth
(87) and is sex-dependent, with
young women possessing a higher production compared with young men
(88). Furthermore, the conversion
of microbial cholesterol to coprostanol in human populations is
currently considered to be bimodal, with high converters converting
almost 100% cholesterol and low converters converting less than
one-third of the fecal neutral sterol content (89,90).
To date, isolated cholesterol-reducing strains have been limited to
the genera Eubacterium (E. coprostanoligenes) and
Bacteroides (Bacteroides sp. strain D8) (91,92),
but a number of remain to be discovered. In rabbit models with
diet-induced hypercholesterolemia, oral administration of coplanar
alkanoic bacteria caused a significant decrease in plasma
cholesterol levels that lasted for >34 days after the last
bacterial feeding (93). Clinical
studies have extensively investigated cholesterol metabolism in the
intestine (87,89,91,94,95).
These studies have indicated an inverse relationship between human
serum cholesterol levels and the ratio of human fecal coprostanol
to cholesterol (96,97). However, these studies used very
small sample sizes, limited variation in sample populations and
lack of diverse population backgrounds, which reduces the
generalizability of the studies. Attempts to isolate the specific
microbial strains responsible for copolymer/cholesterol conversion
in these studies were also unsuccessful, making it difficult to
continue the mechanistic studies that followed. In addition, the
genes or enzymes involved in the conversion of cholesterol to
coplanar alcohols in the intestine remain unknown (98).
SCFAs are metabolites derived from microbial
activity during the fermentation of complex carbohydrates (99,100). These compounds exert influence on
microbiota composition and gut motility, thereby affecting various
host processes (101). The most
abundant SCFAs are acetate, propionate and butyrate (100). Phylum Bacteroidetes members
produce acetate and butyrate, while Phylum Siliques produces
butyrate. SCFAs are also positively correlated with Alistipes
putredinis, Bacteroides spp, Roseburia,
Eubacterium rectale and Faecal prausnitzii (102). In addition, SCFAs play a pivotal
role in preserving intestinal barrier integrity by modulating the
expression of tight junction proteins (103). SCFAs can also reduce serum lipid
levels by blocking cholesterol synthesis and transferring it to the
liver. Therefore, they are considered as a protective factor in the
progression of cardiovascular diseases. The presence of
SCFA-producing bacteria is diminished due to the activation of G
protein-coupled receptor 41(104)
observed in certain cases of cardiovascular diseases (30,105). This reduction is also apparent in
the dysbiosis of the gut microbiota observed in hypertensive
patients (106). Therefore, their
role in vivo and their targets need to be further
examined.
TMAO is identified as a risk factor contributing to
the development of cardiovascular diseases (71,75).
This compound originates from various sources, including red meat,
eggs, fish and vegetables (107).
Compounds such as choline, betaine, phosphatidylcholine, lecithin
and L-carnitine (71,108,109) found in the diet are implicated in
the production of TMAO. A clinical study revealed that elevated
levels of TMAO are linked to an increased risk of death, nonfatal
myocardial infarction, or stroke (110). An in vitro study revealed
the ability of the gut microbiota to produce choline through the
enzyme phospholipase D (111).
TMA molecules, produced by microbiota, can enter the circulation of
the host and subsequently reach hepatocytes. Within these
hepatocytes, they undergo metabolism to form TMAO through the
action of flavin-containing monooxygenases (FMO) encoded by the FMO
gene. These enzymatic reactions occur predominantly in the liver,
kidneys and other tissues (109).
Elevated production of TMAO has been shown to affect blood lipids
(112) and is associated with an
increasing risk of cardiovascular diseases (71,108,113). In situations involving heightened
intestinal permeability, TMAO exhibits associations with C-reactive
protein and endothelial dysfunction. In addition, increased serum
levels of LPS endotoxin (114)
are also linked to TMAO. Moreover, TMAO has been observed to induce
calcium release and increase platelet hyperreactivity (115), potentially influencing the
progression of cardiovascular diseases.
The gut microbiota markedly influences the
production of TMAO. Healthy individuals have a large number of
TMAO-producing microorganisms, with a 2:1 ratio of
Firmicutes to Bacteroidetes (116). TMAO production was found in 102
genomes covering 36 species, including Firmicutes,
Aspergillus and Actinobacteria (107). It has been shown that
Firmicutes and Proteobacter are associated with the
production of TMA (114). One
study found that eight species from Stachybotrys and
Proteobacter consumed >60% of the choline used for TMA
production (117).
Acinetobacter, Sporotrichum, Prevotella
(107) and Micrococcus
(118) are intestinal species
capable of producing higher TMAO than other species and they are
associated with atherosclerotic cardiovascular diseases. Therefore,
metabolites including choline, TMAO and betaine can help predict
the development of cardiovascular diseases. As a potential
therapeutic pathway, probiotic or pharmacological interventions can
be used to inhibit or block specific metabolic pathways and reduce
TMAO-producing microbes (119).
The role of lactobacilli with probiotic effects such
as immunomodulation, absorption promotion and disease prevention in
the intervention of hyperlipidemia and hypertension is of interest.
Previous animal studies have identified a variety of lactobacilli
with antihypertensive effects, the main one being Lactobacillus
swiss (120,121). Experiments in mice suggest that
Lactobacillus rhamnosus can lower cholesterol and improve
non-alcoholic fatty liver disease (122).
Probiotics and their fermented products (e.g.,
yogurt) have been shown in a number of studies to have significant
antihypertensive effects. Khalesi et al (126) showed that blood pressure (both
systolic and diastolic) was significantly lower in individuals
whose intake was ≥1 billion probiotic colonies per day. This
indirect evidence indicated that the gut microbiota plays a crucial
role in maintaining stability in blood pressure. It suggests the
mechanism underlying the hypotensive effect of probiotic products
based on two primary aspects: i) The hypotensive effect of
probiotic products is attributed to the hydrolysis of extracellular
proteases and peptidases (such as carboxypeptidase and
aminopeptidase). This process leads to the liberation of peptides
from food proteins, such as milk protein, which exhibit hypotensive
activity. These released peptides, such as ACE inhibitory peptide
and opioid active peptide, contribute to the observed
antihypertensive effects; ii) Certain probiotic organisms,
particularly Lactobacillus, can transit to the intestinal
tract as live bacteria. These organisms facilitate the intestinal
absorption of specific minerals known to regulate blood pressure.
