Introduction
Pulsed field ablation (PFA) is a novel approach for
cardiac ablation of atrial fibrillation (AF). PFA is a nonthermal
ablative mechanism in which direct current electric energy is
applied to cells, disrupting cell membranes by creating pores and
preferentially ablating myocardial tissue (1). In contrast to all other contemporary
ablative energy sources used in cardiac ablation, such as RF and
cryothermy, PFA reduces the risk of collateral tissue damage
without compromising its myocardial ablative efficacy (2,3).
Given that PFA is a promising ablation method for
eliminating paroxysmal AF, whether PFA can be successfully applied
in more complex situations is unclear. This is the first medical
record of PFA for AF combined with mitral and CTI atrial flutter.
Similar to RF ablation, the present case study revealed that PFA
can be utilized independently to treat complex arrhythmias, without
the aid of other ablation techniques. Concurrently, the present
study is the first, to the best of the authors' knowledge, to
describe a case using a point-to-point PFA ablation technique for
isthmus ablation. This highlights the potential of PFA in treating
a variety of arrhythmias across various regions, including the
mitral isthmus (MI) and other complex areas, by using a
point-to-point PFA ablation technique.
Case report
The present study reports the case of a 76-year-old
woman complaining of palpitations for 3 months. An
electrocardiogram indicated paroxysmal AF. The comorbidities
included hypertension, atrial premature beats, hyperlipidemia and
type 2 diabetes mellitus. Echocardiography revealed a left atrial
diameter of 45 mm and a normal ejection fraction (62%).
Transesophageal echocardiography and cardiac computed tomographic
angiography did not reveal a thrombus in the left atrial appendage.
Cardiac magnetic resonance (CMR) imaging revealed delayed fibrotic
enhancement at the anterior wall of the left atrium (Fig. S1).
The ablation procedure to treat paroxysmal AF was
planned with 3D navigation and mapping system guidance (CARTO™
Version 7; Biosense Webster; Johnson & Johnson). The
ventricular electrode and coronary sinus (CS) electrode were placed
through the right femoral vein. A mapping electrode catheter
invaded the left atrium, and a 3D model of the heart was
established. The PFA ablation system used was an HT Viewer pro (APT
Medical, Inc.). A circular ablation catheter (APT Medical, Inc.),
