Introduction
Deep venous thrombosis (DVT) is a common thrombotic
disease. According to previous reports, the incidence of DVT in
trauma patients without prophylaxis can be as high as 80% (1). Orthopedic trauma patients are at a
high risk for DVT owing to various risk factors and the incidence
of venous thromboembolism in orthopedic trauma patients ranges from
20.81-43.4% (2-5).
Although prophylactic medication can reduce the rate of DVT, the
morbidity and mortality rates of pulmonary embolism caused by DVT
remain high. Studies have shown that ~6% of patients with DVT and
12% of patients with pulmonary embolism succumb within 1 month of
diagnosis, seriously endangering the life and health of patients
(4). The current mechanisms of
interaction between certain therapeutic anticoagulants are diverse
and monitoring changes in coagulation factors is required in some
cases during the use of these medications (6). Therefore, further exploration of the
DVT pathogenesis is required to develop new treatment strategies
(7).
Endothelial cell (EC) damage is a key factor in
TDVT. When the endothelium is traumatized by fractures, trauma,
surgery, or other external factors, an inflammatory response and EC
apoptosis can occur. Simultaneously, it can promote the aggregation
of platelets and white blood cells as well as the activation of the
coagulation system, leading to thrombosis (8). During this process, a number of cells
and molecules, including monocytes, T cells, platelets and
coagulation factors, interact with each other. Excessive expression
of cytokines, such as interleukin IL-6, cyclooxygenase-2 (COX-2)
and tumor necrosis factor-α (TNF-α), can lead to EC damage and
death (9,10).
Toll-like receptor 2 (TLR2) is a receptor that
recognizes microbial components and plays critical roles in
infection and inflammation (11).
Cluster of differentiation 14 (CD14) is an auxiliary protein of
TLR2 that can bind to TLR2 to activate the TLR2 signaling pathway
(12). In ECs, TLR2 and CD14
regulate inflammatory responses through mutual interactions
(13). When pathogens or other
stimuli enter the body, they bind to TLR2 and activate the TLR2
signaling pathway. The involvement of TLR2 leads to the activation
of the nuclear factor-kappa B (NF-κB) pathway, increasing gene
expression and the release of proinflammatory mediators, such as
IL-1β and TNF-α, resulting in local inflammation (13,14).
The expression and activation of TLR2 have been shown to regulate
the function of a number of cell types such as dendritic cells,
monocytes and neutrophils (14).
In addition, TLR2 and CD14 can regulate the apoptosis and
proliferation of ECs, which influence the integrity and stability
of the endothelial barrier (15).
Previous studies showed that TLR2 expression increased in TDVT
samples (16,17). The present study aimed to further
clarify the regulatory mechanism of the TLR2/NF-κB/COX-2 signaling
pathway in TDVT.
Materials and methods
Animals
Specific pathogen-free (SPF) Sprague-Dawley (SD)
experimental rats were purchased from Sleek Venture Experimental
Animals Co., Ltd. [license number: SCXK (Xiang) 2019-0004]. The
experimental unit used the license number SYXK (Gan) 2018-0004. A
total of 30 male SPF SD rats (weighing 200-220 g, 6-to 8-week-old)
were housed at the Laboratory Animal Center of Gannan Medical
University (Ganzhou, China) under standard conditions of
temperature (20-25˚C), humidity (50-60%) and lighting (12-h
light/dark cycle), with free access to food and water. The rats
were allowed to adapt for 3-5 days before the experiment. All
animal experiments were conducted in the animal laboratory of the
Laboratory Animal Center of Gannan Medical University and were
approved by the Ethics Committee of Gannan Medical University
(approval no. LLSC-2022110701). All animal procedures were
performed following the guidelines of the Council on Animal Care
(18). The research complied with
the Five Freedoms (freedom from hunger and thirst, freedom from
discomfort, freedom from pain, injury and disease, freedom to
express normal behavior, freedom from fear and distress) and the 3R
principles (Replacement, Reduction and Refinement) for experimental
animals was observed. The present study strictly followed the
regulations and guidelines set by the ethics committee to ensure
that the welfare and rights of the animals were respected and
protected.
On the day of the experiments, the animals were
anesthetized with intravenous pentobarbital sodium (30 mg/kg). The
rats were anesthetized and maintained under anesthesia throughout
the experiment. At the end of the experiment, deep anesthesia was
induced for sample collection, followed by euthanasia via
intraperitoneal injection of 3% pentobarbital sodium (100 mg/kg) to
ensure a painless and humane procedure. Experimental procedures and
handling were conducted by trained professionals who ensured the
scientific validity and reliability of the experiment.
TDVT model
The TDVT model, widely used in the present study,
was established as follows: 28 male SPF SD rats weighing 200-220 g
were randomly divided into sham operation group, model group, C29
group and Pam3CSK4 group. Except for the sham operation group, all
SD rats were placed in the supine position and anesthetized with 3%
sodium pentobarbital solution at a dose of 1 mg/kg via intravenous
injection. Following anesthesia induction, a self-made traumatic
quantifiable impact device (19)
was used to deliver an instantaneous energy of 5 J striking each
side of the proximal outer thigh of the rats once (1 cm below the
greater trochanter) (19).
Fractures of the femur were confirmed by bone scraping sensation or
abnormal activity and confirmed by X-ray imaging. A window was
created on the surface of the plaster in the superficial femoral
vein area of the inner thigh on both sides to observe the formation
of thrombus. After modeling, the experimental rats were allowed
free access to food and water without the use of hemostatic agents
or antibiotics. At 0, 24, 48 and 96 h post-modeling, rats in the
sham operation and model groups were intraperitoneally injected
with an equal volume of physiological saline; the C29 group
received 5 mg/kg C29 (cat. no. HY-100461; MedChemExpress) (20) and the Pan3CSK4 group received 1
mg/kg Pam3CSK4 (InvivoGen) (21,22),
Based on the results of preliminary experiments, 5 mg/kg of C29 and
1 mg/kg of PAM3CSK4 were selected to achieve a balance between the
mortality rate of experimental animals and experimental
effectiveness.
Collection and measurement of the
venous thrombus
The rats were anesthetized via intraperitoneal
injection of 3% pentobarbital sodium solution (30 mg/kg) and
routine disinfection. At 24 h after the last administration, deep
anesthesia was induced for sample collection, followed by
euthanasia via intraperitoneal injection of 3% pentobarbital sodium
(100 mg/kg). The size, length and weight of deep vein thrombosis
and thrombus formation rate were measured.
