Introduction
Rhinocerebral mucormycosis is an acute and rare
disease caused by infection with the fungi from the
Mucoraceae family, first described by Paltauf in
1885(1). It typically affects
patients with diabetic ketoacidosis or immunosuppression, where its
clinical course is fulminant in the majority of cases (2). It most frequently begins in the nose
and paranasal sinuses, with symptoms resembling those of acute
rhinosinusitis that do not respond to treatment. It then rapidly
progresses and can become fatal if not diagnosed and treated early
(3). Extensive surgical
debridement combined with systemic treatment with intravenous
antifungals is key for controlling this disease (4). However, due to such an invasive
surgical approach, survivors frequently experience significant
sequelae in the maxillofacial region, with extensive involvement in
the maxilla, nose and orbital regions (5), involving loss of vision, difficulty
speaking and eating, hearing problems and aesthetic impairment.
Such maxillectomy defects are typically treated with prosthetic
obturation or autologous tissue reconstruction (6). Since each of these techniques has its
advantages and disadvantages, the optimal approach remains subject
to debate (7). Reconstruction has
the advantage of closing the defect while avoiding the use of a
removable prosthesis, but subjects the patient to surgery with a
high morbidity rate, while an obturator avoids surgery but is
uncomfortable and sometimes very difficult to adapt.
Nevertheless, over the past decade, advances in
medical imaging (8) and
computer-aided design/computer-aided manufacturing (CAD/CAM)
technology (9) have made it
possible to develop novel protocols for designing and manufacturing