which is a 7.5F catheter with 7 electrodes, was selected to carry
out the pulmonary vein isolation (PVI). The successful isolation of
all 4 pulmonary veins was achieved with a median output power
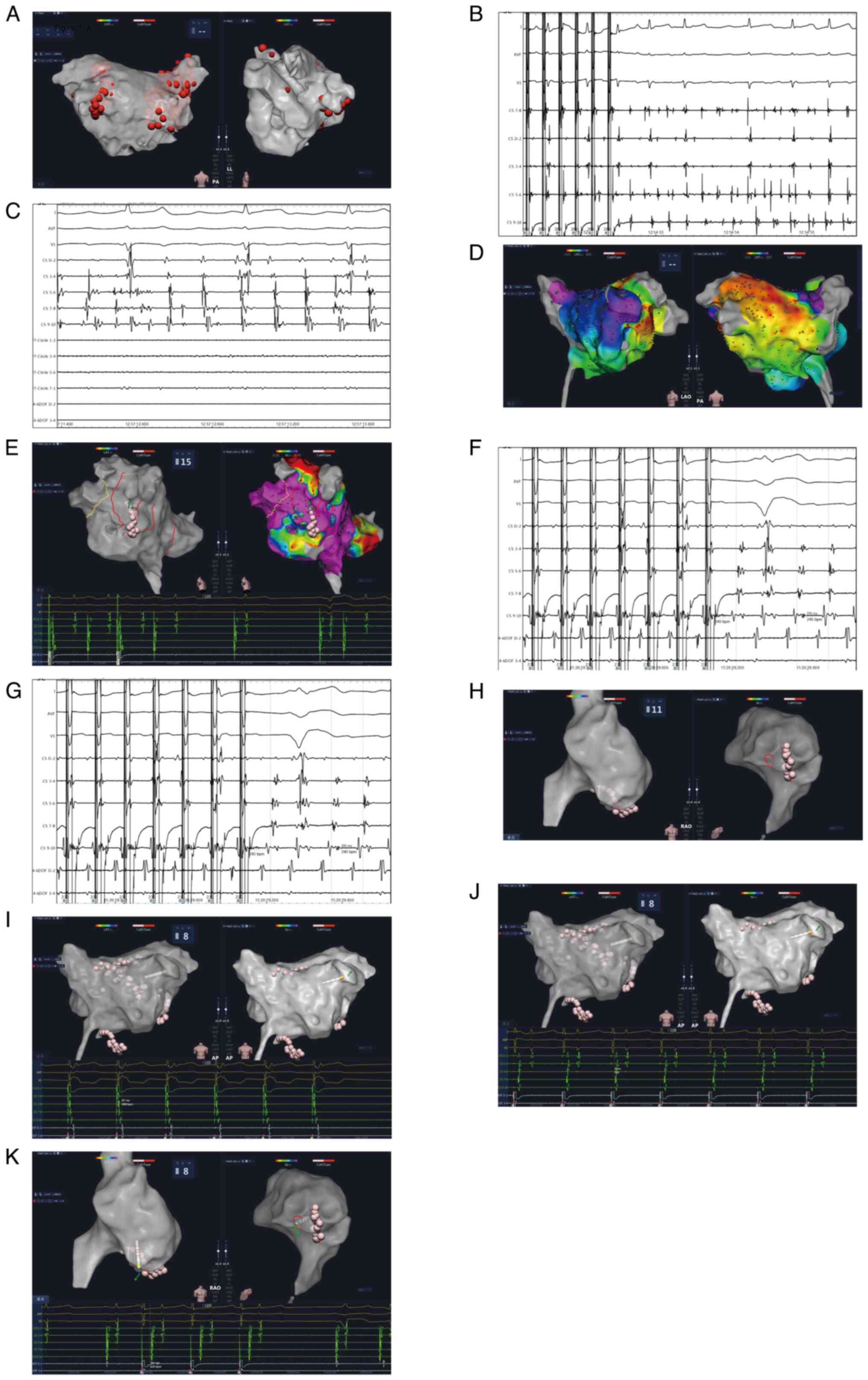
(Fig. 1). AF induced by CS 9-10
with a cycle length of 260 msec was observed after PVI (Fig. 1B), which then soon evolved into an
atrial flutter with a cycle length of 227 msec (Fig. 1C). Remapping was applied, and
electrical excitation was observed around the MI, indicating the
atrial flutter was located at the MI (Fig. 1D). To complete the MI linear
lesion, the ablation catheter was changed to a general pressure
pulse catheter (APT Medical, Inc.), and a point-to-point ablation
technique was used. Medium delivery power was used for ablation.
After termination of the peri-mitral atrial flutter (PMF) (Fig. 1E), another atrial flutter occurred
with a cycle length of 245 msec. The demonstration of transient
entrainment at CS 9-10 (Fig. 1F)
and termination of the atrial flutter at the right atrium free wall
(Fig. 1G) suggested that atrial
flutter is dependent on the CTI. Additional lesions were deployed
to target the CTI (Fig. 1H), after
which the sinus rhythm was ultimately restored. Before the MI
block, the excitation of the atrium pacing at CS1-2 occurred
earlier (Fig. 1I). Following MI
block, the excitation of the atrium pacing at CS9-10 preceded
(Fig. 1J), suggesting the
occurrence of MI block. Pacing was initiated near the lower aspect
of the right atrial free wall, >140 msec from CS9-10, indicating
blockage at the tricuspid valve isthmus (Fig. 1K). A 1-year follow-up confirmed
good sinus rhythm maintenance.
Discussion
PFA is based on the premise of applying ultrarapid
(nanosecond to microsecond) electrical pulses to generate a strong
electrical field, which is subsequently applied to the selected
tissue of interest. PFA was first reported to treat paroxysmal AF
in 22 patients by Reddy et al (1). To date, >400 patients with
paroxysmal AF have been reported to receive PFA treatment in
various studies, with 100% successful PVI and a pooled proportion
of complications of 0.0223(4). A
1-year follow-up of PFA for ablation of paroxysmal AF was
previously reported. Remapping at 2-3 months after PFA revealed PVI
durability in 84.8% of the patients, and 1-year freedom from any
atrial arrhythmia reached 84.5±5.4% (5).
