Introduction
Glioblastoma multiforme (GBM) is one of the most
common and lethal primary brain malignancies in adults, causing a
yearly average of 3.19 new cases per 100,000 individuals (1,2).
Despite the availability of a variety of post-neurosurgical
treatment options, including temozolomide, radiotherapy and certain
targeted drugs, such as bevacizumab, panitumumab and entrectinib,
the prognosis for patients with GBM remains poor. GBM is also the
most lethal primary brain malignancy, causing a 2-year survival
rate of 26-33%, a 4-5% survival rate at 5 years, and a median
survival time of just 15 months (3,4). The
blood-brain barrier (BBB) represents a primary obstacle,
significantly limiting the effectiveness of anticancer drugs in
patients with GBM (5,6). Therefore, it is imperative to
investigate and identify novel potential therapeutic options that
can cross the BBB for managing GBM. Natural small-molecule
compounds offer several advantages, including the wide range of
sources from which they can be obtained, their ability to easily
penetrate the BBB and their capacity to inhibit tumor growth
through multiple mechanisms (7).
In recent years, identifying novel natural
small-molecule compounds for the targeted therapy of GBM is
becoming a field of intense research (8). Peimine is the primary compound
extracted from the Himalayan frillitary lily Bulbus
Fritillariae (BF), which is an established Traditional Chinese
Medicine. BF has been known for >2,000 years for its antitussive
and antiasthma properties, in addition to boasting high therapeutic
efficacy, reported low toxicity and minimal side effects, in
diseases such as osteoarthritis (9). Peimine is a coumarin derivative, with
various pharmacological mechanisms of action, such as
anti-inflammatory, pain inhibitory and anti-asthma actions
(10-12).
It has been previously reported that peimine exhibits anticancer
properties across various malignancies, such as breast, gastric and
prostate cancer (13-15).
However, it remains unclear whether it can exert an inhibitory
effect on GBM.
Therefore, the present study investigated the
potential effects of peimine on GBM both in vitro and in
vivo, whilst also determining its possible underlying mechanism
of action.
Materials and methods
Materials and reagents
Peimine was purchased from Shanghai Yuanye
Bio-Technology Co., Ltd. Unless otherwise stated, all compounds
were dissolved in DMSO. Procell Life Science & Technology Co.,
Ltd. provided the human GBM cell lines U87 (cat. no. CL-0238) and
U251 (cat. no. CL-0237), in addition the normal human brain glial
cell line HEB (cat. no. CL0130). The U87 cell line used was the
U87-MG ATCC (CVCL 0022) cell line (HTB-14) and was authenticated
using STR analysis. U87, U251, HEB and GL261 cells were cultured in
DMEM (Gibco; Thermo Fisher Scientific, Inc.) supplemented with 10%
FBS and 1% penicillin-streptomycin (Gibco; Thermo Fisher
Scientific, Inc.), with high glucose and high glutamine levels. All
cells were cultured at 37˚C in a humidified incubator supplied with
95% air and 5% CO2.
Cell viability assay
In total, 8x103 U87, U251 and HEB cells
were plated in 96-well plates and incubated for 24 h at 37˚C.
Following incubation, AKT activator SC79 (10 µM), AKT inhibitor
MK2206 (10 µM) or peimine (0 or 25 µM) were added to the wells. The
treatment time was 1, 24 and 24 h at 37˚C, respectively.
Subsequently, 10 µl MTT solution was added to each well and
incubated for 4 h at 37˚C. To dissolve the formazan crystals, 100
µl DMSO was added. Next, the plates were vortexed at room
temperature for 10 min. The Bio-Tek ELX800 Multi-Mode Reader
(BioTek Instruments, Inc.) was used to measure absorbance at 490
nm.
Colony formation assay
Trypsin was used to lift U87 cells during the
logarithmic growth phase. The cell suspension was then resuspended
using 1 ml DMEM and counted. In a 6-well plate, 1,000 cells were
then plated in each well for each experimental group. The cells
were cultured for 14 days at 37˚C or until the majority of the
single clones had ≥50 cells. Every 3 days, the medium was changed
and the condition of the cells was routinely checked. After the
14-day growth period, cells were once again washed with PBS and
imaged under a microscope. After fixing each well for 30 min with 1
ml 4% paraformaldehyde at 26˚C, the cells were washed again with
PBS. The cells were then stained for 20 min with 1 ml at 26˚C
crystal violet staining solution. Subsequently, the cells were
washed several times with PBS at 26˚C, dried and imaged using a
digital camera (16).
Wound healing assay
In 6-well plates, U87 cells in the logarithmic
growth phase were trypsinized, before 3x105 were
resuspended and seeded into each well. Cells were cultured until
the confluence reached ~95% at 37˚C, then a 200-µl pipette tip was
used to create a wound in the monolayer of cells. The culture media
was removed from the wells, the monolayer of cells was washed twice
and then 0, 25 or 50 µM of 2 ml peimine solution was added. Images
of wound closure were captured at 0 and 24 h. After 24 h at 37˚C,
the culture media was aspirated, the wells were washed twice with
PBS and then imaged using an X71 fluorescence microscope (Olympus
Corporation).
Cell migration and invasion
assays
Using the Transwell inserts (0.4-µm pore size;
Corning, Inc.), the effects of peimine on the invasion and
migration of U87 cells were evaluated. For migration assays, a
total of 5x103 cells per well in 200 µl serum-free media
was added into the upper chamber of a Transwell insert in a 24-well
plate. A total of 600 µl DMEM containing the 0, 25 or 50 µM peimine
was added to the lower chamber. For invasion assays, Matrigel
(Corning, Inc.) diluted 1:8 (60 µl) was first added to the upper
chamber of the insert and once it had solidified on a cell culture
incubator for 1 h at 37˚C, cells were added to the upper chamber
and media was added to the lower chamber in the same manner as the
migration assay. Cells were incubated for 24 h at 37˚C, before
cells that had passed through the Transwell membrane were fixed for
20 min using 4% paraformaldehyde at 26˚C and then stained for 20
min using 1% crystal violet at 26˚C. Images were taken using an X71
fluorescent microscope (Olympus Corporation) and data was analyzed
with Image J software (version 1.53e; National Institutes of
Health).
Reactive oxygen species (ROS)
analysis
The production of ROS is inevitable for aerobic
organisms, which occurs at a controllable rate in healthy cells.
Under oxidative stress conditions, ROS production increases
sharply, causing changes in membrane lipids, proteins and nucleic
acids. The oxidative damage of these biomolecules is associated
with cancer. Dichloro fluorescein (DCF), the fluorescent oxidized
product of 2',7'-dichlorofluorescin diacetate (DCFDA;
MilliporeSigma), was used to measure intracellular ROS levels.
First, 1.5x105 U87 cells were plated into 6-well plates
and pre-treated for 6 h at 37˚C with either DMEM or 3 mM
N-acetyl-L-cysteine (MilliporeSigma). Peimine (0, 25 or 50 µM) was
then added to the media and cells were treated for a further 8 h at
37˚C, after which the media was replaced with fresh complete media
containing 2 µg DCFDA for 6 min at 37˚C in the dark. Using an X71
fluorescent microscope, the proportion of apoptotic cells was
determined.