personalized implants that can aid in the reconstruction of these
maxillary defects. These implants, known as personalized
subperiosteal implants (PSIs), were first described in
1943(10). Due to recent
technological advances, numerous modifications, including
reductions in size and thickness, and new connections, have
improved the design and manufacturing processes of such PSIs
(11,12), enabling such sequelae to be treated
with notable results, with fewer exposures and better prosthetic
management (13). The present case
documents the rehabilitation of a patient with rhino-orbit-cerebral
mucormycosis sequela in the maxilla, using a removable
implant-supported prosthesis attached to a PSI.
Case report
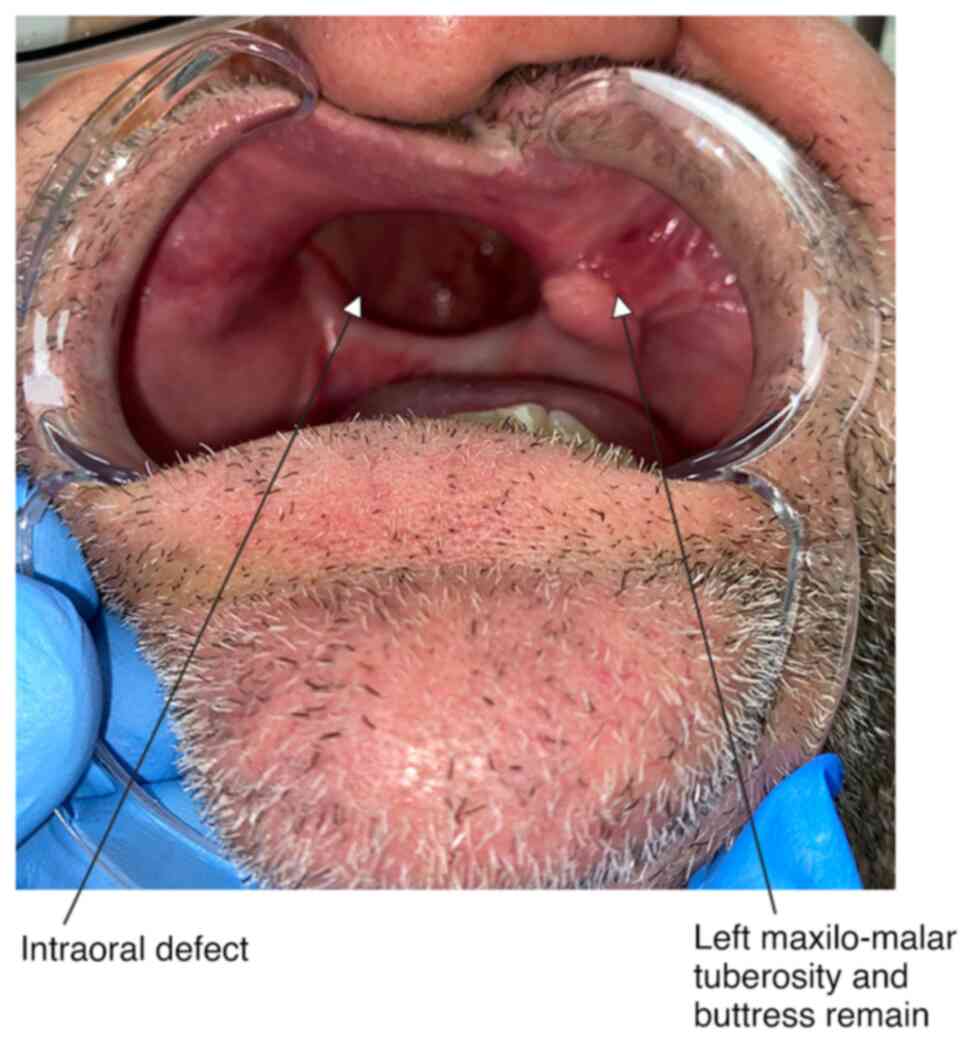
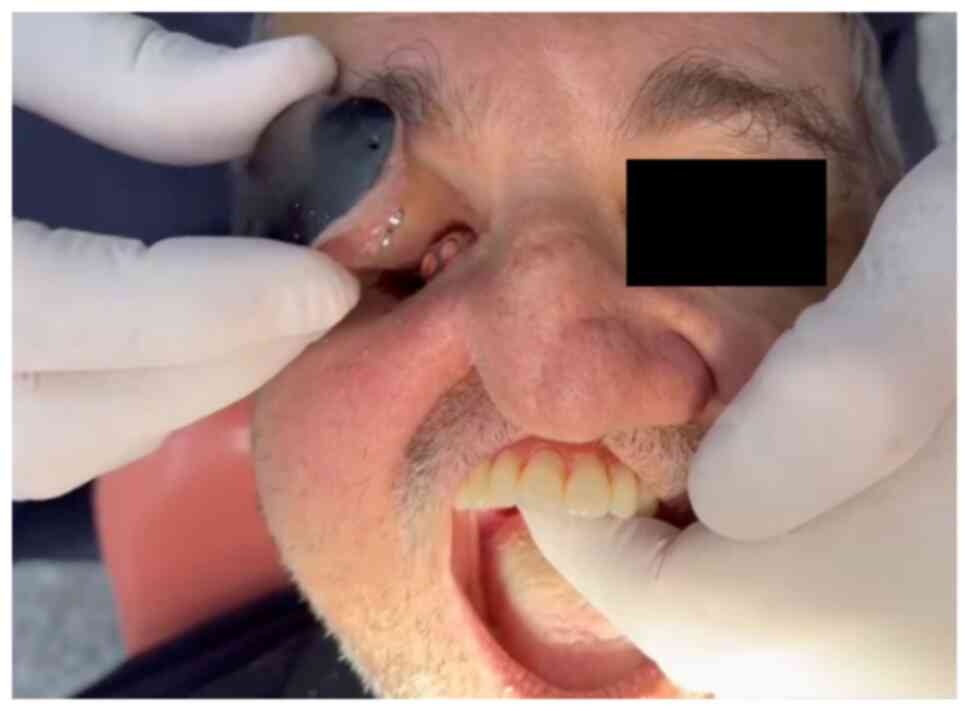
A 53-year-old male patient, a former smoker without
any other relevant medical history, with a IIIb maxillary defect
(14), right orbital exenteration
and bilateral ethmoidectomy due to rhinocerebral mucormycosis, was
referred to the Virgen Macarena University Hospital (Seville,
Spain) in December 2021 from the University Hospital of Badajoz
(Badajoz, Spain) after the failure of reconstruction using a
microvascular fibula flap due to internal jugular vein thrombosis
on postoperative day 3 (Fig. 1).
Upon arrival at the Virgen Macarena University Hospital 1 year
after the fibula flap failure in December 2021, the patient
exhibited severe difficulties in oral intake and speech. In
addition, the patient presented with significant aesthetic damage,
with a sunken mid-facial third and microstomia due to the scar on
the lip from previous surgeries (maxillectomy and deferred
microvascular fibula flap reconstruction).
