Introduction
Bone defects are major problems in trauma
orthopedics, leading to non-union and limb disability, making the
prognosis of the patient unsatisfactory. It causes severe damage to
the physical and mental health of the patient, with a huge economic
burden on both patients and society (1). Fracture healing is divided into four
major overlapping phases: Inflammation, bridging osseous callus
formation, callus mineralization and bone remodeling (2). Functional reduction of osteoblasts is
an important cause of bone defects. Therefore, pharmacological or
physical interventions that induce osteoblast differentiation and
proliferation, thereby promoting bone formation, are potential
strategies for treating bone defects.
Low-frequency-pulsed electromagnetic field (LPEMF)
is a noninvasive and inexpensive method that has shown efficacy
against a wide range of diseases of the skeletal muscle system
(3,4). LPEMF may inhibit osteoclast
differentiation in vitro (5,6),
accelerate bone defect repair and shorten the healing time of bone
defects by promoting osteogenic differentiation of bone marrow
stromal cells (BMSCs) (2). BMSCs
have multidirectional differentiation potential and are the main
source of osteoblasts (7).
Osteogenic differentiation of BMSCs is the physiological basis of
bone defect repair and promoting osteogenic differentiation of
BMSCs can substantially shorten the time required for bone defect
repair (8). Therefore,
investigating the underlying mechanisms of LPEMF in osteoblasts is
essential for understanding its efficacy in treating
osteoblast-related diseases.
Connexin 43 (Cx43) is the most abundant gap junction
protein in bone (9). Cx43 is the
most widely expressed connexin among bone cell types, encoded by
Gja1 gene (10) and
expressed at the cell surface, where it mediates cell communication
and allows small molecules and ions to pass between cells (9,11).
Cx43 plays important roles in bone formation and homeostasis.
Osteoblasts regulate cell proliferation, differentiation and
metabolic functions via intercellular communication (9,10,12-14).
Cx43 is also expressed in cartilage and is necessary for
mineralization and osteoclast formation, suggesting that Cx43 may
play an important role in multiple stages of fracture healing
(15-17).
In three-dimensional culture, overexpression of Cx43 in BMSCs
enhances the size and spatial distribution of gap junctions,
intercellular communication and expression of osteogenic markers
(18).
LPEMF has the ability to promote the osteogenic
differentiation of BMSCs, accelerate the process of bone defect
repair and shorten the healing time of bone defects. However, the
mechanism of LPEMF on Cx43 expression in promoting the osteogenic
differentiation of BMSCs remains to be elucidated. The present
study provided a comprehensive and systematic annotation of the
role of Cx43 in the regulation of the osteogenic differentiation of
BMSCs by LPEMF through in vitro experiments at the molecular
and cellular levels to explore the mediation of Cx43 and provide a
target for the treatment of bone defects.
Materials and methods
Isolation and culture of BMSCs
Primary rat BMSCs were isolated from specific
pathogen free (SPF) four to six-week-old rats weighing 100-150 g
Sprague-Dawley, which were provided by the SPF grade animal house
of Shantou University Medical College (Guangdong, China). A total
of 30 rats, 50% male and 50% female, were libitum fed in the same
environment were maintained at an indoor temperature of 22˚C,
relative humidity of 18-22%, and a 12-h light/dark cycle. All
animal experimental procedures were approved by the Institutional
Ethics Committee for Animal Experimentation of Ganzhou People's
Hospital (Ganzhou, China). Rats were anesthetized by
intraperitoneal injection of 3% pentobarbital sodium (30 mg/kg) and
euthanized by cervical dislocation. The two lower limbs were
rapidly dissociated and disinfected by immersion in 75% ethanol for
5-10 min, under aseptic conditions. Subsequently, the femoral bone
marrow was lavaged and the lavage fluid was inoculated into a 10-cm
dish using the whole bone marrow adherent culture method (19). Briefly, the cells were seeded onto
flasks for culturing in a CO2 cell incubator. The
culture medium was maintained at 37˚C and 5% CO2 in
Dulbecco's modified Eagle's medium (Thermo Fisher Scientific, Inc.)
containing 10% fetal bovine serum (FBS; Thermo Fisher Scientific,
Inc.) and 0.5 mg/ml penicillin/streptomycin. Cell morphology and
growth were observed daily using an inverted microscope. When the
cell confluence reached 70-90%, a subculture was carried out and
the cells were cultured to the third generation for subsequent
experiments.
LPEMF exposure
To evaluate the proliferation and osteogenic
differentiation behavior of BSMCs under different intensity
gradients of LPEMF, the cells were continuously exposed to
sinusoidal LPEMF (20, 40, 60, 80 and 100 Hz) of five different
gradient signals with a density of 1 mT in the incubator. A pulsed
electromagnetic field in vitro cell intervention system
(DG1022U; Rigol Technologies, Inc.) generated electromagnetic field
signals at a predetermined frequency. The magnetic field was
generated using a pair of 60-turn Helmholtz coils. The coil
diameter was 300 mm and the spacing was 122.5 mm. A Petri dish was
placed at the center of the coil. The control cells were placed in
another incubator under the same conditions, but without LPEMF.
BMSCs osteogenic differentiation
culture
When P3 cells were cell confluence reached 70-90%,
they were subcultured and inoculated at 5,000-10,000
cells/cm2. The cells were incubated overnight in a
humidified incubator at 37˚C, with 5% CO2 and 95% air
and then replaced with an osteogenic induction medium (alpha-MEM;
(Gibco; Thermo Fisher Scientific, Inc.; cat. no. C12571500BT)
supplemented with 10% fetal bovine serum (Gibco; Thermo Fisher
Scientific, Inc.; cat. no. 12664025), 50 µM vitamin C
(MilliporeSigma; cat. no. A4544), 10 mM beta-sodium
glycerophosphate (MilliporeSigma; cat. no. G9422) and 100 nM
dexamethasone (MilliporeSigma; cat. no. D1756) for further culture.
The osteogenic induction medium was replaced once every 2-3 days
and cell growth was observed under a light microscope every day.
The cells were cultured until they were terminated at 9 days and
tested for ALP activity. After 14 days of cell culture, total RNA
and total protein were extracted for PCR and Western blotting
experiments. Cell cultures were terminated at 14 days and Alizarin
red S staining was performed.