Mitochondrial transmembrane potential
assay
Membrane permeability JC-1 staining is widely used
in apoptosis studies to monitor mitochondrial health. JC-1 staining
reagent showed potential-dependent accumulation in mitochondria. At
low concentration, it existed as monomer and produced green
fluorescence at ~529 nm. Carbonyl cyanide 3-chlorophenylhydrazone
(CCCP; Biosharp Life Sciences) was selected as the positive
control. U87 cells (2.5x104) were treated with 0, 25 or
50 µM peimine for 24 h at 37˚C. Next, cells were collected,
centrifuged for 5 min at 26˚C (1,400 x g) and resuspended in PBS.
CCCP (10 mM) was added to the cell culture medium at a ratio of
1:1,000, diluted to 10 µM, and the cells were treated with 200 µl
for 20 min at 37˚C. The cells were then centrifuged again,
resuspended in binding buffer and incubated for 30 min at 37˚C in
the dark with 200 µl the JC-1 probe (Biosharp Life Sciences). Cells
were then resuspended in 500 µl pre-cooled PBS and rinsed twice. An
X71 fluorescent microscope was used to calculate the proportion of
apoptotic cells.
Annexin V-FITC/PI staining assay
U87 cells (2.5x104) in 24-well plates
(Costar; Corning, Inc.) were treated with 0, 25 or 50 µM of peimine
for 24 h at 37˚C. Cells were then harvested, centrifuged for 5 min
at 26˚C (1,400 x g) and resuspended in PBS. After re-suspending in
binding buffer, 1.5x105 U87 cells were stained using the
Annexin V-FITC/PI kit (cat. no. BL1884A; Biosharp Life Sciences)
for 20 min at 37˚C. The percentage of apoptotic cells was then
determined using an X71 fluorescence microscope. Staining quality
[apoptotic cells were double positive (Annexin
V+/PI+) by FITC and PI binding staining] was
analyzed by Image J software (version 1.53e; National Institutes of
Health).
GBM subcutaneous xenograft model
Hubei University of Science and Technology Animal
Ethics Committee approved the animal experiments (approval no.
2022-11-027) and all procedures adhered to national and
international standards for the care and use of animals in
research. GL261 GBM cells (cat. no. CTCC-400-0404; Zhejiang Meisen
Cell Technology Co., Ltd.) were used to establish the mouse model.
In total, 5-week-old male C57BL/6 mice (n=3/group; total, n=9)
weighing 20-24 g were selected for the study. GL261 cells were
subcutaneously injected into the right back of each mouse with
5x106 cells in 100 µl sterile PBS. After the tumor
reached a mean volume of ~100 mm³, mice were divided into the
following three groups at random: Control; low-dose (20 mg/kg); and
high-dose (40 mg/kg) (17). Body
weights were recorded daily and tumor volumes were measured every 2
days using the following formula: Volume=a x b2 x 0.52,
where a is the length and b is the width in mm. Mice in the
low-dose and high-dose groups were treated intragastrically every 2
days with 20 or 40 mg/kg peimine, respectively. Control mice
received saline instead of peimine. The mice were sacrificed under
anesthesia (intraperitoneal injection of sodium pentobarbital 100
mg/kg) after 14 days. Death was confirmed by a lack of response to
a toe pinch, lack of breathing and lack of heartbeat. Samples from
the tumors and livers in the animals were then collected for
additional examination.
H&E staining
Liver tissues from mice were fixed using 4%
paraformaldehyde for 24 h at 26˚C, dehydrated and embedded in
paraffin. The samples were fixed in 10% formalin for 12 h at 4˚C,
and then embedded in paraffin for subsequent sectioning. The 4-µm
specimens were first placed in distilled water and then in an
aqueous solution of hematoxylin for staining for ~10 min at room
temperature. After which, the slices were placed into ammonia and
acid water for several seconds, and then rinsed in running water
for 1 h. The sections were placed in distilled water for several
seconds, after which they were dehydrated in alcohol at
concentrations of 90 and 70% for 10 min each. Subsequently, the
sections were stained with eosin staining solution for 2-3 min at
room temperature. After staining, the samples were dehydrated with
100% alcohol and placed in xylene. The sections were sealed and
placed in an incubator for drying. H&E staining (cat. no.
BL700A; Biosharp Life Sciences) was performed on the liver tissues
for toxicological analysis using light microscropy.
Western blot analysis
Treated U87 cells were first collected, washed three
times with ice-cold PBS and then lysed using RIPA lysis buffer
(cat. no. P0013B; Beyotime Institute of Biotechnology) supplemented
with protease and phosphatase inhibitors (Beyotime Institute of
Biotechnology) for 30 min. The resultant lysate was centrifuged and
the supernatant was collected (4˚C, 15 min and 14,000 x g). A BCA
assay kit (Beyotime Institute of Biotechnology) was used to measure
the protein content according to the manufacturer's protocol.
Loading buffer was added to the protein samples and then boiled at
100˚C for 10 min. Next, the protein samples (40 µg/lane) were
loaded on 8-10% SDS gels, resolved using SDS-PAGE, transferred onto
PVDF membranes and the membranes were blocked (5% skimmed milk for
1 h at 26˚C). Following blocking, the membranes were incubated with
the indicated primary antibodies overnight at 4˚C. The following
day, the membranes were washed, and incubated with the
HRP-conjugated secondary antibodies at 26˚C. Signals were
visualized using ECL plus reagent (Beyotime Institute of
Biotechnology). GAPDH was used as the loading control. The
following antibodies were used: p53 (1:1,000; cat. no. R380701),
Bax (1:1,000; cat. no. R22708), Bcl-2 (1:1,000; cat. no. R23309),
Caspase 3 (1:1,000; cat. no. R23315), Cleaved-Caspase 3 (1:1,000;
cat. no. 341034) (all Chengdu Zen-Bioscience Co., Ltd.),
phosphorylated (p)-PI3K (1:1,000; cat. no. AP1463), PI3K (1:1,000;
cat. no. A19742), p-AKT (1:1,000; cat. no. AP1259), AKT (1:1,000;
cat. no. A17909), GAPDH (1:1,000; cat. no. 390035) and HRP Goat
Anti-Mouse IgG (1:1,000, cat. no. AS003) (all ABclonal Biotech Co.,
Ltd.). The blot densities were quantified using ImageJ software
(version 1.53e; National Institutes of Health).
Statistical analysis
Experimental data were analyzed by Image J (version
1.53e) and SPSS 27.0 software (IBM Corp.). GraphPad prism 8.0
software (Dotmatics) was used for visualization. Data are presented
as the mean ± SD. Data were analyzed using a one-way ANOVA followed
by Tukey's post hoc test using SPSS 27.0 software (IBM Corp.).
P<0.05 was considered to indicate a statistically significant
difference.