Through this action, they demonstrate antihypertensive effects.
Therefore, the application of probiotics to modify the composition
of the gut microbiota is a potential therapeutic target for
cardiovascular diseases.
Dietary interventions to correct intestinal
dysbiosis could be a therapeutic strategy for cardiovascular
disease. Indeed, a heart-healthy diet, abundant in vegetables and
high in fiber, is regarded as advantageous for the cardiovascular
system (127). Certainly,
lifestyle can markedly impact bacterial transmission, particularly
environmental sanitation practices and drinking water treatment
methods. These aspects play a crucial role in influencing
alterations observed in the gut microbiota. The Mediterranean diet
is a combination of fish, beans, vegetables, fruits, nuts, olive
oil and a moderate amount of red wine (128,129). This diet has become particularly
popular in recent years and is considered to prevent cardiovascular
disease and reduce cardiovascular mortality in both men and women
(130,131). For most individuals, a
Mediterranean-style diet is an effective and viable way to prevent
heart disease and other health problems.
The microbiota can be regulated with antibiotics and
used to restore the microbiome and prevent cardiovascular disease.
A study showed that oral vancomycin reduced infarct size and
improved post-infarct cardiac function in rats (72). Furthermore, in an experimental
mouse model, antibiotics reduced bacterial translocation,
inflammation and myocardial injury (132). In one study, a 69-year-old
patient with a long history (44 years) of hypertension and
resistant hypertension showed reduced blood pressure after
combination antibiotic therapy (133). Moreover, a two-month dietary
intervention involving a broad-spectrum antibiotic cocktail
successfully reversed vascular dysfunction induced by a Western
diet in mice. This reversal represents a crucial preclinical step
in preventing the progression of cardiovascular disease (134). In addition, a study on rats
showed that vancomycin administration was associated with a
reduction in myocardial infarct size and improved recovery of
mechanical function after ischemia, while effectively reducing the
total number and group of intestinal microbes (72).
Collectively, various clinical and animal studies
have strongly indicated the involvement of gut microbiota
composition in acute myocardial infarction. However, the specific
direction of microbiota alterations and the potential metabolic or
inflammatory pathways involved remain poorly understood.
The relevance of gut microbiota to disease is in
its infancy and, similarly, the relevance to cardiovascular disease
is still at an early stage and a number of key questions need to be
explored. Increasing evidence indicates an association between the
gut microbiome and the incidence of cardiovascular disease. Studies
suggest that the microbiota interacts with the host through
multiple pathways. Abnormal gut microbiota composition or microbial
metabolites may be responsible for altered cardiovascular disease
risk and its associated pathological changes (Fig. 1). Thus, the potential role of the
gut microbiota could be utilized in the future to develop novel
therapeutic strategies for the prevention and treatment of
cardiovascular disease.
As a potential therapeutic strategy, the
feasibility of gut microbiota transplantation has been demonstrated
in short-term studies, but long-term trials are needed to evaluate
the safety and efficacy. Modulation of gut microbiota as a
potential therapeutic target for cardiovascular disease needs to be
further evaluated and the therapeutic indications and specific
strategies need to be further demonstrated.
Not applicable.
Funding: The present study was funded by China National Natural
Science Funding (grant no. 81902937), Hubei University of Science
and Technology ENT special project (grant no. 2020WG06) for SYL,
Hubei University of Science and Technology ENT special project (No.
2) for NZF and (grant no. 2020XZ30) for SDW, Hubei province Key R
and D plan (grant no. 2022BCE011) for NZF.
Not applicable.
ZFN, LL, KQC and GLW conceived and supervised the
present study. SWX, YC, YL and DWS prepared the first draft of the
manuscript. XPY, ZWu, YLS, ZhiW, JL and ZFN reviewed the manuscript
for important intellectual content. All authors have read and
approved the final manuscript. Data authentication is not
applicable.
Not applicable.
Not applicable.
The authors declare that they have no competing
interests.
|
1
|
Barreto HC and Gordo I: Intrahost
evolution of the gut microbiota. Nat Rev Microbiol. 21:590–603.
2023.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Guarner F and Malagelada JR: Gut flora in
health and disease. Lancet. 361:512–519. 2003.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Shayya NW, Foote MS, Langfeld LQ, Du K,
Bandick R, Mousani S, Bereswill S and Heimesaat MM: Human
microbiota associated IL-10-/- mice: A valuable enterocolitis model
to dissect the interactions of Campylobacter jejuni with host
immunity and gut microbiota. Eur J Microbiol Immunol (Bp).
12:107–122. 2023.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Couvillion SP, Danczak RE, Cao X, Yang Q,
Keerthising TP, McClure RS, Bitounis D, Bunret MC, Fansler SJ,
Richardson RE, et al: Graphene oxide exposure alters gut microbial
community composition and metabolism in an in vitro human model.
NanoImpact. 30(100463)2023.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Mishra SP, Wang B, Jain S, Ding J, Rejeski
J, Furdui CM, Kitzman DW, Taraohder S, Brechot C, Kumar A and Yadav
H: A mechanism by which gut microbiota elevates permeability and
inflammation in obese/diabetic mice and human gut. Gut.
72:1848–1865. 2023.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Vallino L, Garavaglia B, Visciglia A,
Amoruso A, Pane M, Ferraresi A and Lsidoro C: Cell-free
Lactiplantibacillus plantarum OC01 supernatant suppresses
IL-6-induced proliferation and invasion of human colorectal cancer
cells: Effect on β-Catenin degradation and induction of autophagy.
J Tradit Complement Med. 13:193–206. 2023.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Hooper LV and Gordon JI: Commensal
host-bacterial relationships in the gut. Science. 292:1115–1118.