Cell Counting Kit-8 (CCK-8) assay
To evaluate cell proliferation, BMSCs were
inoculated into 96-well plates at a density of 1x104
cells per well and incubated in a CO2 cell incubator
(37˚C, 5% CO2) for 4 h. Cells were cultured 24 h after
electromagnetic field treatment for 5 days and 10 µl CCK8 (Dojindo
Laboratories, Inc.) dye solution was added to each well. After 2 h,
the absorbance of each well was measured at 450 nm according to the
manufacturer's instructions. Each data point was obtained as the
mean of five wells.
ALP activity detection
ALP activity is an early marker of bone formation
(20). After cells were incubated
for the indicated times, cells were washed once with PBS at 4˚C,
lysed with 1% Triton X-100 and the lysates collected. After
centrifugation for 5 min at 4˚C and 1,174 x g, the supernatant was
collected. Color substrate (50 µl) and 50 µl of the group sample or
sample buffer (blank control) was added to the 96-well plate, then
placed on a shaker for mixing. After incubation at 37˚C for 10 min,
100 µl of the reaction stop solution was added to each well. The
absorbance of each well was measured at 405 nm by using a
microplate reader.
Alizarin red S staining and
quantification
After 14 days of cell culture under different
conditions, the medium was removed and 1 ml PBS was added to each
well. The cells were gently washed once, fixed with 2 ml anhydrous
ethanol for 30 min and cleaned once with ultra-pure water. Alizarin
red S solution (1 ml, 0.1%) was added after the cell surface dried.
The bed was shaken at 22˚C room temperature for 15 min, the
Alizarin red S solution was removed, the cells were rinsed again
with ultra-pure water three times and the staining of calcium
nodules was observed under a light microscope after the cells were
dried naturally. After Alizarin Red S staining was completed, 2 ml
of 10% CPC sodium phosphate buffer solution was added to each well
and incubated at 22˚C room temperature for 15 min to elute Alizarin
Red S. The Alizarin red S eluent was diluted 10 times with
ultra-pure water and 100 µl diluent was absorbed into 96-well
plates with five wells per group. The absorbance of each well was
measured at 540 nm using a microplate analyzer.
RNA extraction and reverse
transcription-quantitative (RT-q) PCR
After treatment, total RNA was extracted from each
group of cells using TRIzol® reagent (Thermo Fisher
Scientific, Inc.) and the RNA concentration was measured using a
NanoDrop spectrophotometer (Thermo Fisher Scientific, Inc.). Next,
mRNA was reverse-transcribed into cDNA using a reverse
transcription system (Toyobo Life Science) according to the
manufacturer's protocol. Then, cDNA was used as a template to
perform qPCR (BeyoFast SYBR Green qPCR Mix; Beyotime Institute of
Biotechnology) under the following thermal cycler conditions: 95˚C
for 5 sec, followed by 45 cycles, including denaturation at 95˚C
for 30 sec in an ABI 7500 real-time PCR system (Applied Biosystems;
Thermo Fisher Scientific, Inc.); further it was annealed at 60˚C
for 30 sec and extended at 72˚C for 30 sec. GAPDH was used as an
internal control, and the relative expression level of mRNA was
calculated using the 2-ΔΔCq method (21). Primers were designed and
synthesized by BGI Biological Co. and the sequences are listed in
Table I.
 | Table IPrimers used for quantitative
PCR. |
Table I
Primers used for quantitative
PCR.
| Target gene | Oligonucleotide
sequence |
|---|
| Runx2 |
5'-GGGACCGTCCACTGTCACTTTAA-3'
(Forward) |
| |
5'-TACAAGTGGCCAGGTTCAACGA-3'
(Reverse) |
| BSP |
5'-GCTATGAAGGCTACGAGGGTCAGGATTAT-3'
(Forward) |
| |
5'-GGGTATGTTAGGGTGGTTAGCAATGGTGT-3'
(Reverse) |
| Bglap |
5'-AGGTGGTGAATAGACTCCG-3' (Forward) |
| |
5'-GGCTGTGCCGTCCATACTT-3' (Reverse) |
| Osx |
5'-GGAGGCACAAAGAAGCCATA-3' (Forward) |
| |
5'-GGGAAAGGGTGGGTAGTCAT-3' (Reverse) |
| GAPDH |
5'-TCCTGCACCACCAACTGCTTAG-3'
(Forward) |
| |
5'-AGTGGCAGTGATGGCATGGACT-3'
(Reverse) |
Western blotting
Western blotting was performed according to standard
procedures. Briefly, proteins were extracted from cells or tissues
using RIPA lysis buffer (Beyotime Institute of Biotechnology; cat.
no. P0013B). Protein concentrations were determined using the BCA
Protein Assay Kit (Solarbio, Beijing, China). After denaturation
~20 µg of protein per lane was separated by 10% sodium dodecyl
sulfate polyacrylamide gel, proteins were transferred to
polyvinylidene fluoride (PVDF) membranes (0.45 µm; MilliporeSigma).
The PVDF membranes were blocked with Tris-buffered saline (TBS)
containing 5% nonfat milk for 1 h at room temperature.
Subsequently, it was incubated with primary antibodies [rabbit
anti-Cx43 (1:1,000; Cell Signaling Technology; cat. no. 3512),
rabbit anti-GAPDH (1:1,000; Panera; cat. no. SF-PA005)] overnight
at 4˚C, then washed with TBST (0.1% Tween20) and incubated with the
corresponding secondary antibody (Jackson ImmunoResearch; cat. no.
111-035-003) for 2 h. A chemiluminescence system (ECL Kit; Biosharp
Life Sciences) was used to observe the protein bands and digital
images were captured, which were then analyzed using ImageJ v6.0
(National Institutes of Health).
Statistical analysis
All data were analyzed using SPSS 19.0 software (IBM
Corp.) and the results were expressed as mean ± standard deviation.
The t-test was used for comparisons between two groups and one-way
ANOVA followed by Tukey's post hoc test was used for comparisons
between multiple groups. P<0.05 was considered to indicate a
statistically significant difference. All experiments were
independently repeated at least three times.