Following the unsuccessful reconstruction attempt,
placing an obturator was considered for this patient. However, this
option was dismissed due to the lack of support provided by the
bone and soft-tissue defect. The significant collapse of the lip
and nose posed challenges in manufacturing any type of prosthesis,
compounded by the absence of support from intraoral tissues,
leading to the rejection of this therapeutic option, even as a
provisional solution. Soft-tissue reconstruction surgery using a
microvascularized forearm flap to cover the defect was proposed,
but having already undergone two surgical operations, for
mucormycosis and the failed attempt at reconstruction, the patient
did not want further reconstructive surgery, so expressed a
preference for an alternative reconstructive approach rather than
another microvascularized graft. Given the patient's rejection of
any reconstructive therapeutic options and the impossibility of
placing an obturator, PSI was then suggested as a support for the
prosthetic obturator.
To reconstruct the maxillary defect, a PSI (Avinent
Implant System S.L.U) with connections for a removable
implant-supported prosthesis was proposed, which would provide
occlusion with the mandible and close the oronasal-antral
communication defect.
A virtual simulation of the obturator and maxillary
prosthesis was first performed, using 3-matic Medical®
17.0 software (Materialise), based on the defect and the opposing
dental arch (Fig. 2). Acquiring
digital impressions with an intraoral scanner was not feasible due
to the lack of intraoral references. Consequently, analog
impressions were obtained instead using heavy silicone in two
stages. Initially, impressions were made from the orbital defect
towards the oral cavity, before impressions were then made from the
oral cavity towards the orbital defect. Integrating the two
impressions facilitated duplication of the defect, enabling the
creation of a prototype with which to conduct the facial CT scan
used for PSI planning [CT machine model: Revolution CT; supplier,
GE Healthcare; Imaging parameters: Scan mode, helical; collimation,
0.625 mm; slice thickness, 1 mm; reconstruction interval, 0.5 mm;
tube voltage, 120 kVp; tube current, 100-200 mA (automatically
adjusted by the system); field of view, 220 mm; reconstruction
matrix, 512x512; reconstruction filter, bone plus (high-resolution
bone kernel); reconstruction mode, multiplanar and 3D; rotation
time, 0.4 sec; pitch, 0.8; contrast, no (contrast is generally not
used for bone evaluations); patient position, supine, head first].
Radiopaque markers were placed on the resulting prosthesis
prototype, along with locator-type connections on the orbital end
to ensure proper prosthesis placement during the facial CT scan of
the patient (Fig. 3). A cone beam
CT scan of the prosthesis was also conducted (Kodak Carestream CS
Imaging version 8.0.25; DICOM files voxel size 76x76x76 µm). Images
from both CT scans were segmented using Mimics 25.0 (Materialise)
and merged using 3-matic Medical® 17.0 software
(Materialise). In this manner, an implant model that conformed
seamlessly to the unique contours of the remaining bone of the
patient was crafted.
Using the latter software, a PSI made of a sintered
Grade V titanium alloy (Ti6AI4V) with a thickness of 0.8 mm was
designed together with Avinent Implant System S.L.U engineers and
manufactured by Avinent Implant System S.L.U using an EOS M290
printer (EOS GmbH). The PSI was then meticulously tailored to
accommodate both the defect and the adjacent anatomical structures
where it is anchored. The implant included six universal external
hex connections. The connection area was reinforced by increasing
the implant thickness to 1.2 mm.
The present case employed a Weber-Ferguson approach
(15) to access the malar area and
the remaining right infraorbital rim. On the left side, an
intraoral approach was used due to the lesser necessity for
exposure of the left malar for the proper fixation of the PSI. The
implant was fixed to both zygomatic bones and to the left
maxillo-zygomatic buttress using self-drilling osteosynthesis
screws with a thickness of 1.9 mm and a length of 6 mm (Fig. 4), leaving part of the PSI exposed
in the oral cavity. No intraoperative complications or mucositis in
the 2nd quadrant area were encountered. Only the immediate
postoperative fabrication of a silicone protector for the area
exposed to the PSI was needed to prevent mucosal contact with the
upper lip. No provisional prosthesis was considered to avoid
interference with soft tissue healing. At 2 weeks after placement,
a removable implant-supported prosthesis was designed using six
locators, completely obturating the defect and allowing the patient
to speak and eat normally, whilst facilitating the hygiene of both
the prosthesis and the oropharyngeal mucosa (Figs. 5 and 6). The present case designated 2 weeks as
the time frame between PSI placement surgery and the prosthetic
phase to avoid interference with tissue cicatrization. After
checking for correct cicatrization, the prosthetic phase
commenced.