Cell culture and grouping
Human umbilical vein EC (HUVECs; immortalized human
umbilical vein EC Line Certificate of STR Analysis:FH1122, STR
20170721-05)were purchased from Shanghai Fuheng Biology Co, Ltd.
and cultured in RPMI-1640 (cat. no. 22400089; Gibco; Thermo Fisher
Scientific, Inc.) supplemented with 10% fetal bovine serum (cat.
no. 10099141; Gibco; Thermo Fisher Scientific, Inc.) and 1%
penicillin/streptomycin (cat. no. P1400-100; Beijing Solarbio
Science & Technology Co., Ltd.) in a 5% CO2
incubator at 37˚C. At the logarithmic growth stage, the cells were
divided into control, oxygen-glucose deprivation (OGD) model
(model), Pam3CSK4 and C29 groups. All the cells were subjected to
OGD, with the exception of those in the control group. The HUVECs
were treated with Pam3CSK4 (1 µmol/l) or C29 (1 µmol/l) for 1 h
before OGD (23). Meanwhile, an
equal volume of RMPI-1640 was added to the control and OGD groups.
To induce OGD, the cells were rinsed and incubated in glucose-free
RPMI 1640 (cat. no. 11879020; Gibco; Thermo Fisher Scientific,
Inc.) saturated with 95% N2 and 5% CO2 for 6
h. The cells were then rinsed and maintained under normal
conditions for 24 h.
Cell counting kit 8 (CCK8)
HUVECs at the logarithmic growth stage were seeded
into 96-well plates (3,000 cells/well) and treated with Pam3CSK4
(0.1, 1.0 and 10 µmol/l) or C29 (0.1, 1.0 and 10 µmol/l) for 24, 48
and 72 h. CCK8 proliferation assay was performed using a CCK8 Kit
(cat. no. CA1210; Beijing Solarbio Science & Technology Co.,
Ltd.) according to the manufacturer's instructions. Each well was
treated with 10 µl of CCK8 for 4 h and the absorbance was measured
at 450 nm using a microplate reader (VLBLATD2 Multifunctional
enzyme marker; Thermo Fisher Scientific, Inc.). Each group had six
wells and the experiment was repeated thrice.
Annexin V-FITC/PI flow cytometry
HUVECs were seeded into six-well plates at a density
of 5x104/ml, followed by the addition of Pam3CSK4 (1
µmol/l) or C29 (1 µmol/l) for 24 h. The cells were collected and
stained using an Annexin V-FITC/PI kit (cat. no. C1062L; Beyotime
Institute of Biotechnology) according to the manufacturer's
protocol. The HUVECs were exposed to 5 µl of Annexin V-FITC and 10
µl of PI solution for 15 min in the dark. After staining, the cells
were run on a Gallios flow cytometer (Beckman Coulter, Inc.) and
the data were analyzed using the Kaluza software (perpetual A82959;
Beckman Coulter, Inc.).
Reverse transcription-quantitative
(RT-q) PCR
Total RNA from fresh deep vein tissues from model
animals and HUVECs (1x106/well in 6-well plates) was
extracted using TRIzol® reagent (Thermo Fisher
Scientific, Inc.) according to the manufacturer's protocol.
Additionally, 1 µg RNA was reverse transcribed into cDNA using the
HI Script Q RT SuperMix (Vazyme Biotech Co., Ltd.) according to the
manufacturer's instructions. RT-qPCR was performed using the cDNA
templates and SYBR (Takara Bio, Inc.) in a Bio-Rad CFX96 system
(Bio-Rad Laboratories, Inc.). Specific primers for TLR2, TF and
IL-6 were designed and synthesized according to the GenBank
(National Center for Biotechnology Information) sequences. RT-PCR
was performed using Maxima SYBR Green qPCR Master Mix (cat. no.
K0251; Thermo Fisher Scientific, Inc.). RT-PCR was performed using
Maxima SYBR Green qPCR Master Mix (cat. no. K0251; Thermo Fisher
Scientific, Inc.). The PCR cycling conditions were: denaturation at
95˚C for 30 sec, annealing at 60˚C for 30 sec and extension at 72˚C
for 30 sec, for a total of 40 cycles. Gene expression levels were
calculated using the 2-ΔΔCq method (24), normalized to GAPDH. The experiments
were replicated three times. The primer sequences are listed in
Table I.
 | Table ISequences of primers used for RT-PCR
analysis. |
Table I
Sequences of primers used for RT-PCR
analysis.
| Gene | Primer | Sequence
(5'-3') | PCR Products |
|---|
| GAPDH | Forward |
TCAAGAAGGTGGTGAAGCAGG | 115 bp |
| | Reverse |
TCAAAGGTGGAGGAGTGGGT | |
| TLR2 | Forward |
TGTGAAGAGTGAGTGGTGCA | 208 bp |
| | Reverse |
TACCCAAAATCCTTCCCGCT | |
| IL-6 | Forward |
AGGAGACTTGCCTGGTGAAA | 180 bp |
| | Reverse |
CAGGGGTGGTTATTGCATCT | |
| COX-2 | Forward |
CTCCTGTGCCTGATGATTGC | 169 bp |
| | Reverse |
AACTGATGCGTGAAGTGCTG | |
| P-selectin | Forward |
TGCCAGAATCGCTACACAGA | 181 bp |
| | Reverse |
TATCAGCCCAGTTCTCAGCC | |
| TF | Forward |
ACCCCAACTGGTGATGAAAG | 200 bp |
| | Reverse |
GAATGGCTGTTGTTGTAAATG | |
Western blot analysis
Total protein was extracted from HUVECs and vessel
tissues using radioimmunoprecipitation assay buffer (Beijing
Solarbio Science & Technology Co., Ltd.) supplemented with
protease and phosphatase inhibitors (Thermo Fisher Scientific,
Inc.). The protein concentration was determined using the BCA
Protein Assay Kit (cat. no. 71285-M; MilliporeSigma) following the
manufacturer's protocol. For western blot analysis, 50 µg of lysate
was loaded onto sodium dodecyl sulfate-polyacrylamide gel
electrophoresis gels, transferred onto polyvinylidene difluoride
membranes (MilliporeSigma). Equal amounts of proteins were resolved
on 10% SDS-PAGE gels and blotted to polyvinylidene difluoride
membranes. The membranes were blocked in 5% not-fat dry milk at
room temperature for 1 h and incubated overnight with primary
antibodies (1:1,000) against TLR2 (cat. no. ab213676; Abcam,
Cambridge, UK), NF-kB p65 (cat. no. ab16502; Abcam) and IL-6 (cat.
no. ab233706; Abcam). After retrieval of the primary antibody, the
membranes were washed thrice with 1XTris-buffered saline with 0.1%
Tween 20 for 5 min each. Secondary antibodies conjugated with
horseradish peroxidase were diluted 1:1,000 according to the
corresponding specifications. Incubation with secondary antibodies
was conducted at 4˚C overnight or at room temperature for 4 h.