Results
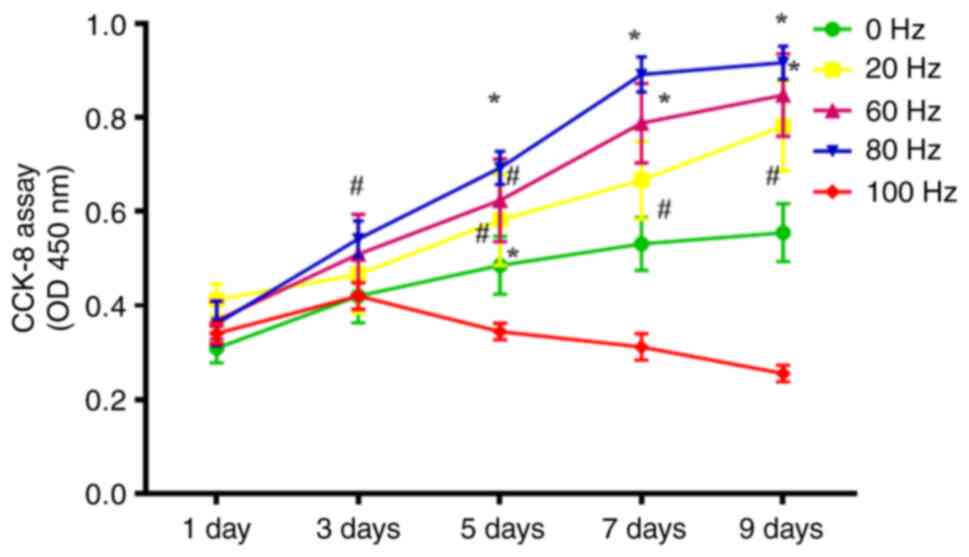
Effect of different frequencies of
LPEMF treatment on the proliferation ability of BMSCs
Evaluation of the effect of LPEMF on BMSCs
proliferation in vitro: BMSCs were treated with different
intensity gradients (0, 20, 40, 60, 80 and 100 Hz) of LPEMF for 24
h and the viability of BMSCs treated with LPEMF for 24 h was
determined using CCK-8. The results showed that the proliferation
viability of BMSCs increased with increasing magnetic field
strength from 0 to 80 Hz peaking at 80 Hz (P<0.05). However,
when the intensity reached 100 Hz, the proliferation viability of
BMSCs was significantly inhibited and exhibited toxic side effects
(Fig. 1). Therefore, the 100 Hz
magnetic field intensity was removed in subsequent experiments as
the research condition and 80 Hz magnetic field stimulation was
used as the optimal stimulation intensity for the LPEMF treatment
of BMSCs.
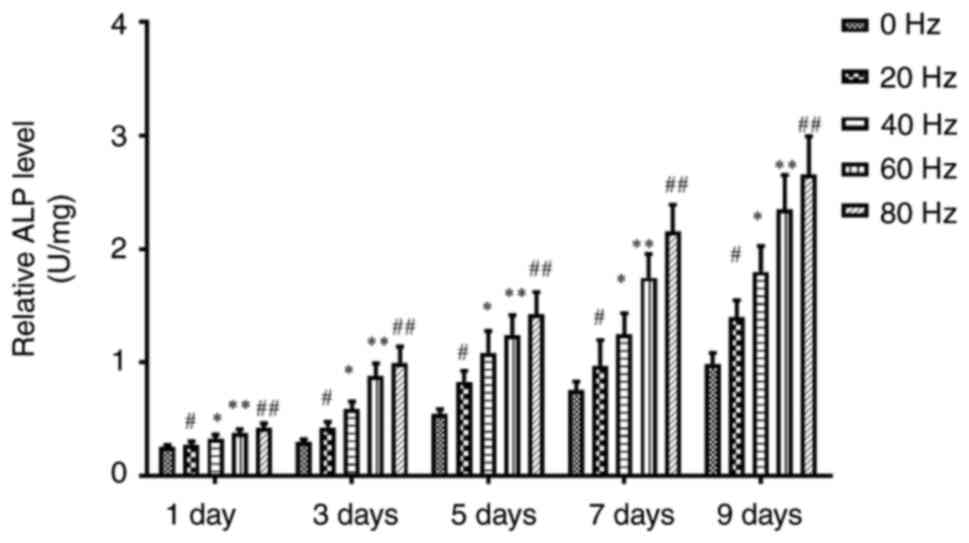
Effect of LPEMF treatment on ALP
activity during osteogenic differentiation of BMSCs
To test whether LPEMF can promote the osteogenic
differentiation of BMSCs, the ALP activity of each group was
measured. The results showed that, compared with the control group,
the ALP activity of BMSCs pretreated with LPEMF at different
frequencies was enhanced on day 9 after osteogenic induction
culture and the intensity showed a dose-dependent trend, with
significant differences among all groups (Fig. 2).
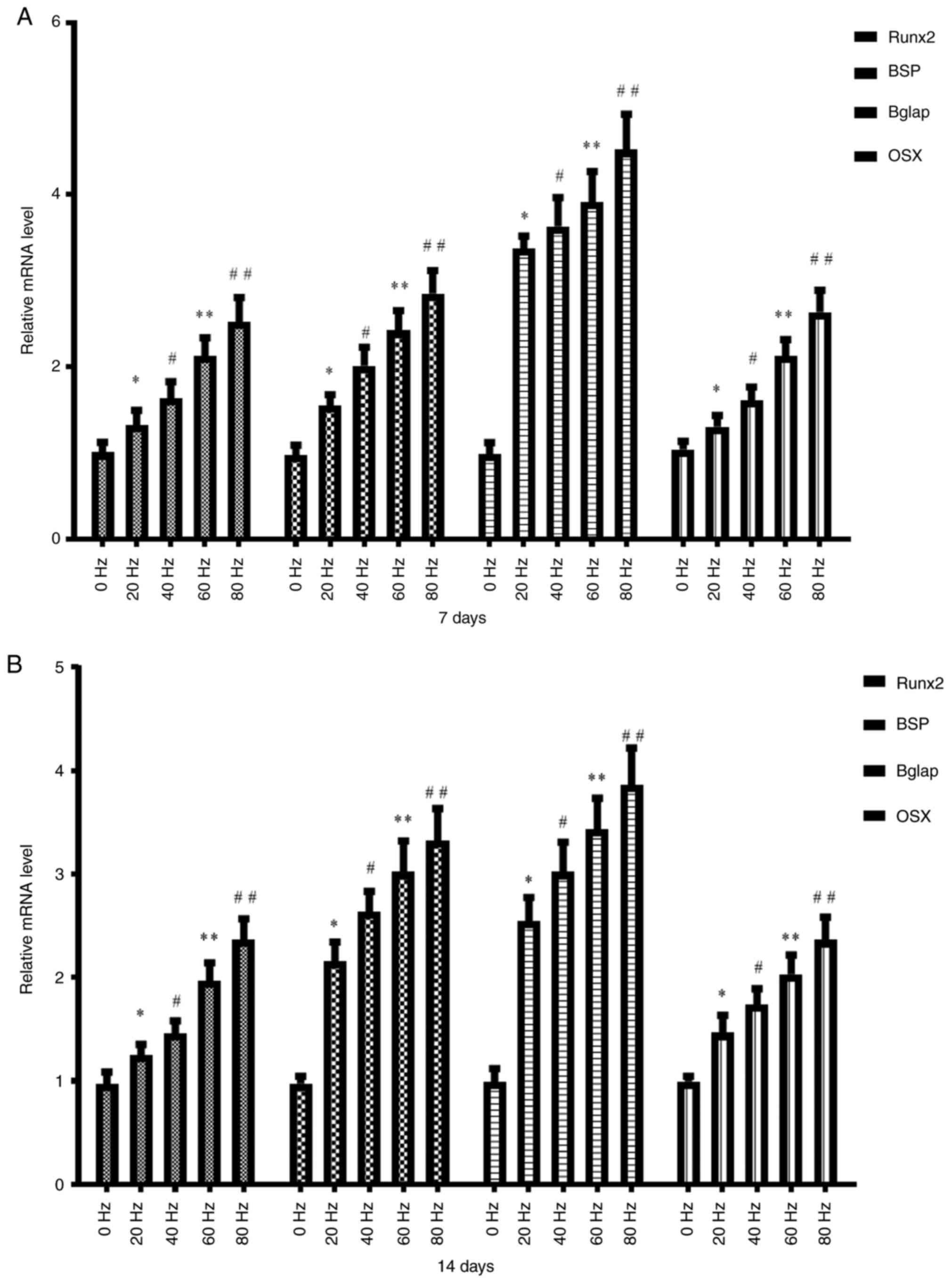
Effect of LPEMF treatment on the
expression of osteogenic marker genes during osteogenic
differentiation of BMSCs