Results
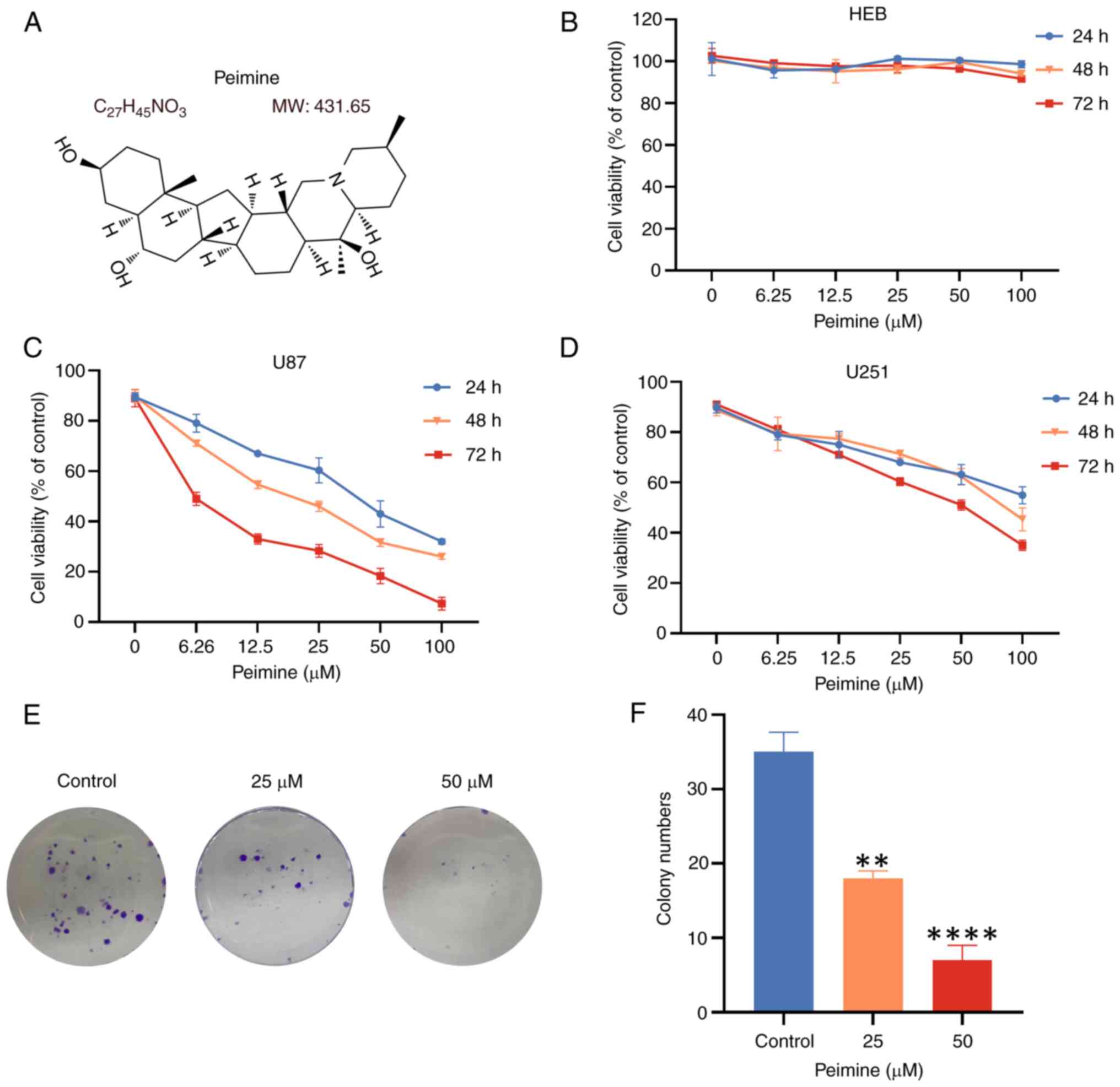
Peimine inhibits GBM cell
proliferation
MTT assays were performed to assess the viability of
HEB, U87 and U251 cells following treatment with various
concentrations of peimine (0, 6.25, 12.5, 25, 50 or 100 µM;
Fig. 1B-D). The results showed a
dose-dependent reduction of GBM cell viability caused by peimine.
Compared with the control group, with the increase of drug
concentration, GBM cell viability was inhibited to an marked
degree, but there were no obvious effects of peimine on HEB cells
(Fig. 1B). In U87 and U251 cells,
the IC50 values were found to be 21.3 and 92.8 µM,
respectively after 48 h of treatment. By contrast, the
IC50 value was 39.9 µM in U87 cells treated for 24 h. As
the IC50 of U87 cells is much lower than that of U251
cells, for subsequent experiments, 25 and 50 µM peimine was used
for U87 cells based on the IC50 value, with a treatment
duration of 24 h.
A colony formation assay was next used to assess the
proliferation of U87 cells. Treatment of U87 cells with varying
concentrations of peimine resulted in a dose-dependent decrease in
colony formation. Notably, significant differences in colony
formation ability were observed in cells treated with 25 and 50 µM
peimine compared with that in cells treated with 0 µM peimine,
demonstrating that peimine had an inhibitory effect on the growth
of GBM cell colonies (Fig. 1E and
F). These findings suggest the
inhibitory effects of peimine on GBM cell proliferation, with
minimal toxicity and side effects observed in HEB cells. This
suggests that peimine exhibits selectivity for cancerous cells
whilst maintaining a safe profile on normal cells, highlighting it
as a promising candidate for further drug development.
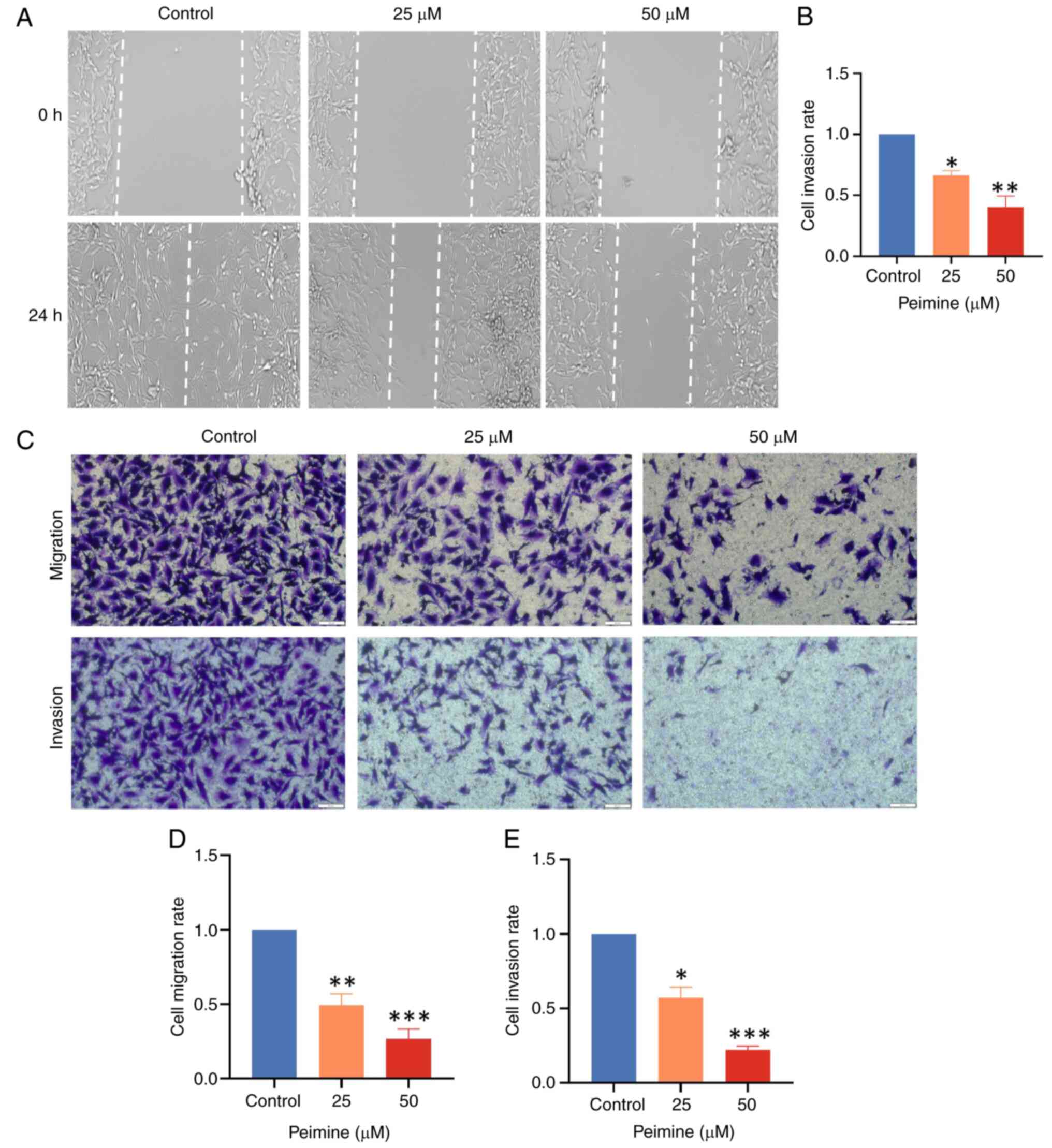
Peimine inhibits GBM cell migration
and invasion
Results from wound healing assays showed significant
inhibition of U87 cell migration following 24 h of treatment with
25 and 50 µM peimine (Fig. 2A and
B), where peimine dose-dependently
reduced migration compared with that in the control group.