In January 2024, 18 months after treatment, the
patient was doing well, the PSI remained exposed in the oral cavity
without causing any problems and the prosthesis was functioning
properly, with no mobility of the PSI and good adjustment of the
prosthesis, ensuring that the defect was filled and the patient
could therefore speak and eat normally.
Discussion
Mucormycosis is a fungal infection that is
contracted through the inhalation of fungus spores. It typically
affects immunocompromised patients, leading to severe conditions
that can be life-threatening (3,16,17),
but it is rare for healthy individuals to be affected (16), as in the present case study. In
recent years, an increase in cases has been described, particularly
associated with the use of corticosteroids during the coronavirus
disease-19 pandemic (18-20).
Treatment with antifungals and early surgical intervention are
crucial for the survival of the patient (17).
Defects associated with the surgical treatment of
rhinocerebral mucormycosis present a therapeutic challenge for
maxillofacial surgeons. Difficult-to-reconstruct sequelae are often
encountered in the maxilla, nose and orbit (14). A combination of surgical and
prosthetic rehabilitation is preferred in cases of large midfacial
defects to adequately restore the patient's functional and
aesthetic needs (21). However,
there remains to be a lack of valid recommendations regarding the
optimal procedure, especially in terms of the quality of life
(22,23).
When the situation of the patient does not allow for
reconstruction, the least invasive solution is to treat these
sequelae using an obturator (22),
which provides notable results for patients who are not good
candidates for major reconstruction surgery or who reject this type
of surgery. Specifically, it entails closing the communication
between the oral cavity and the sinuses and nose, allowing the
patient to eat and speak whilst using the prosthesis. In such
cases, this can provide a temporary solution until the defect has
undergone reconstruction or a definitive solution in patients where
communication cannot be closed off (24,25).
The prosthesis is typically retained by metal hooks anchored to the
remaining teeth, if there are any (26). In terms of the multiple
classifications of obturator use in existence, depending on the
type of maxillary defect, there is a consensus that the main
challenge for patients is the stability and retention of such
devices (27). The use of CAD/CAM
in the design and manufacture of obturators has improved their
adaptation to the intraoral defect (28).
When the option of a removable obturator is not
feasible, such as in patients with larger and more complex defects
where prosthesis adaptation and fit are not straightforward, it is
necessary to find anchor points that can allow for proper closure
and stable fixation. PSIs can provide such anchor points by
attaching them to areas adjacent to the defect, such as the nasal
and zygomatic buttresses, which are typically preserved in these
types of sequelae (10). PSIs are
customized to the bone anatomy of the patient and include the
prosthetic connections in the implant design itself (11,12,29-32).
The use of PSIs in oral cavity defect reconstruction is well
established, which is frequently combined with microvascularized
flaps (33) to reconstruct soft
tissues and close the oromaxillary communication. In these cases,
the PSI replaces the placement of endosseous implants, as it
provides the connections necessary for optimal prosthetic
rehabilitation. However, its use as an alternative to
reconstruction (13), as in the
case described in the present report, is less developed. Although
advances in locoregional and microvascular reconstruction have led
to successful surgical outcomes, not all patients are candidates
for surgical reconstruction (34).
The patient in the present case was referred from another center
after a failed microvascular fibula graft and declined a second
reconstructive surgery operation.
The option of placing an obturator was considered
but dismissed, even as a provisional solution, due to the lack of
support provided by the bone and soft-tissue defect. Given the
patient's refusal of any reconstructive therapeutic options and the
impossibility of placing an obturator, PSI was then suggested as a
support for the prosthetic obturator.
The advantages of using a PSI, compared with
reconstructing the defect, are that it avoids a second bone and
soft-tissue reconstruction, which would entail greater morbidity
and a new donor area, simplifies the surgery and avoids a third
surgical operation to place conventional implants (33). PSI surgery provides a solution that
then only awaits the prosthetic rehabilitation. However, with this
option the patient must then always use the prosthesis as an
obturator to cover and close off the defect. In addition, one zone
of the PSI will remain exposed to the oral cavity where it is
unknown how it will develop in the long term.