The present study examined the effect of LPEMF
treatment on the expression of osteogenic marker genes during
osteogenic differentiation of BMSCs. Following LPEMF treatment at
different frequencies, BMSC osteogenic differentiation was induced
and cultured for 7 and 14 days and the transcription levels of the
osteogenic marker genes Runx2, BSP, Bglap and
Osx in each group were measured. The results showed that the
transcription levels of osteogenic marker genes on day 7 (Fig. 3A) and 14 (Fig. 3B) were enhanced with an increase in
the LPEMF frequency and there was a significant difference between
the groups with different frequencies (P<0.05). After cells were
treated with LPEMF at the same frequency, the transcription levels
of osteogenic marker genes on day 14 were higher than those on day
7 (P<0.05).
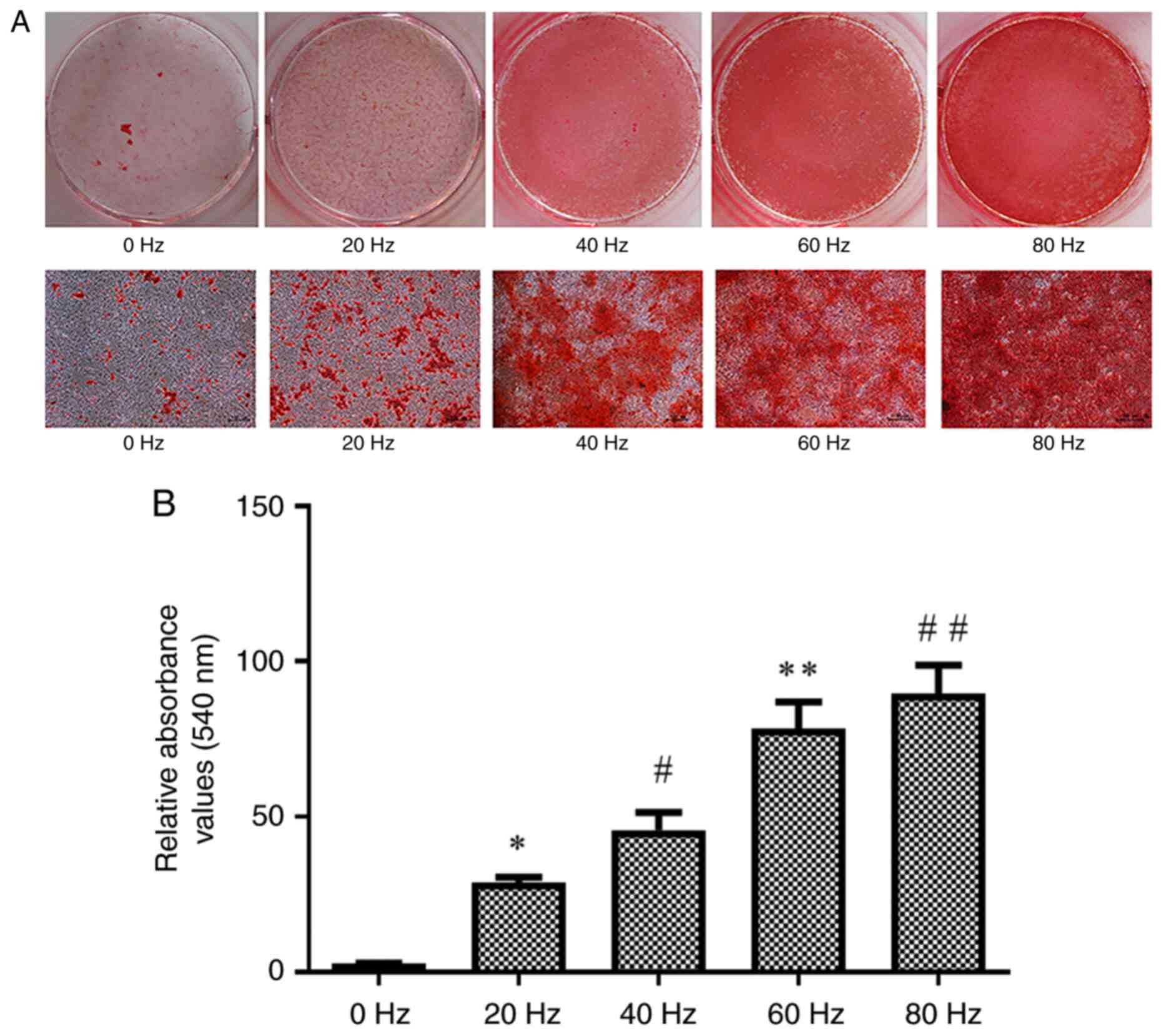
Effect of LPEMF treatment on
osteogenic mineralization of BMSCs
The effect of LPEMF treatment on osteogenic
mineralization of BMSCs was investigated. BMSCs from each group
were terminated after being cultured under osteogenic inductive
conditions for 14 days after treatment with LPEMF at different
frequencies and mineralization was assessed using Alizarin Red S
staining. The gross specimens are shown (Fig. 4A). The formation of calcium nodules
was lowest in the control group and numerous calcium nodules were
observed in the other groups. The amount of calcium nodule
deposition increased with an increase in LPEMF frequency. Alizarin
Red S quantitative results showed that the number of calcium
nodules significantly increased with an increase in frequency in
each group (P<0.05; Fig.