Secondary antibodies (1:3,000; cat. nos. 31460 and 31430; Thermo
Fisher Scientific, Inc.) were incubated at room temperature for 1
h. Proteins were visualized using an ECL reagent (cat. no. 34580;
Thermo Fisher Scientific, Inc.) Densitometry analysis was performed
using Quantity One Software and quantified relative to the loading
control, β-actin. Image analysis was performed using Image-Pro Plus
software (version 6.0; Media Cybernetics, Inc.).
Immunofluorescence staining
NF-κB translocation in the vein tissues and HUVECs
was determined using an NF-κB Activation, Nuclear Translocation
Assay Kit (cat. no. SN368; Beyotime Institute of Biotechnology)
according to the manufacturer's instructions. HUVECs were seeded
onto slides at a density of 1x104 cells per slide. After
fixation with 4% paraformaldehyde for 15 min at room temperature
(approximately 25˚C). Cells were permeabilized with 0.1% Triton
X-100 in PBS for 10 min at room temperature. he sections and cells
were blocked with 3% bovine serum albumin (BSA; cat. no. A7030;
MilliporeSigma) for 1 h. The samples were incubated with primary
antibodies, including an NF-κB antibody (1:200, cat. no. ab16502;
Abcam) and TLR2 antibody (1:200, cat. no. ab213676; Abcam) for 1 h
at room temperature. They were then washed thrice with
phosphate-buffered saline (PBS) for 5 min each time. The samples
were incubated with a secondary antibody solution (1:500, goat
anti-rabbit IgG, cat. no. A-11008; Invitrogen) for 1 h at room
temperature. The sections and cells were treated with DAPI solution
(1 µg/ml, cat. no. D9542; MilliporeSigma) for 5 min at room
temperature. An antifluorescence quencher (cat. no. P0126; Beyotime
Institute of Biotechnology) was added. Images were captured under a
laser confocal fluorescence microscope (FV1200; Olympus
Corporation) at a magnification of 40x and analyzed using Image-Pro
Plus software (version 6.0; Media Cybernetics, Inc.).
Hematoxylin and eosin (H&E)
For histological examination, tissues were fixed
with 4% paraformaldehyde at room temperature for 24 h, washed with
70% alcohol, dehydrated through a graded ethanol series (70, 80,
95, and 100%, 10 min each), and cleared in xylene (two changes, 10
min each). Tissues were embedded in paraffin and cut into 4 µm
sections. Sections were deparaffinized in xylene (two changes, 10
min each), rehydrated through a graded ethanol series (100, 95, 80,
and 70%, 5 min each), and rinsed in distilled water. Hematoxylin
staining was performed at room temperature for 5 min, rinsed in
running tap water for 5 min, followed by eosin staining at room
temperature for 2 min. Sections were then dehydrated through a
graded ethanol series and cleared in xylene. Images were acquired
under a light microscope (DM 2500; Leica Microsystems GmbH).
Statistical analysis
Results were presented as mean ± SD and calculated
using the SPSS 21.0 software (IBM Corp.) and GraphPad Prism 8.0
(Dotmatics). One-way ANOVA test was employed followed by Tukey post
hoc test. P<0.05 was considered to indicate a statistically
significant difference.
Results
TLR2 activation promotes DVT whereas
TLR2 inhibition of reduces DVT in a TDVT model
A DVT model was first established using the
quantitative impact and gypsum fixation method (plaster cast) and
X-ray examination was performed to examine the effect (Fig. 1A). After successful modeling, the
number of thrombus formation cases in each group as well as the
length and weight of deep vein thrombosis were compared (Table II). Inflammation was evaluated via
hematoxylin and eosin staining of the tissue samples (Fig. 1B). The number of thrombus formation
cases, as well as the weight and length of the thrombi, were higher
in the Pam3CSK4 group compared with the model and C29 groups
(Fig. 1C-E). This indicated that
TLR2 activation increases the incidence of DVT in TDVT model rats,
whereas TLR2 inhibition reduces DVT incidence.
 | Figure 1Establishment of an animal model to
simulate TDVT formation using quantitative impact method. (A)
Representative image of the TDVT model established in rats (X-ray
image). (B) Histological sections of rat femoral veins after
modeling and hematoxylin and eosin staining. The images at the top
shows the gross specimen, displaying the extracted deep vein
without thrombosis, the deep vein with thrombosis, and the thrombus
within it, all after fixation with paraformaldehyde. (C) The rates
of DVT formation in different groups, where the sham group did not
form thrombi, the model group had a thrombus formation rate of
42.8%, the pam3csk4 group had a thrombus formation rate of 85.7%
and the C29 group had a thrombus formation rate of 28.5%. (D) The
length of thrombus formed in different groups as observed in gross
specimens, where there was a statistically significant difference
in thrombus length between the sham group and the pam3csk4 group
(P<0.05), between the model group and the pam3csk4 group
(P<0.05) and between the sham group and the pam3csk4 group
(P<0.05). (E) The weight of thrombus formed in different groups
as observed in gross specimens, where there was a statistically
significant difference in thrombus weight between the sham group
and the pam3csk4 group (P<0.05), between the model group and the
pam3csk4 group (P<0.05) and between the sham group and the
pam3csk4 group (P<0.05). All data were expressed as the mean ±
SD. One-way ANOVA followed by Tukey's test, *P<0.05,
**P<0.01, n=7. TDVT, traumatic deep vein
thrombosis. |
 | Table IIThrombosis rate in each group. |
Table II
Thrombosis rate in each group.
| Group | Cases of
thrombosis | Cases without
thrombosis | Total | Thrombosis rate
(%) |
|---|
| Sham | 0 | 7 | 7 | 0 |
| Model | 3 | 4 | 7 | 42.8 |
| Pam3csk4 | 6 | 1 | 7 | 85.7 |
| C29 | 2 | 5 | 7 | 28.6 |
| Total | 12 | 16 | 28 | |
TLR2 expression is increased in the
TDVT model group and is positively correlated with NF-κB
phosphorylation
TLR2 expression was higher in the model group and
NF-κB phosphorylation was positively correlated with TLR2
expression. To verify the relationship between TLR2 and its
downstream molecules in the TDVT model, immunofluorescence
staining, PCR and western blotting of TDVT tissues were conducted.
Immunofluorescence staining of TLR2 in the vascular tissues of the
animal model showed that TLR2 expression was significantly higher
in the model group than in the sham group. In addition, TLR2
expression was significantly higher in the model + Pam3CSK4 group
than in the model + C29 group (Fig.
2A and C). The present study
also found a positive correlation between NF-κB expression and TLR2
expression as well as intergroup differences in expression
(Fig. 2B and D). qPCR revealed that compared with those
in the model group, the vascular EC of rats in the model + Pam3CSK4
and model + C29 groups had an increased transcription of TLR2 and
the differences were significant (P<0.05; Fig. 2E). These results were confirmed by
western blotting, which showed that TLR2 expression was positively
correlated with P-65 and phosphorylated (p-)P65 expression in the
TDVT animal model (Fig. 2F-I).