Similarly, Transwell assay results showed
significantly reduced U87 cell migration and invasion following the
24 h peimine treatment in a dose-dependent manner (Fig. 2C and D). These results were consistent with the
results of the wound healing assays. Together, these results
suggest the inhibitory effect of peimine on the invasion and
migration of GBM cells.
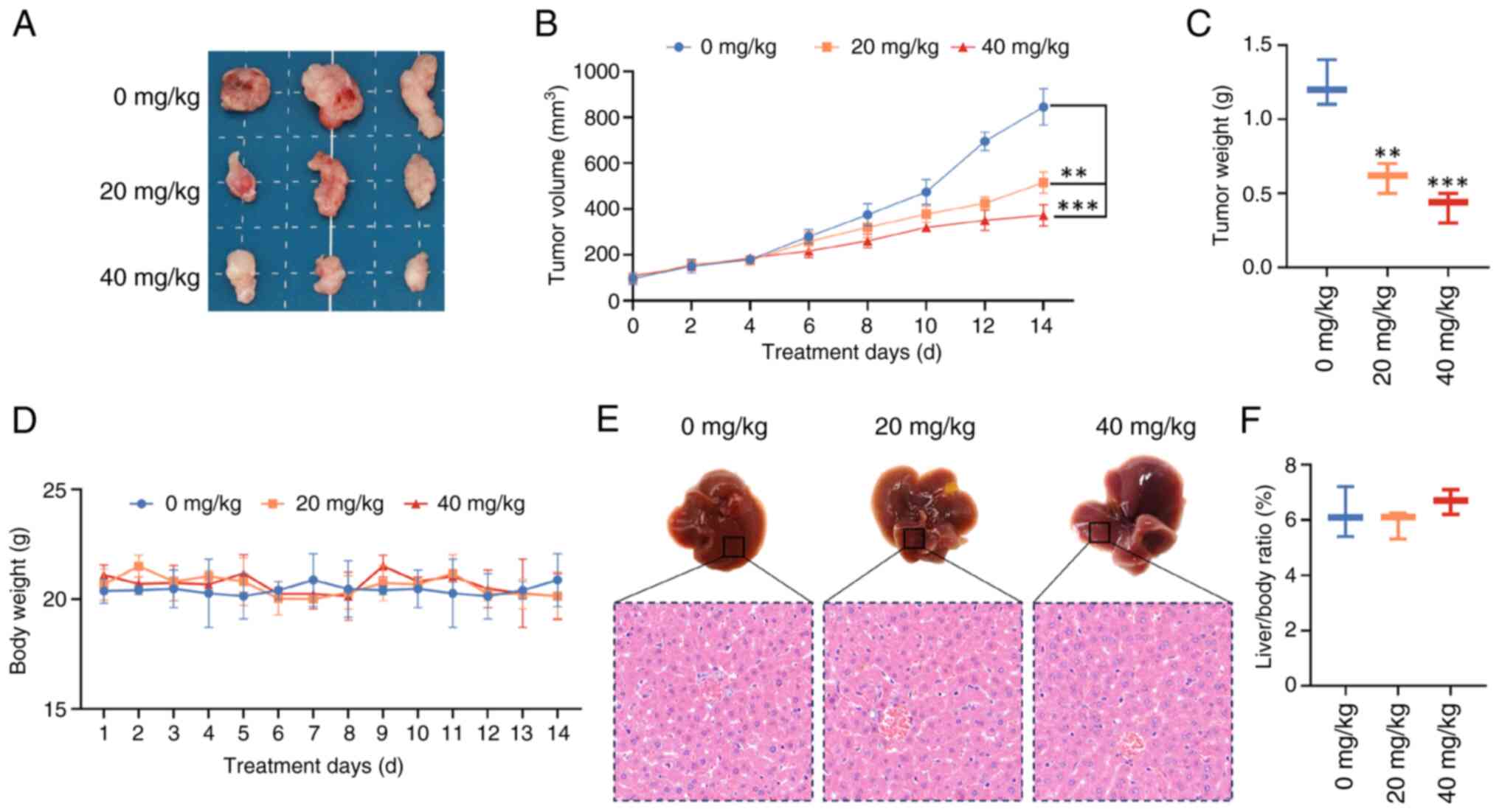
Peimine exhibits anti-GBM activity in
vivo
The possible inhibitory effects of Peimine on GBM
in vivo was assessed using a mouse xenograft model. Tumor
size, volume and weight were found to be markedly reduced in both
the low (20 mg/kg) and high (40 mg/kg) dose groups compared with
those in the control group (Fig.
3A-C). Peimine did not considerably alter the body weight of
the mice, where at therapeutic doses, no negative side effects
(such as marked weight loss) were noted (Fig. 3D-F). Additionally, as shown in
Fig. 3E and F, there were no marked variations in the
liver/body ratio, liver morphology or the sectional tissue
morphology between the peimine-treated group and the control group.
These results suggest that peimine can effectively reduce GBM
growth in vivo whilst maintaining favorable safety profiles,
highlighting its potential as a therapeutic option for the
treatment of GBM.
