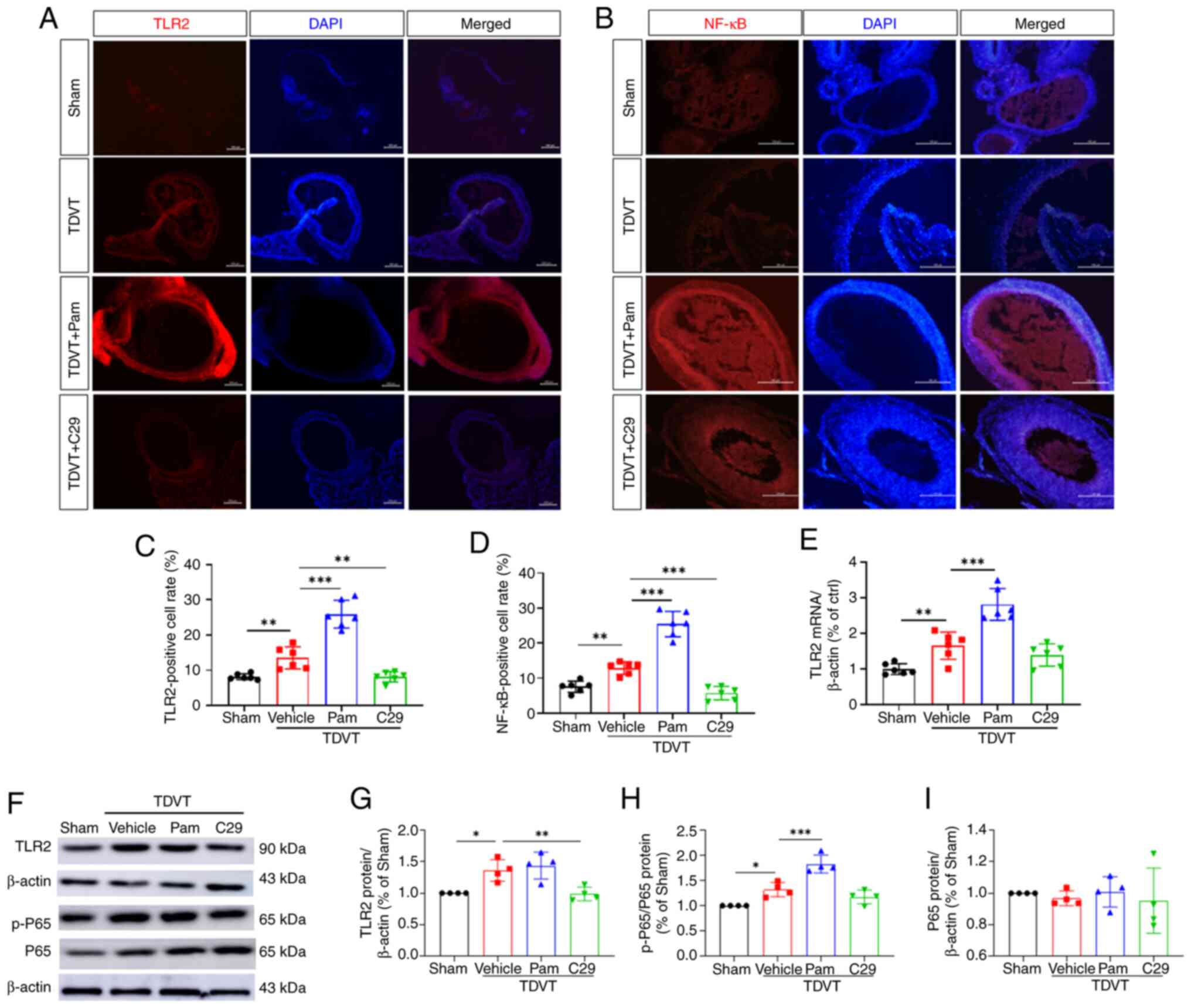
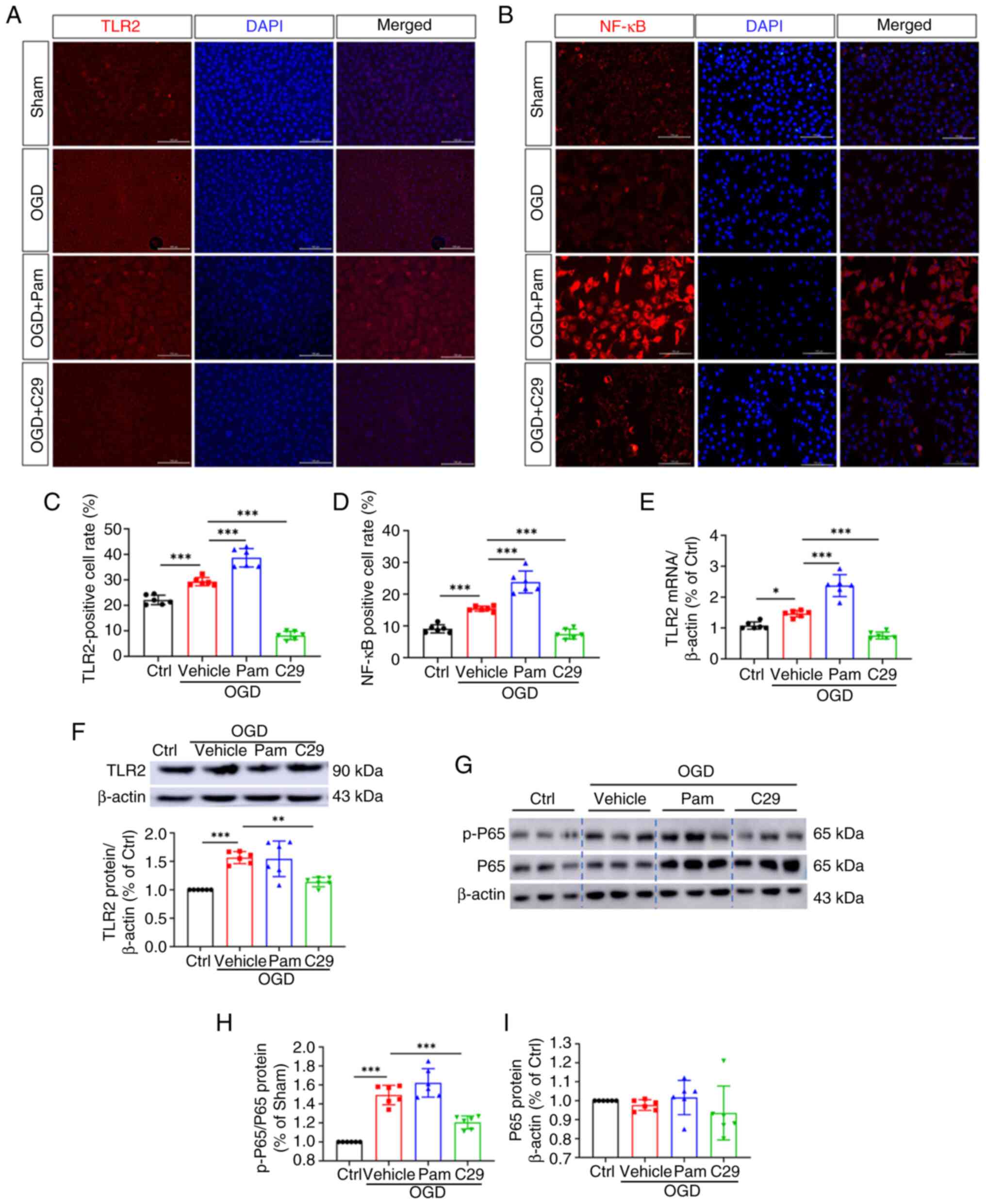
|
1
|
Scolaro JA, Taylor RM and Wigner NA:
Venous thromboembolism in orthopaedic trauma. J Am Acad Orthop
Surg. 23:1–6. 2015.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Wu L and Cheng B: Analysis of
perioperative risk factors for deep vein thrombosis in patients
with femoral and pelvic fractures. J Orthop Surg Res.
15(597)2020.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Fan J, Zhou F, Xu X, Zhang Z, Tian Y, Ji
H, Guo Y, Lv Y, Yang Z and Hou G: Clinical predictors for deep vein
thrombosis on admission in patients with intertrochanteric
fractures: A retrospective study. BMC Musculoskelet Disord.
22(328)2021.PubMed/NCBI View Article : Google Scholar
|
|
4
|
He LX, Xie JY, Lv J, Liu H, Liao DB, Wang
GL, Ning N and Zhou ZK: Quality evaluation of clinical practice
guidelines for thromboprophylaxis in orthopaedic trauma based on
AGREE II and AGREE-REX: A systematic review protocol. BMJ Open.