2001.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Dethlefsen L, McFall-Ngai M and Relman DA:
An ecological and evolutionary perspective on human-microbe
mutualism and disease. Nature. 449:811–818. 2007.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Maciel-Fiuza MF, Muller GC, Campos DMS,
Costa PSS, Peruzzo J, Bonamigo RR, Veit T and Vianna FSL: Role of
gut microbiota in infectious and inflammatory diseases. Front
Microbiol. 14(1098386)2023.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Zhao J, Hu Y, Qian C, Hussain M, Liu S,
Zhang A, He R and Sun P: The interaction between mushroom
polysaccharides and gut microbiota and their effect on human
health: A review. Biology (Basel). 12(122)2023.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Belvoncikova P, Splichalova P, Videnska P
and Gardlik R: The human mycobiome: Colonization, composition and
the role in health and disease. J Fungi. 8(1046)2022.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Ley RE, Lozupone CA, Hamady M, Knight R
and Gordon JI: Worlds within worlds: Evolution of the vertebrate
gut microbiota. Nat Rev Microbiol. 6:776–788. 2008.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Faust K, Sathirapongsasuti JF, Izard J,
Segata N, Gevers D, Raes J and Huttenhower C: Microbial
co-occurrence relationships in the human microbiome. PLoS Comput
Biol. 8(e1002606)2012.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Kwa WT, Sundarajoo S, Toh KY and Lee J:
Application of emerging technologies for gut microbiome research.
Singapore Med J. 64:45–52. 2023.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Sauceda C, Bayne C, Sudqi K, Gonzalez A,
Dulai PS, Knight R, Gonzalez DJ and Gonzalez C: Stool multi-omics
for the study of host-microbe interactions in inflammatory bowel
disease. Gut Microbes. 14(2154092)2022.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Minj J, Riordan J, Teets C,
Fernholz-Hartman H, Tanggono A, Lee Y, Chauvin T, Carbonero F and
Solverson P: Diet-induced rodent obesity is prevented and the fecal
microbiome is improved with elderberry (Sambucus nigra ssp.
canadensis) juice powder. J Agric Food Chem. 72:12555–12565.
2024.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Rochoń J, Kalinowski P, Szymanek-Majchrzak
K and Grąt M: Role of gut-liver axis and glucagon-like peptide-1
receptor agonists in the treatment of metabolic
dysfunction-associated fatty liver disease. World J Gastroenterol.
30:2964–2980. 2024.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Zhang Y, Zheng T, Ma D, Shi P, Zhang H, Li
J and Sun Z: Probiotics Bifidobacterium lactis M8 and Lactobacillus
rhamnosus M9 prevent high blood pressure via modulating the gut
microbiota composition and host metabolic products. mSystems.
8(e0033123)2023.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Yan D, Si W, Zhou X, Yang M, Chen Y, Chang
Y, Lu Y, Liu J, Wang K, Yan M, et al: Eucommia ulmoides bark
extract reduces blood pressure and inflammation by regulating the
gut microbiota and enriching the Parabacteroides strain in
high-salt diet and N(omega)-nitro-L-arginine methyl ester induced
mice. Front Microbiol. 18(967649)2022.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Mao Y, Kong C, Zang T, You L, Wang LS,
Shen L and Ge JB: Impact of the gut microbiome on atherosclerosis.
mLife. 3:167–175. 2024.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Glorieux G, Nigam SK, Vanholder R and
Verbeke F: Role of the microbiome in gut-heart-kidney cross talk.
Circ Res. 132:1064–1083. 2023.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Hijová E: Benefits of biotics for
cardiovascular diseases. Int J Mol Sci. 24(6292)2023.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Liao L, Huang J, Zheng J, Ma X, Huang L
and Xu W: Gut microbiota in Chinese and Japanese patients with
cardiovascular diseases: A systematic review and meta-analysis. Ann
Saudi Med. 43:105–114. 2023.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Zhang H, Li H, Pan B, Zhang S, Su X, Sun
W, Zhang T, Zhang Z, Lv S and Cui H: Integrated 16S rRNA Sequencing
and untargeted metabolomics analysis to reveal the protective
mechanisms of polygonatum sibiricum polysaccharide on type 2
diabetes mellitus model rats. Curr Drug Metab.
10(1389200224666230406114012)2023.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Song Z, Song R, Liu Y, Wu Z and Zhang X:
Effects of ultra-processed foods on the microbiota-gut-brain axis:
The bread-and-butter issue. Food Res Int.
167(112730)2023.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Sajdel-Sulkowska EM: Neuropsychiatric
ramifications of COVID-19: Short-chain fatty acid deficiency and
disturbance of microbiota-gut-brain axis signaling. Biomed Res Int.
2021(7880448)2021.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Shantsila E, Kamphuisen PW and Lip GY:
Circulating microparticles in cardiovascular disease: Implications
for atherogenesis and atherothrombosis. J Thromb Haemost.
8:2358–2368. 2010.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Philippova M, Suter Y, Toggweiler S,
Schoenenberger AW, Joshi MB, Kyriakakis E, Erne P and Resink TJ:
T-cadherin is present on endothelial microparticles and is elevated
in plasma in early atherosclerosis. Eur Heart J. 32:760–771.
2011.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Markin AM, Markina YV, Bogatyreva AI,
Tolstik TV, Chakal DA, Breshenkov DG and Charchyan ER: The role of
cytokines in cholesterol accumulation in cells and atherosclerosis
progression. Int J Mol Sci. 24(6426)2023.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Karlsson FH, Fåk F, Nookaew I, Tremaroli
V, Fagerberg B, Petranovic D, Bäckhed F and Nielsen J: Symptomatic
atherosclerosis is associated with an altered gut metagenome. Nat
Commun. 3(1245)2012.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Khalili L, Centner AM and Salazar G:
Effects of berries, phytochemicals, and probiotics on
atherosclerosis through gut microbiota modification: A
meta-analysis of animal studies. Int J Mol Sci.
24(3084)2023.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Kumar T, Dutta RR, Velagala VR, Ghosh B
and Mudey A: Analyzing the complicated connection between
intestinal microbiota and cardiovascular diseases. Cureus.