Such personalized solutions render it possible to
connect the area of residual bone, where the implant will be fixed,
to the area where connections are needed for the prosthesis that
the patient will wear. Reconstructing the soft tissues without
needing bone reconstruction for endosseous implant placement, as
previously proposed by Korn et al (35), would be a valid option for closing
the oronasal-antral communication caused by bone loss. However, the
present case shows that the PSI option is equally valid when the
patient either cannot or will not undergo reconstruction. In the
present case, the difficulties lay in the lack of bone support in
the maxilla-orbit and the failure of previous attempts at
soft-tissue reconstruction.
The PSI needs to be meticulously tailored to
accommodate both the defect and the adjacent anatomical structures
where it is anchored (35). In
instances where bone quality is compromised, the objective in the
present case was to devise the implant in a manner conducive to
anchoring it in regions distal to the defect, leveraging the
presence of cortical and trabecular bone architecture to ensure the
robust stability of the PSI. Through strategic design and surgical
approaches, a PSI capable of adapting to a spectrum of defects,
even those as severe as those exhibited by the present patient, was
successfully manufactured.
To the best of our knowledge, there is no previous
reference in the literature regarding patients with low bone
quality in whom a PSI has been placed to support an obturator with
part of the PSI framework exposed to the oral cavity without a
soft-tissue covering. In any event, since the majority of the PSI
is fixed to the nasal and malar buttresses, where the bone is
highly cortical, observations from the present case resulted in a
hypothesis that the fixation can remain stable even in patients
with poor maxillary bone quality.
We consider that the PSI provides sufficient
stability to support a removable implant-supported prosthesis that
can occlude the defect without the need for soft-tissue
reconstruction. The stable fixation minimizes obturator mobility
and fit issues, in addition to being removable to allow the area to
be cleaned. Planning and fabricating the implant from a prosthetic
perspective allows the incorporation of 3D connections that are
ideal for achieving the most precise functional and aesthetic fit
for the prosthesis, even in extensive and complex defects such as
those faced in the present case.
Unlike a subperiosteal implant used for bone
atrophy, the present implant was thicker in the area that is
exposed to the oral cavity, reaching critical thicknesses of 1.5 mm
in areas that were considered important for force distribution,
instead of the usual 0.8-mm thickness of these PSIs. To the best of
our knowledge, minimum thickness data for this type of implant were
not found in the literature. Regarding the fixation of the implant
with osteosynthesis screws, a similar diameter to that described by
Korn et al (35) was used,
specifically between 1.9 and 2.2 mm. Furthermore, in the present
case, it was hypothesized that more screws needed to be placed than
in conventional PSI cases and a more distant anchorage was
required, utilizing the nasal and zygomatic buttresses and
extending to the zygomatic arch if necessary, as indicated by Korn
et al (35) for a higher
Brown's class 1 case.
Although an intraoral surgical approach is adequate
for the rehabilitation of severe maxillary atrophies in cases of
PSI to ensure optimal fixation (31), in other situations, such as the
present case, it is necessary to combine the intraoral approach
with an extraoral counterpart to gain access to stable bony
fixation areas for PSI, such as the malar, zygomatic arch and
orbital rim.
In cases such as that of the present patient, the
traditional alternative would have been a microvascularized flap to
provide external bone and soft tissues, followed by rehabilitation
with osseointegrated implants. The cost of the PSI does exceed that
of serial osteosynthesis plates for securing the flap bone,
including plates combined with osseointegrated implants. However,
since the costs associated with the surgical procedure and hospital
stay were substantially lower with the PSI option, the expense was
significantly lower overall compared with the traditional
alternative. A cost-effectiveness analysis will be needed to
confirm this hypothesis in the future.
In conclusion, taking into account the limitations
of the present case, it may be concluded that PSIs are a valid
option for prosthetic rehabilitation in patients with extensive
defects of the maxilla. In some cases, they may even remove the
need for reconstruction. However, further studies will be necessary
to evaluate the medium and long-term performance of the area of the
PSI that is exposed to the oral cavity.
Acknowledgements
The authors wish to thank Mrs. Mary Georgina
Hardinge (Jaume I University, Castellon, Spain) for English
language editing assistance.
Funding
Funding: Avinent Implant System S.L.U. provided financial and
administrative support for English language editing. No other
funding was received.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
JPH and JHL performed the surgery and were
responsible for study design. ATP was responsible for PSI design.
EMC performed the surgery. DMG was responsible for the
manufacturing and design of the prototype and the prosthesis, while
GCC helped with the prosthesis design. ARM helped to design the
study. JHL and JPH confirm the authenticity of all the raw data.