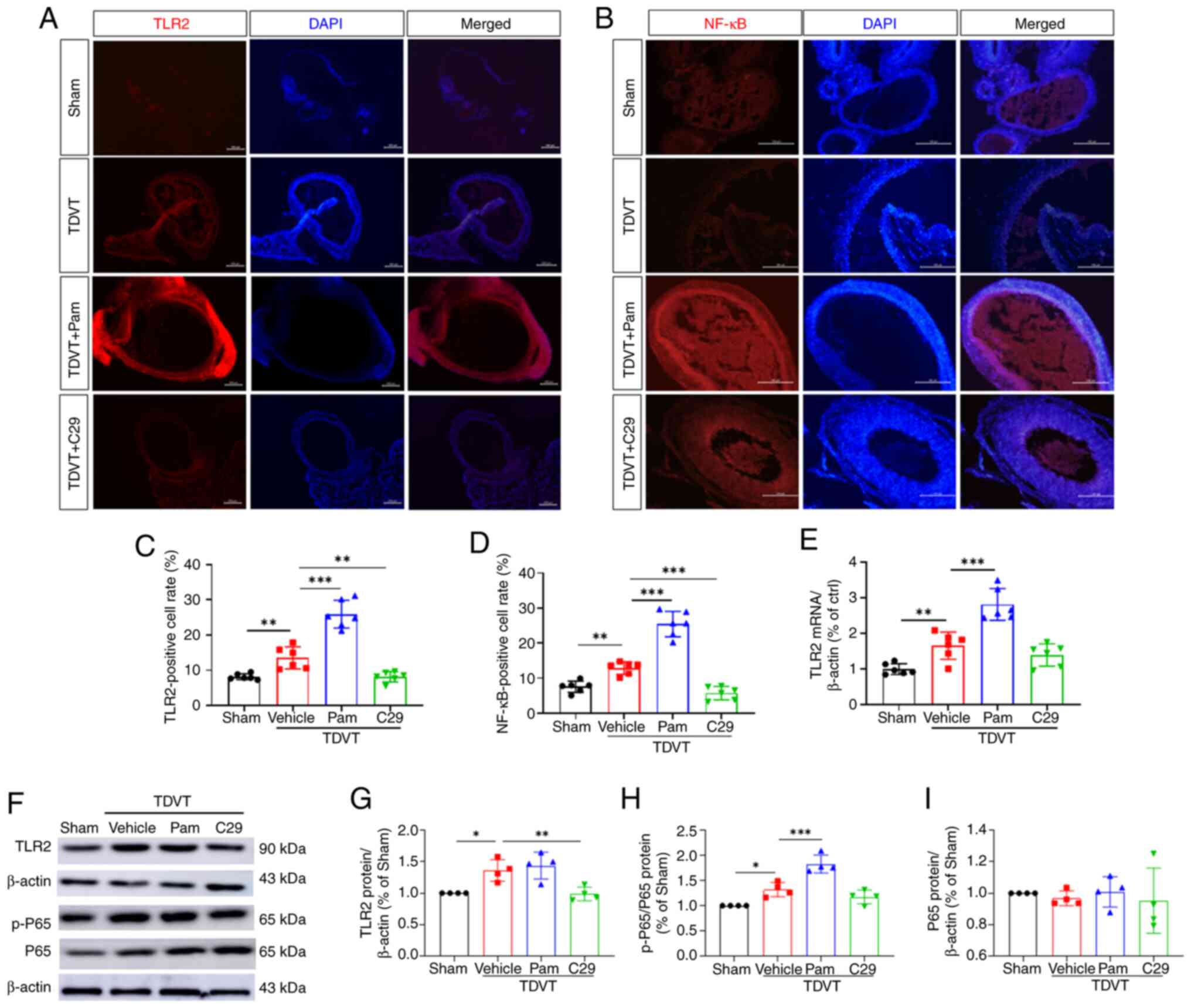 | Figure 2TLR2/NF-κB signaling were activated
in the deep veins of the TDVT model animals. (A and B)
Immunofluorescence results of TLR2 and NF-κB in the deep vein
tissues, magnification, x20. (C and D) TLR2 and NF-κB positive cell
rate. (E) Relative mRNA expression of TLR2 in the deep vein
tissues. (F-I) Relative protein levels of TLR2, p-P65 and P65 in
the deep vein tissues. All data were expressed as the mean ± SD.
One-way ANOVA followed by Tukey post hoc test,
*P<0.05, **P<0.01 and
***P<0.001. TLR2, Toll-like receptor; NF-κB, nuclear
factor kappa B; TDVT, traumatic deep vein thrombosis; p-,
phosphorylated; Pam, Pam3CSK4. |
The downstream molecules of TLR2/NF-κB
in the TDVT model are IL-6, COX-2 and P-selectin
To validate the relationship between TLR2 and its
downstream molecules in the TDVT model, the PCR and western
blotting data from the tissues of the TDVT animal model were
analyzed. It was found that TLR2 expression was significantly
higher in the model group than in the sham group, along with a
significant increase in the gene expression of IL-6, COX-2 and
P-selectin. In the model + Pam3CSK4 group, the gene expression
levels of IL-6, COX-2, P-selectin and TF were significantly higher
than those in the model + C29 group (Fig. 3A and D). Western blotting analysis revealed
that the protein levels of IL-6 and COX-2 in the TDVT model were
higher than those in the sham group. Furthermore, TLR2 activation
led to an increase in protein levels, whereas TLR2 inhibition
resulted in a significant decrease in protein levels (Fig. 3E-G). Based on these results, it was
considered that IL-6, COX-2 and P-selectin are the downstream
molecules of TLR2/NF-κB in the TDVT model.
Validation of changes in the TDVT
model caused by EC
To validate the specific cellular changes
responsible for alterations in thrombus formation in the TDVT
model, the present study focused on EC injury induced by
inflammation as a key factor in TDVT pathogenesis. EC are an
important component of the vascular wall that maintain the
integrity and normal blood flow of the vessel by controlling
platelet activity and clotting factors in the blood. The present
study used HUVECs and established an OGD model for analysis. The
appropriate drug concentration for experimentation was determined
using the CCK8 method. Pam3CSK4 at 1 µmol/l showed inhibitory
effects on HUVECs and this effect increased with increasing
Pam3CSK4 concentration. At low concentrations, C29 promoted HUVEC
proliferation, but it peaked at 1 µmol/l and then declined.
Pam3CSK4 and C29 both had the most significant promoting or
inhibitory effects at 1 µmol/l and the differences compared with
the control group were significant (Fig. 4A). In addition, the effects of TLR2
activation and inhibition on EC apoptosis under OGD conditions were
observed. It was found that the apoptosis rate in the vehicle group
was significantly higher than that in the control group but was
lower than that in the Pam3CSK4 (Fig.