14(e28165)2022.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Shi X, Wu H, Liu Y, Huang H, Liu L, Yang
Y, Jiang T, Zhou M and Dai M: Inhibiting vascular smooth muscle
cell proliferation mediated by osteopontin via regulating gut
microbial lipopolysaccharide: A novel mechanism for paeonol in
atherosclerosis treatment. Front Pharmacol.
13(936677)2022.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Liu S, He F, Zheng T, Wan S, Chen S, Yang
F, Xu X and Pei X: Ligustrum robustum alleviates atherosclerosis by
decreasing serum TMAO, modulating gut microbiota, and decreasing
bile acid and cholesterol absorption in mice. Mol Nutr Food Res.
65(e2100014)2021.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Zhen J, Zhou Z, He M, Han HX, Lv EH, Wen
PB, Liu X, Wang YT, Cai XC, Tian JQ, et al: The gut microbial
metabolite trimethylamine N-oxide and cardiovascular diseases.
Front Endocrinol (Lausanne). 14(1085041)2023.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Canyelles M, Tondo M, Cedó L, Farràs M,
Escolà-Gil JC and Blanco-Vaca F: Trimethylamine N-Oxide: A link
among diet, gut microbiota, gene regulation of liver and intestine
cholesterol homeostasis and HDL function. Int J Mol Sci.
19(3228)2018.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Shi C, Pei M, Wang Y, Chen Q, Cao P, Zhang
L, Guo J, Deng W, Wang L, Li X and Gong Z: Changes of
flavin-containing monooxygenases and trimethylamine-N-oxide may be
involved in the promotion of non-alcoholic fatty liver disease by
intestinal microbiota metabolite trimethylamine. Biochem Biophys
Res Commun. 594:1–7. 2022.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Trenteseaux C, Gaston AT, Aguesse A,
Poupeau G, Coppet P, Andriantsitohaina R, Laschet J, Amarger V,
Krempf M, Nobecourt-Dupuy E and Ouguerram K: Perinatal
hypercholesterolemia exacerbates atherosclerosis lesions in
offspring by altering metabolism of trimethylamine-n-oxide and bile
acids. Arterioscler Thromb Vasc Biol. 37:2053–2063. 2017.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Schleifer KH and Kandler O: Peptidoglycan
types of bacterial cell walls and their taxonomic implications.
Bacteriol Rev. 36:407–477. 1972.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Popescu NI, Girton A, Burgett T, Lovelady
K and Coggeshall KM: Monocyte procoagulant responses to anthrax
peptidoglycan are reinforced by proinflammatory cytokine signaling.
Blood Adv. 3:2436–2447. 2019.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Xie Y, Li Y, Cai X, Wang X and Li J:
Interleukin-37 suppresses ICAM-1 expression in parallel with NF-κB
down-regulation following TLR2 activation of human coronary artery
endothelial cells. Int Immunopharmacol. 38:26–30. 2016.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Dong C, Zhang M, Song S, Wei F, Qin L, Fan
P, Shi Y, Wang X and Wang R: A small subunit of Geranylgeranyl
Diphosphate synthase functions as an active regulator of carotenoid
synthesis in nicotiana tabacum. Int J Mol Sci.
24(992)2023.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Jo HE, Son SY and Lee CH: Comparison of
metabolome and functional properties of three Korean cucumber
cultivars. Front Plant Sci. 13(882120)2022.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Bocchini M, D'Amato R, Ciancaleoni S,
Fontanella MC, Palmerini CA, Beone GM, Onofri A, Negri V, Marconi
G, Albertini E and Businelli D: Soil Selenium (Se) biofortification
changes the physiological, biochemical and epigenetic responses to
water stress in Zea mays L. by inducing a higher drought tolerance.
Front Plant Sci. 9(389)2018.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Wang Y, Zheng Y, Liu Y, Shan G, Zhang B,
Cai Q, Lou J and Qu Y: The lipid-lowering effects of fenugreek gum,
hawthorn pectin, and burdock inulin. Front Nutr.
10(1149094)2023.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Tahri K, Grill JP and Schneider F:
Bifidobacteria strain behavior toward cholesterol: Coprecipitation
with bile salts and assimilation. Curr Microbiol. 33:187–193.
1996.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Bordoni A, Amaretti A, Leonardi A,
Boschetti E, Danesi F, Matteuzzi D, Roncaglia L, Raimondi S and
Rossi M: Cholesterol-lowering probiotics: In vitro selection and in
vivo testing of bifidobacteria. Appl Microbiol Biotechnol.
97:8273–8281. 2013.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Burnier M and Damianaki A: Hypertension as
cardiovascular risk factor in chronic kidney disease. Circ Res.
132:1050–1063. 2023.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Mutengo KH, Masenga SK, Mweemba A, Mutale
W and Kirabo A: Gut microbiota dependant trimethylamine N-oxide and
hypertension. Front Physiol. 14(1075641)2023.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Honour JW, Borriello SP, Ganten U and
Honour P: Antibiotics attenuate experimental hypertension in rats.
J Endocrinol. 105:347–350. 1985.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Yang Z, Wang Q, Liu Y, Wang L, Ge Z, Li Z,
Feng S and Wu C: Gut microbiota and hypertension: Association,
mechanisms and treatment. Clin Exp Hypertens.
45(2195135)2023.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Li J, Zhao F, Wang Y, Chen J, Tao G, Tian
G, Wu S, Liu W, Cui Q, Geng B, et al: Gut microbiota dysbiosis
contributes to the development of hypertension. Microbiome.
5(14)2017.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Jin J, Gao L, Zou X, Zhang Y, Zheng Z,
Zhang X, Li J, Tian Z, Wang X, Gu J, et al: Gut dysbiosis promotes
preeclampsia by regulating macrophages and trophoblasts. Circ Res.
131:492–506. 2022.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Gomez-Arango LF, Barrett HL, McIntyre HD,
Callaway LK, Morrison M and Nitert MD: SPRING Trial Group.
Increased systolic and diastolic blood pressure is associated with
altered gut microbiota composition and butyrate production in early
pregnancy. Hypertension. 68:974–981. 2016.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Lucas SE, Walton SL, Colafella KM, Mileto
SJ, Lyras D and Denton KM: Antihypertensives and antibiotics:
Impact on intestinal dysfunction and hypertension. Hypertension.