4B).
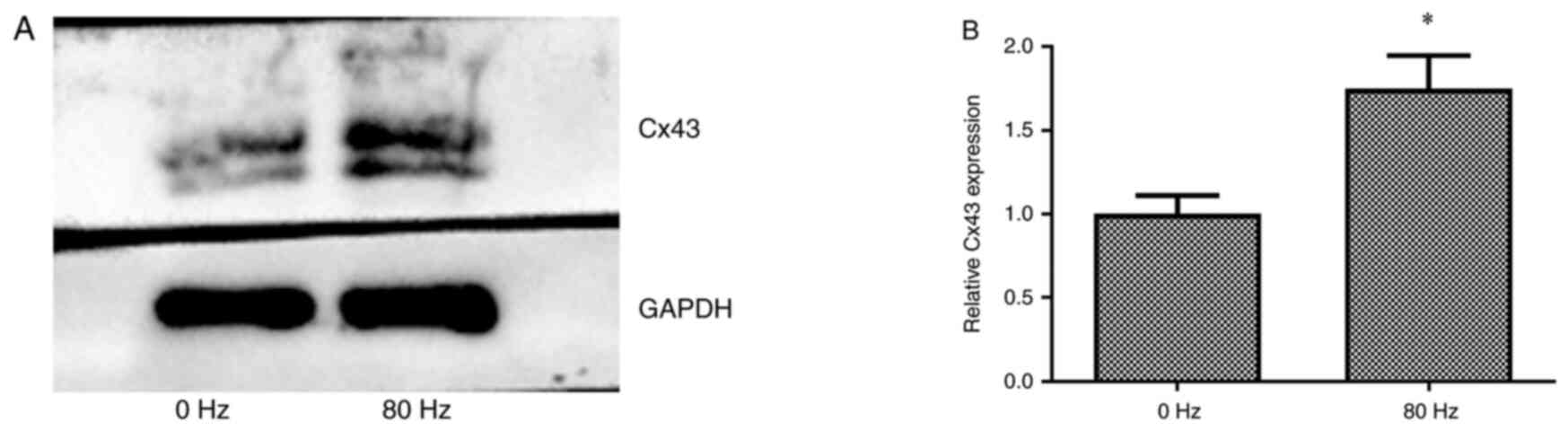
Effect of LPEMF treatment on Cx43
expression during osteogenic differentiation of BMSCs
To detect the expression of Cx43 following LPEMF
treatment during the process of osteogenic differentiation of
BMSCs, Cx43 expression levels in BMSCs treated with LPEMF at 0 and
80 Hz for 14 days after osteogenic induction culture were
determined using western blotting. The results showed that Cx43
expression was significantly enhanced in the 80 Hz LPEMF-treated
group compared with that of the 0 Hz LPEMF-treated group
(P<0.05; Fig. 5).
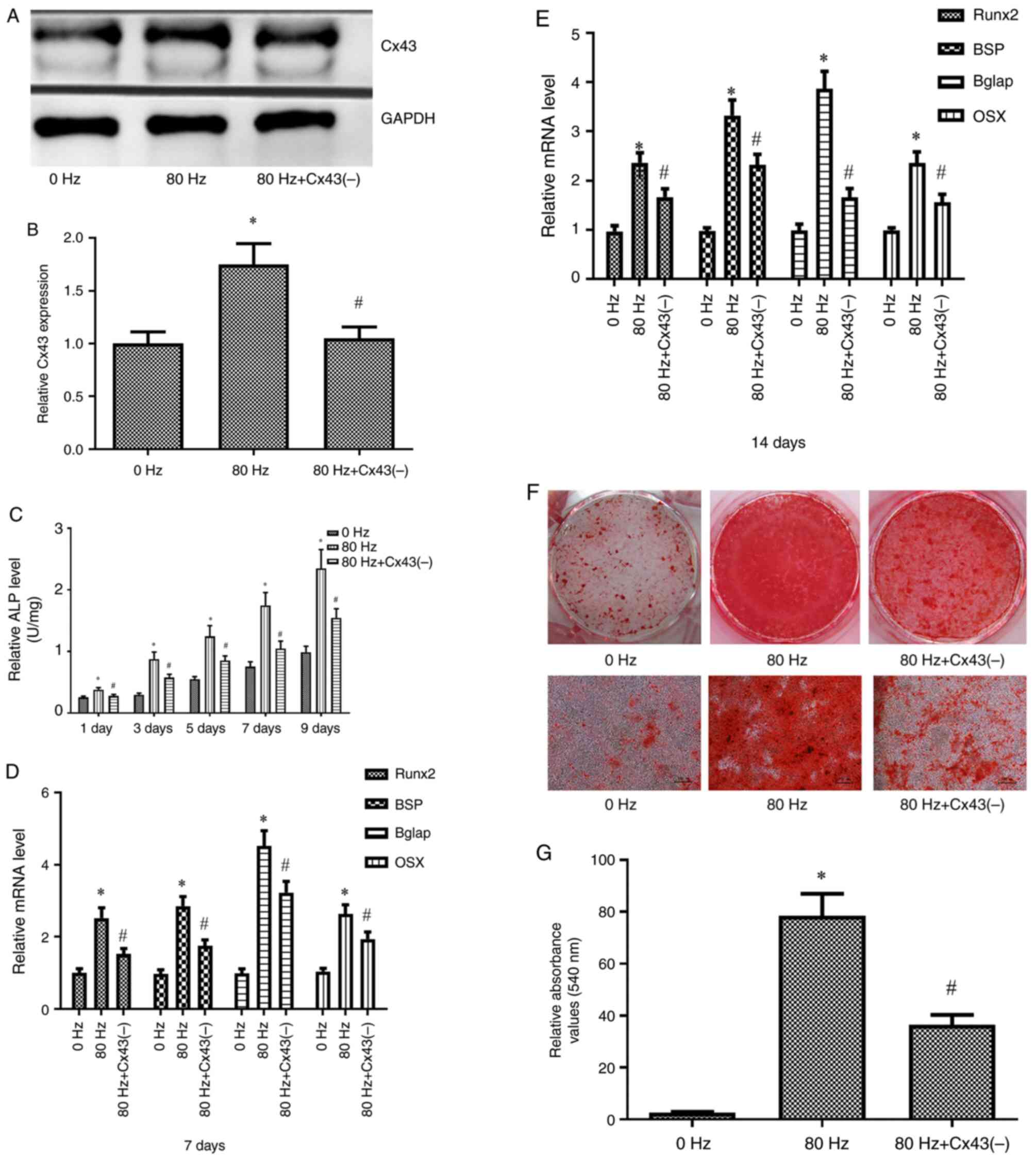
Inhibition of Cx43 expression
attenuates the role of LPEMF in promoting osteogenic
differentiation of BMSCs
To verify the role of Cx43 in promoting the
osteogenic differentiation of BMSCs by LPEMF, BMSCs were
transfected with a CX43-specific lentivirus vector to inhibit the
expression of Cx43. Transfection efficiency was detected using
western blotting and the results showed that Cx43 expression was
significantly inhibited in BMSCs after transfection with the Cx43
specific lentivirus vector. Following Cx43 inhibition, BMSCs were
treated with an 80 Hz LPEMF. The indicators of osteogenic
differentiation of BMSCs were significantly lower than those of
Cx43 without inhibition (P<0.05; Fig. 6).