4B-D).
The downstream molecules of TLR2/NF-κB
in the OGD model are IL-6, COX-2 and P-selectin
To verify the relationship between TLR2 and its
downstream molecules in the HUVEC OGD model, PCR analysis weas
performed. It was found that IL-6 expression increased in the
vehicle group compared with the sham group and that the gene
expression of IL-6, COX-2, P-selectin and TF increased
significantly. In the model + Pam3CSK4 group, the gene expression
of IL-6, COX-2, P-selectin and TF was significantly higher than
that in the model + C29 group (Fig.
5A-D). Western blotting revealed that the protein levels of
IL-6 and COX-2 in the TDVT model were higher than those in the sham
group. TLR2 activation increased their protein levels, whereas TLR2
inhibition significantly decreased their levels (Fig. 5E-G). These results indicate that
IL-6, COX-2 and P-selectin are the downstream molecules of
TLR2/NF-κB.
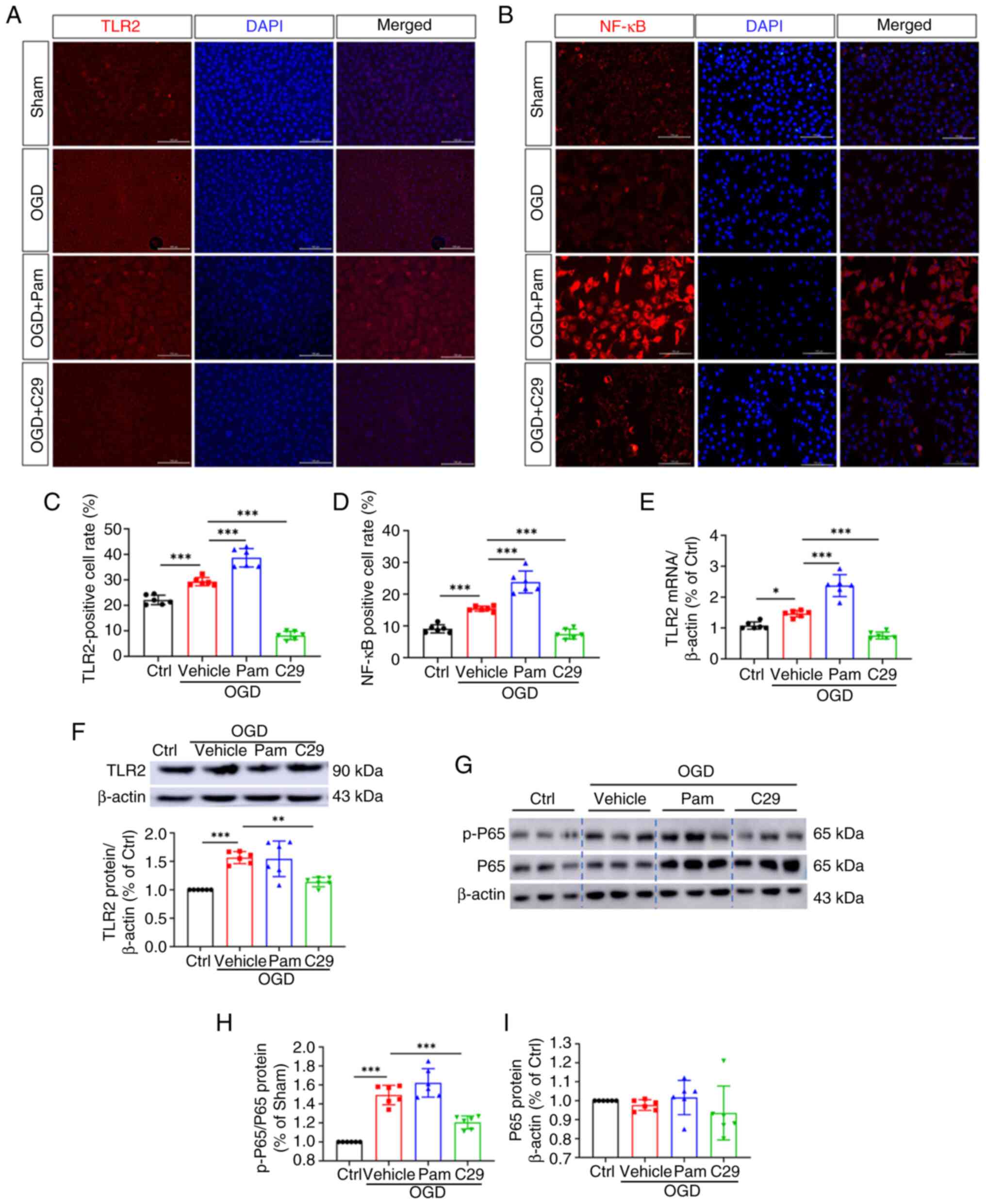 | Figure 5TLR2/NF-κB signaling were activated
in HUVECs after OGD injury. Immunofluorescence results of (A) TLR2
and (B) NF-κB in HUVECs (magnification, x100), (C and D) TLR2 and
NF-κB positive cell rate. (E) Relative mRNA levels of TLR2 in
HUVECs. (F-I) Relative protein levels of TLR2, p-P65, P65 in
HUVECs. All data were expressed as the mean ± SD. One-way ANOVA
followed by Tukey test, *P<0.05,
**P<0.01 and ***P<0.001, n=6. TLR2,
Toll-like receptor 2; NF-κB, nuclear factor kappa B; OGD, oxygen
glucose deprivation; HUVECs, human umbilical vein endothelial
cells; Ctrl, control; Pam, Pam3CSK4. |
Immunofluorescence reveals that IL-6,
COX-2 and P-selectin are the downstream molecules of TLR2/NF-κB in
the OGD model
To investigate the relationship between TLR2 and its
downstream molecules in the HUVEC OGD model, immunofluorescence
staining, PCR and western blotting analysis were performed.
Immunofluorescence staining of TLR2 in the vein tissue of the
cellular OGD model showed that TLR2 expression significantly
increased in the vehicle group compared with that in the sham
group. TLR2 expression in the OGD + Pam3CSK4 group was
significantly higher than that in the OGD + C29 group (Fig. 5A and C). Meanwhile, NF-κB expression was
positively associated with TLR2 expression and showed intergroup
differences (Fig. 5B and D). qPCR showed that the transcription
level of TLR2 in the EC of the model + Pam3CSK4 group was greater
than that in the model group. The transcription levels of TLR2, P65
and p-P65 genes in the EC of the model + C29 group were higher than
that in the model group. The differences between the two groups
were significant (P<0.05; Fig.