11:1393–1402. 2023.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Kyoung J and Yang T: Depletion of the gut
microbiota enhances the blood pressure-lowering effect of
captopril: Implication of the gut microbiota in resistant
hypertension. Hypertens Res. 45:1505–1510. 2022.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Chen XF, Ren SC, Tang G, Wu C, Chen X and
Tang XQ: Short-chain fatty acids in blood pressure, friend or foe.
Chin Med J (Engl). 134:2393–2394. 2021.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Dinakis E, O'Donnell JA and Marques FZ:
The gut-immune axis during hypertension and cardiovascular
diseases. Acta Physiol (Oxf). 20(e14193)2024.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Spencer AG, Labonte ED, Rosenbaum DP,
Plato CF, Carreras CW, Leadbetter MR, Kozuka K, Kohler J, Koo-McCoy
S, He L, et al: Intestinal inhibition of the Na+/H+ exchanger 3
prevents cardiorenal damage in rats and inhibits Na+
uptake in humans. Sci Transl Med. 6(277ra36)2014.PubMed/NCBI View Article : Google Scholar
|
|
60
|
Ohman KP, Karlberg BE, Nilsson OR and
Wettre S: Captopril in primary hypertension. Effects related to the
renin-angiotensin-aldosterone and kallikrein-kinin systems. Acta
Med Scand Suppl. 646:98–105. 1981.PubMed/NCBI
|
|
61
|
Andrade JM, de Farias Lelis D, Mafra V and
Cota J: The angiotensin converting enzyme 2 (ACE2), gut microbiota,
and cardiovascular health. Protein Pept Lett. 24:827–832.
2017.PubMed/NCBI View Article : Google Scholar
|
|
62
|
Perlot T and Penninger JM: ACE2-from the
renin-angiotensin system to gut microbiota and malnutrition.
Microbes Infect. 15:866–873. 2013.PubMed/NCBI View Article : Google Scholar
|
|
63
|
Zhang QL, Chen XH, Zhou SJ, Lei YQ, Huang
JS, Chen Q and Cao H: Relationship between disorders of the
intestinal microbiota and heart failure in infants with congenital
heart disease. Front Cell Infect Microbiol.
13(1152348)2023.PubMed/NCBI View Article : Google Scholar
|
|
64
|
Desai D, Desai A, Jamil A, Csendes D,
Gutlapalli SD, Prakash K, Swarnakari KM, Bai M, Manoharan MP, Raja
R and Khan S: Re-defining the gut heart axis: A systematic review
of the literature on the role of gut microbial dysbiosis in
patients with heart failure. Cureus. 15(e34902)2023.PubMed/NCBI View Article : Google Scholar
|
|
65
|
Xu X, Hu H, Zeng H, Li B, Yin Q, Jiang Y,
Zang L, Zhao C and Qian G: Sinisan ameliorates colonic injury
induced by water immersion restraint stress by enhancing intestinal
barrier function and the gut microbiota structure. Pharm Biol.
61:598–609. 2023.PubMed/NCBI View Article : Google Scholar
|
|
66
|
Tousoulis D, Guzik T, Padro T, Duncker DJ,
Luca GD, Eringa E, Vavlukis M, Antonopoulos AS, Katsimichas T,
Cenko E, et al: Mechanisms, therapeutic implications, and
methodological challenges of gut microbiota and cardiovascular
diseases: A position paper by the ESC working group on coronary
pathophysiology and microcirculation. Cardiovasc Res.
118:3171–3182. 2022.PubMed/NCBI View Article : Google Scholar
|
|
67
|
Cui X, Su Y, Huang X, Chen J, Ma J, Liao P
and He X: Combined analysis of plasma metabolome and intestinal
microbiome sequencing to explore jiashen prescription and its
potential role in changing intestine-heart axis and effect on
chronic heart failure. Front Cardiovasc Med.
10(1147438)2023.PubMed/NCBI View Article : Google Scholar
|
|
68
|
Zong X, Fan Q, Yang Q, Pan R, Zhuang L, Xi
R, Zhang R and Tao R: Trimethyllysine, a trimethylamine N-oxide
precursor, predicts the presence, severity, and prognosis of heart
failure. Front Cardiovasc Med. 9(907997)2022.PubMed/NCBI View Article : Google Scholar
|
|
69
|
Li X, Fan Z, Cui J, Li D, Lu J, Cui X, Xie
L, Wu Y, Lin Q and Li Y: Trimethylamine N-Oxide in heart failure: A
meta-analysis of prognostic value. Front Cardiovasc Med.
16(817396)2022.PubMed/NCBI View Article : Google Scholar
|
|
70
|
Wang Z, Tang WH, Buffa JA, Fu X, Britt EB,
Koeth RA, Levison BS, Fan Y, Wu Y and Hazen SL: Prognostic value of
choline and betaine depends on intestinal microbiota-generated
metabolite trimethylamine-N-oxide. Eur Heart J. 35:904–910.
2014.PubMed/NCBI View Article : Google Scholar
|
|
71
|
Wang Z, Klipfell E, Bennett BJ, Koeth R,
Levison BS, Dugar B, Feldstein EB, Britt EB, Fu X, Chung YM, et al:
Gut flora metabolism of phosphatidylcholine promotes cardiovascular
disease. Nature. 472:57–63. 2011.PubMed/NCBI View Article : Google Scholar
|
|
72
|
Lam V, Su J, Koprowski S, Hsu A, Tweddell
JS, Rafiee P, Gross GJ, Salzmman NH and Baker JE: Intestinal
microbiota determine severity of myocardial infarction in rats.
FASEB J. 26:1727–1735. 2012.PubMed/NCBI View Article : Google Scholar
|
|
73
|
Lam V, Su J, Hsu A, Gross GJ, Salzman NH
and Baker JE: Intestinal microbial metabolites are linked to
severity of myocardial infarction in rats. PLoS One.