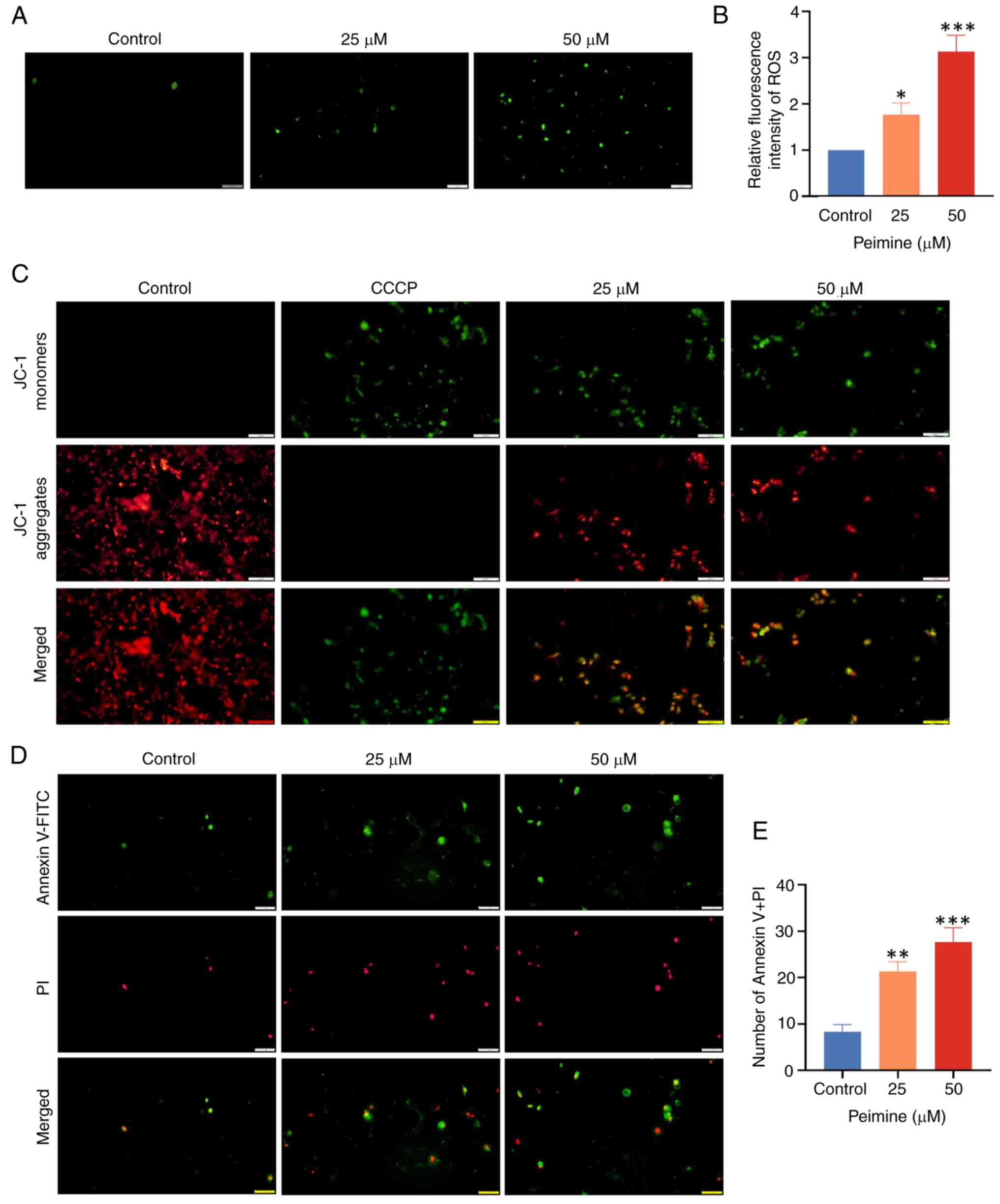
Peimine induces GBM cell
apoptosis
ROS serves a pivotal role in apoptosis, the
excessive accumulation which in cells can induce apoptosis. As
shown in Fig. 4A and B, based on DCFH-DA staining.
Quantification of fluorescent images revealed a significant
increase in cellular ROS levels induced by peimine, indicative of
increased oxidative damage. The mitochondrial membrane potential is
typically altered during early apoptosis. The JC-1 probe was
therefore next used to measure this change (Fig. 4C). CCCP, an apoptosis inducer
serving as a mitochondrial oxidative phosphorylation uncoupling
agent, was used as a positive control to denote a reduction of the
mitochondrial membrane potential. As the peimine concentration
increased, the quantity of JC-1 monomers (green) in GBM cells was
increased, whilst that of JC-1 aggregates (red) was decreased,
suggesting the induction of early apoptosis. Furthermore, U87 cell
apoptosis induction was assessed further using Annexin V-FITC/PI
staining (Fig. 4D and E). These results collectively suggest
that peimine can induce apoptosis in GBM cells.
Peimine induces PI3K/AKT-dependent
apoptosis in GBM cells
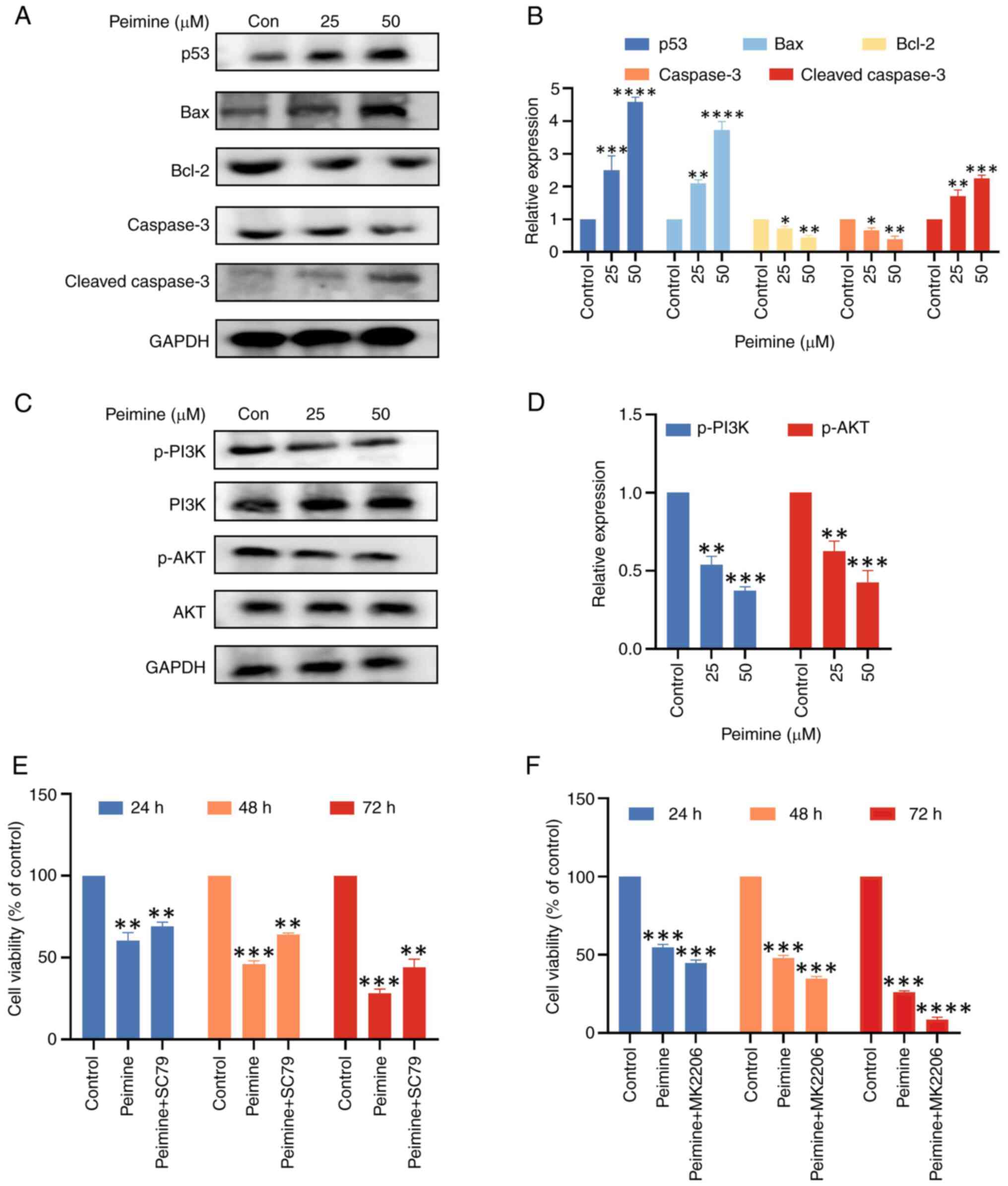
Western blot analysis was used to assess the effect
of peimine on the expression of proteins associated with apoptosis
in U87 cells (Fig. 5A and B). Following 24 h treatment with 25 and
50 µM peimine, Bcl-2 expression was found to be downregulated in a
dose-dependent manner, whereas p53 and Bax expression were found to
be upregulated. Furthermore, with 25 and 50 µM peimine, there was
an increase in Cleaved-Caspase 3 expression.