CMR was used to evaluate atrial structure and
fibrosis. CMR is a noninvasive imaging modality that allows for
detailed tissue characterization, provides high spatial resolution
images and enables the visualization of ablation lesions. Cardiac
MRI remains the gold standard for fibrosis assessment (6). In particular, late gadolinium
enhancement MRI appears to be a promising alternative for
pre-ablation scar visualization and quantification (7). The degree of left atrial fibrosis
prior to ablation predicts prognosis; the more atrial fibrosis
there is, the more likely the patient is for an atrial arrhythmia
to recur after ablation (8).
Therefore, several scholars have proposed MRI-guided fibrosis
ablation, but the results have not been satisfactory (9). This may be because the ablation did
not cover the preexisting left atrial fibrosis adequately (10). CMR has more frequently been studied
in AF ablation but less in atrial flutter ablation. This is likely
because the typical atrial flutter-dependent anatomy is relatively
fixed and does not need to be localized with the additional aid of
imaging. Catheter ablation can be successfully performed for
atypical AF via a mapping system.
The concept of MI was first described when Luria
et al (11) noted that
inadvertent damage to a narrow ‘isthmus’ of myocardium between the
lateral mitral annulus and the left inferior pulmonary vein (LIPV)
could lead to intra-atrial conduction block. MI ablation is
challenging from both an efficacy and a safety standpoint, as it
may be associated with significant complications. There are several
reasons for MI ablation difficulty. First, the thickness of the
myocardium ranges from 1.4 to 7.7 mm at the level of the LIPV, from
1.2 to 4.4 mm in the mid-isthmus region, and up to 3.2 mm in the
mitral annulus (12). The vastly
divergent myocardial thickness limits the ease of bidirectional
block through point-by-point ablation. Second, the CS and
circumflex artery near the mitral annulus can reduce conductive
heating of the sub epicardium and act as a ‘heat sink’, thereby
limiting lesion transmurality (13). Third, the proximity of the left
circumflex artery and CS increases the possibility of coronary
injury, while ablation, myocardial sleeves and the vein of Marshall
may act as epicardial bridges preventing MI blockage despite
endocardial ablation.
The most widespread ablation strategy for PMF is MI
ablation, with endpoints of PMF termination and blockage across the
line (14). Reported success rates
are widely distributed [56-96% for MI block (14-17)
and 88-100% for PMF termination (17,18)]. Despite acute bidirectional MI
conduction block, the recovery of conduction can reach 73%, which
may lead to AT recurrence (19).
Several studies have revealed that MI block has little impact on
arrhythmia recurrence in patients with PMF after ablation for AF
(18,20). Poor lesion durability was mostly
recorded with thermal ablation. However, it was difficult to
determine the reason why MI RF ablation strategies fail, but it
could possibly be due to this approach being mechanistically
ineffective or because mitral lines are typically not durable.
While the present case report illustrated the
success of PFA for isthmus ablation, it also highlighted the
challenges associated with this procedure. Although PFA has
demonstrated safety and efficacy in preventing atrial arrhythmias
(5), the risk of coronary spasms
has emerged as a concern, particularly when energy is applied near
coronary arteries (21).
Gunawardene et al (22)
reported successful MI ablation with PFA, but coronary artery spasm
occurred in one patient and was resolved by nitroglycerin after
eight PFA applications. Additionally, a case of ventricular
fibrillation was encountered during tricuspid isthmus ablation with
PFA, leading to emergency defibrillation and coronary artery
dilatation (23). Another hurdle
is the lack of homogeneity in PFA systems, with varying parameter
settings among manufacturers, such as FARAPULSE™ (Boston
Scientific), CENTAURI™ (Galaxy Medical) and PulseSelect™
(Medtronic), which makes it challenging for operators to fully
comprehend their surgical tools. This lack of standardization may
contribute to heterogeneity in clinical trials conducted by
different manufacturers.
The present study provided the first case of AF, MI
and CTI PFA. MI and CTI blockade with a median dose of power
without any complications during the procedure was successfully
achieved. Circular catheters were utilized to isolate pulmonary
veins and transition to pressure catheters for point-to-point
linear ablation of the MI and CTI. Previous studies commonly
employed patterned or circular catheters for isthmus ablation,
followed by additional point ablation using RF catheters (22,24).
The present study suggested the efficacy of employing PFA for
point-to-point ablation in challenging areas. Considering the
enhanced selectivity of PFA for tissue damage and relatively
greater tolerance for catheter stability, using PFA may prove to be
a safer, more effective, and easily implementable approach for
ablation in areas such as the roof line and posterior line beyond
the isthmus region.