11(e0160840)2016.PubMed/NCBI View Article : Google Scholar
|
|
74
|
Gan XT, Ettinger G, Huang CX, Burton JP,
Haist JV, Rajapurohitam V, Sidaway JE, Martin G, Gloor GB, Swann
JR, et al: Probiotic administration attenuates myocardial
hypertrophy and heart failure after myocardial infarction in the
rat. Circ Heart Fail. 7:491–499. 2014.PubMed/NCBI View Article : Google Scholar
|
|
75
|
Tang WH and Hazen SL: The contributory
role of gut microbiota in cardiovascular disease. J Clin Invest.
124:4204–4211. 2014.PubMed/NCBI View Article : Google Scholar
|
|
76
|
Bäckhed F, Ding H, Wang T, Hooper LV, Koh
GY, Nagy A, Semenkovich CF and Gordon JI: The gut microbiota as an
environmental factor that regulates fat storage. Proc Natl Acad Sci
USA. 101:15718–15723. 2004.PubMed/NCBI View Article : Google Scholar
|
|
77
|
Jones BV, Begley M, Hill C, Gahan CG and
Marchesi JR: Functional and comparative metagenomic analysis of
bile salt hydrolase activity in the human gut microbiome. Proc Natl
Acad Sci USA. 105:13580–13585. 2008.PubMed/NCBI View Article : Google Scholar
|
|
78
|
Chiang JY: Bile acids: Regulation of
synthesis. J Lipid Res. 50:1955–1966. 2009.PubMed/NCBI View Article : Google Scholar
|
|
79
|
Ferrell JM, Boehme S, Li F and Chiang JY:
Cholesterol 7α-hydroxylase-deficient mice are protected from
high-fat/high-cholesterol diet-induced metabolic disorders. J Lipid
Res. 57:1144–1154. 2016.PubMed/NCBI View Article : Google Scholar
|
|
80
|
Li T, Francl JM, Boehme S, Ochoa A, Zhang
Y, Klaassen CD, Erickson SK and Chiang JY: Glucose and insulin
induction of bile acid synthesis: Mechanisms and implication in
diabetes and obesity. J Biol Chem. 287:1861–1873. 2012.PubMed/NCBI View Article : Google Scholar
|
|
81
|
Broeders EP, Nascimento EB, Havekes B,
Brans B, Roumans KH, Tailleux A, Schaart G, Kouach M, Charton J,
Deprez B, et al: The bile acid chenodeoxycholic acid increases
human brown adipose tissue activity. Cell Metab. 22:418–426.
2015.PubMed/NCBI View Article : Google Scholar
|
|
82
|
Kumar PS, Mason MR, Brooker MR and O'Brien
K: Pyrosequencing reveals unique microbial signatures associated
with healthy and failing dental implants. J Clin Periodontol.
39:425–433. 2012.PubMed/NCBI View Article : Google Scholar
|
|
83
|
Mayerhofer CCK, Ueland T, Broch K, Vincent
RP, Cross GF, Dahl CP, Aukrust P, Gullestad L, Hov JR and Trøseid
M: Increased Secondary/Primary bile acid ratio in chronic heart
failure. J Card Fail. 23:666–671. 2017.PubMed/NCBI View Article : Google Scholar
|
|
84
|
Dam H: The formation of coprosterol in the
intestine: The action of intestinal bacteria on cholesterol.
Biochem J. 28:820–825. 1934.PubMed/NCBI View Article : Google Scholar
|
|
85
|
Lichtenstein AH: Intestinal cholesterol
metabolism. Ann Med. 22:49–52. 1990.PubMed/NCBI View Article : Google Scholar
|
|
86
|
Illman RJ, Storer GB and Topping DL: White
wheat flour lowers plasma cholesterol and increases cecal steroids
relative to whole wheat flour, wheat bran and wheat pollard in
rats. J Nutr. 123:1094–1100. 1993.PubMed/NCBI View Article : Google Scholar
|
|
87
|
Midtvedt AC and Midtvedt T: Conversion of
cholesterol to coprostanol by the intestinal microflora during the
first two years of human life. J Pediatr Gastroenterol Nutr.
17:161–168. 1993.PubMed/NCBI View Article : Google Scholar
|
|
88
|
Benno P, Midtvedt K, Alam M, Collinder E,
Norin E and Midtvedt T: Examination of intestinal conversion of
cholesterol to coprostanol in 633 healthy subjects reveals an age-
and sex-dependent pattern. Microbial Ecology in Health and Disease.
17:200–204. 2005.
|
|
89
|
Midtvedt T, Lingaas E, Carlstedt-Duke B,
Höverstad T, Midtvedt AC, Saxerholt H, Steinbakk M and Norin KE:
Intestinal microbial conversion of cholesterol to coprostanol in
man. Influence of antibiotics. APMIS. 98:839–844. 1990.PubMed/NCBI View Article : Google Scholar
|
|
90
|
Veiga P, Juste C, Lepercq P, Saunier K,
Béguet F and Gérard P: Correlation between faecal microbial
community structure and cholesterol-to-coprostanol conversion in
the human gut. FEMS Microbiol Lett. 242:81–86. 2005.PubMed/NCBI View Article : Google Scholar
|
|
91
|
Gerard P, Lepercq P, Leclerc M, Gavini F,
Raibaud P and Juste C: Bacteroides sp. strain D8, the first
cholesterol-reducing bacterium isolated from human feces. Appl
Environ Microbiol. 73:5742–5749. 2007.PubMed/NCBI View Article : Google Scholar
|
|
92
|
Ren D, Li L, Schwabacher AW, Young JW and
Beitz DC: Mechanism of cholesterol reduction to coprostanol by
Eubacterium coprostanoligenes ATCC 51222. Steroids. 61:33–40.
1996.PubMed/NCBI View Article : Google Scholar
|
|
93
|
Li L, Baumann CA, Meling DD, Sell JL and
Beitz DC: Effect of orally administered Eubacterium
Coprostanoligenes ATCC 51222 on plasma cholesterol concentration in
laying hens. Poult Sci. 75:743–745. 1996.PubMed/NCBI View Article : Google Scholar
|
|
94
|
Rosenfeld RS, Fukushima DK, Hellman L and
Gallagher TF: The transformation of cholesterol to coprostanol. J
Biol Chem. 211:301–311. 1954.PubMed/NCBI
|
|
95
|
Antharam VC, McEwen DC, Garrett TJ, Dossey
AT, Li EC, Kozlov AN, Mesbah Z and Wang GP: An integrated
metabolomic and microbiome analysis identified specific gut
microbiota associated with fecal cholesterol and coprostanol in
clostridium difficile infection. PLoS One.