6E). Similar results were obtained in western blotting, which
showed a positive correlation between TLR2 expression and the gene
expression of P65 and p-P65 in the TDVT animal model (Fig. 6F-I).
 | Figure 6Downstream molecules of TLR2/NF-κB in
the OGD model. Relative mRNA expressions of (A) IL-6, (B) COX-2,
(C) P-selectin and (D) TF in different groups of HUVECs. (E-G)
Relative protein levels of IL-6 and COX-2 in different groups of
HUVECs. All data were expressed as the mean ± SD. One-way ANOVA
followed by Tukey test, *P<0.05,
**P<0.01 and ***P<0.00, n=6. Toll-like
receptor 2; NF-κB, nuclear factor kappa B; OGD, oxygen glucose
deprivation; COX-2, cyclooxygenase-2; HUVECs, human umbilical vein
endothelial cells; Ctrl, control; Pam, Pam3CSK4; Tissue Factor. |
Discussion
TVDT is a prevalent complication of severe trauma or
surgery and is a serious threat to human health. Currently, the
diagnosis and treatment of DVT remain at the stage of coagulation
function examination and post-onset treatment. The consequences of
the onset of TVDT impose a heavy burden on patients. Therefore,
identifying the pathogenesis of TDVT is an important research
direction for improving its diagnosis and treatment. Studies have
shown that DVT and inflammation have similar cellular signaling
pathways and that inflammation significantly contributes to the
development of TVDT (2,23). TLR2/NF-κB is a common inflammatory
signaling pathway. TLR2 is a potential target for inflammatory
injury in various EC and this mechanism may be related to the
enhanced TLR2 activation of NF-κB (3).
Vascular EC are multifunctional cells on the inner
side of the vascular wall that have important regulatory functions
in various physiological and pathological processes, such as
thrombosis, inflammation, immunity, angiogenesis and substance
transport (3). Their basic
function is to maintain unobstructed blood flow in blood vessels
(24). Recent studies have found
that in a mouse model of DVT, a process of acute to chronic
inflammation in the vein walls occurs (5). These results suggested that
inflammation is closely associated with DVT. When inflammation
causes the activation of inflammatory cytokines, the release of a
large number of inflammatory mediators damages EC in the vascular
wall (3). Simultaneously, large
amounts of TF, von Willebrand factor, adhesion molecules and
cytokines are released (6) and the
anti-adhesion, anticoagulant, vasodilator and anti-inflammatory
phenotypes are transformed into activated states, thus promoting
adhesion, coagulation and inflammation. In addition, EC injury can
cause leukocyte aggregation and platelet adhesion, aggregation and
activation, which causes blood to be in a hypercoagulable state,
leading to an imbalance in the coagulation and fibrinolysis
systems, thus promoting the occurrence and development of DVT
(8,11). Therefore, the structural and
functional integrity of the vascular EC largely determines
thrombosis.
Evidence has shown that TLR2 may be a potential
target for inflammatory injury in various EC (11). Other studies have suggested that
the expression of COX-2 is regulated by the NF-κB signaling pathway
(9). Studies on TLR2 signaling
molecules have mostly focused on atherosclerosis (9,10),
osteoarthritis (25) and tumors
(26). Some studies have analyzed
changes in the expression of TLR2, NF-κB and COX-2 in DVT samples
using bioinformatics analysis (10,11,26)
and preliminarily found that the three molecules are closely
related to thrombosis.
TLRs are transmembrane proteins that belong to the
pattern recognition receptor family and are highly evolutionarily
conserved (27). Studies have
shown that TLR2 and TLR4 are the most important molecules involved
in TLR-mediated inflammatory responses, with TLR2 being mainly
distributed in immune cells such as monocytes, macrophages and EC
(11,13). When the blood flow status in the
blood vessel changes (such as in a case of turbulence), the TLR2
distributed in EC is easily activated and its expression increases
rapidly (14). The inflammatory
response induced by TLRs (except TLR3) is widely considered to
occur through a classical signaling pathway that starts from a
conserved intracellular sequence of TLRs, the Toll/IL-1 receptor
homologous region (14,28), which can activate mitogen-activated
protein kinase (MAPK) and IκB kinase, further activating the
expression of inflammatory factors induced by NF-κB (11,29).
NF-κB is a downstream molecule of TLR2, wherein
transcription-dependent NF-κB binds to MYD88 and MAL to activate
related kinases, further phosphorylating IKB and releases the P50
and p65 subunits of the NF-κB molecule, thereby causing an
inflammatory reaction and facilitating gene transcription (11,27).
In an independent pathway of NF-κB transcription, nuclear
translocation of activated NF-κB can directly cause inflammatory
reactions and guide the transcription of related genes. In
addition, NF-κB can be activated by a variety of stimulants, such
as cytokines, growth factors, bacterial or viral products and
reactive oxygen species (30). The
activated NF-κB, its double-stranded DNA and the MAPK system
further activate EC, promoting the expression of inflammatory
mediators such as ICAM-1, VCAM-I and E-selectin and increasing the
expression of inflammatory mediators such as TNF-α, IL-1, IL-6,
IL-8 and IL-17, which amplify the inflammatory effect (31-33).
TNF-α, IL-1 and CD40 can induce the expression of TF in EC and
monocytes (29,34) and NF-κB can mediate the activation
of TF in vascular smooth muscle cells and venous vascular EC, thus
playing an important role in venous thrombosis (32).
COX-2 is an inducible enzyme that is generally not
expressed in normal tissues but is highly expressed when cells are
stimulated by cancer-promoting agents, inflammatory mediators,
cytokines and growth factors (9).
COX-2 has NF-κB binding sites that mediate COX-2 expression
(9). The latter promotes the
oxidative stress response, leading to the accumulation of reactive
oxygen species and can convert arachidonic acid into prostaglandins
(PGE2), which are important factors in inflammatory diseases
(10). PGE2 is a key
proinflammatory factor that induces the release of chemokines from
inflammatory cells, recruits inflammatory cells and cooperates with
lipopolysaccharides to induce the expression of IL-6 and IL-1 in
macrophages. PGE2 expression promotes platelet activation and
thrombosis (10).
As aforementioned, TLR2/NF-κB is a common
inflammatory signaling pathway. TLR2 has been shown to be a
potential target for inflammatory injury in various EC (32). The mechanism may be related to
enhanced TLR2 activation of NF-κB.
In the present study, the thrombus formation rate in
the Pam3CSK4 group (85.71%) was significantly higher than those in
the model (57.14%) and TLR2 inhibition (28.57%) groups. These
results indicated that the TLR2 agonist Pam3CSK4 promoted TDVT
development, whereas the TLR2 inhibitor C29 inhibited TDVT
development. This suggests that TLR2 may be involved in an
important mechanism in TVDT and may promote venous thrombus
formation. Literature review and preliminary bioinformatics
analysis revealed that the key downstream molecules of TLR2 may
include NF-κB, COX-2, IL-6 and TF (9-11).