Furthermore, to elucidate the molecular mechanism
underlying peimine-mediated repression of proliferation and
metastasis, the PI3K/AKT signaling cascade was next investigated,
also using western blot analysis. The results showed that after
treating U87 cells with peimine for 24 h, the levels of PI3K and
AKT phosphorylation were found to be significantly decreased, in a
dose-dependent manner (Fig. 5C and
D).
To determine whether peimine can mediate apoptosis
through AKT signaling, AKT signaling was activated or inhibited in
cells. Rescue experiments were first conducted using 10 µM SC79, an
AKT agonist. The results showed that the proliferative effects
induced by peimine (25 µM) over 24, 48 and 72 h were nullified
following pretreatment with SC79 for 1 h (Fig. 5E); however, this nullification was
not significant. To further explore the mechanism of peimine on AKT
signaling in vitro, the relationship between peimine
activity and AKT pathway was explored using 10 µM MK2206, an AKT
inhibitor (Fig. 5F). The
combination of peimine and AKT inhibitor MK2206 had a stronger
effect on the proliferation of U87 cells than peimine alone.
Together, these results highlight the role of the PI3K/AKT
signaling cascade in mediating the anti-tumor activities in GBM
cells of peimine.
Discussion
GBM is an invasive primary malignant tumor that is
difficult to treat, prone to relapse and exhibits a high mortality
rate (18,19). Natural small-molecule compounds
derived from plants used in Chinese herbal medicines are
increasingly becoming an important source for the treatment of
malignancies (20). The
constituent components of Chinese herbal medicines, such as
quercetin, lycorine and isobavachalcone, are also increasingly
being studied for the identification of novel compounds to manage
GBM (21-23).
The limited toxicities and side effects compared
with conventional treatments, such as radiotherapy and
chemotherapy, with additional capabilities of limiting tumor
proliferation and apoptosis, have fueled further research in this
field (17). Despite its
established efficacy in inducing cell death and inhibiting
angiogenesis, their precise mechanisms of action remain to be fully
elucidated. Researchers have found that eugenol enhances the
chemotherapeutic potential of gemcitabine and induces
anticarcinogenic and anti-inflammatory activity in human cervical
cancer cells (24). The
synergistic use of chemotherapeutic drugs with natural components
can enhance the pharmacological effects of chemotherapy, to improve
efficacy and concurrently reduce the side effects (25-28).
The process of apoptosis, a type of programmed cell
death (29,30), causes major alterations in the
appearance and activity of cells prior to their death (31,32).
Apoptotic protein regulation and the activation of complex
signaling pathways control this coordinated process (33,34).
Peimine was shown in the present study to induce apoptosis via
activation of the mitochondrial pathway, whilst also reducing the
migration, invasion and proliferation of GBM cells. Peimine
specifically decreased the mitochondrial membrane potential,
promoting Bax, a key step for the irreversible initiation of the
caspase cascade. The PI3K/AKT signaling pathway is crucial to the
onset and progression of GBM (35,36).
Activation of this pathway has been previously associated with the
development of GBM by promoting tumor cell proliferation and
metastasis, preventing apoptosis and facilitating cell cycle
progression (37). In addition,
the PI3K/AKT signaling pathway serves as a target of several
antitumor drugs, such as peimine, where it is intricately involved
in both treatment and resistance mechanisms (38,39).
MK2206 is a selective allosteric inhibitor of the AKT signaling
pathway. MK2206 specifically targets and inhibits AKT activity by
binding to its allosteric site, leading to a decrease in its
phosphorylation and subsequent downstream signaling. This
inhibition can potentially induce apoptosis in cancer cells and
enhance the efficacy of other therapeutic agents, making MK2206 a
candidate for targeted cancer therapy, particularly in tumors where
the PI3K/AKT pathway is aberrantly activated. In this study, the
combination of peimine and AKT inhibitor MK2206 further inhibited
the proliferation of U87 cells. Clinical trials and research
studies are ongoing to evaluate its effectiveness and safety in
breast, gastric and prostate cancer among others, and in
combination with other treatments (40-43).
The effects of peimine on GBM were investigated in
the present study using multiple experimental approaches, including
MTT assays, Transwell assays, measurement of ROS levels and western
blot analysis. These methods collectively provided insights into
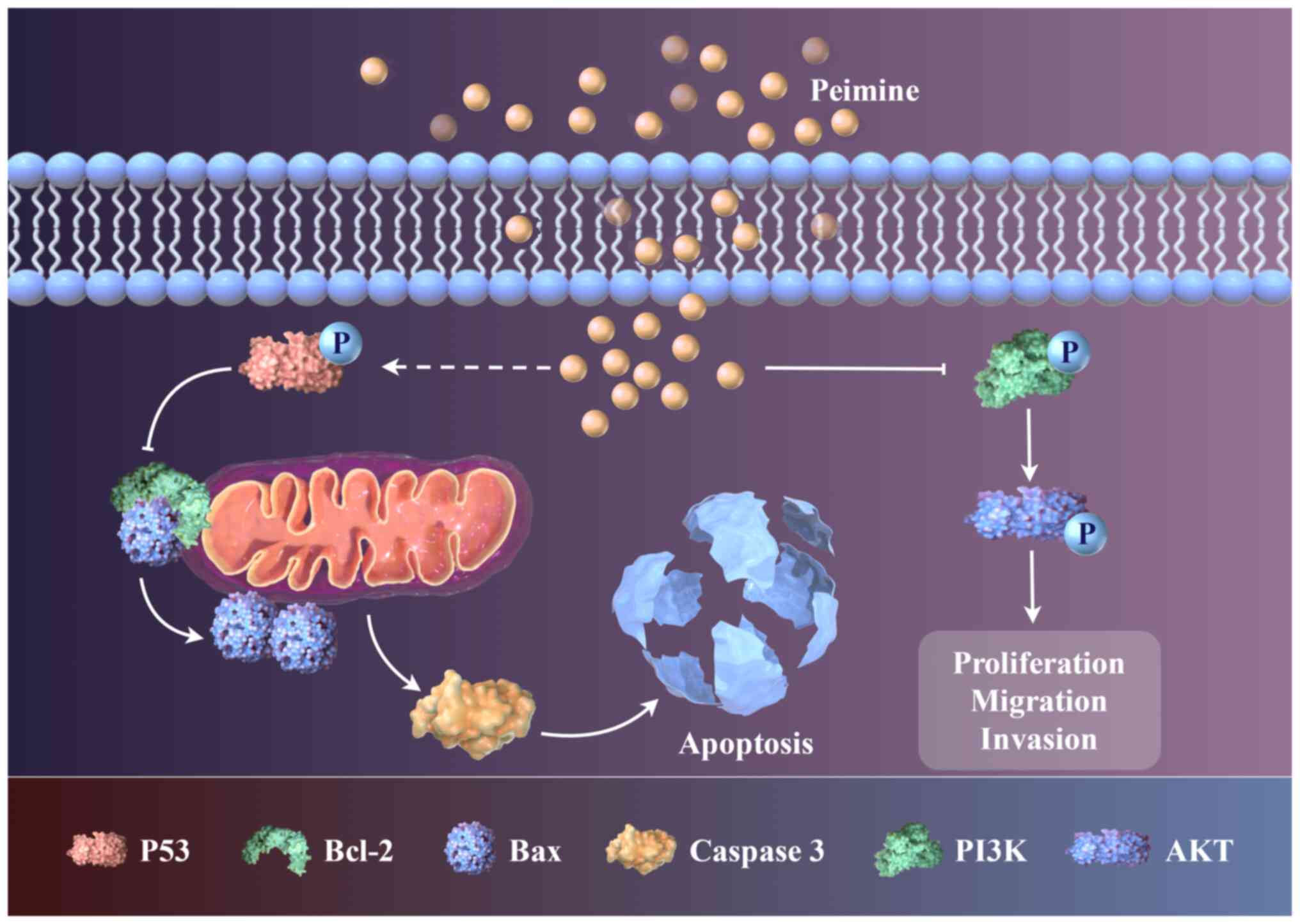
the inhibitory mechanisms of peimine against GBM. Notably, the
results suggested that peimine regulated the PI3K/AKT signaling
pathway by inducin apoptosis to exert its effects (Fig. 6). Peimine was shown to induce
apoptosis in GBM cells, prompting further exploration of its
mechanism and potential therapeutic significance in GBM treatment.