11(e0148824)2016.PubMed/NCBI View Article : Google Scholar
|
|
96
|
Lye HS, Rusul G and Liong MT: Removal of
cholesterol by lactobacilli via incorporation and conversion to
coprostanol. J Dairy Sci. 93:1383–1392. 2010.PubMed/NCBI View Article : Google Scholar
|
|
97
|
Tahri K, Grill JP and Schneider F:
Involvement of trihydroxyconjugated bile salts in cholesterol
assimilation by bifidobacteria. Curr Microbiol. 34:79–84.
1997.PubMed/NCBI View Article : Google Scholar
|
|
98
|
Gérard P: Metabolism of cholesterol and
bile acids by the gut microbiota. Pathogens. 3:14–24.
2013.PubMed/NCBI View Article : Google Scholar
|
|
99
|
Nutting CW, Islam S and Daugirdas JT:
Vasorelaxant effects of short chain fatty acid salts in rat caudal
artery. Am J Physiol. 261:H561–H567. 1991.PubMed/NCBI View Article : Google Scholar
|
|
100
|
Macfarlane GT and Macfarlane S:
Fermentation in the human large intestine: Its physiologic
consequences and the potential contribution of prebiotics. J Clin
Gastroenterol. 45 (Suppl):S120–S127. 2011.PubMed/NCBI View Article : Google Scholar
|
|
101
|
Musso G, Gambino R and Cassader M:
Interactions between gut microbiota and host metabolism
predisposing to obesity and diabetes. Annu Rev Med. 62:361–380.
2011.PubMed/NCBI View Article : Google Scholar
|
|
102
|
David LA, Maurice CF, Carmody RN,
Gootenbreg DB, Button JE, Wolfe BE, Ling AV, Devlin AS, Varma Y,
Fischbach MA, et al: Diet rapidly and reproducibly alters the human
gut microbiome. Nature. 505:559–563. 2014.PubMed/NCBI View Article : Google Scholar
|
|
103
|
Krishnan S, Alden N and Lee K: Pathways
and functions of gut microbiota metabolism impacting host
physiology. Curr Opin Biotechnol. 36:137–145. 2015.PubMed/NCBI View Article : Google Scholar
|
|
104
|
Pluznick J: A novel SCFA receptor, the
microbiota, and blood pressure regulation. Gut Microbes. 5:202–207.
2014.PubMed/NCBI View Article : Google Scholar
|
|
105
|
Yang T, Santisteban MM, Rodriguez V, Li E,
Ahmari N, Carvajal JM, Zadeh M, Gong M, Qi Y, Zubcevic J, et al:
Gut dysbiosis is linked to hypertension. Hypertension.
65:1331–1340. 2015.PubMed/NCBI View Article : Google Scholar
|
|
106
|
Jama HA, Snelson M, Schutte AE, Muir J and
Marques FZ: Recommendations for the use of dietary fiber to improve
blood pressure control. Hypertension. 81:1450–1459. 2024.PubMed/NCBI View Article : Google Scholar
|
|
107
|
Falony G, Vieira-Silva S and Raes J:
Microbiology meets big data: The case of gut microbiota-derived
Trimethylamine. Annu Rev Microbiol. 69:305–321. 2015.PubMed/NCBI View Article : Google Scholar
|
|
108
|
Koeth RA, Wang Z, Levison BS, Buffa JA,
Org E, Sheehy BT, Britt EB, Fu X, Wu Y, Li L, et al: Intestinal
microbiota metabolism of L-carnitine, a nutrient in red meat,
promotes atherosclerosis. Nat Med. 19:576–585. 2013.PubMed/NCBI View Article : Google Scholar
|
|
109
|
Zeisel SH and Warrier M: Trimethylamine
N-oxide, the microbiome, and heart and kidney disease. Annu Rev
Nutr. 37:157–181. 2017.PubMed/NCBI View Article : Google Scholar
|
|
110
|
Tang WHW, Wang Z, Levison BS, Koeth RA,
Britt EB, Fu X, Wu Y and Hazen SL: Intestinal microbial metabolism
of phosphatidylcholine and cardiovascular risk. N Engl J Med.
368:1575–1584. 2013.PubMed/NCBI View Article : Google Scholar
|
|
111
|
Chittim CL, del Campo AM and Balskus EP:
Gut bacterial phospholipase Ds support disease-associated
metabolism by generating choline. Nat Microbiol. 4:155–163.
2019.PubMed/NCBI View Article : Google Scholar
|
|
112
|
Allayee H and Hazen SL: Contribution of
gut bacteria to lipid levels: Another metabolic role for microbes?
Circ Res. 117:750–754. 2015.PubMed/NCBI View Article : Google Scholar
|
|
113
|
Ghazalpour A, Cespedes I, Bennett BJ and
Allayee H: Expanding role of gut microbiota in lipid metabolism.
Curr Opin Lipidol. 27:141–147. 2016.PubMed/NCBI View Article : Google Scholar
|
|
114
|
Al-Obaide MAI, Singh R, Datta P,
Rewers-Felkins KA, Salguero MV, Al-Obaidi I, Kottapalli KR and
Vasylyeva TL: Gut microbiota-dependent trimethylamine-N-oxide and
serum biomarkers in patients with T2DM and advanced CKD. J Clin
Med. 6(86)2017.PubMed/NCBI View Article : Google Scholar
|
|
115
|
Zhu W, Gregory JC, Org E, Buffa JA, Gupte
N, Wang Z, Li L, Fu X, Wu Y, Mehrabian M, et al: Gut microbial
metabolite TMAO enhances platelet hyperreactivity and thrombosis
risk. Cell. 165:111–124. 2016.PubMed/NCBI View Article : Google Scholar
|
|
116
|
Ley RE, Turnbaugh PJ, Klein S and Gordon
JI: Microbial ecology: Human gut microbes associated with obesity.