By establishing a rat model of TDVT to simulate in vivo
conditions, the OGD experiment mimicked the ischemic-hypoxic
environment of vascular EC during thrombus formation. It was
observed that with an increase in TLR2 expression in the in
vivo and in vitro experiments, NF-κB p-NF-κB (p-P65)
expression increased. In the HUVEC OGD model, TLR2 expression
increased under ischemic-hypoxic conditions and NF-κB translocated
from the cytoplasm to the nucleus. Key molecules in the TLR2
signaling pathway, TLR2/NF-κB/COX-2/IL-6, changed with TLR2
regulation. Therefore, it was hypothesized that
TLR2/NF-κB/COX-2/IL-6 is one of the signaling pathways involved in
TVDT. In the in vivo experiments, it was also observed that,
compared with the control group, the levels of TLR2, IL-6, COX-2
and p-P65 were significantly higher in the model group. The
thrombus formation rate in the Pam3CSK4 group was higher and the
levels of TLR2, IL-6 and COX-2 increased. In addition, the levels
of the aforementioned indicators in the C29 group were
significantly relatively lower compared with those in the control
group. Thus, it can be inferred that TLR2, IL-6 and COX-2 are
upregulated during DVT.
The present study observed a positive correlation
between the expression of IL-6 and COX-2 and the activation and
inhibition of TLR2. In in vivo experiments, IL-6 and COX-2
levels significantly increased when TLR2 was activated and were
positively correlated with thrombosis. It was hypothesized that
IL-6, COX-2 and other molecules play important roles as signal
transducers and chemokines in DVT.
To gain a comprehensive understanding of the
mechanisms underlying TDVT formation, future research endeavors
should encompass a broader examination of changes within the
systemic environment. By investigating the interplay of these
factors, the intricate pathways involved in thrombus formation can
be more clearly elucidated. Such investigations will pave the way
for the development of more effective prevention and treatment
strategies for this clinically significant condition.
In conclusion, increased TLR2 expression was found
in the TDVT model. TLR2 was positively correlated with the
molecular contents of IL-6, NF-κB and COX-2. TLR2/NF-κB/COX-2 is
one of the possible signaling pathways in TDVT. Further research on
the signaling pathways in TDVT is of great significance for an
in-depth understanding of the condition as well as the selection of
therapeutic targets and development of new drugs. It may also
provide new clues for clinical prophylactic medication and
treatment of TDVT, which has important theoretical and practical
significance.
Acknowledgements
The authors thank Dr Limei Zhang, Dr Xuanfeng Wang
(Department of Pathology of Gannan Medical University, Ganzhou,
China) and Professor Minhong Zhang (Affiliated Hospital of Gannan
Medical University, Ganzhou, China) for technical support and for
supporting histological processing, sectioning and staining and
Xiaozhu Wu (Affiliated Hospital of Gannan Medical University,
Ganzhou, China) for taking X-rays.
Funding
Funding: The present study was supported by grants from the
National Natural Science Foundation of China (grant no. 82160375),
the Natural Science Foundation of Jiangxi Province (grant no.
20202BABL206035), the Science and Technology Program of Jiangxi
Provincial Administration of Traditional Chinese Medicine (grant
no. 2021A374), the Science and Technology Planning Project of
Jiangxi Provincial Health Commission in 2023 (grant no. 202312146)
and the Ganzhou Municipal Science and Technology Plan Project
(grant no. 2023LNS26841).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
TG conceived and designed the experiments, performed
the experiments, analyzed the data, prepared figures and tables, LX
wrote and reviewed drafts of the article and approved the final
draft. JX and JZ prepared figures and performed analysis and
interpretation of data. MY and ZH prepared figures and contributed
to manuscript revisions. TG and JM confirm the authenticity of all
the raw data. JM and XZ, as corresponding authors, edited and
approved the final draft. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
All animal procedures were performed following the
guidelines of the Council on Animal Care and were approved by the
Gannan Medical Ethics Committee (Ganzhou, China; approval no.
LLSC-2022110701).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Scolaro JA, Taylor RM and Wigner NA:
Venous thromboembolism in orthopaedic trauma. J Am Acad Orthop
Surg. 23:1–6. 2015.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Wu L and Cheng B: Analysis of
perioperative risk factors for deep vein thrombosis in patients
with femoral and pelvic fractures. J Orthop Surg Res.
15(597)2020.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Fan J, Zhou F, Xu X, Zhang Z, Tian Y, Ji
H, Guo Y, Lv Y, Yang Z and Hou G: Clinical predictors for deep vein
thrombosis on admission in patients with intertrochanteric
fractures: A retrospective study. BMC Musculoskelet Disord.
22(328)2021.PubMed/NCBI View Article : Google Scholar
|
|
4
|
He LX, Xie JY, Lv J, Liu H, Liao DB, Wang
GL, Ning N and Zhou ZK: Quality evaluation of clinical practice
guidelines for thromboprophylaxis in orthopaedic trauma based on
AGREE II and AGREE-REX: A systematic review protocol. BMJ Open.
12(e59181)2022.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Whiting PS, White-Dzuro GA, Greenberg SE,
VanHouten JP, Avilucea FR, Obremskey WT and Sethi MK: Risk factors
for deep venous thrombosis following orthopaedic trauma surgery: An
analysis of 56,000 patients. Arch Trauma Res.
5(e32915)2016.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Di Minno A, Frigerio B, Spadarella G,
Ravani A, Sansaro D, Amato M, Kitzmiller JP, Pepi M, Tremoli E and
Baldassarre D: Old and new oral anticoagulants: Food, herbal
medicines and drug interactions. Blood Rev. 31:193–203.
2017.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Alhassan S, Pelinescu A, Gandhi V, Naddour
M, Singh AC and Bihler E: Clinical presentation and risk factors of
venous thromboembolic disease. Crit Care Nurs Q. 40:201–209.
2017.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Colling ME, Tourdot BE and Kanthi Y:
Inflammation, infection and venous thromboembolism. Circ Res.
128:2017–2036. 2021.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Du X, He S, Jiang Y, Wei L and Hu W:
Adiponectin prevents islet ischemia-reperfusion injury through the
COX2-TNFα-NF-κB-dependent signal transduction pathway in mice. J
Endocrinol. 218:75–84. 2013.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Lee SY, Kim MH, Kim SH, Ahn T, Kim SW,
Kwak YS, Cho IH, Nah SY, Cho SS, Park KM, et al: Korean Red Ginseng
affects ovalbumin-induced asthma by modulating IL-12, IL-4, and
IL-6 levels and the NF-κB/COX-2 and PGE(2) pathways. J Ginseng Res.
45:482–489. 2021.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Pinheiro CR, Coelho AL, de Oliveira CE,
Gasparoto TH, Garlet GP, Silva JS, Santos CF, Cavassani KA,
Hogaboam CM and Campanelli AP: Recognition of Candida albicans by
gingival fibroblasts: The role of TLR2, TLR4/CD14, and MyD88.