Although peimine shows promise as an anticancer agent, additional
animal experiments,for instance pharmacokinetics and clinical
trials, are warranted to fully elucidate and confirm its
therapeutic potential. Future research should delve deeper into
other signaling pathways involved, such as angiogenesis pathways,
and the potential crosstalk between them, and conduct in
vivo experiments to determine the value of peimine as a
targeted small-molecule drug for GBM treatment to determine its
precise mechanism of action.
In conclusion, the results of the present study
showed that peimine, a novel small-molecule, was a potential
therapeutic option for the management of GBM. Peimine inhibited GBM
cell proliferation, migration and invasion in vitro in a
dose-dependent manner and prevented tumor growth without
significant drug toxicity in vivo. It is important to note
that the present study focused on peimine treatment in mouse models
of GBM, but did not assess its expression in human tissues, which
is a significant limitation. Overall, the results of the present
study showed peimine may hold promise as a novel small-molecule
therapeutic agent for the management of GBM.
Acknowledgements
Not applicable.
Funding
Funding: The present study was funded by The Science and
Technology Research Project of the Hubei Education Department
(grant no. B2019161) and the National Natural Science Foundation of
China (grant no. 31900853).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
JL and JY conceived and designed the study,
performed the experiments, gathered and evaluated the data, and
wrote and edited the manuscript. SC, FL ZihW, ZiyW, ZiqW and CS
performed the experiments and collected and analyzed the data. LL
assisted with study conception and design, and contributed to the
revision of the manuscript. All authors have read and approved the
final manuscript. All authors confirm the authenticity of the raw
data.
Ethics approval and consent to
participate
Hubei University of Science and Technology Animal
Ethics Committee approved the animal experiments (approval no.
2022-11-027) and all procedures adhered to national and
international standards for the care and use of animals in
research.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Tamimi AF and Juweid M: Epidemiology and
outcome of glioblastoma. In: Glioblastoma. De Vleeschouwer S (ed).
Codon Publications, Brisbane, AU, 2017.
|
|
2
|
Batash R, Asna N, Schaffer P, Francis N
and Schaffer M: Glioblastoma multiforme, diagnosis and treatment;
recent literature review. Curr Med Chem. 24:3002–3009.
2017.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Omuro A and DeAngelis LM: Glioblastoma and
other malignant gliomas: A clinical review. JAMA. 310:1842–1850.
2013.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Siegel RL, Miller KD, Wagle NS and Jemal
A: Cancer statistics, 2023. CA Cancer J Clin. 73:17–48.
2023.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Dréan A, Goldwirt L, Verreault M, Canney
M, Schmitt C, Guehennec J, Delattre JY, Carpentier A and Idbaih A:
Blood-brain barrier, cytotoxic chemotherapies and glioblastoma.
Expert Rev Neurother. 16:1285–1300. 2016.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Schaff LR and Mellinghoff IK: Glioblastoma
and other primary brain malignancies in adults: A review. JAMA.
329:574–587. 2023.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Fang XH, Zou MY, Chen FQ, Ni H, Nie SP and
Yin JY: An overview on interactions between natural product-derived
β-glucan and small-molecule compounds. Carbohydr Polym.
261(117850)2021.PubMed/NCBI View Article : Google Scholar
|
|
8
|
McKinnon C, Nandhabalan M, Murray SA and
Plaha P: Glioblastoma: Clinical presentation, diagnosis, and
management. BMJ. 374(n1560)2021.PubMed/NCBI View Article : Google Scholar
|
|
9
|
An YL, Wei WL and Guo DA: Application of
analytical technologies in the discrimination and authentication of
herbs from Fritillaria: A review. Crit Rev Anal Chem. 54:1775–1796.
2024.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Yi PF, Wu YC, Dong HB, Guo Y, Wei Q, Zhang
C, Song Z, Qin QQ, Lv S, Wu SC and Fu BD: Peimine impairs
pro-inflammatory cytokine secretion through the inhibition of the
activation of NF-κB and MAPK in LPS-induced RAW264.7 macrophages.
Immunopharmacol Immunotoxicol. 35:567–572. 2013.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Xu J, Zhao W, Pan L, Zhang A, Chen Q, Xu
K, Lu H and Chen Y: Peimine, a main active ingredient of
Fritillaria, exhibits anti-inflammatory and pain suppression
properties at the cellular level. Fitoterapia. 111:1–6.
2016.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Zhang L, Cui M and Chen S: Identification
of the molecular mechanisms of peimine in the treatment of cough
using computational target fishing. Molecules.