12(e59181)2022.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Whiting PS, White-Dzuro GA, Greenberg SE,
VanHouten JP, Avilucea FR, Obremskey WT and Sethi MK: Risk factors
for deep venous thrombosis following orthopaedic trauma surgery: An
analysis of 56,000 patients. Arch Trauma Res.
5(e32915)2016.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Di Minno A, Frigerio B, Spadarella G,
Ravani A, Sansaro D, Amato M, Kitzmiller JP, Pepi M, Tremoli E and
Baldassarre D: Old and new oral anticoagulants: Food, herbal
medicines and drug interactions. Blood Rev. 31:193–203.
2017.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Alhassan S, Pelinescu A, Gandhi V, Naddour
M, Singh AC and Bihler E: Clinical presentation and risk factors of
venous thromboembolic disease. Crit Care Nurs Q. 40:201–209.
2017.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Colling ME, Tourdot BE and Kanthi Y:
Inflammation, infection and venous thromboembolism. Circ Res.
128:2017–2036. 2021.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Du X, He S, Jiang Y, Wei L and Hu W:
Adiponectin prevents islet ischemia-reperfusion injury through the
COX2-TNFα-NF-κB-dependent signal transduction pathway in mice. J
Endocrinol. 218:75–84. 2013.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Lee SY, Kim MH, Kim SH, Ahn T, Kim SW,
Kwak YS, Cho IH, Nah SY, Cho SS, Park KM, et al: Korean Red Ginseng
affects ovalbumin-induced asthma by modulating IL-12, IL-4, and
IL-6 levels and the NF-κB/COX-2 and PGE(2) pathways. J Ginseng Res.
45:482–489. 2021.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Pinheiro CR, Coelho AL, de Oliveira CE,
Gasparoto TH, Garlet GP, Silva JS, Santos CF, Cavassani KA,
Hogaboam CM and Campanelli AP: Recognition of Candida albicans by
gingival fibroblasts: The role of TLR2, TLR4/CD14, and MyD88.
Cytokine. 106:67–75. 2018.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Koc M, Siklova M, Sramkova V, Štěpán M,
Krauzová E, Štich V and Rossmeislová L: Signs of deregulated gene
expression are present in both CD14(+) and CD14(-) PBMC from
non-obese men with family history of T2DM. Front Endocrinol
(Lausanne). 11(582732)2021.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Staller S, Lindsay AK, Ramos ED, Thomas P
and Srinivasan M: Changes in salivary microbial sensing proteins
CD14 and TLR2 with aging. Clin Oral Investig. 24:2523–2528.
2020.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Aguilar-Briseno JA, Upasani V, Ellen BMT,
Moser J, Pauzuolis M, Ruiz-Silva M, Heng S, Laurent D, Choeung R,
Dussart P, et al: TLR2 on blood monocytes senses dengue virus
infection and its expression correlates with disease pathogenesis.
Nat Commun. 11(3177)2020.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Reinhardt C: The gut microbiota as an
influencing factor of arterial thrombosis. Hamostaseologie.
39:173–179. 2019.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Yao M, Fang C, Wang Z, Guo T, Wu D, Ma J,
Wu J and Mo J: miR-328-3p targets TLR2 to ameliorate oxygen-glucose
deprivation injury and neutrophil extracellular trap formation in
HUVECs via inhibition of the NF-κB signaling pathway. PLoS One.
19(e299382)2024.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Wang Z, Fang C, Yao M, Wu D, Chen M, Guo T
and Mo J: Research progress of NF-kappaB signaling pathway and
thrombosis. Front Immunol. 14(1257988)2023.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Griffin G: Establishing a Three Rs
programme at the Canadian Council on Animal Care. Altern Lab Anim.
37 (Suppl 2):S63–S67. 2009.PubMed/NCBI View Article : Google Scholar
|
|
19
|
X Z: Establishment of a new animal model
of traumatic limb deep vein thrombosis and related studies,
2004.
|
|
20
|
Jeong JJ, Woo JY, Kim KA, Han MJ and Kim
DH: Lactobacillus pentosus var. plantarum C29 ameliorates
age-dependent memory impairment in Fischer 344 rats. Lett Appl
Microbiol. 60:307–314. 2015.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Lee SJ, Baek SE, Jang MA and Kim CD: SIRT1
inhibits monocyte adhesion to the vascular endothelium by
suppressing Mac-1 expression on monocytes. Exp Mol Med. 51:1–12.