All authors participated in the clinical case and contributed to
writing the manuscript. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
The present clinical case is part of the
‘CLIN25_Impact on quality of life and observational clinical
follow-up of Avinent Subperiosteal Personalized Implants’ study,
approved by the Virgen Macarena and Virgen del Rocio University
Hospital Ethics Committee of Seville (approval no. 01082023).
Patient consent for publication
The patient gave written informed consent for the
publication of any associated data and accompanying images.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Paltauf A: Mycosis mucorina. Archiv F
Pathol Anat. 102:543–564. 1885.
|
|
2
|
O'Neill BM, Alessi AS, George EB and Piro
J: Disseminated rhinocerebral mucormycosis: A case report and
review of the literature. J Oral Maxillofac Surg. 64:326–333.
2006.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Arnáiz-García ME, Alonso-Peña D,
González-Vela Mdel C, García-Palomo JD, Sanz-Giménez-Rico JR and
Arnáiz-García AM: Cutaneous mucormycosis: Report of five cases and
review of the literature. J Plast Reconstr Aesthet Surg.
62:e434–e441. 2009.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Rangel-Guerra RA, Martínez HR, Sáenz C,
Bosques-Padilla F and Estrada-Bellmann I: Rhinocerebral and
systemic mucormycosis. Clinical experience with 36 cases. J Neurol
Sci. 143:19–30. 1996.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Oh WS and Roumanas E: Dental
implant-assisted prosthetic rehabilitation of a patient with a
bilateral maxillectomy defect secondary to mucormycosis. J Prosthet
Dent. 96:88–95. 2006.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Lenox ND and Kim DD: Maxillary
reconstruction. Oral Maxillofac Surg Clin North Am. 25:215–222.
2013.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Moreno MA, Skoracki RJ, Hanna EY and
Hanasono MM: Microvascular free flap reconstruction versus palatal
obturation for maxillectomy defects. Head Neck. 32:860–868.
2010.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Colombo M, Mangano C, Mijiritsky E, Krebs
M, Hauschild U and Fortin T: Clinical applications and
effectiveness of guided implant surgery: A critical review based on
randomized controlled trials. BMC Oral Health.
17(150)2017.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Joda T, Zarone F and Ferrari M: The
complete digital workflow in fixed prosthodontics: A systematic
review. BMC Oral Health. 17(124)2017.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Dahl C: If the opportunity for
implantation in the jaw of metal skeletons as the base or retention
for fixed or removable dentures. Odontol Tidskr. 51:440–449.
1943.
|
|
11
|
Mommaerts MY: Evolutionary steps in the
design and biofunctionalization of the additively manufactured
sub-periosteal jaw implant ‘AMSJI’ for the maxilla. Int J Oral
Maxillofac Surg. 48:108–114. 2019.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Tofé-Povedano Á, Hernández JP, López JH,
García DM, González-Moguena VA and Rollón-Mayordomo Á: Design
modifications in subperiosteal implants to avoid complications.
Presentation of a case series study and literature review. Rev Esp
Cir Oral Maxilofac. 45:57–63. 2023.
|
|
13
|
Kondaka S, Singh VD, Vadlamudi C and
Bathala LR: Prosthetic rehabilitation of untailored defects using
patient-specific implants. Dent Res J (Isfahan).
19(83)2022.PubMed/NCBI
|
|
14
|
Cordeiro PG and Chen CM: A 15-year review
of midface reconstruction after total and subtotal maxillectomy:
Part II. Technical modifications to maximize aesthetic and
functional outcomes. Plast Reconstr Surg. 129:139–147.
2012.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Andi KA, Holmes SB and Hutchison IL:
Infraorbital orbitotomy: Modification of the Weber-Ferguson
approach. Br J Oral Maxillofac Surg. 48:44–45. 2010.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Adjari A, Zolfagharypoor A, Firouzifar M
and Akbarpour M: Rhinocerebral mucormycosis in immunocompetent
patients: A case report and review of literature. Infection.
52:673–684. 2024.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Ferguson BJ: Mucormycosis of the nose and
paranasal sinuses. Otolaryngol Clin North Am. 33:349–365.