Discussion
The present study established an in vitro
osteogenic differentiation model for LPEMF-induced BMSCs. It showed
that the proliferation, osteogenic differentiation and
mineralization of BMSCs were enhanced in a magnetic field
intensity-dependent manner by LPEMF intervention.
BMSCs are the main cell source of osteoblasts in
fracture healing. They are adult stem cells with self-renewal,
proliferation and multidirectional differentiation potential and
can differentiate into osteoblasts, adipocytes, chondrocytes, nerve
cells and myoblasts (22,23). The osteogenic differentiation of
BMSCs is a continuous and dynamic process. Generally, osteogenic
differentiation of BMSCs is divided into three stages: Osteoblast
precursor cells, immature osteoblasts and osteoblasts. Massive
deposition of mineralized matrix marks the formation of osteoblasts
and the main component of the mineralized matrix is calcium salt
(24). Therefore, the present
study investigated the mechanism of LPEMF healing from a
cytological perspective and used BMSCs to study the process of
LPEMF-promoting BMSC osteogenic differentiation from multiple
aspects and at different stages. LPEMF has been used to promote
fracture healing for more than half a century and Wang et al
(25) showed that it plays a
positive role in promoting bone regeneration. However, the
mechanism through which LPEMF promotes bone regeneration remains to
be elucidated. Currently, there are theories, such as ‘non-thermal
effect’ (26), ‘window effect’
hypothesis (27), local blood flow
increase theory, calcium ion deposition theory (28), mediating mechanism of second
messenger molecule (29) and
endocrinoid effect of pulsed electromagnetic field (30).
ALP is an enzyme secreted by BMSCs during the early
stages of osteogenic differentiation and its activity reaches its
peak before the mineralization stage. Through the hydrolysis of
pyrophosphate, ALP provides phosphate ions and hydroxyl radicals
for the deposition of hydroxyapatite crystals, thus forming a
mineralized bone matrix (31). The
proliferation of osteoblasts isolated from ALP knockout mice was
normal, but there was no normal mineral salt deposition following
culture. The quantitative measurement of ALP activity is often used
as an indicator of changes in the strength of early osteogenic
differentiation (20). Runx2
activation controls the initiation of the osteogenic
differentiation switch in BMSCs and promotes osteoblast maturation
(32). Runx2 gene defects cause
BMSCs to have no osteogenic differentiation ability and endometrial
and endochondral osteogenic functions are lost in Runx2 knockout
mice, suggesting osteoblast maturation disorder (33). Runx2 binds to osteoblastic
heterozygous elements to regulate the expression of osteoblastic
extracellular matrix protein genes Bglap, osteopontin, BSP and
Col1α1 and participate in the synthesis of extracellular matrix
(34). Osx is a key gene that
controls BMSCs during osteoblast differentiation. Osx was first
discovered during the process of osteogenic differentiation induced
by BMP-2 in C2C12 myoblasts and regulates the expression of Bglap,
osteopontin, bone salivariin, type I collagen and other genes
(35). BSP and Bglap are secreted
by mature osteoblasts, which together with osteopontin and type I
collagen participate in the synthesis of osteoids and deposition of
calcium salts and other minerals (36). The increased secretion of BSP and
osteocalcin signals the transformation of immature osteoblasts into
mature osteoblasts and can be used as indicators of middle and late
osteogenic differentiation of BMSCs (36-38).
Alizarin red S forms a complex with calcium ion chelation and is an
orange-red deposition. Alizarin red S staining is commonly used to
detect the maturity of BMSCs during the late stage of osteogenic
differentiation (39). In the
present study, ALP activity and Runx2, Osx, BSP and Bglap were used
as markers of osteogenic differentiation of BMSCs. Alizarin Red S
staining was used to detect calcium nodules and reflect the level
of osteogenic differentiation in BMSCs. The results of the present
study showed that LPEMF stimulated the proliferation of BMSCs in a
frequency-dependent manner in the range of 0-80 Hz, whereas at 100
Hz, LPEMF inhibited the growth of BMSCs, presenting a toxic side
effect. Therefore, it can be concluded that a magnetic field with a
frequency <80 Hz is safe for stimulating BMSCs. Following LPEMF
treatment, the activity of ALP and expression of the osteogenic
marker genes Runx2, BSP, Bglap and Osx notably increased during
BMSCs osteogenic differentiation. The mineralization level of BMSCs
was also notably increased, suggesting that LPEMFs can promote bone
formation.
Cx43 is a connexin encoded by Gja1 that is widely
expressed in bone tissue cells (40). Cx43 is an essential gene for the
survival, proliferation, differentiation and other physiological
processes of osteoblasts and interference with Cx43 expression can
significantly inhibit the differentiation of osteoblasts (40-42).