Nature. 444:1022–1023. 2006.PubMed/NCBI View Article : Google Scholar
|
|
117
|
Liu TX, Niu HT and Zhang SY: Intestinal
microbiota metabolism and atherosclerosis. Chin Med J (Engl).
128:2805–2811. 2015.PubMed/NCBI View Article : Google Scholar
|
|
118
|
Wang Z, Roberts AB, Buffa JA, Levison BS,
Zhu W, Oeg E, Gu X, Huang Y, Zamanian-Daryoush M, Culley MK, et al:
Non-lethal inhibition of gut microbial Trimethylamine production
for the treatment of atherosclerosis. Cell. 163:1585–1595.
2015.PubMed/NCBI View Article : Google Scholar
|
|
119
|
Martin FP, Wang Y, Sprenger N, Yap IKS,
Lundsedt T, Lek P, Rezzi S, Ramadan Z, van Bladeren P, Fay LB, et
al: Probiotic modulation of symbiotic gut microbial-host metabolic
interactions in a humanized microbiome mouse model. Mol Syst Biol.
4(157)2008.PubMed/NCBI View Article : Google Scholar
|
|
120
|
Nakamura Y, Masuda O and Takano T:
Decrease of tissue angiotensin I-converting enzyme activity upon
feeding sour milk in spontaneously hypertensive rats. Biosci
Biotechnol Biochem. 60:488–489. 1996.PubMed/NCBI View Article : Google Scholar
|
|
121
|
Nakamura Y, Yamamoto N, Sakai K and Takano
T: Antihypertensive effect of sour milk and peptides isolated from
it that are inhibitors to angiotensin I-converting enzyme. J Dairy
Sci. 78:1253–1257. 1995.PubMed/NCBI View Article : Google Scholar
|
|
122
|
Kim B, Park KY, Ji Y, Park S, Holzapfel W
and Hyun CK: Protective effects of Lactobacillus rhamnosus GG
against dyslipidemia in high-fat diet-induced obese mice. Biochem
Biophys Res Commun. 473:530–536. 2016.PubMed/NCBI View Article : Google Scholar
|
|
123
|
Derrien M and van Hylckama Vlieg JE: Fate,
activity, and impact of ingested bacteria within the human gut
microbiota. Trends Microbiol. 23:354–366. 2015.PubMed/NCBI View Article : Google Scholar
|
|
124
|
Deng M, Zhang S, Wu S, Jiang Q, Teng W,
Luo T, Ouyang Y, Liu J and Gu B: Lactiplantibacillus plantarum N4
ameliorates lipid metabolism and gut microbiota structure in high
fat diet-fed rats. Front Microbiol. 7(1390293)2024.PubMed/NCBI View Article : Google Scholar
|
|
125
|
Ebel B, Lemetais G, Beney L, Cachon R,
Sokol H, Langella P and Gervais P: Impact of probiotics on risk
factors for cardiovascular diseases. A review. Crit Rev Food Sci
Nutr. 54:175–189. 2014.PubMed/NCBI View Article : Google Scholar
|
|
126
|
Khalesi S, Sun J, Buys N and Jayasinghe R:
Effect of probiotics on blood pressure: A systematic review and
meta-analysis of randomized, controlled trials. Hypertension.
64:897–903. 2014.PubMed/NCBI View Article : Google Scholar
|
|
127
|
De Filippis F, Pellegrini N, Vannini L,
Jeffery IB, Storia AL, Laghi L, Serrazanetti D, Cagno RD, Ferrocino
I, Lazzi C, et al: High-level adherence to a Mediterranean diet
beneficially impacts the gut microbiota and associated metabolome.
Gut. 65:1812–1821. 2016.PubMed/NCBI View Article : Google Scholar
|
|
128
|
Willett WC, Sacks F, Trichopoulou A,
Drescher G, Ferro-Luzzi A, Helsing E and Trichopoulos D:
Mediterranean diet pyramid: A cultural model for healthy eating. Am
J Clin Nutr. 61:1402S–1406S. 1995.PubMed/NCBI View Article : Google Scholar
|
|
129
|
Sofi F, Abbate R, Gensini GF and Casini A:
Accruing evidence on benefits of adherence to the Mediterranean
diet on health: An updated systematic review and meta-analysis. Am
J Clin Nutr. 92:1189–1196. 2010.PubMed/NCBI View Article : Google Scholar
|
|
130
|
Lopez-Garcia E, Rodriguez-Artalejo F, Li
TY, Fung TT, Li S, Willett WC, Rimm EB and Hu FB: The
Mediterranean-style dietary pattern and mortality among men and
women with cardiovascular disease. Am J Clin Nutr. 99:172–180.
2014.PubMed/NCBI View Article : Google Scholar
|
|
131
|
Cani PD, Delzenne NM, Amar J and Burcelin
R: Role of gut microflora in the development of obesity and insulin
resistance following high-fat diet feeding. Pathol Biol (Paris).
56:305–309. 2008.PubMed/NCBI View Article : Google Scholar
|
|
132
|
Zhou X, Li J, Guo J, Geng B, Ji W, Zhao Q,
Li J, Liu X, Liu J, Guo Z, et al: Gut-dependent microbial
translocation induces inflammation and cardiovascular events after
ST-elevation myocardial infarction. Microbiome.
6(66)2018.PubMed/NCBI View Article : Google Scholar
|
|
133
|
Qi Y, Aranda JM, Rodriguez V, Raizada MK
and Pepine CJ: Impact of antibiotics on arterial blood pressure in
a patient with resistant hypertension-A case report. Int J Cardiol.
201:157–158. 2015.PubMed/NCBI View Article : Google Scholar
|
|
134
|
Battson ML, Lee DM, Jarrell DK, Hou S,
Ecton KE, Weir TL and Gentile CL: Suppression of gut dysbiosis
reverses Western diet-induced vascular dysfunction. Am J Physiol
Endocrinol Metab. 314:E468–E477. 2018.PubMed/NCBI View Article : Google Scholar
|