Cytokine. 106:67–75. 2018.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Koc M, Siklova M, Sramkova V, Štěpán M,
Krauzová E, Štich V and Rossmeislová L: Signs of deregulated gene
expression are present in both CD14(+) and CD14(-) PBMC from
non-obese men with family history of T2DM. Front Endocrinol
(Lausanne). 11(582732)2021.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Staller S, Lindsay AK, Ramos ED, Thomas P
and Srinivasan M: Changes in salivary microbial sensing proteins
CD14 and TLR2 with aging. Clin Oral Investig. 24:2523–2528.
2020.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Aguilar-Briseno JA, Upasani V, Ellen BMT,
Moser J, Pauzuolis M, Ruiz-Silva M, Heng S, Laurent D, Choeung R,
Dussart P, et al: TLR2 on blood monocytes senses dengue virus
infection and its expression correlates with disease pathogenesis.
Nat Commun. 11(3177)2020.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Reinhardt C: The gut microbiota as an
influencing factor of arterial thrombosis. Hamostaseologie.
39:173–179. 2019.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Yao M, Fang C, Wang Z, Guo T, Wu D, Ma J,
Wu J and Mo J: miR-328-3p targets TLR2 to ameliorate oxygen-glucose
deprivation injury and neutrophil extracellular trap formation in
HUVECs via inhibition of the NF-κB signaling pathway. PLoS One.
19(e299382)2024.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Wang Z, Fang C, Yao M, Wu D, Chen M, Guo T
and Mo J: Research progress of NF-kappaB signaling pathway and
thrombosis. Front Immunol. 14(1257988)2023.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Griffin G: Establishing a Three Rs
programme at the Canadian Council on Animal Care. Altern Lab Anim.
37 (Suppl 2):S63–S67. 2009.PubMed/NCBI View Article : Google Scholar
|
|
19
|
X Z: Establishment of a new animal model
of traumatic limb deep vein thrombosis and related studies,
2004.
|
|
20
|
Jeong JJ, Woo JY, Kim KA, Han MJ and Kim
DH: Lactobacillus pentosus var. plantarum C29 ameliorates
age-dependent memory impairment in Fischer 344 rats. Lett Appl
Microbiol. 60:307–314. 2015.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Lee SJ, Baek SE, Jang MA and Kim CD: SIRT1
inhibits monocyte adhesion to the vascular endothelium by
suppressing Mac-1 expression on monocytes. Exp Mol Med. 51:1–12.
2019.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Liao X, He J, Wang R, Zhang J, Wei S, Xiao
Y, Zhou Q, Zheng X, Zhu Z, Zheng Z, et al: TLR-2 agonist Pam3CSK4
has no therapeutic effect on visceral leishmaniasis in BALB/c mice
and may enhance the pathogenesis of the disease. Immunobiology.
228(152725)2023.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Mo J, Zheng T, Lei L, Dai P, Liu J, He H,
Shi J, Chen X, Guo T, Yuan B and Ji G: MicroRNA-1253 suppresses
cell proliferation migration and invasion of osteosarcoma by
targeting MMP9. Technol Cancer Res Treat.
20(1533033821995278)2021.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Yu J, Jin Y, Xu C, Fang C, Zhang Z, Chen L
and Xu G: Downregulation of miR-125a-5p promotes endothelial
progenitor cell migration and angiogenesis and alleviates deep vein
thrombosis in mice via upregulation of MCL-1. Mol Biotechnol.
65:1664–1678. 2023.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Sykora D, Firth C, Girardo M, Bhatt S,
Tseng A, Chamberlain A, Liedl D, Wennberg P and Shamoun FE:
Peripheral artery disease and the risk of venous thromboembolism.
VASA. 51:365–371. 2022.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Zhou M, Zhang T, Zhang B, Zhang X, Gao S,
Zhang T, Li S, Cai X and Lin Y: A DNA nanostructure-based
neuroprotectant against neuronal apoptosis via inhibiting toll-like
receptor 2 signaling pathway in acute ischemic stroke. ACS Nano.
16:1456–1470. 2022.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Won Y, Yang JI, Park S and Chun JS:
Lipopolysaccharide binding protein and CD14, cofactors of toll-like
receptors, are essential for low-grade inflammation-induced
exacerbation of cartilage damage in mouse models of posttraumatic
osteoarthritis. Arthritis Rheumatol. 73:1451–1460. 2021.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Porta C, Consonni FM, Morlacchi S,
Sangaletti S, Bleve A, Totaro MG, Larghi P, Rimoldi M, Tripodo C,
Strauss L, et al: Tumor-Derived Prostaglandin E2 Promotes p50
NF-κB-Dependent differentiation of monocytic MDSCs. Cancer Res.
80:2874–2888. 2020.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Hojo K, Tamai R, Kobayashi-Sakamoto M and
Kiyoura Y: Etidronate down-regulates Toll-like receptor (TLR) 2
ligand-induced proinflammatory cytokine production by inhibiting
NF-κB activation. Pharmacol Rep. 69:773–778. 2017.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Makni L, Zidi S, Barbiroud M, Ahmed AB,
Gazouani E, Mezlini A, Stayoussef M and Yacoubi-Loueslati B:
Increased risks between TLR2 (-196 to -174 ins/del) and TLR3
1377C>T variants and head and neck cancers in Tunisia. Cent Eur
J Immunol. 44:144–149. 2019.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Bishayi B, Bandyopadhyay D, Majhi A and
Adhikary R: Effect of exogenous MCP-1 on TLR-2 neutralized murine
macrophages and possible mechanisms of CCR-2/TLR-2 and MCP-1
signalling during Staphylococcus aureus infection. Immunobiology.
220:350–362. 2015.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Li S, Yang Z, Tian H, Ren S, Zhang W and
Wang A: Effects of dietary carbohydrate/lipid ratios on
non-specific immune responses, antioxidant capacity, hepatopancreas
and intestines histology, and expression of TLR-MAPK/NF-κB
signaling pathway-related genes of Procambarus clarkii. Fish
Shellfish Immunol. 124:219–229. 2022.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Song R, Ao L, Zhao KS, Zheng D, Venardos
N, Fullerton DA and Meng X: Soluble biglycan induces the production
of ICAM-1 and MCP-1 in human aortic valve interstitial cells
through TLR2/4 and the ERK1/2 pathway. Inflamm Res. 63:703–710.
2014.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Zheng X, Liu H, Ma M, Ji J, Zhu F and Sun
L: Anti-thrombotic activity of phenolic acids obtained from Salvia
miltiorrhiza f. alba in TNF-α-stimulated endothelial cells via the
NF-κB/JNK/p38 MAPK signaling pathway. Arch Pharm Res. 44:427–438.
2021.PubMed/NCBI View Article : Google Scholar
|




