25(1105)2020.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Zhang T, Liu GY, Cao JL, Li YN, Xue H, Wu
HT and Jin CH: Peimine-induced apoptosis and inhibition of
migration by regulating reactive oxygen species-mediated
MAPK/STAT3/NF-κB and Wnt/β-catenin signaling pathways in gastric
cancer MKN-45 cells. Drug Dev Res. 83:1683–1696. 2022.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Sun J, Li J, Kong X and Guo Q: Peimine
inhibits MCF-7 breast cancer cell growth by modulating inflammasome
activation: Critical roles of MAPK and NF-κB signaling. Anticancer
Agents Med Chem. 23:317–327. 2023.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Tan H, Zhang G, Yang X, Jing T, Shen D and
Wang X: Peimine inhibits the growth and motility of prostate cancer
cells and induces apoptosis by disruption of intracellular calcium
homeostasis through Ca2+/CaMKII/JNK pathway. J Cell
Biochem. 121:81–92. 2020.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Chen J, Yu Y, Li H, Hu Q, Chen X, He Y,
Xue C, Ren F, Ren Z, Li J, et al: Long non-coding RNA PVT1 promotes
tumor progression by regulating the miR-143/HK2 axis in gallbladder
cancer. Mol Cancer. 18(33)2019.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Chen K, Lv ZT, Zhou CH, Liang S, Huang W,
Wang ZG, Zhu WT, Wang YT, Jing XZ, Lin H, et al: Peimine suppresses
interleukin-1β-induced inflammation via MAPK downregulation in
chondrocytes. Int J Mol Med. 43:2241–2251. 2019.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Tanaka S, Louis DN, Curry WT, Batchelor TT
and Dietrich J: Diagnostic and therapeutic avenues for
glioblastoma: No longer a dead end? Nat Rev Clin Oncol. 10:14–26.
2013.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Le Rhun E, Preusser M, Roth P, Reardon DA,
van den Bent M, Wen P, Reifenberger G and Weller M: Molecular
targeted therapy of glioblastoma. Cancer Treat Rev.
80(101896)2019.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Zhao W, Zheng XD, Tang PY, Li HM, Liu X,
Zhong JJ and Tang YJ: Advances of antitumor drug discovery in
traditional Chinese medicine and natural active products by using
multi-active components combination. Med Res Rev. 43:1778–1808.
2023.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Sevastre AS, Costachi A, Tataranu LG,
Brandusa C, Artene SA, Stovicek O, Alexandru O, Danoiu S, Sfredel V
and Dricu A: Glioblastoma pharmacotherapy: A multifaceted
perspective of conventional and emerging treatments (review). Exp
Ther Med. 22(1408)2021.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Wang W, Yuan X, Mu J, Zou Y, Xu L, Chen J,
Zhu X, Li B, Zeng Z, Wu X, et al: Quercetin induces
MGMT+ glioblastoma cells apoptosis via dual inhibition
of Wnt3a/β-catenin and Akt/NF-κB signaling pathways. Phytomedicine.
118(154933)2023.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Shen J, Zhang T, Cheng Z, Zhu N, Wang H,
Lin L, Wang Z, Yi H and Hu M: Lycorine inhibits glioblastoma
multiforme growth through EGFR suppression. J Exp Clin Cancer Res.
37(157)2018.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Luo H, Vong CT, Chen H, Gao Y, Lyu P, Qiu
L, Zhao M, Liu Q, Cheng Z, Zou J, et al: Naturally occurring
anti-cancer compounds: Shining from Chinese herbal medicine. Chin
Med. 14(48)2019.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Zhao L, Zhang H, Li N, Chen J, Xu H, Wang
Y and Liang Q: Network pharmacology, a promising approach to reveal
the pharmacology mechanism of Chinese medicine formula. J
Ethnopharmacol. 309(116306)2023.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Hussain A, Brahmbhatt K, Priyani A, Ahmed
M, Rizvi TA and Sharma C: Eugenol enhances the chemotherapeutic
potential of gemcitabine and induces anticarcinogenic and
anti-inflammatory activity in human cervical cancer cells. Cancer
Biother Radiopharm. 26:519–527. 2011.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Liu Y, Yang S, Wang K, Lu J, Bao X, Wang
R, Qiu Y, Wang T and Yu H: Cellular senescence and cancer: Focusing
on traditional Chinese medicine and natural products. Cell Prolif.
53(e12894)2020.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Wang X, Li J, Chen R, Li T and Chen M:
Active ingredients from Chinese medicine for combination cancer
therapy. Int J Biol Sci. 19:3499–3525. 2023.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Zhang P, Jiang Y, Ye X, Zhang C and Tang
Y: PDK1 inhibition reduces autophagy and cell senescence through
the PI3K/AKT signalling pathway in a cigarette smoke mouse
emphysema model. Exp Ther Med. 25(223)2023.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Wong RS: Apoptosis in cancer: From
pathogenesis to treatment. J Exp Clin Cancer Res.
30(87)2011.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Wu Y, Chang J, Ge J, Xu K, Zhou Q, Zhang
X, Zhu N and Hu M: Isobavachalcone's alleviation of pyroptosis
contributes to enhanced apoptosis in glioblastoma: Possible
involvement of NLRP3. Mol Neurobiol. 59:6934–6955. 2022.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Bertheloot D, Latz E and Franklin BS:
Necroptosis, pyroptosis and apoptosis: An intricate game of cell
death. Cell Mol Immunol. 18:1106–1121. 2021.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Ketelut-Carneiro N and Fitzgerald KA:
Apoptosis, pyroptosis, and necroptosis-oh my! The many ways a cell
can die. J Mol Biol. 434(167378)2022.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Attwaters M: Persisting through apoptosis.
Nat Rev Mol Cell Biol. 23(697)2022.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Newton K, Strasser A, Kayagaki N and Dixit
VM: Cell death. Cell. 187:235–256. 2024.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Zhang L, Liu Z, Dong Y and Kong L: E2F2
drives glioma progression via PI3K/AKT in a PFKFB4-dependent
manner. Life Sci. 276(119412)2021.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Chautard E, Ouédraogo ZG, Biau J and
Verrelle P: Role of Akt in human malignant glioma: From oncogenesis
to tumor aggressiveness. J Neurooncol. 117:205–215. 2014.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Zhang X, Li Z, Wei C, Luo L, Li S, Zhou J,
Liang H, Li Y and Han L: PLK4 initiates crosstalk between cell
cycle, cell proliferation and macrophages infiltration in gliomas.
Front Oncol. 12(1055371)2022.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Liu F, Chen G, Zhou LN, Wang Y, Zhang ZQ,
Qin X and Cao C: YME1L overexpression exerts pro-tumorigenic
activity in glioma by promoting Gαi1 expression and Akt activation.
Protein Cell. 14:223–229. 2023.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Ren J, Zheng S, Zhang L, Liu J, Cao H, Wu
S, Xu Y and Sun J: MAPK4 predicts poor prognosis and facilitates
the proliferation and migration of glioma through the AKT/mTOR
pathway. Cancer Med. 12:11624–11640. 2023.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Tang YQ, Li ZW, Feng YF, Yang HQ, Hou CL,
Geng C, Yang PR, Zhao HM and Wang J: MK2206 attenuates
atherosclerosis by inhibiting lipid accumulation, cell migration,
proliferation, and inflammation. Acta Pharmacol Sin. 43:897–907.
2022.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Xiang RF, Wang Y, Zhang N, Xu WB, Cao Y,
Tong J, Li JM, Wu YL and Yan H: MK2206 enhances the cytocidal
effects of bufalin in multiple myeloma by inhibiting the AKT/mTOR
pathway. Cell Death Dis. 8(e2776)2017.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Cui X, Zhao J, Li G, Yang C, Yang S, Zhan
Q, Zhou J, Wang Y, Xiao M, Hong B, et al: Blockage of EGFR/AKT and
mevalonate pathways synergize the antitumor effect of temozolomide
by reprogramming energy metabolism in glioblastoma. Cancer Commun
(Lond). 43:1326–1353. 2023.PubMed/NCBI View Article : Google Scholar
|