2019.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Liao X, He J, Wang R, Zhang J, Wei S, Xiao
Y, Zhou Q, Zheng X, Zhu Z, Zheng Z, et al: TLR-2 agonist Pam3CSK4
has no therapeutic effect on visceral leishmaniasis in BALB/c mice
and may enhance the pathogenesis of the disease. Immunobiology.
228(152725)2023.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Mo J, Zheng T, Lei L, Dai P, Liu J, He H,
Shi J, Chen X, Guo T, Yuan B and Ji G: MicroRNA-1253 suppresses
cell proliferation migration and invasion of osteosarcoma by
targeting MMP9. Technol Cancer Res Treat.
20(1533033821995278)2021.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Yu J, Jin Y, Xu C, Fang C, Zhang Z, Chen L
and Xu G: Downregulation of miR-125a-5p promotes endothelial
progenitor cell migration and angiogenesis and alleviates deep vein
thrombosis in mice via upregulation of MCL-1. Mol Biotechnol.
65:1664–1678. 2023.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Sykora D, Firth C, Girardo M, Bhatt S,
Tseng A, Chamberlain A, Liedl D, Wennberg P and Shamoun FE:
Peripheral artery disease and the risk of venous thromboembolism.
VASA. 51:365–371. 2022.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Zhou M, Zhang T, Zhang B, Zhang X, Gao S,
Zhang T, Li S, Cai X and Lin Y: A DNA nanostructure-based
neuroprotectant against neuronal apoptosis via inhibiting toll-like
receptor 2 signaling pathway in acute ischemic stroke. ACS Nano.
16:1456–1470. 2022.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Won Y, Yang JI, Park S and Chun JS:
Lipopolysaccharide binding protein and CD14, cofactors of toll-like
receptors, are essential for low-grade inflammation-induced
exacerbation of cartilage damage in mouse models of posttraumatic
osteoarthritis. Arthritis Rheumatol. 73:1451–1460. 2021.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Porta C, Consonni FM, Morlacchi S,
Sangaletti S, Bleve A, Totaro MG, Larghi P, Rimoldi M, Tripodo C,
Strauss L, et al: Tumor-Derived Prostaglandin E2 Promotes p50
NF-κB-Dependent differentiation of monocytic MDSCs. Cancer Res.
80:2874–2888. 2020.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Hojo K, Tamai R, Kobayashi-Sakamoto M and
Kiyoura Y: Etidronate down-regulates Toll-like receptor (TLR) 2
ligand-induced proinflammatory cytokine production by inhibiting
NF-κB activation. Pharmacol Rep. 69:773–778. 2017.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Makni L, Zidi S, Barbiroud M, Ahmed AB,
Gazouani E, Mezlini A, Stayoussef M and Yacoubi-Loueslati B:
Increased risks between TLR2 (-196 to -174 ins/del) and TLR3
1377C>T variants and head and neck cancers in Tunisia. Cent Eur
J Immunol. 44:144–149. 2019.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Bishayi B, Bandyopadhyay D, Majhi A and
Adhikary R: Effect of exogenous MCP-1 on TLR-2 neutralized murine
macrophages and possible mechanisms of CCR-2/TLR-2 and MCP-1
signalling during Staphylococcus aureus infection. Immunobiology.
220:350–362. 2015.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Li S, Yang Z, Tian H, Ren S, Zhang W and
Wang A: Effects of dietary carbohydrate/lipid ratios on
non-specific immune responses, antioxidant capacity, hepatopancreas
and intestines histology, and expression of TLR-MAPK/NF-κB
signaling pathway-related genes of Procambarus clarkii. Fish
Shellfish Immunol. 124:219–229. 2022.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Song R, Ao L, Zhao KS, Zheng D, Venardos
N, Fullerton DA and Meng X: Soluble biglycan induces the production
of ICAM-1 and MCP-1 in human aortic valve interstitial cells
through TLR2/4 and the ERK1/2 pathway. Inflamm Res. 63:703–710.
2014.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Zheng X, Liu H, Ma M, Ji J, Zhu F and Sun
L: Anti-thrombotic activity of phenolic acids obtained from Salvia
miltiorrhiza f. alba in TNF-α-stimulated endothelial cells via the
NF-κB/JNK/p38 MAPK signaling pathway. Arch Pharm Res. 44:427–438.
2021.PubMed/NCBI View Article : Google Scholar
|