2000.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Deek AJ, Boukovalas S, Rathfoot CJ and
Gotcher JE: Rhinocerebral Mucormycosis as a Sequelae of COVID-19
Treatment: A case report & literature review. J Oral Maxillofac
Surg. 80:333–340. 2022.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Maassarani D, Bassil GF, Nehme M, Nassar
A, Ghanime G and Sleiman Z: Rhinocerebral Mucormycosis: An emerging
threat in the Era of COVID-19. Cureus. 14(e28057)2022.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Al-Tawfiq JA, Alhumaid S, Alshukairi AN,
Temsah MH, Barry M, Al Mutair A, Rabaan AA, Al-Omari A, Tirupathi
R, AlQahtani M, et al: COVID-19 and mucormycosis superinfection:
The perfect storm. Infection. 49:833–853. 2021.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Robb GL, Marunick MT, Martin JW and
Zlotolow IM: Midface reconstruction: Surgical reconstruction versus
prosthesis. Head Neck. 23:48–58. 2001.PubMed/NCBI
|
|
22
|
Cao Y, Yu C, Liu W, Miao C, Han B, Yang J,
Li L and Li C: Obturators versus flaps after maxillary oncological
ablation: A systematic review and best evidence synthesis. Oral
Oncol. 82:152–161. 2018.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Buurman DJM, Speksnijder CM, de Groot RJ,
Kessler P and Rieger JM: Mastication in maxillectomy patients: A
comparison between reconstructed maxillae and implant supported
obturators: A cross-sectional study. J Oral Rehabil. 47:1171–1177.
2020.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Rieger JM, Tang JA, Wolfaardt J, Harris J
and Seikaly H: Comparison of speech and aesthetic outcomes in
patients with maxillary reconstruction versus maxillary obturators
after maxillectomy. J Otolaryngol Head Neck Surg. 40:40–47.
2011.PubMed/NCBI
|
|
25
|
Keyf F: Obturator prostheses for
hemimaxillectomy patients. J Oral Rehabil. 28:821–829.
2001.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Rathee M, Divakar S, Jain P, Alam M and
Singh S: Post maxillectomy rehabilitation and amelioration of
quality of life of post-COVID rhinocerebral mucormycosis patients
using obturator: A case series. J Family Med Prim Care.
11:7476–7482. 2022.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Hazra R, Srivastava A and Kumar D:
Obturators: A proposed classification and its associated
techniques. J Indian Prosthodont Soc. 23:192–197. 2023.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Jiao T, Zhu C, Dong X and Gu X:
Rehabilitation of maxillectomy defects with obturator prostheses
fabricated using computer-aided design and rapid prototyping: A
pilot study. Int J Prosthodont. 27:480–486. 2014.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Herce-López J, Pingarrón MDC,
Tofé-Povedano Á, García-Arana L, Espino-Segura-Illa M, Sieira-Gil
R, Rodado-Alonso C, Sánchez-Torres A and Figueiredo R: Customized
subperiosteal implants for the rehabilitation of atrophic jaws: A
consensus report and literature review. Biomimetics (Basel).
9(61)2024.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Cerea M and Dolcini GA: Custom-Made direct
metal laser sintering titanium subperiosteal implants: A
retrospective clinical study on 70 patients. Biomed Res Int.
2018(5420391)2018.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Mommaerts MY: Additively manufactured
sub-periosteal jaw implants. Int J Oral Maxillofac Surg.
46:938–940. 2017.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Gellrich NC, Zimmerer RM, Spalthoff S,
Jehn P, Pott PC, Rana M and Rahlf B: A customised digitally
engineered solution for fixed dental rehabilitation in severe bone
deficiency: A new innovative line extension in implant dentistry. J
Craniomaxillofac Surg. 45:1632–1638. 2017.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Cebrián Carretero JL, Del Castillo Pardo
de Vera JL, Montesdeoca García N, Garrido Martínez P, Pampín
Martínez MM, Aragón Niño I, Navarro Cuéllar I and Navarro Cuéllar
C: Virtual surgical planning and customized subperiosteal titanium
maxillary implant (CSTMI) for three dimensional reconstruction and
dental implants of maxillary defects after oncological resection:
Case series. J Clin Med. 11(4594)2022.PubMed/NCBI View Article : Google Scholar
|
|
34
|
O'Connell DA and Futran ND: Reconstruction
of the midface and maxilla. Curr Opin Otolaryngol Head Neck Surg.
18:304–310. 2010.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Korn P, Gellrich NC, Jehn P, Spalthoff S
and Rahlf B: A new strategy for patient-specific implant-borne
dental rehabilitation in patients with extended maxillary defects.
Front Oncol. 11(718872)2021.PubMed/NCBI View Article : Google Scholar
|