Supplementary Material
Cardiac magnetic resonance image
before ablation, indicating late gadolinium enhancement in the
anterior wall.
Acknowledgements
Not applicable.
Funding
Funding: The present study was supported by Chengdu High-Level
Key Clinical Specialty Construction Project.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
The manuscript was written by JLH, with ZZ, GSY, and
DL performing the ablation. JLH participated in the operation,
collected operation data, edited pictures, reviewed relevant
literature and followed up patients. ZZ organized the operation and
provided the design for this article. ZZ and GSY interpreted the
data. JLH and SQX confirm the authenticity of all the raw data.
JLH, SQX and XCY participated in submitting the manuscript. HXL
organised the operation, was the chief operator and provided
technical support. JLH and SQX reviewed the manuscript. YXY and GJH
participated in the ablation, and HXL provided technical support.
All authors read and approved the final version of the
manuscript.
Ethics approval and consent to
participate
The present study has been approved by the Research
Ethical Committee of The Third People's Hospital of Chengdu
(Chengdu, China; approval no. 2023CD-045-07).
Patient consent for publication
Written informed consent was obtained from the
patient for publication of patient data and associated images.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Reddy VY, Koruth J, Jais P, Petru J, Timko
F, Skalsky I, Hebeler R, Labrousse L, Barandon L, Kralovec S, et
al: Ablation of atrial fibrillation with pulsed electric fields: An
ultra-rapid, tissue-selective modality for cardiac ablation. JACC
Clin Electrophysiol. 4:987–995. 2018.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Koruth JS, Kuroki K, Kawamura I, Brose R,
Viswanathan R, Buck ED, Donskoy E, Neuzil P, Dukkipati SR and Reddy
VY: Pulsed field ablation versus radiofrequency ablation:
Esophageal injury in a novel porcine model. Circ Arrhythm
Electrophysiol. 13(e008303)2020.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Kuck KH, Brugada J, Fürnkranz A, Metzner
A, Ouyang F, Chun KR, Elvan A, Arentz T, Bestehorn K, Pocock SJ, et
al: Cryoballoon or radiofrequency ablation for paroxysmal atrial
fibrillation. N Engl J Med. 374:2235–2245. 2016.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Shaheen N, Shaheen A and Ramadan A:
Efficacy and Safety of Novel Pulsed Field Ablation (PFA) Technique:
A Systematic review and Meta-analysis. Authorea: August 30,
2022.
|
|
5
|
Reddy VY, Dukkipati SR, Neuzil P, Anic A,
Petru J, Funasako M, Cochet H, Minami K, Breskovic T, Sikiric I, et
al: Pulsed field ablation of paroxysmal atrial fibrillation: 1-year
outcomes of IMPULSE, PEFCAT, and PEFCAT II. JACC Clin
Electrophysiol. 7:614–627. 2021.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Obeng-Gyimah E and Nazarian S:
Advancements in imaging for atrial fibrillation ablation: Is there
a potential to improve procedural outcomes? J Innov Card Rhythm
Manag. 11:4172–4178. 2020.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Siebermair J, Kholmovski EG and Marrouche
N: Assessment of left atrial fibrosis by late gadolinium
enhancement magnetic resonance imaging: Methodology and clinical
implications. JACC Clin Electrophysiol. 3:791–802. 2017.PubMed/NCBI View Article : Google Scholar
|
|
8
|
McGann C, Akoum N, Patel A, Kholmovski E,
Revelo P, Damal K, Wilson B, Cates J, Harrison A, Ranjan R, et al:
Atrial fibrillation ablation outcome is predicted by left atrial
remodeling on MRI. Circ Arrhythm Electrophysiol. 7:23–30.
2014.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Marrouche NF, Wazni O, McGann C, Greene T,
Dean JM, Dagher L, Kholmovski E, Mansour M, Marchlinski F, Wilber
D, et al: Effect of MRI-guided fibrosis ablation vs conventional
catheter ablation on atrial arrhythmia recurrence in patients with
persistent atrial fibrillation: The DECAAF II randomized clinical
trial. JAMA. 327:2296–2305. 2022.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Akoum N, Wilber D, Hindricks G, Jais P,
Cates J, Marchlinski F, Kholmovski E, Burgon N, Hu N, Mont L, et
al: MRI assessment of ablation-induced scarring in atrial
fibrillation: Analysis from the DECAAF study. J Cardiovasc
Electrophysiol. 26:473–480. 2015.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Luria DM, Nemec J, Etheridge SP, Compton
SJ, Klein RC, Chugh SS, Munger TM, Shen WK, Packer DL, Jahangir A,
et al: Intra-atrial conduction block along the mitral valve annulus
during accessory pathway ablation: Evidence for a left atrial
‘isthmus’. J Cardiovasc Electrophysiol. 12:744–749. 2001.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Becker AE: Left atrial isthmus: Anatomic
aspects relevant for linear catheter ablation procedures in humans.