In a three-dimensional osteogenic induction culture of BMSCs, the
overexpression of Cx43 increased the number and spatial
distribution of GJIC and enhanced the transcription level of
osteogenic marker genes (43).
Osteoblast differentiation of BMSCs is regulated by various
signaling pathways. The present study hypothesized that LPEMF may
promote BMSC osteoblast differentiation by regulating Cx43 gene
expression. The results showed that Cx43 expression was
significantly increased in BMSCs treated with 80 Hz LPEMF for 14
days after osteogenic induction. According to the present study,
LPEMF may promote osteogenic differentiation of BMSCs by
upregulating ALP activity, expression of osteogenic marker genes
and calcium salt formation and Cx43 upregulation may be involved in
LPEMF promoting the osteogenic differentiation of BMSCs; however,
the specific mechanism is still not completely clear and needs to
be further verified by more cell and animal experiments. In tissue
engineering, BMSCs have the characteristic of directional
differentiation into osteoblasts and are often used as the seed
cells of bone cells. Using a pulsed electromagnetic field to
stimulate osteogenesis has the advantages of being non-invasive,
having no complications and suitable for popularization and
application. The application of LPEMF to promote the osteogenic
differentiation of BMSCs will provide a new treatment for clinical
bone nonunion and bone defects.
In conclusion, the present study concluded that when
the LPEMF frequency was <80 Hz, the proliferation, osteogenic
differentiation and mineralization of BMSCs were enhanced in a
magnetic field intensity-dependent manner. LPEMF enhanced the
osteogenic differentiation of BMSCs by regulating ALP activity and
osteogenic marker gene expression. The upregulation of Cx43
expression may be involved in the process by which LPEMF promotes
the osteogenic differentiation of BMSCs. This provided an
experimental basis for the treatment of non-unions and osteoporosis
caused by bone defects. These findings suggested that LPEMF may be
an effective strategy for the treatment of osteoblast-related
diseases.
Acknowledgements
Not applicable.
Funding
Funding: The present study was supported by the funding of the
2023 Technology + Medical joint plan project-Key research and
development plan-Ganzhou People's Hospital (grant nos. 2023LNS17521
and 2023LNS17517) and the Science and Technology planning project
of Jiangxi Provincial Administration of Traditional Chinese
Medicine (grant no. 2019A430).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
FL, HG and ZLu conceived the study. FL performed the
experiments. ZLu, HG and ZLi analyzed the data. FL wrote the
manuscript. FL, HG and ZLu confirm the authenticity of all the raw
data. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ganzhou
People's Hospital Animal Care and Use Committee (Ganzhou, China;
approval no. 20211210038).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
El-Rashidy AA, Roether JA, Harhaus L,
Kneser U and Boccaccini AR: Regenerating bone with bioactive glass
scaffolds: A review of in vivo studies in bone defect models. Acta
Biomater. 62:1–28. 2017.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Schindeler A, McDonald MM, Bokko P and
Little DG: Bone remodeling during fracture repair: The cellular
picture. Semin Cell Dev Biol. 19:459–466. 2008.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Jing D, Li F, Jiang M, Cai J, Wu Y, Xie K,
Wu X, Tang C, Liu J, Guo W, et al: Pulsed electromagnetic fields
improve bone microstructure and strength in ovariectomized rats
through a Wnt/Lrp5/β-catenin signaling-associated mechanism. PLoS
One. 8(e79377)2013.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Hannemann PFW, Mommers EHH, Schots JPM,
Brink PRG and Poeze M: The effects of low-intensity pulsed
ultrasound and pulsed electromagnetic fields bone growth
stimulation in acute fractures: A systematic review and
meta-analysis of randomized controlled trials. Arch Orthop Trauma
Surg. 134:1093–1106. 2014.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Chang K, Chang WHS, Tsai MT and Shih C:
Pulsed electromagnetic fields accelerate apoptotic rate in
osteoclasts. Connect Tissue Res. 47:222–228. 2006.PubMed/NCBI View Article : Google Scholar
|
|
6
|
He Z, Selvamurugan N, Warshaw J and
Partridge NC: Pulsed electromagnetic fields inhibit human
osteoclast formation and gene expression via osteoblasts. Bone.
106:194–203. 2018.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Huo SC and Yue B: Approaches to promoting
bone marrow mesenchymal stem cell osteogenesis on orthopedic
implant surface. World J Stem Cells. 12:545–561. 2020.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Liang J, Li W, Zhuang N, Wen S, Huang S,
Lu W, Zhou Y, Liao G, Zhang B and Liu C: Experimental study on bone
defect repair by BMSCs combined with a light-sensitive material:
g-C3N4/rGO. J Biomater Sci Polym Ed.
32:248–265. 2021.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Donahue HJ: Gap junctions and biophysical
regulation of bone cell differentiation. Bone. 26:417–422.
2000.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Batra N, Kar R and Jiang JX: Gap junctions
and hemichannels in signal transmission, function and development
of bone. Biochim Biophys Acta. 1818:1909–1918. 2012.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Unger VM, Kumar NM, Gilula NB and Yeager
M: Three-dimensional structure of a recombinant gap junction
membrane channel. Science. 283:1176–1180. 1999.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Saez JC, Berthoud VM, Branes MC, Martinez
AD and Beyer EC: Plasma membrane channels formed by connexins:
Their regulation and functions. Physiol Rev. 83:1359–1400.
2003.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Liao Y, Day KH, Damon DN and Duling BR:
Endothelial cell-specific knockout of connexin 43 causes
hypotension and bradycardia in mice. Proc Natl Acad Sci USA.
98:9989–9994. 2001.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Plotkin LI: Connexin 43 hemichannels and
intracellular signaling in bone cells. Front Physiol.
5(131)2014.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Lecanda F, Warlow PM, Sheikh S, Furlan F,
Steinberg TH and Civitelli R: Connexin43 deficiency causes delayed
ossification, craniofacial abnormalities, and osteoblast
dysfunction. J Cell Biol. 151:931–944. 2000.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Tonon R and D'Andrea P: The functional
expression of connexin 43 in articular chondrocytes is increased by
interleukin 1beta: Evidence for a Ca2+-dependent mechanism.