J Cardiovasc Electrophysiol. 15:809–812. 2004.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Wittkampf FH, van Oosterhout MF, Loh P,
Derksen R, Vonken EJ, Slootweg PJ and Ho SY: Where to draw the
mitral isthmus line in catheter ablation of atrial fibrillation:
Histological analysis. Eur Heart J. 26:689–695. 2005.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Jaïs P, Hocini M, Hsu LF, Sanders P,
Scavee C, Weerasooriya R, Macle L, Raybaud F, Garrigue S, Shah DC,
et al: Technique and results of linear ablation at the mitral
isthmus. Circulation. 110:2996–3002. 2004.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Yokokawa M, Sundaram B, Garg A,
Stojanovska J, Oral H, Morady F and Chugh A: Impact of mitral
isthmus anatomy on the likelihood of achieving linear block in
patients undergoing catheter ablation of persistent atrial
fibrillation. Heart Rhythm. 8:1404–1410. 2011.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Ernst S, Schlüter M, Ouyang F, Khanedani
A, Cappato R, Hebe J, Volkmer M, Antz M and Kuck KH: Modification
of the substrate for maintenance of idiopathic human atrial
fibrillation: Efficacy of radiofrequency ablation using
nonfluoroscopic catheter guidance. Circulation. 100:2085–2092.
1999.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Ammar S, Luik A, Hessling G, Bruhm A,
Reents T, Semmler V, Buiatti A, Kathan S, Hofmann M, Kolb C, et al:
Ablation of perimitral flutter: Acute and long-term success of the
modified anterior line. Europace. 17:447–452. 2015.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Bai R, Di Biase L, Mohanty P, Dello Russo
A, Casella M, Pelargonio G, Themistoclakis S, Mohanty S, Elayi CS,
Sanchez J, et al: Ablation of perimitral flutter following catheter
ablation of atrial fibrillation: Impact on outcomes from a
randomized study (PROPOSE). J Cardiovasc Electrophysiol.
23:137–144. 2012.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Sawhney N, Anand K, Robertson CE, Wurdeman
T, Anousheh R and Feld GK: Recovery of mitral isthmus conduction
leads to the development of macro-reentrant tachycardia after left
atrial linear ablation for atrial fibrillation. Circ Arrhythm
Electrophysiol. 4:832–837. 2011.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Latcu DG, Squara F, Massaad Y, Bun SS,
Saoudi N and Marchlinski FE: Electroanatomic characteristics of the
mitral isthmus associated with successful mitral isthmus ablation.
Europace. 18:274–280. 2016.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Reddy VY, Petru J, Funasako M, Kopriva K,
Hala P, Chovanec M, Janotka M, Kralovec S and Neuzil P: Coronary
arterial spasm during pulsed field ablation to treat atrial
fibrillation. Circulation. 146:1808–1819. 2022.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Gunawardene MA, Schaeffer BN, Jularic M,
Eickholt C, Maurer T, Akbulak RÖ, Flindt M, Anwar O, Hartmann J and
Willems S: Coronary spasm during pulsed field ablation of the
mitral isthmus line. JACC Clin Electrophysiol. 7:1618–1620.
2021.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Della Rocca DG, Del Monte A, Bala G,
Pannone L, Ströker E, Monaco C, Almorad A, Sieira J, Sorgente A, de
Asmundis C and Chierchia GB: Transient Inferior ST-Segment
elevation and ventricular fibrillation after cavotricuspid isthmus
pulsed-field ablation. JACC Clin Electrophysiol. 9:704–706.
2023.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Kueffer T, Seiler J, Madaffari A, Mühl A,
Asatryan B, Stettler R, Haeberlin A, Noti F, Servatius H, Tanner
HJ, et al: Pulsed-field ablation for the treatment of left atrial
reentry tachycardia. J Interv Card Electrophysiol. 66:1431–1440.
2023.PubMed/NCBI View Article : Google Scholar
|