Biorheology. 39:153–160. 2002.PubMed/NCBI
|
|
17
|
Ransjö M, Sahli J and Lie A: Expression of
connexin 43 mRNA in microisolated murine osteoclasts and regulation
of bone resorption in vitro by gap junction inhibitors. Biochem
Biophys Res Commun. 303:1179–1185. 2003.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Loiselle AE, Paul EM, Lewis GS and Donahue
HJ: Osteoblast and osteocyte-specific loss of Connexin43 results in
delayed bone formation and healing during murine fracture healing.
J Orthop Res. 31:147–154. 2013.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Meirelles Lda S and Nardi NB: Murine
marrow-derived mesenchymal stem cell: Isolation, in vitro
expansion, and characterization. Br J Haematol. 123:702–711.
2003.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Wennberg C, Hessle L, Lundberg P, Mauro S,
Narisawa S, Lerner UH and Millán JL: Functional characterization of
osteoblasts and osteoclasts from alkaline phosphatase knockout
mice. J Bone Miner Res. 15:1879–1888. 2000.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Phinney DG, Kopen G, Isaacson RL and
Prockop DJ: Plastic adherent stromal cells from the bone marrow of
commonly used strains of inbred mice: Variations in yield, growth,
and differentiation. J Cell Biochem. 72:570–585. 1999.PubMed/NCBI
|
|
23
|
Gurusamy N, Alsayari A, Rajasingh S and
Rajasingh J: Adult stem cells for regenerative therapy. Prog Mol
Biol Transl Sci. 160:1–22. 2018.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Muruganandan S and Sinal CJ: The impact of
bone marrow adipocytes on osteoblast and osteoclast
differentiation. IUBMB Life. 66:147–155. 2014.PubMed/NCBI View
Article : Google Scholar
|
|
25
|
Wang T, Yang L, Jiang J, Liu Y, Fan Z,
Zhong C and He C: Pulsed electromagnetic fields: Promising
treatment for osteoporosis. Osteoporos Int. 30:267–276.
2019.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Weber C, Thai V, Neuheuser K, Groover K
and Christ O: Efficacy of physical therapy for the treatment of
lateral epicondylitis: A meta-analysis. BMC Musculoskelet Disord.
16(223)2015.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Ibiwoye MO, Powell KA, Grabiner MD,
Patterson TE, Sakai Y, Zborowski M, Wolfman A and Midura RJ: Bone
mass is preserved in a critical-sized osteotomy by low energy
pulsed electromagnetic fields as quantitated by in vivo
micro-computed tomography. J Orthop Res. 22:1086–1093.
2004.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Yildiz M, Cicek E, Cerci SS, Cerci C, Oral
B and Koyu A: Influence of electromagnetic fields and protective
effect of CAPE on bone mineral density in rats. Arch Med Res.
37:818–821. 2006.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Lohmann CH, Schwartz Z, Liu Y, Li Z, Simon
BJ, Sylvia VL, Dean DD, Bonewald LF, Donahue HJ and Boyan BD:
Pulsed electromagnetic fields affect phenotype and connexin 43
protein expression in MLO-Y4 osteocyte-like cells and ROS 17/2.8
osteoblast-like cells. J Orthop Res. 21:326–334. 2003.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Nelson FRT, Brighton CT, Ryaby J, Simon
BJ, Nielson JH, Lorich DG, Bolander M and Seelig J: Use of physical
forces in bone healing. J Am Acad Orthop Surg. 11:344–354.
2003.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Coleman JE: Structure and mechanism of
alkaline phosphatase. Annu Rev Biophys Biomol Struct. 21:441–483.
1992.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Otto F, Thornell AP, Crompton T, Denzel A,
Gilmour KC, Rosewell IR, Stamp GW, Beddington RS, Mundlos S, Olsen
BR, et al: Cbfa1, a candidate gene for cleidocranial dysplasia
syndrome, is essential for osteoblast differentiation and bone
development. Cell. 89:765–771. 1997.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Komori T, Yagi H, Nomura S, Yamaguchi A,
Sasaki K, Deguchi K, Shimizu Y, Bronson RT, Gao YH, Inada M, et al:
Targeted disruption of Cbfa1 results in a complete lack of bone
formation owing to maturational arrest of osteoblasts. Cell.
89:755–764. 1997.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Franceschi RT and Xiao G: Regulation of
the osteoblast-specific transcription factor, Runx2: Responsiveness
to multiple signal transduction pathways. J Cell Biochem.
88:446–454. 2003.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Zou L, Zou X, Li H, Mygind T, Zeng Y, Lü N
and Bünger C: Molecular mechanism of osteochondroprogenitor fate
determination during bone formation. Adv Exp Med Biol. 585:431–441.
2006.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Gordon JA, Tye CE, Sampaio AV, Underhill
TM, Hunter GK and Goldberg HA: Bone sialoprotein expression
enhances osteoblast differentiation and matrix mineralization in
vitro. Bone. 41:462–473. 2007.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Nakashima K, Zhou X, Kunkel G, Zhang Z,
Deng JM, Behringer RR and de Crombrugghe B: The novel zinc
finger-containing transcription factor osterix is required for
osteoblast differentiation and bone formation. Cell. 108:17–29.
2002.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Oldknow KJ, Macrae VE and Farquharson C:
Endocrine role of bone: Recent and emerging perspectives beyond
osteocalcin. J Endocrinol. 225:R1–R19. 2015.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Gregory CA, Gunn WG, Peister A and Prockop
DJ: An Alizarin red-based assay of mineralization by adherent cells
in culture: Comparison with cetylpyridinium chloride extraction.
Anal Biochem. 329:77–84. 2004.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Gramsch B, Gabriel HD, Wiemann M, Grümmer
R, Winterhager E, Bingmann D and Schirrmacher K: Enhancement of
connexin 43 expression increases proliferation and differentiation
of an osteoblast-like cell line. Exp Cell Res. 264:397–407.
2001.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Merrifield PA and Laird DW: Connexins in
skeletal muscle development and disease. Semin Cell Dev Biol.
50:67–73. 2016.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Plotkin LI, Laird DW and Amedee J: Role of
connexins and pannexins during ontogeny, regeneration, and
pathologies of bone. BMC Cell Biol. 17 (Suppl
1)(S19)2016.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Rosselló RA, Wang Z, Kizana E, Krebsbach
PH and Kohn DH: Connexin 43 as a signaling platform for increasing
the volume and spatial distribution of regenerated tissue. Proc
Natl Acad Sci USA. 106:13219–13224. 2009.PubMed/NCBI View Article : Google Scholar
|